Abstract
Objective:
To identify prescriber characteristics that predict antibiotic high-prescribing behavior to inform statewide antimicrobial stewardship interventions.
Design:
Retrospective analysis of 2016 IQVIA Xponent, formerly QuintilesIMS, outpatient retail pharmacy oral antibiotic prescriptions in Tennessee.
Setting:
Statewide retail pharmacies filling outpatient antibiotic prescriptions.
Participants:
Prescribers who wrote at least 1 antibiotic prescription filled at a retail pharmacy in Tennessee in 2016.
Methods:
Multivariable logistic regression, including prescriber gender, birth decade, specialty, and practice location, and patient gender and age group, to determine the association with high prescribing.
Results:
In 2016, 7,949,816 outpatient oral antibiotic prescriptions were filled in Tennessee: 1,195 prescriptions per 1,000 total population. Moreover, 50% of Tennessee’s outpatient oral antibiotic prescriptions were written by 9.3% of prescribers. Specific specialties and prescriber types were associated with high prescribing: urology (odds ratio [OR], 3.249; 95% confidence interval [CI], 3.208–3.289), nurse practitioners (OR, 2.675; 95% CI, 2.658–2.692), dermatologists (OR, 2.396; 95% CI, 2.365–2.428), physician assistants (OR, 2.382; 95% CI, 2.364–2.400), and pediatric physicians (OR, 2.340; 95% CI, 2.320–2.361). Prescribers born in the 1960s were most likely to be high prescribers (OR, 2.574; 95% CI, 2.532–2.618). Prescribers in rural areas were more likely than prescribers in all other practice locations to be high prescribers. High prescribers were more likely to prescribe broader-spectrum antibiotics (P < .001).
Conclusions:
Targeting high prescribers, independent of specialty, degree, practice location, age, or gender, may be the best strategy for implementing cost-conscious, effective outpatient antimicrobial stewardship interventions. More information about high prescribers, such as patient volumes, clinical scope, and specific barriers to intervention, is needed.
Antibiotic overprescribing is a leading cause of antibiotic resistance. The Centers for Disease Control and Prevention (CDC) predicts that at least 2 million people in the United States are infected with a resistant pathogen and that at least 23,000 people die from these infections each year.1 In 2015, the first assessment of US outpatient antibiotic use, analyzing 2011 data, showed that the highest per capita antibiotic prescription rates were in the Southeast region; Tennessee had the third-highest outpatient antibiotic prescription rate.2 Subsequent national outpatient antibiotic use annual analyses showed minimal reduction in per capita antibiotic prescription in the Southeast region, and Tennessee remained one of the highest outpatient antibiotic-prescribing states as of 2016.3
In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, which called for a reduction of inappropriate outpatient antibiotic use by 50% by 2020.4 Recent published studies have estimated that inappropriate antibiotic use rates range from 14% to 45%, depending on the location of the patient visit and the specific diagnosis given.5-8
Prior studies have shown that physician characteristics predicting higher antibiotic prescription rates include male gender; longer time since graduation from medical school; practice in smaller, higher-volume practices; and for US physicians, practice in the South.9-11
To date, no published data regarding prescriber characteristics that predict higher outpatient antibiotic prescription rates on a US state or county level are available. These data are necessary to inform public health and health systems interventions to reduce high rates of outpatient antibiotic use.
In this retrospective analysis of 2016 Tennessee outpatient antibiotic prescriptions, we aimed to understand more clearly whether type of prescriber, prescriber age, gender, specialty, and/or practice location are associated with higher outpatient antibiotic prescription rates. We intend to use this information to focus future outpatient antimicrobial stewardship resources to corroborate prescription data; to better understand inappropriate outpatient antibiotic use prescribing patterns; to explore prescriber barriers and challenges; and to create and implement effective, statewide outpatient antimicrobial stewardship interventions. Our study results can assist other states and healthcare systems in the evaluation of current outpatient antibiotic use to inform the development of local outpatient antimicrobial stewardship programs.
Methods
The Tennessee Department of Health (DOH) purchased the 2016 IQVIA Xponent (IQVIA, Durham, NC, formerly QuintilesIMS) dataset of outpatient antibiotic prescriptions written in Tennessee. IQVIA Xponent uses a patented method that incorporates 100% of wholesale distribution data and, for 2016 data, 90% of retail pharmacy sales data to estimate 100% of dispensed prescriptions.12 The data purchased by Tennessee DOH excluded mail-order pharmacy, federal facility pharmacy, and inpatient-healthcare-facility–associated pharmacy prescriptions.
All antifungal, antiviral, nonantibacterial, and non–orally administered prescriptions were excluded. Urinary analgesics listing methenamine as the only active antibiotic were excluded. All antibiotics were grouped into 12 categories, including a division between narrow- and broad-spectrum penicillins and a division between first- or second-generation and third-generation cephalosporins.
Tennessee population data from 2016 were obtained from the Tennessee DOH Office of Policy and Data Management, derived from the US Census, Annual Estimates of the Resident Population.13 Patients were classified as pediatric patients (aged < 20 years) or adults (aged ≥ 20 years), consistent with prior similar analyses.2,3 Counties were classified by 2013 National Center for Health Statistics (NCHS) urban–rural classification, based on the Office of Management and Budget’s metropolitan statistical areas: NCHS-1, large, central metropolitan counties (population > 1 million with at least 250,000 residents of principal city), NCHS-2, large, fringe metropolitan counties (>1 million population and not classified as NCHS-1), NCHS-3, medium metropolitan counties (population 250,000–999,999), NCHS-4, small metropolitan counties (population < 250,000), NCHS-5, micropolitan areas (population 10,000–50,000), and NCHS-6, noncore counties (outside all other areas).14 More than 182 individual prescriber specialties listed were categorized into 17 specialty groups. In this dataset, nurse practitioners and physician assistants from all specialties were listed as separate specialty groups and were not included in other specialties. For this reason, nurse practitioners and physician assistants are listed as individual specialties in our analysis.
All antibiotic prescriptions written by trainees were excluded from prescriber-level characteristics analysis because trainees in Tennessee prescribe under an institutional training license. Trainee prescriptions were included when analyzing patient-level characteristics.
High prescribers were identified by creating a Pareto chart in which each individual prescriber’s total antibiotic prescriptions, ordered from highest to lowest, were plotted against the cumulative total statewide antibiotic prescriptions. The highest individual prescribers, who collectively accounted for 50% of the cumulative statewide antibiotic prescriptions, were identified as the high-prescribing group. High prescribers who had no specific demographic details associated with their individual prescribing identifier (N = 11, 0.4%) were considered to represent institutionally aggregated entities and were excluded from our analysis of the high-prescribing group.
Antibiotic prescriptions are reported as prescriptions per 1,000 population within the specific patient population or geographic area described. When reporting antibiotic prescriptions per 1,000 population, the patient’s reported county (ie, the filling pharmacy location in this dataset) was used, except when reporting prescriptions per 1,000 population by prescriber characteristic, for which the prescriber’s practice location county was used. When calculating prescriptions per prescriber, the prescriber denominator was restricted to specialty or specialty grouping.
Statistical significance of prescription rates by patient characteristics was calculated using negative binomial regression. For prescriber characteristics, averages were reported for gender, NCHS classification of prescriber practice location, specialty, and birth decade.
We performed a multivariable logistic regression to compare “high prescribers” to “non–high prescribers.” A simple logistic regression was performed for each independent variable of prescriber gender, practice location, specialty, and birth decade, and for patient gender and age group, which were then included in the multivariable logistic regression model.
To compare high prescriber prescriptions to non–high-prescriber prescriptions by antibiotic group, we calculated a ratio for each antibiotic group. The χ2 test was used to detect whether there was an overall statistically significant difference between the prescribing patterns of the 2 groups.
Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC). This study was approved by the Vanderbilt University Institutional Review Board.
Results
In 2016, 7,949,816 outpatient antibiotic prescriptions were filled in Tennessee: a rate of 1,195 antibiotic prescriptions per 1,000 total population. Of those prescriptions, female patients had a significantly higher antibiotic prescription rate (1,445 prescriptions per 1,000 population vs 928 for male patients) (P < .001). The highest rates of antibiotic prescription were observed in age groups 0–2 years (1,575) and 65+ years (1,525) (P < .001) (Table 1).
Table 1.
Outpatient Antibiotic Prescriptions by Patient Gender and Agea
| Variable | Population, No. (%) |
Total Prescriptions, No. (%) |
Rate per 1,000 Population |
|---|---|---|---|
| Tennessee | 6,651,120 | 7,949,816 | 1,195 |
| Female | 3,408,659 (51.2) | 4,939,913 (62.1) | 1,449 |
| Male | 3,242,461 (48.8) | 3,008,014 (37.8) | 928 |
| P < .001 | |||
| Pediatrics | 1,664,796 | 1,939,633 | 1,165 |
| Female | 815,548 (49.0) | 1,018,585 (52.5) | 1,249 |
| Male | 849,248 (51.0) | 920,160 (47.4) | 1,084 |
| P = .046 | |||
| Adults | 4,986,324 | 6,009,806 | 1,205 |
| Female | 2,593,111 (52.0) | 3,921,099 (65.2) | 1,512 |
| Male | 2,393,213 (48.0) | 2,087,709 (34.7) | 872 |
| P < .001 | |||
| Age | |||
| 0–2 y | 243,636 (3.7) | 383,689 (4.8) | 1,575 |
| 3–9 y | 578,798 (8.7) | 747,578 (9.4) | 1,292 |
| 10–19 y | 842,362 (12.7) | 808,366 (10.2) | 960 |
| 20–39 y | 1,767,333 (26.6) | 1,792,943 (22.6) | 1,014 |
| 40–64 y | 2,171,960 (32.7) | 2,619,686 (33.0) | 1,206 |
| ≥65 | 1,047,031 (15.7) | 1,597,177 (20.1) | 1,525 |
| P < .001 |
Negative binomial regression for rates was used to calculate P values.
The 5 most commonly prescribed antibiotic groups were narrow-spectrum penicillins (20.2%), macrolides (16.5%), fluoroquinolones (11.4%), extended-spectrum penicillins (10.4%), and first- and second-generation cephalosporins (8.6%). The 5 most frequently prescribed antibiotics for pediatric patients accounted for 79.7% of all pediatric antibiotic prescriptions. By comparison, the 5 most frequently prescribed adult antibiotics only accounted for 55.4% of all adult antibiotic prescriptions (Table 2).
Table 2.
Most Prescribed Antibiotic Groups and Individual Antibioticsa
| Antibiotic Group | Total Prescriptions, No. (%) |
Rate per 1,000 Populationb |
|---|---|---|
| Narrow-spectrum penicillin | 1,604,065 (20.2) | 241 |
| Macrolide | 1,308,726 (16.5) | 197 |
| Fluoroquinolone | 903,155 (11.4) | 136 |
| Extended-spectrum penicillin | 823,093 (10.4) | 124 |
| First- & second-generation Cephalosporins | 684,156 (8.7) | 103 |
| Trimethoprim-sulfamethoxazole | 635,596 (8.0) | 96 |
| Tetracyclines | 582,875 (7.3) | 88 |
| Third-generation plus cephalosporins | 498,192 (6.3) | 75 |
| Lincosamides | 324,210 (4.1) | 49 |
| Urinary agents | 274,352 (3.5) | 41 |
| Metronidazole | 261,609 (3.3) | 39 |
| Other | 49,797 (0.6) | 8 |
| 10 most frequently prescribed antibiotics statewidec | ||
| Amoxicillin | 1,410,638 (17.7) | 212 |
| Azithromycin | 1,221,726 (15.4) | 184 |
| Amoxicillin-Clavulanate | 823,093 (10.4) | 124 |
| Trimethoprim-Sulfamethoxazole | 635,596 (8.0) | 96 |
| Cephalexin | 588,936 (7.4) | 89 |
| Ciprofloxacin | 542,675 (6.8) | 82 |
| Cefdinir | 468,959 (5.9) | 71 |
| Doxycycline | 460,663 (5.8) | 70 |
| Levofloxacin | 352,316 (4.4) | 53 |
| Clindamycin | 324,210 (4.1) | 49 |
| 5 most frequently antibiotics prescribed to pediatric patientsd | ||
| Amoxicillin | 661,821 (34.1) | 398 |
| Azithromycin | 294,701 (15.2) | 177 |
| Cefdinir | 252,546 (13.0) | 152 |
| Amoxicillin-Clavulanate | 214,810 (11.1) | 129 |
| Cephalexin | 121,631 (6.3) | 73 |
| 5 most frequently antibiotics prescribed to adult patientse | ||
| Azithromycin | 926,991 (15.4) | 186 |
| Amoxicillin | 748,740 (12.6) | 150 |
| Amoxicillin-clavulanate | 608,255 (10.1) | 122 |
| Ciprofloxacin | 526,862 (8.8) | 106 |
| Trimethoprim-Sulfamethoxazole | 517,955 (8.6) | 104 |
Source: IQVIA Xponent.
The total rate per 1,000 population for antibiotic group prescriptions adds to 1,197, as opposed to 1,195 reported as the state’s prescriptions per 1,000 population, due to rounding after each group’s antibiotic prescription rate was calculated.
The top 10 percentages are of total state antibiotic count. They add to 85.9%.
The top 5 pediatric antibiotics make up 79.7% of all pediatric antibiotic prescriptions.
The top 5 adult antibiotics make up 55.4% of all adult antibiotic prescriptions.
In 2016, there were 42,471 licensed prescribers in Tennessee (Mary Katherine Bratton, e-mail communication, March 2019). In our dataset, there were 32,168 (75.7%) prescribers (excluding trainees who practice under an institutional license) who wrote at least 1 outpatient antibiotic oral prescription in 2016. Female prescribers (7,221, 22.4%) wrote 1,960,725 prescriptions (24.9%), an average of 272 prescriptions per female prescriber. Male prescribers (12,815, 40.0%) wrote 3,383,789 prescriptions (42.9%), an average of 264 prescriptions per male prescriber. Prescribers who did not report a gender (12,132, 37.8%) wrote 2,542,867 prescriptions (32.2%), an average of 210 prescriptions per prescriber. Prescribers in medium metropolitan (NCHS-3) areas (8,864, 27.6%) wrote the most antibiotic prescriptions (2,131,804, 27.0%), an average of 241 prescriptions per prescriber. By contrast, prescribers practicing in noncore (NCHS-6) rural areas (1,511, 4.7%) wrote 689,149 prescriptions (8.7%) but averaged the highest prescriptions per prescriber with 456 (Table 3).
Table 3.
Prescriptions by Prescriber Gender, Practice Location, Specialty and Birth Decade
| Prescribers, No. (%) |
Total Prescriptions, No. (%) |
Average Prescriptions Per Prescriber |
|
|---|---|---|---|
| All Prescribers | 32,168a | 7,887,380 (100) | 245 |
| Prescriber gender | |||
| Female | 7,221 (22.4) | 1,960,725 (24.9) | 272 |
| Male | 12,815 (40.0) | 3,383,789 (42.9) | 264 |
| Not specified | 12,132 (37.7) | 2,542,867 (32.2) | 210 |
| Practice locationb | |||
| NCHS-1 | 11,960 (37.2) | 2,043,017 (25.9) | 171 |
| NCHS-2 | 4,086 (12.7) | 1,210,669 (15.3) | 296 |
| NCHS-3 | 8,864 (27.6) | 2,131,804 (27.0) | 241 |
| NCHS-4 | 3,089 (9.6) | 840,351 (10.7) | 272 |
| NCHS-5 | 2,658 (8.3) | 972,390 (12.3) | 366 |
| NCHS-6 | 1,511 (4.7) | 689,149 (8.7) | 456 |
| Prescriber specialty | |||
| Nurse practitioner | 7,382 (22.9) | 2,416,018 (30.6) | 327 |
| Family medicine | 2,427 (7.5) | 1,227,865 (15.6) | 506 |
| Internal medicine | 2,698 (8.4) | 784,869 (10.0) | 291 |
| Physician assistant | 1,639 (5.1) | 679,820 (8.6) | 415 |
| Pediatrics | 1,211 (3.8) | 608,054 (7.7) | 502 |
| Dentists | 3,084 (9.6) | 601,012 (7.6) | 195 |
| Medicine subspecialty | 2,268 (7.1) | 251,893 (3.2) | 111 |
| Other | 6,340 (19.7) | 235,417 (3.0) | 37 |
| Emergency medicine | 741 (2.3) | 234,177 (3.0) | 316 |
| Obstetrics/ Gynecology |
963 (3.0) | 198,435 (2.5) | 206 |
| Other surgery | 1,890 (5.9) | 169,644 (2.2) | 90 |
| Urology | 269 (0.8) | 153,536 (1.9) | 571 |
| Dermatology | 226 (0.7) | 119,784 (1.5) | 530 |
| Otolaryngology | 247 (0.8) | 81,281 (1.0) | 329 |
| Oral & maxillofacial surgery | 145 (0.5) | 80,065 (1.0) | 552 |
| Pediatric subspecialty | 638 (2.0) | 45512 (0.6) | 71 |
| Prescriber birth year by decade | |||
| Missing birth year | 14,119 (43.9) | 3,056,527 (38.8) | 216 |
| Prior to 1940 | 410 (1.3) | 45,800 (0.6) | 112 |
| 1940s | 2,089 (6.5) | 413,877 (5.2) | 198 |
| 1950s | 5,481 (17.0) | 1,453,016 (18.3) | 265 |
| 1960s | 5,392 (16.8) | 1,664,177 (21.1) | 309 |
| 1970s | 4345 (13.5) | 1,177,678 (14.9) | 271 |
| 1980s | 332 (1.0) | 76,304 (1.0) | 230 |
Trainees were excluded from this analysis because trainees practice under an institutional license.
Counties were classified by 2013 National Center for Health Statistics (NCHS) urban–rural classification, based on the Office of Management and Budget’s metropolitan statistical areas: NCHS-1, large, central metropolitan counties (population > 1 million with at least 250,000 residents of principal city), NCHS-2, large, fringe metropolitan counties (>1 million population and not classified as NCHS-1), NCHS-3, medium metropolitan counties (population 250,000–999,999), NCHS-4, small metropolitan counties (population < 250,000), NCHS-5, micropolitan areas (population 10,000–50,000), and NCHS-6, noncore counties (outside all other areas).
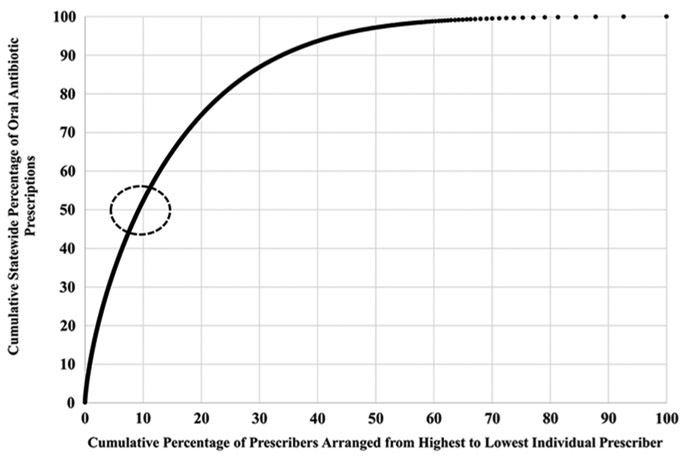
Of all specialties, nurse practitioners (7,382, 22.9% of all prescribers) wrote the most antibiotic prescriptions with 2,416,018 (30.6%). However, urologists (269, 0.8% of all prescribers), who wrote only 153,536 (1.9%) prescriptions, had the highest average prescriptions per prescriber, with 571. Family medicine physicians (2,427, 7.5%) wrote 1,227,865 prescriptions (15.6%), the second-most prescriptions, with an average of 506 prescriptions per prescriber (Table 3). Of the state’s total antibiotic prescriptions, 50% were written by 9.3% of all prescribers (Fig. 1). These 9.3% of prescribers all wrote 770 or more prescriptions during 2016 and were subsequently identified as “high prescribers.”
Fig. 1.
Cumulative percentage of individual prescriber antibiotic prescriptions contributing to cumulative percentage of 2016 Tennessee oral outpatient antibiotic prescriptions. Prescriptions per individual prescriber were added cumulatively from highest individual prescriber to the lowest. The dotted red circle indicates the point at which 50% of the state’s total antibiotic prescriptions are accounted for and corresponds to 9.3% of all prescribers included.
The high-prescriber group consisted of 2,994 prescribers: 778 female (26.0%), 1,293 male (43.2%), and 923 unspecified (30.8%). The greatest number of high prescribers were from medium-population metropolitan (NCHS-3) counties (789, 26.4%), representing 8.9% of all prescribers practicing in NCHS-3 counties. By contrast, 329 high prescribers (11.0%) practiced in rural (NCHS-6) areas, which represented 21.7% of all prescribers practicing in NCHS-6 counties (Table 4).
Table 4.
Comparison of High Prescribers to Non-High Prescribers by Prescriber Gender, Practice Location, Specialty, and Birth Decade, and Patient Gender and Age
| Prescriber Characteristicab | High Prescribers N (%)cd |
Non-High Prescribers N (%)d |
Unadjusted Odds Ratioe |
Adjusted Odds Ratiof |
95% Confidence Interval |
|---|---|---|---|---|---|
| All prescribers | 2994 (9.3) | 29,163 (90.7) | |||
| Gender | |||||
| Male | 1,293 (10.1) | 11,522 (89.9) | Reference | Reference | Reference |
| Female | 778 (10.8) | 6,443 (89.2) | 1.008 | 0.487 | 0.485–0.490 |
| Not Specified | 923 (7.6) | 11,188 (92.4) | 0.908 | 0.407 | 0.404–0.411 |
| Prescriber practice locationg | |||||
| NCHS-1 | 688 (5.8) | 11,270 (94.2) | 0.345 | 0.450 | 0.447–0.453 |
| NCHS-2 | 487 (11.9) | 3,594 (88.1) | 0.512 | 0.540 | 0.536–0.544 |
| NCHS-3 | 789 (8.9) | 8,074 (91.1) | 0.407 | 0.455 | 0.453–0.458 |
| NCHS-4 | 301 (9.7) | 2,787 (90.3) | 0.506 | 0.553 | 0.549–0.557 |
| NCHS-5 | 400 (15.1) | 2,256 (84.9) | 0.624 | 0.663 | 0.658–0.668 |
| NCHS-6 | 329 (21.8) | 1,182 (78.2) | Reference | Reference | Reference |
| Prescriber specialty | |||||
| Internal medicine | 285 (10.6) | 2,413 (89.4) | Reference | Reference | Reference |
| Urology | 87 (32.3) | 182 (67.7) | 2.970 | 3.249 | 3.208–3.289 |
| Nurse practitioner | 1,076 (14.6) | 6,306 (85.4) | 1.809 | 2.675 | 2.658–2.692 |
| Dermatology | 53 (23.5) | 173 (75.5) | 2.032 | 2.396 | 2.365–2.428 |
| Physician assistant | 303 (18.5) | 1336 (81.5) | 1.775 | 2.382 | 2.364–2.400 |
| Pediatrics | 322 (26.6) | 889 (73.4) | 2.684 | 2.340 | 2.320–2.361 |
| Oral & maxillofacial surgery | 45 (31.0) | 100 (69.0) | 2.105 | 2.026 | 1.994–2.059 |
| Family medicine | 560 (23.1) | 1,867 (76.9) | 2.007 | 1.945 | 1.933–1.957 |
| Otolaryngology | 28 (11.3) | 219 (88.7) | 0.971 | 0.856 | 0.843–0.869 |
| Emergency medicine | 56 (7.6) | 685 (92.4) | 0.511 | 0.513 | 0.508–0.519 |
| Pediatric specialty | 8 (1.3) | 630 (98.7) | 0.413 | 0.347 | 0.339–0.355 |
| Obstetrics/ Gynecology | 35 (3.6) | 928 (96.4) | 0.312 | 0.331 | 0.327–0.335 |
| Medicine specialty | 31 (1.4) | 2,237 (98.6) | 0.260 | 0.261 | 0.258–0.264 |
| Dentists | 76 (2.5) | 3,008 (97.5) | 0.198 | 0.189 | 0.187–0.191 |
| Other surgery | 17 (0.9) | 1,873 (99.1) | 0.144 | 0.126 | 0.124–0.128 |
| Other | 12 (0.2) | 6,317 (99.8) | 0.122 | 0.130 | 0.128–0.132 |
| Prescriber birth year by decade | |||||
| Missing birth year | 1,145 (8.1) | 12,963 (91.9) | 1.769 | 2.145 | 2.108–2.183 |
| Prior to 1940 | 14 (3.4) | 396 (96.6) | 1.253 | 1.205 | 1.172–1.238 |
| 1940s | 141 (6.7) | 1,948 (93.3) | 1.568 | 1.912 | 1.878–1.947 |
| 1950s | 548 (10.0) | 4,933 (90.0) | 1.783 | 2.136 | 2.101–2.173 |
| 1960s | 679 (12.6) | 4,713 (87.4) | 2.150 | 2.574 | 2.532–2.618 |
| 1970s | 444 (10.2) | 3,901 (89.8) | 1.656 | 1.832 | 1.801–1.863 |
| 1980s | 23 (6.9) | 309 (93.1) | Reference | Reference | Reference |
Source: IQVIA Xponent.
Trainees were excluded from this analysis because trainees practice under an institutional license.
11 prescribers from “other” were excluded due to lack of demographic information.
Percentage of prescribers in that characteristic group that are high and non–high prescribers, respectively.
All unadjusted odds ratios were statistically significant with a P < .001 with exception of the unadjusted odds ratio for Otolaryngology, which had a P < .001.
All adjusted odds ratios were statistically significant with a P < .001.
Counties were classified by 2013 National Center for Health Statistics (NCHS) urban–rural classification, based on the Office of Management and Budget’s metropolitan statistical areas: NCHS-1, large, central metropolitan counties (population > 1 million with at least 250,000 residents of principal city), NCHS-2, large, fringe metropolitan counties (>1 million population and not classified as NCHS-1), NCHS-3, medium metropolitan counties (population 250,000–999,999), NCHS-4, small metropolitan counties (population < 250,000), NCHS-5, micropolitan areas (population 10,000–50,000), and NCHS-6, noncore counties (outside all other areas).
Within the high-prescribing group, nurse practitioners had the highest number of high prescribers at 1,076 (35.9%); however, this accounted for only 14.6% of all prescribing nurse practitioners. In contrast, 87 urologists comprised 3% of all high prescribers but 32.3% of all prescribing urologists. Similarly, 45 high prescribers (2%) were oral and maxillofacial surgeons, which represented 31.0% of all prescribing oral and maxillofacial surgeons (Table 4).
In the multivariable logistic regression, female prescribers were significantly less likely than males to be high prescribers (odds ratio [OR], 0.487; 95% confidence interval [CI], 0.485–0.490). Compared to prescribers practicing in rural (NCHS-6) areas, prescribers practicing in all other, more urban, counties were associated with less likelihood of being a high prescriber. The top 5 specialties most likely to be associated with being a high prescriber when compared to internal medicine were urologists (OR, 3.249; 95% CI, 3.208–3.289), nurse practitioners (OR, 2.675; 95% CI, 2.658–2.692), dermatologists (OR, 2.396; 95% CI, 2.365–2.428), physician assistants (OR, 2.382; 95% CI, 2.364–2.400), and pediatricians (OR, 2.340; 95% CI, 2.320–2.361). Compared to physicians born in the 1980s, the youngest prescribers in this dataset, all other prescriber birth decades were associated with an increased likelihood of being a high prescriber, most notably in prescribers born in the 1960s (OR, 2.574; 95% CI, 2.531–2.618) (Table 4).
High prescribers also prescribed more broad-spectrum antibiotics than their non–high-prescribing counterparts. High prescribers accounted for 69.0% of third-generation cephalosporin prescriptions, 57.4% of macrolide prescriptions, and 56.0% of extended-spectrum penicillin prescriptions. Interestingly, high prescribers prescribed fluoroquinolones (49.3%) less often than non–high prescribers. High prescribers prescribed all other narrower-spectrum antibiotic groups less often than non–high prescribers (Table 5).
Table 5.
Comparison of Prescriptions for Antibiotic Groups Between High and Non–High Prescribersa
| Antibiotic Group | No. of Prescriptions Written by High Prescribers, No. (%)b |
No. of Prescriptions Written by Non–High Prescribers, No. (%)b |
Ratio of Prescriptions between High and Non–High Prescribers |
|---|---|---|---|
| Third-generation cephalosporins | 341,088 (69.0) | 153,459 (31.0) | 2.22 |
| Macrolides | 745,888 (57.4) | 554,293 (42.6) | 1.35 |
| Extended-spectrum penicillins | 455,771 (56.0) | 357,865 (44.0) | 1.27 |
| Fluoroquinolones | 431,399 (48.3) | 462,626 (51.7) | 0.93 |
| Trimethoprim- sulfamethoxazole | 299,242 (47.7) | 328,328 (52.3) | 0.91 |
| Tetracyclines | 275,373 (47.7) | 301,801 (52.3) | 0.91 |
| Narrow-spectrum penicillins | 736,383 (46.3) | 852,812 (53.7) | 0.86 |
| Urinary agents | 118,734 (43.9) | 152,037 (56.1) | 0.78 |
| First- and second-generation cephalosporins | 311,155 (45.9) | 366,011 (54.1) | 0.85 |
| Lincosamides | 112,843 (35.5) | 204,931 (64.5) | 0.55 |
| Metronidazole | 82,159 (32.0) | 174,224 (68.0) | 0.47 |
| Other | 14,106 (28.7) | 34,969 (71.3) | 0.40 |
| P < .001 |
High prescriber and non–high-prescriber groups each wrote for 50% of total antibiotics.
Percentages are the contribution of high and non–high prescribers, respectively, to the total prescriptions for that antibiotic group.
Discussion
Most antibiotic use occurs in the ambulatory setting.15 Outpatient antimicrobial stewardship is a priority, as evidenced by its inclusion as a 2021 Joint Commission requirement.16 As clinics and healthcare systems focus more on establishing and optimizing outpatient programs, data on prescriber characteristics that may predict high prescribing are vital to marshalling limited resources effectively. To our knowledge, ours is the first publication to describe prescriber characteristics associated with high-prescribing behavior at a state level.
We found that 9.3% of all Tennessee prescribers are responsible for 50% of Tennessee’s outpatient antibiotic prescriptions, a dramatically disproportionate number of the total antibiotic prescriptions. These high prescribers were more likely to prescribe broader-spectrum antibiotics, including more oral third-generation cephalosporins (69.0%), macrolides (57.4%), and extended-spectrum penicillins (56.0%) prescriptions and less narrow-spectrum penicillins (46.3%) and first- or second-generation cephalosporins (45.9%) prescriptions than their non–high-prescribing counterparts (P < .001) (Table 5). This trend has been described previously, though to a lesser degree. Aabenhus et al17 evaluated outpatient antibiotic prescribing in general medicine practices in Denmark and found that 10% of high-prescribing practices, not individual prescribers, accounted for 15% of total antibiotic prescriptions and 18% of “critically important antibiotics,” defined as macrolides, fluoroquinolones, and cephalosporins.
In our study, the highest volume of high prescribers by specialty were nurse practitioners, family medicine doctors, pediatric doctors, physician assistants, and internal medicine doctors. Prescribers in the specialties of urology, nurse practitioner, dermatology, physician assistant, and pediatrics were more likely to be high prescribers than internal medicine prescribers. Previously, urology, dermatology, and family medicine physicians have been shown to be high prescribers of antibiotics compared to other specialties.2,18,19
Nationally, pediatric antibiotic prescriptions have declined; however, Tennessee pediatricians were more likely to be high antibiotic prescribers (OR, 2.340; 95% CI, 2.320–2.361) compared to internal medicine prescribers.3,20,21 Dentists have been reported to prescribe 10%–13% of outpatient antibiotics nationally, but they accounted for 7.6% of antibiotics in Tennessee and were less likely to be high prescribers (adjusted OR, 0.189; 95% CI, 0.187–0.191) than internal medicine prescribers.2,22,23 The cause of this divergence from the national trend is unclear.
In our dataset, physicians born in the 1960s were most likely to be high prescribers compared to those born in the 1980s, the youngest group in this dataset (OR, 2.574; 95% CI, 2.531–2.618). Prior studies have demonstrated that physicians more removed from training are more likely to prescribe antibiotics, although these studies did not specifically quantify age of the physician or years since graduation.9-11 Interestingly, there was not a linear correlation with time from training or age; prescribers born in the 1940s and 1950s demonstrated slightly less propensity to be high prescribers than those born in the 1980s. The same pattern was revealed in a study by Blommaert et al24 in which physicians ages 45–54 in 2008–2009 were much more likely than younger physicians and somewhat more likely than older physicians to prescribe broad-spectrum antibiotics.
Our study has several limitations. The biggest limitation is that information about the total number of patient visits per prescriber and the indication for patient visits was unavailable. This information would have allowed for the calculation of the proportion of visits with an antibiotic prescription, adjustment of prescriber antibiotic use accounting for patient volume, and analysis and conclusions about antibiotic overuse.
The dataset used in this study excluded prescriptions filled via mail order, federal facility, hospital-associated, and other healthcare-facility outpatient pharmacies. As of 2015, mail-in and nonretail pharmacies (which include federal facilities and long-term care facilities) accounted for 18.6% of outpatient antibiotic expenditures: 4.4% from mail-in and 14.2% from nonretail pharmacies.15 Expenditures likely correlate with use; therefore, the number of antibiotic prescriptions would likely increase if these data were included. This limitation might affect the ability to identify all high prescribers within our state, but it likely represents a very small minority who only practice in those settings.
The data included prescribers who wrote at least 1 antibiotic prescription filled in Tennessee in 2016. We know the total number of licensed, potential Tennessee prescribers for 2016, but we do not know the total licensed prescribers by specialty, number actively practicing, nor number of prescribers in full-time versus part-time practice. This lack of data may have affected prescriptions per prescriber results and interpretation. Additionally, nurse practitioners and physician assistants comprise 28.1% of prescribers, but they were not further categorized by practice specialty, which somewhat limited our ability to explore antibiotic prescribing differences among specialty fields and prescriber degrees.
Despite these limitations, the data highlight a central dilemma when developing outpatient antimicrobial stewardship programs: who do you target? Although targeting high-prescribing specialties or groups with a higher percentage of high prescribers, like urology, allows for a more focused, specialized approach to antimicrobial stewardship interventions, the overall antibiotic use impact is less because of the targeted group’s size. Alternatively, targeting groups with larger numbers of prescribers but smaller overall proportions of high prescribers, like nurse practitioners, requires flexible antimicrobial stewardship interventions that consider a broader range of patient presentations and antibiotic indications and require more resources to reach more prescribers; however, if successful, the overall antibiotic use impact can be greater because of a larger group antibiotic use footprint. Here, we introduce a potential third approach: focus on a small but impactful group of high prescribers regardless of specialty, practice location, experience, or gender. Identifying and focusing on a relatively small group with high antibiotic use, in which effective antimicrobial stewardship interventions could reduce outpatient antibiotic prescribing significantly, is a more efficient use of limited resources. Additionally, this strategy would span prescriber specialties, gender, age groups, and practice locations. The challenge with this approach is finding universal antimicrobial stewardship interventions that are relevant to a diverse prescriber group and that can be deployed easily.
To date, no published studies have evaluated outpatient antimicrobial stewardship interventions on the state level. Prior, smaller studies have demonstrated success with locally implemented interventions, although data on sustained success are limited.25 We intend to determine whether our strategy can be implemented effectively. Our next steps, some already underway, include analyzing subsequent years’ data to assess trends and to evaluate whether high prescribers are consistent from year to year. We will then conduct a pilot study in which we will partner with a portion of representative high prescribers to corroborate the data, to identify and correct data gaps, and to ascertain individual information about patient volume and antibiotic indications. Additionally, we will assess prescribers’ perceived challenges to reducing inappropriate antibiotic use. Based on these findings, we intend to develop and study the impact of various antimicrobial stewardship interventions, including offering different types and deliveries of antimicrobial stewardship education, providing individualized antibiotic use data, and/or developing antibiotic prescribing decision support tools to prescribers. Although we recognize the challenges inherent in this approach, we are hopeful that with continued support and creativity, we can develop and implement successful antimicrobial stewardship interventions for all high prescribers across Tennessee. We look forward to sharing the results of our process with other public and private healthcare entities.
In conclusion, these findings offer an initial approach with which other states, healthcare systems, and independent practices, can identify groups on which to focus initial outpatient antimicrobial stewardship program development. Further analysis is needed to understand what drives high prescribing and how to develop cost-effective, relevant, and impactful outpatient antimicrobial stewardship interventions that can be used at a local, regional, state, and/or national level and that can span prescriber differences.
Acknowledgments.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. S.K. received grant support from the National Institute of Allergy and Infectious Diseases Childhood Infection Research Program (ChIRP), National Institute of Health (grant no. 1T32AI095202-07). M.S. received initial grant support from the Centers for Disease Control and Prevention as a recipient of the Leadership in Epidemiology, Antimicrobial Stewardship and Public Health (LEAP) fellowship, sponsored by Society for Healthcare Epidemiology of America (SHEA), Infectious Diseases Society of America (IDSA) and Pediatric Infectious Diseases Society (PIDS). M.S. subsequently received support from the Office of Academic Affiliations, Department of Veterans’ Affairs, VA National Quality Scholars Program, with use of the facilities at the VA Tennessee Valley Healthcare System, Nashville, Tennessee. Y.O., C.E., P.T., and M.K. received grant support from the Centers for Disease Control and Prevention through the Epidemiology and Laboratory Capacity Cooperative Agreement (federal grant no. 5 NU50CK000386-05-00).
Footnotes
PREVIOUS PRESENTATION: These data were presented at the Society for Healthcare Epidemiology of America Spring Meeting in an oral abstract on April 26, 2019, in Boston, Massachusetts.
Conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Patient Safety Atlas. Centers for Disease Control and Prevention website; https://gis.cdc.gov/grasp/PSA/index.html. Published 2019. Accessed July 5, 2019. [Google Scholar]
- 2.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015;60:1308–1316. [DOI] [PubMed] [Google Scholar]
- 3.King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis 2019. doi: 10.1093/cid/ciz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National action plan for combating antibiotic-resistant bacteria, 2015. The White House website; https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Published 2015. Accessed July 5, 2019. [Google Scholar]
- 5.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016;315:1864–1873. [DOI] [PubMed] [Google Scholar]
- 6.Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open 2018;1:e180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palms DL, Hicks LA, Bartoces M. Comparison of antibiotic prescribing in retail clinics, urgent care centers, emergency departments, and traditional ambulatory care settings in the United States. JAMA Intern Med 2018;178:1267–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patient: ICD-10-CM based cross sectional study. BMJ 2019;364:K5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming-Dutra KE, Bartoces M, Roberts RM, Hicks LA. Characteristics of primary care physicians associated with high outpatient antibiotic prescribing volume. Open Forum Infect Dis 2018;5:ofx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadieux G, Tamblyn R, Dauphinee D, Libman M. Predictors of inappropriate antibiotic prescribing among primary care physicians. CMAJ 2007;177:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone S, Gonzales R, Maselli J, Lowenstein SR. Antibiotic prescribing for patients with colds, upper respiratory tract infections and bronchitis: a national study of hospital-based emergency departments. Ann Emerg Med 2000;36:320–327. [DOI] [PubMed] [Google Scholar]
- 12.Boardman C, inventor; IMS Health Incorporated, assignee. System and method for estimating product distribution using a product specific universe. US patent 7,174,304 February 6, 2007.
- 13.Population counts by age group, sex, race and ethnicity, estimates 2016. Tennessee Department of Health, Division of Policy, Planning and Assessment website; https://www.tn.gov/content/dam/tn/health/documents/TN_Population_by_Agegrp_Sex_Race_Ethnicity_-_2016.pdf. Published 2016. Accessed September 2018. [Google Scholar]
- 14.Ingram DD, Franco SJ. 2013 NCHS Urban–rural classification scheme for counties Vital Health Stat 2, pp. 1–73. Centers for Disease Control and Prevention website; http://www.cdc.gov/nchs/data/series/sr_02/sr02_166.pdf. Published 2014. Accessed September 2018. [PubMed] [Google Scholar]
- 15.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin Infect Dis 2018;66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antimicrobial stewardship in ambulatory health care. R3 Report. The Joint Commission website; https://www.jointcommission.org/assets/1/18/R3_23_Antimicrobial_Stewardship_AMB_6_14_19_FINAL2.pdf. Published 2019. Accessed July 5, 2019. [Google Scholar]
- 17.Aabenhus R, Siersma V, Sandholdt H, Køster-Rasmussen R, Hansen MP, Bjerrum L. Identifying practice-related factors for high-volume prescribers of antibiotics in Danish general practice. J Antimicrob Chemother 2017;72:2385–2391. [DOI] [PubMed] [Google Scholar]
- 18.Lebentrau S, Gilfrich C, Vetterlein MW, Schumacher H, et al. Impact of the medical specialty on knowledge regarding multidrug-resistant organisms and strategies toward antimicrobial stewardship. Int Urol Nephrol 2017;49:1311–1318. [DOI] [PubMed] [Google Scholar]
- 19.Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni ML. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infect Control Hosp Epidemiol 2015;36:153–159. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein JA, Stille C, Nordin J, et al. Reduction in antibiotic use among US children, 1996–2000. Pediatrics 2003;112:620–627. [DOI] [PubMed] [Google Scholar]
- 21.McCaig LF, Hicks LA, Roberts RM, Fairlie TA. Office-related antibiotic prescribing for persons aged ≤14 years—United States, 1993–1994 to 2007–2008. Morb Mortal Wkly Rep 2011;60:1153–1156. [PubMed] [Google Scholar]
- 22.Roberts RM, Bartoces M, Thompson SE, Hicks LA. Antibiotic prescribing by general dentists in the United States, 2013. J Am Dent Assoc 2017;148:172–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durkin MJ, Hsueh K, Sallah YH, et al. An evaluation of dental antibiotic prescribing practices in the United States. J Am Dent Assoc 2017;148:878–86.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blommaert A, Coenen S, Gielen B, Goossens H, Hens N, Beutels P. Patient and prescriber determinants for the choice between amoxicillin and broader-spectrum antibiotics: a nationwide prescription-level analysis. J Antimicrob Chemother 2013;86:2383–2392. [DOI] [PubMed] [Google Scholar]
- 25.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;4:CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]