Abstract
Purpose
To determine the frequency and severity of general and ear nose throat (ENT)- related symptoms, especially smell and/or loss of sense of taste (STL) in COVID-19 disease, as well as to investigate the recovery process of STL.
Materials and methods
Patients with a positive COVID-19 diagnosis were given a questionnaire consisting of general questions (age, sex, date of symptoms, smoking history, concomitant diseases), questions about the most obvious symptom at presentation (one option only), the severity and frequency of symptoms (general and ENT), and STL (recovery time and degree of recovery).
Results
The study population consisted of 172 patients, 18–65 years old (mean age, 37.8 ± 12.5 years; 51.2% female; 76.2% nonsmokers). Cough (n = 30, 17.4%) and loss of sense of smell (n = 18, 10.4%) were the most obvious general and ENT symptoms, respectively. Eighty-eight patients (51.2%) reported loss of sense of smell, and 81 patients (47.1%) reported loss of sense of taste. The mean recovery times for loss of sense of smell and loss of sense of taste were 8.02 ± 6.41 and 8.20 ± 7.07 days, respectively. The loss of sense of smell and loss of sense of taste were the unique symptoms in four (4.54%) and one (1.23%) patients, respectively.
Conclusion
STL is a common symptom in COVID-19 and may be the first and/or only symptom of this disease. In patients presenting with STL complaints, surveillance for possible COVID-19 disease and screening tests will facilitate the struggle against the disease.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Loss of sense of smell, Loss of sense of taste
1. Introduction
The virus responsible for COVID-19 disease, SARS-CoV-2, is a novel member of the coronavirus family that appeared in the Hubai region of China in late 2019 and rapidly became a pandemic affecting the world. Although this virus can cause severe respiratory failure and even death in infected patients, it has spread rapidly and continues to spread among people because it can cause mild or no symptoms in the majority of cases [1,2]. The most effective method for preventing the spread of the virus is the early detection and isolation of infected individuals.
The most common symptoms of COVID-19 are fever, cough, myalgia, fatigue, and difficulty breathing. In addition, ear, nose, and throat (ENT) symptoms, including loss of sense of smell and/or loss of sense of taste (STL) have been reported as symptoms caused by the virus [3,4]. Rhinoviruses, Epstein–Barr virus, parainfluenza virus, and some coronaviruses have been shown to cause upper respiratory infections, nasal congestion, and rhinorrhea, and may result in STL. Although the pathophysiology of STL developing after infection with these viruses is not yet clear, it has been suggested to be due to olfactory epithelial damage by the virus or its spread to the central nervous system [5,6]. There are increasing reports of SARS-CoV-2 causing STL both anecdotally and in the peer-reviewed medical literature. However, it has been reported that infection with SARS-CoV-2 may cause STL without nasal discharge and/or nasal congestion or any other symptoms in some patients, unlike other viruses that infect the upper respiratory tract [7,8]. The behavior of SARS-CoV-2 in some patients is contrary to observations in other patients. This prevents suspicion of the disease clinically, delays diagnosis and isolation of patients infected with the virus and, therefore, makes it difficult to deal with the disease. Therefore, knowing the full range of possible symptoms associated with the virus is the first and most important step in identifying infected patients.
The present study was performed to determine the frequency and severity of the general symptoms of patients who were laboratory-proven to be infected with SARS-CoV-2, as well as the frequency and severity of ENT-related symptoms, especially STL, and the recovery process of STL symptoms.
2. Materials and methods
Analysis of patients' symptoms focusing on STL with confirmed polymerase chain reaction (PCR)-positive testing for the SARS-CoV-2 viral genome was conducted during the COVID-19 outbreak in Turkey. Patients treated at the Istanbul Aydin University, Faculty of Medicine, Istanbul, Turkey, were included in the study. In addition, many other patients and health workers (nurses, physicians, etc.) with confirmed positive PCR test results were voluntarily enrolled in the study. The study protocol was approved by the Ethics Committee of Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine (number: 83045809-604.01.02). All subjects provided informed consent to participation in the study. The exclusion criteria were as follows: age < 18 years old, failure to complete the whole questionnaire, STL before the COVID-19 outbreak, no confirmed positive PCR test result, recent commencement of treatment with new drugs, a history of chronic nasal problems and recent head injury or brain and/or nose operations, severe respiratory failure, or in the intensive care unit. Therefore, we mainly studied patients with mild-to-moderate symptoms with a positive COVID-19 test result without severe respiratory failure.
2.1. Clinical data and classification of symptoms
A questionnaire survey was administered among the patients in the outpatient room or by telephone for some outpatients and health workers with SARS-CoV-2 infection. In addition, an online questionnaire (Professional Survey Monkey, San Mateo, CA) was sent by e-mail to patients who could not be reached by telephone. The questionnaire consisted of general questions regarding age, sex, date of symptoms, smoking history and concomitant diseases; questions about the most obvious symptoms at presentation (one option only) and questions about severity and frequency of symptoms during illness (general and ENT symptoms including STL associated with SARS-CoV-2 infection); and questions regarding recovery time and degree of recovery of STL. General and ENT symptoms were assessed. Symptoms were classified as none, mild, moderate, or severe. In addition, patients with and without loss of sense of smell were divided into two groups and the characteristics were compared between the groups. For evaluation of the STL recovery, patients with STL were contacted by telephone 20 days after diagnosis and assessed again. Questions regarding the degree of recovery of STL were evaluated as none, mild, moderate, and complete recovery. The mean time of STL recovery was evaluated.
2.2. Statistical analysis
Descriptive statistics are presented as the mean, standard deviation, median, highest and lowest values, frequency, and ratio. The Kolmogorov–Smirnov test was used to measure the distribution of variables. The Mann–Whitney U test was performed to analyze the quantitative independent data. The chi-square test was performed for analysis of independent qualitative variables. Statistical analyses were performed using SPSS version 26.0 (SPSS, Chicago, IL). In all analyses, p < 0.05 was taken to indicate statistical significance.
3. Results
A total of 175 patients answered all questions during the study period. Three patients were excluded from the analysis—two had chronic STL due to chronic rhinosinusitis, and one had undergone nose surgery 10 days before the COVID-19 outbreak. Therefore, the final analysis was performed in 172 patients. The patients ranged in age from 18 to 65 years (37.8 ± 12.5 years), 51.2% were female, and 76.2% were nonsmokers. The mean time to diagnosis after onset of symptoms was 4.3 ± 3.2 days (Table 1 ). The most prevalent comorbidities in these patients were allergic rhinitis in 8%, high blood pressure in 5%, depression in 4%, asthma in 3%, diabetes in 3%, renal disease in 2%, thyroid diseases in 2%, heart problems in 2%, and autoimmune diseases in 2%. There were no significant associations between comorbidities and STL.
Table 1.
Clinical features of patients and most obvious COVID-19 symptoms at presentation.
| Minimum-Maximum | Mean ± SD | Number % | ||
|---|---|---|---|---|
| Age (years) | 18.0–65.0 | 37.8 ± 12.5 | ||
| Date of symptoms (days) | 1.0–11.0 | 4.3 ± 3.2 | ||
| Gender | Female | 88 | 51.2% | |
| Male | 84 | 48.8% | ||
| Smoking history | (−) | 131 | 76.2% | |
| (+) | 41 | 23.8% | ||
| The most obvious symptoms | ||||
| Need to blow nose | 2 | 1.16% | ||
| Nasal obstruction | 3 | 1.74% | ||
| Sneezing | 0 | 0% | ||
| Runny nose | 2 | 1.16% | ||
| Cough | 30 | 17.4% | ||
| Post nasal discharge | 1 | 0.58% | ||
| Dizziness | 0 | 0% | ||
| Ear fullness | 0 | 0% | ||
| Fatigue | 25 | 14.5% | ||
| Ear pain | 0 | 0% | ||
| Facial pain/pressure | 0 | 0% | ||
| Loss of sense of taste | 11 | 6.40% | ||
| Loss of sense of smell | 18 | 10.4% | ||
| Fever | 24 | 13.1% | ||
| Sore throat | 4 | 2.32% | ||
| Headache | 17 | 9.88% | ||
| Myalgia | 16 | 9.30% | ||
| Difficulty breathing | 15 | 8.72% | ||
| Diarrhea | 4 | 2.32% | ||
| Total | 172 | 100% | ||
3.1. General and ENT- related symptoms
The most obvious symptoms of patients at presentation were cough (n = 30, 17.4%), fatigue (n = 25, 14.5%), fever (n = 24, 13.1%), loss of sense of smell (n = 18, 10.4%), headache (n = 17, 9.88%), myalgia (n = 16, 9.8%), difficulty breathing (n = 15, 8.72%), loss of sense of taste (n = 11, 6.40%), sore throat (n = 4, 2.32%), diarrhea (n = 4, 2.32%), nasal obstruction (n = 3, 1.74%), need to blow the nose (n = 2, 1.16%), rhinorrhea (n = 2, 1.16%), and postnasal discharge (n = 1, 0.58%) (Table 1).
The severity and frequency of general symptoms during Covid-19 disease are shown in Table 2 . Cough, fatigue, fever, headache, myalgia, and difficulty breathing were the most common symptoms. The severity and frequency of ENT symptoms during COVID-19 disease are shown in Table 3 .
Table 2.
The severity and frequency of general symptoms.
| Symptoms | Severity of symptoms | Number | % |
|---|---|---|---|
| Cough | None | 57 | 33.1% |
| Mild | 14 | 8.1% | |
| Moderate | 19 | 11.0% | |
| Severe | 82 | 47.7% | |
| Dizziness | None | 143 | 83.1% |
| Mild | 25 | 14.5% | |
| Moderate | 4 | 2.3% | |
| Fatigue | None | 50 | 29.1% |
| Mild | 28 | 16.30% | |
| Moderate | 32 | 18.6% | |
| Severe | 62 | 36.0% | |
| Fever | None | 84 | 48.8% |
| Mild | 16 | 9.3% | |
| Moderate | 20 | 11.6% | |
| Severe | 52 | 30.2% | |
| Headache | None | 75 | 43.6% |
| Mild | 32 | 18.60% | |
| Moderate | 41 | 23.8% | |
| Severe | 24 | 14.0% | |
| Myalgia | None | 47 | 27.3% |
| Mild | 26 | 15.1% | |
| Moderate | 44 | 25.6% | |
| Severe | 55 | 32.0% | |
| Difficulty breathing | None | 95 | 55.2% |
| Mild | 47 | 27.3% | |
| Moderate | 18 | 10.5% | |
| Severe | 12 | 7.0% | |
| Diarrhea | None | 89 | 51.7% |
| Mild | 48 | 27.9% | |
| Moderate | 20 | 11.6% | |
| Severe | 15 | 8.7% |
Table 3.
The severity and frequency of ear nose throat- related symptoms.
| Symptoms | Severity of symptoms | Number | % |
|---|---|---|---|
| Need to blow nose | None | 121 | 70.3% |
| Mild | 24 | 14.0% | |
| Moderate | 17 | 9.9% | |
| Severe | 10 | 5.8% | |
| Nasal obstruction | None | 80 | 46.5% |
| Mild | 46 | 26.7% | |
| Moderate | 22 | 12.8% | |
| Severe | 24 | 14.0% | |
| Sneezing | None | 97 | 56.4% |
| Mild | 61 | 35.5% | |
| Moderate | 8 | 4.7% | |
| Severe | 6 | 3.5% | |
| Runny nose | None | 106 | 61.6% |
| Mild | 51 | 29.7% | |
| Moderate | 6 | 3.5% | |
| Severe | 9 | 5.2% | |
| Post nasal discharge | None | 121 | 70.3% |
| Mild | 18 | 10.5% | |
| Moderate | 22 | 12.8% | |
| Severe | 11 | 6.4% | |
| Ear fullness | None | 141 | 82.0% |
| Mild | 20 | 11.6% | |
| Moderate | 4 | 2.3% | |
| Severe | 6 | 3.5% | |
| Ear pain | None | 137 | 79.7% |
| Mild | 17 | 9.9% | |
| Moderate | 8 | 4.7% | |
| Severe | 10 | 5.8% | |
| Facial pain/pressure | None | 129 | 75.0% |
| Mild | 33 | 19.2% | |
| Moderate | 8 | 4.7% | |
| Severe | 2 | 1.2% | |
| Loss of sense of taste | None | 91 | 52.9% |
| Mild | 11 | 6.4% | |
| Moderate | 18 | 10.5% | |
| Severe | 52 | 30.2% | |
| Loss of sense of smell | None | 84 | 48.8% |
| Mild | 2 | 1.2% | |
| Moderate | 24 | 14.0% | |
| Severe | 62 | 36.0% | |
| Sore throat | None | 86 | 50.0% |
| Mild | 57 | 33.1% | |
| Moderate | 21 | 12.2% | |
| Severe | 8 | 4.7% |
3.2. Outcomes regarding loss of sense of smell
Eighty-eight patients (51.2% of total; 44 females, 50%) were reported to have loss of sense of smell related to SARS-CoV-2 infection. Among them, loss of sense of smell was mild in one (1.2%) patient, moderate in 24 (14%), and severe in 62 (36.0%) (Table 3). The loss of sense of smell was the only symptom in four (4.54%) patients, and these patients did not develop any other symptoms during the period of the disease. The loss of sense of smell appeared after, before, or simultaneously with the appearance of other symptoms of SARS-CoV-2 infection in 23 (26.1%), 19 (21.5%), and 46 (52.2%) cases, respectively. Forty-five (51.1%) patients with loss of sense of smell had neither nasal obstruction nor rhinorrhea.
3.3. Comparison of groups with and without loss of sense of smell
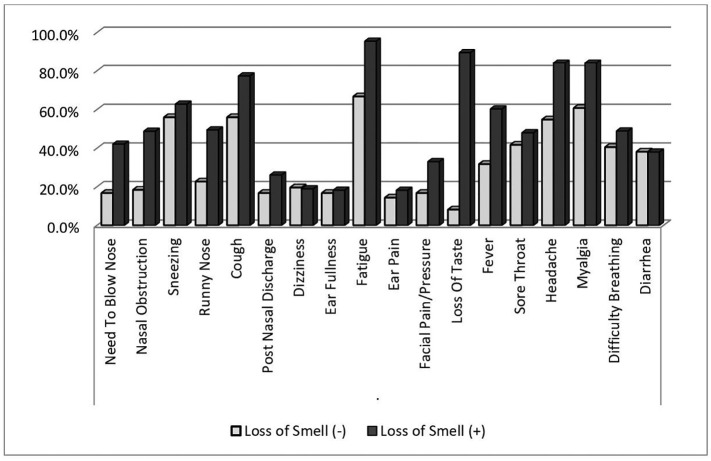
The mean age of patients was significantly younger in the loss of sense of smell group than the no loss of sense of smell group (35.0 ± 11.3 years vs. 40.8 ± 13.1 years, respectively, p < 0.05). The rate of smoking was higher in the loss of sense of smell group than the no loss of sense of smell group but the difference was not statistically significant (26 [29.5%] vs. 15 [17.9%], respectively, p = 0.072). There was a significant positive association between loss of sense of smell and taste (p < 0.001). In addition, the rates of the symptoms of the need to blow the nose, nasal obstruction, rhinorrhea, fatigue, myalgia, fever, facial pain, and headache were significantly higher in the loss of sense of smell group than the no loss of sense of smell group (all p < 0.05). There were no statistically significant associations of other symptoms with or without loss of sense of smell (all p > 0.05) (Fig. 1 ). Similar results were observed for loss of sense of taste (p < 0.001).
Fig. 1.
Comparison of groups with and without loss of sense of smell in term of association with other symptoms of COVID-19.
3.4. Recovery status of loss of sense of smell
Nineteen (21.6%) patients with loss of sense of smell showed no improvement 20 days after diagnosis. The loss of sense of smell improved within 20 days in a total of 69 (78.4%) patients, with 16 (18.2%) showing mild recovery, 33 (37.5%) patients showing moderate recovery, and 20 (22.7%) patients showing complete recovery. The average time for recovery of the sense of smell was 8.02 ± 6.41 days (Table 4 ).
Table 4.
Recovery time and status of the loss of sense of smell and taste.
| Degree of recovery | Number | % | Mean ± SD | |
|---|---|---|---|---|
| Recovery status of loss of sense of smell | None | 19 | 21.6% | |
| Mild recovery | 16 | 18.2% | ||
| Moderate recovery | 33 | 37.5% | ||
| Complete recovery | 20 | 22.7% | ||
| Recovery time for loss of sense of smell (days) | 8.02 ± 6.41 | |||
| Recovery status of loss of sense of taste | None | 18 | 22.2% | |
| Mild recovery | 17 | 21.0% | ||
| Moderate recovery | 27 | 33.3% | ||
| Complete recovery | 19 | 23.4% | ||
| Recovery time for loss of sense of taste (days) | 8.20 ± 7.07 |
3.5. Outcomes of loss of sense of taste
Eighty-one patients (47.1% of total; 43 females, 53.08%) had loss of sense of taste related to SARS-CoV-2 infection. Among them, loss of sense of taste was mild in 11 (6.4%) patients, moderate in 18 (10.5%), and severe in 52 (30.2%) (Table 3). The loss of sense of taste was the only symptom in one (1.23%) of these patients. While nine (11.1%) of the patients who described a loss of sense of taste did not describe loss of sense of smell, the remaining 72 (88.8%) patients described loss of the senses of both smell and taste together. Among these latter 72 patients, the loss of sense of taste appeared after (n = 14, 19.4%), before (n = 6, 8.33%), or at the same time as the appearance of the loss of sense of smell (n = 52, 72.2%).
3.6. Recovery status of loss of sense of taste
Eighteen (22.2%) patients with loss of sense of smell showed no improvement 20 days after diagnosis. A total of 63 (77.8%) patients described improvement in loss of sense of taste within 20 days, with 17 (21.0%) patients showing mild recovery, 27 (33.3%) patients showing moderate recovery, and 19 (23.4%) patients showing complete recovery. The average time for recovery of the sense of taste was 8.20 ± 7.07 days (Table 4).
4. Discussion
Patients infected with SARS-CoV-2 present with a wide range of ENT-related and/or general symptoms, with new symptoms added to the list every day. In recent weeks, both published articles and anecdotal reports on COVID-19 have indicated that the virus may also cause STL. Here, we investigated the frequency and severity of ENT-related and general symptoms in patients with confirmed SARS-CoV-2 infection, the relationships between STL and other symptoms, and the recovery process of STL.
The rapid increase in the number of patients infected with SARS-CoV-2 and in deaths due to infection place a major burden on the healthcare systems of countries struggling with the disease. Success against COVID-19 disease requires early detection and isolation of infected patients. Therefore, it is very important to determine the probabilities of all symptoms caused by the disease [1,2]. Previous studies showed that COVID-19 disease causes a number of general symptoms, such as difficulty in breathing, myalgia, fatigue, fever, and cough. In more severe cases, it may lead to viral pneumonia and acute respiratory distress syndrome (ARDS), which may require hospitalization for treatment and follow-up. However, it may still result in death despite treatment [3,4].
In a meta-analysis involving five studies evaluating general and upper respiratory tract symptoms of patients hospitalized due to COVID-19 disease in Asian countries, it was reported that 85.6% of patients had fever, 68.7% had cough, and 39.4% had fatigue as the main symptom on admission to hospital, while 12.4% of patients had pharyngodynia, and 3.7% had nasal congestion and upper respiratory tract symptoms. The authors stated that rhinorrhea and sore throat are very rare in patients infected with SARS-CoV-2, and STL was not seen. They warned that other upper respiratory tract symptoms, especially STL, are rare as the articles they examined included hospitalized SARS-CoV-2-positive patients and, therefore, symptoms such as STL, nasal congestion, and sore throat may be complaints in patients infected with SARS-CoV-2 [9]. Similarly, in a study comparing the loss of sense of smell in hospitalized vs. non-hospitalized SARS-CoV-2-positive patients, the rate of loss of sense of smell was approximately five times higher among outpatients than inpatients. The authors argued that this difference may be due to the reduced sensitivity to loss of sense of smell in patients due to factors such as respiratory distress in patients hospitalized for COVID-19 disease [10].
More than 80% of patients infected with SARS-CoV-2 survive the disease with mild symptoms, and patients with mild presentation can show a number of very different symptoms [11]. In a study including only SARS-CoV-2 positive patients with mild symptoms in Italy in which the participants were asked about their general symptoms over the telephone, and the Sino-nasal Outcome Test-22 (SNOT-22) was performed, the most common symptoms were fever (55.9%), cough (60.4%), and fatigue (68.3%). The most common upper respiratory tract symptoms were nasal congestion (41.1%), sore throat (31.2%), and STL (64.4%) [12]. In the present study in patients with mild or moderate symptoms, the most common general symptoms were myalgia (72.7%), fatigue (70.9%), cough (66.8%), headache (56.4%), fever (51.1%). and difficulty breathing (44.8%). The most frequent ENT-related symptoms of the patients were nasal obstruction (53.5%), loss of sense of smell (51.2%), sore throat (50.2%), loss of sense of taste (47.1%), and rhinorrhea (38.5%).
It is well known that STL may develop after viral upper respiratory infections. Rhinoviruses, coronaviruses, Epstein-Barr virus, and parainfluenza viruses are known to cause STL [5,6]. With the spread of SARS-CoV-2 especially to European countries and the USA, the number of patients developing loss of sense of smell has been shown to increase. The American Ear Nose and Throat Academy stated that STL is one of the symptoms of COVID-19 disease, and the COVID-19 disease screening test should be performed in patients with newly developed STL [13]. During the same period, Hopkins argued that there is strong evidence that SARS-CoV-2 causes STL [14].
The first report including information about STL caused by SARS-CoV-2 was of a study conducted in China by Mao et al. emphasizing the neurological symptoms of the disease. The authors stated that approximately 6% of patients infected with SARS-CoV-2 had loss of sense of smell and that loss of sense of smell may be the first symptom before onset of complicated neurological symptoms [15]. In the articles published in the subsequent weeks, higher rates were reported for the frequency of STL symptoms in patients infected with SARS-CoV-2. In a meta-analysis conducted by Tong et al. investigating the frequency of STL occurrence in patients positive for SARS-CoV-2, they found that the average frequency of loss of sense of smell in 10 studies was 52.73% (29.64%–75.23%), and in nine studies that the average frequency of loss of sense of taste was 43.93%. (20.46%–68.95%) [16].
In a study conducted on the Internet in patients with upper respiratory tract infection who had been tested for SARS-CoV-2, 68% of patients who were confirmed to have SARS-CoV-2 by PCR described loss of sense of smell and 71% described loss of sense of taste, while 16% and 17% of patients negative for SARS-CoV-2 described loss of the sense of smell and taste, respectively [17]. The frequency of STL has been reported be at least 10 times higher in SARS-CoV-2-positive patients than in uninfected individuals, and there is a strong association between loss of sense of smell and loss of taste. In the above study, loss of sense of smell was the main reason for admission to hospital in 22% of SARS-CoV-2-positive patients, and 72.5% of patients who received no treatment for STL recovered, with the majority recovering within the first 2 weeks [17]. The data obtained with the COVID-19 Anosmia Reporting Tool developed by the American Academy of Otolaryngology-Head and Neck Surgery for clinicians indicated that 73% of 237 SARS-CoV-2-positive patients had loss of sense of smell at admission to hospital, and 26.6% of these patients had loss of sense of smell as the main complaint at the time of admission to hospital. It was reported that 85% of the patients who described loss of sense of smell recovered within the first 10 days, and the average recovery time was 7.2 days [18]. In a study conducted in 417 SARS-CoV-2-positive patients with mild or moderate symptoms, 85.6% of patients described loss of sense of smell, 88.8% described loss of taste, and 79.6% of those with loss of sense of smell had anosmia and 78.4% reported that they had ageusia. The report also stated that 67.8% of patients with loss of sense of smell and 78.9% of patients with loss of sense of taste recovered to various degrees within an average of 8 days [19]. In the present study, we found that 36% of the patients who described various degrees of loss of sense of taste (52.2% of the total) had anosmia. In addition, we found that 30.2% of the patients who described various degrees of loss of sense of taste (47.1% of the total) had ageusia. Among our patients who described loss of sense of smell, 78.4% improved to various degrees within an average of 8.02 ± 6.41 days. In 77.8% of our patients who described loss of taste, we found that the loss of sense of taste improved to various degrees within an average of 8.20 ± 7.07 days.
Lechien et al. reported that STL was the main symptom in 11.8% of patients and that 79.7% of patients with STL described only loss of sense of smell without complaints of nasal obstruction or rhinorrhea in their study [19]. In the present study, the main reason for admission to hospital was loss of sense of smell in 18 (10.4%) patients and loss of sense of taste in 11 (6.4%) patients. In addition, 51.1% of our patients who described loss of sense of smell did not describe nasal congestion or rhinorrhea.
The pathogenesis of STL caused by SARS-CoV-2 virus is not yet clear. However, direct extension through the nasal mucosa via angiotensin-converting enzyme 2 receptor and extension to the olfactory bulb of SARS-CoV-2 are thought to be involved (16–19). Despite all the unknown factors regarding the pathogenesis of STL caused by SARS-CoV-2, our study showed that SARS-CoV-2 can cause STL in infected patients, that STL may be the first and only symptom independent of other upper respiratory complaints in SARS-CoV-2-positive patients, and that the majority of the patients developing STL due to SARS-CoV-2 show rapid improvement.
The present study had several limitations. First, in our study, objective diagnostic tests were not used to evaluate the severity and frequency of the STL symptoms and improvement of STL but, instead, subjective tests were used to determine the degree and improvement of the symptoms. Although objective testing is more sensitive for determining the degree of loss of sense of smell and of recovery, self-reported loss of sense of smell is relatively accurate (90%) [20]. Second, the present study excluded hospitalized patients with COVID-19 disease or those in intensive care units with severe symptoms. Therefore, our study was not representative of all patients infected with SARS-CoV-2. However, there are a number of ethical problems with the administration of symptom questionnaires in patients with severe illness in hospital or intensive care units. Third, although the majority of our patients described improvement of the symptoms of STL within the first 20 days, long-term follow-up results were not available for patients who did not describe recovery within 20 days. Fourth, the medical treatments received, especially in patients describing STL, were not included in our study. All of these limitations should be taken into account in future studies, and there is a need for large-scale studies using internationally validated diagnostic tests for STL in patients infected with SARS-CoV-2 and long-term follow-up of the recovery process of STL.
Patients infected with SARS-CoV-2 can present to hospitals with different general and ENT symptoms. STL is a common condition, especially in patients with mild and moderate symptoms, and it may appear without the development of other general and ENT-related symptoms associated with COVID-19 disease or it may be the first and only symptom of COVID-19 disease. In patients with STL occurring during the COVID-19 outbreak, screening tests performed due to suspected COVID-19 disease will allow early diagnosis and isolation of patients, which will make it easier to deal with the disease.
Declaration of competing interest
None.
Acknowledgments
Acknowledgments
Conflict of Interest Statement: There is not any financial and personal relationships with other people or organisations. Also there is not any funding source.
Author contributions
ES and DT; conception and design of the work, collecting data, analysis, writing manuscript, final approvel of manuscript. EB, EEA, SCE and MB; design of the work, collecting data, analysis, drafting the work, final approvel of the manuscript. All co-authors take full responsibility for all aspects of the study and the final manuscript.
References
- 1.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. Feb 24. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y.C., Chen C.S., Chan Y.J. Overview of the novel coronavirus (2019-nCoV): the pathogen of severe specific contagious pneumonia (SSCP) J Chin Med Assoc. 2020 [Google Scholar]
- 5.Suzuki M., Saito K., Min W.P. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 7.Sayin I., Yasar K.K., Yazici ZM. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedaghat A.R., Gengler I., Speth M.M. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820926464. May 5:194599820926464. [DOI] [PubMed] [Google Scholar]
- 9.Lovato A., Filippis Cd. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020 doi: 10.1177/0145561320920762. Apr 13:145561320920762. [DOI] [PubMed] [Google Scholar]
- 10.Yan C.H., Faraji F., Parajapati D.P. Self-reported Olfactory Loss Associates With Outpatient Clinical Course in COVID-19. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinato G., Fabbris C., Polesel J. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.6771. Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Otolaryngology- Head and Neck Surgery. Anosmia, hyposmia, and dysgeusia symptoms of coronavirus disease. https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease. Published March 22,2020. Accessed April 5, 2020.
- 14.Hopkins C. Loss of sense of smell as marker of COVID-19 infection. https://www.entuk.org/categories/covid-19. Accessed April 5, 2020.
- 15.Mao L., Jin H., Wang M. Hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong J.Y., Wong A., Zhu D. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820926473. May 5:194599820926473. [DOI] [PubMed] [Google Scholar]
- 17.Yan C.H., Faraji F., Prajapati D.P. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22579. Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye R., Chang C.W.D., Kazahaya K. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820922992. Apr 28:194599820922992. [DOI] [PubMed] [Google Scholar]
- 19.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild- to- moderate forms of the coronavirus disease (COVID- 19):a multicenter European study. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05965-1. Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehling E., Nordin S., Espeseth T. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011;26(3):260–269. doi: 10.1093/arclin/acr019. Apr. [DOI] [PubMed] [Google Scholar]