Abstract
Salmonella typhimurium (S. typhimurium) represents an important global public health problem and has the ability to survive under desiccation conditions in foods and food processing facilities for years. The aim of this study was to investigate the effects of Allium sativum (A. sativum) and Cuminum cyminum (C. cyminum) essential oils (EOs) against planktonic growth, biofilm formation and quorum sensing (QS) of S. Typhimurium isolates, the strong biofilm producers. The major components of EOs were determined by gas chromatography–mass spectrometry (GC–MS). Biofilm formation of S. Typhimurium isolates was measured by crystal violet staining. Then, the effects of the EOs on the planktonic cell growth (using determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)), measurement of the synergistic effects of EOs (using checkerboard method), biofilm formation (using microtiter-plate test and scanning electron microscope (SEM)), and expression of QS and cellulose synthesis genes (using quantitative real-time PCR) were assessed. Finally, tetrazolium-based colorimetric (MTT) assay was used to examine EOs cytotoxicity on the Vero cell line. GC–MS analysis showed that terpineol, carene and pinene in C. cyminum EO and sulfur compounds in A. sativum EO were the major components of the plant extract. The Geometric mean of MIC values of the A. sativum and C. cyminum were 0.66 and 2.62 μL mL−1, respectively. The geometric means of the fractional inhibitory concentration index (FICi) for both EOs were calculated as 1.05. The qPCR results showed that MIC/2 concentrations of both EOs significantly down-regulated of QS (sdiA and luxS) and cellulose synthesis (csgD and adrA) genes. Scanning electron microscopy showed the EOs reduced the amount of S. Typhimurium mature biofilm. In general, we showed that C. cyminum and A. sativum EOs can be considered as the potential agents against planktonic and biofilm form of S. Typhimurium without any concern of cytotoxic effect at 4 MIC concentrations on the eukaryotic Vero cells.
Keywords: Salmonella typhimurium, Biofilm, Quorum sensing, Allium sativum, Cuminum cyminum
Introduction
Salmonella enterica serovar Typhimurium can cause infections in humans and animals. Salmonellosis is one of the most important food-borne diseases in the world that has various economic and health effects on human societies. Poultry meat and its derivatives are the best sources of high-quality proteins, transmitting Salmonella infections in different communities (Dar et al. 2017).
Recently, the antibiotic resistance Salmonellatyphimurium (S. typhimurium) is increasing worldwide, especially those related to β-lactams and macrolide (Shaikh et al. 2015). One of the reasons for resistance to antibiotics is biofilm formations. Biofilms are the bacterial colonies, attached to biotic or abiotic surfaces. The attachment occurs via production of extracellular matrix, called exopolysaccharide (Watnick and Kolter 2000). Due to the matrix, biofilm formation protects bacteria from antimicrobial agents and increases their resistance to disinfectants and antibiotics (Ebrahimi et al. 2016). Compared with planktonic counterpart, bacteria living in the biofilm statuses are often up to 1000 times more resistant to antibiotics (Caraher et al. 2007; Ebrahimi et al. 2014).
Quorum sensing (QS) is the mechanism of cell-to-cell communication in the biofilm masses and plays an important role in biofilm formation. QS mechanisms include: (i) acylhomoserine lactone (AHL) QS system (in Gram negative bacteria), (ii) autoinducing peptide (AIP) QS system (in Gram positive bacteria) (iii) autoinducer-2(AI-2) QS system (in both Gram negative and positive bacteria). Salmonella enterica serovar Typhimurium harbors QS systems activated by autoinducer 2 (AI-2) (Brackman and Coenye 2015). sdiA and luxS genes, QS genes, play important roles in the production of fully developed biofilms in Salmonella enterica. The luxS gene encodes the AI-2 proteins (i.e., LuxS protein) (Sperandio et al. 2011; Bai and Rai 2011).
In recent years, QS inhibitors (QSI) compounds are considered as the novel prospective targets for antimicrobial therapies(Rasamiravaka et al. 2015). Natural broad QSI compounds have been found in edible vegetables, fruits, seaweeds, marine sponges (Adonizio et al. 2006; Packiavathy et al. 2012; Brackman and Coenye 2015). Some of the above-mentioned components have been extracted from aromatic plants, such as carvacrol (a natural terpene extracted from thyme or oregano) (Burt et al. 2014), casbane diterpene (ethanolic extract of Croton nepetaefolius) (Carneiro et al. 2010) and naphthalene derivative (extracted from Trachyspermum ammi seeds) (Chaieb et al. 2011). These components are deemed as biofilm formation inhibitors.
Allium sativum (A. sativum) and Cuminum cyminum (C. cyminum) are widely used as food additives in various communities. These plants have also been used as a medicine, as they comprise different antimicrobial components (Palombo 2011). The current study aimed to study to investigate the antibacterial (bacteriostatic and bactericidal activities), anti-biofilm and anti-QS of these plants against S. typhimurium biofilms.
Materials and methods
Bacterial strains and biofilm assay
A total of 78 S. typhimurium strains which had been previously isolated from chicken meat (38 isolates) and human (40 isolates) between November 2015 and April 2017 (Tehran, Iran) were included in this project. All bacterial isolates were evaluated for biofilm formation by end-smooth 96-cell micro-plates as explained by Tendolkar and colleagues (Tendolkar et al. 2004). In the following, ten isolates that were strong biofilm producers were selected to determine the minimum inhibitory concentration (MIC) of EOs.
Plant materials and essential oils extraction
The bulbs of C. cyminum family Apiaceae and bulbs of A. sativum (Garlic) family Liliaceae were obtained from Shahid Beheshti Plant Institute, Tehran, Iran. 50 g of each plant was submitted to hydro-distillation in a Clevenger-type apparatus for 4 h according to the standard procedure (El Gendy et al. 2015). The obtained EOs were stored in a dark glass bottle and kept at 4 °C prior to further analysis. The amount of oil obtained from each plant material was calculated as: .
Gas chromatography–mass spectrometry analysis
The EOs were analyzed by gas chromatography–mass spectrometry (GC–MS) analysis using Hewlett Packard 6890 gas chromatograph system. GC/MS analysis was done on a Thermoquest–Finnigan Trace GC–MS equipped with a DB-5 (5% phenyl) methylpolysiloxane column (60 m × 0.25 mm, film thickness 0.25 μm). Helium was used as the carrier gas at 1 mL/min, and 1 μL of the sample was injected for analysis (Al-Rubaye et al. 2017).
Determination of the MIC and MBC
To do this experiment, the MIC and minimum bactericidal concentration (MBC) of the plant essential oils were evaluated according to the guidelines of National Committee for Clinical Laboratory Standard. Briefly, 100 μL of the bacterial inocula (corresponding to 0.5 of the McFarland) and 100 μL of the twofold serial dilutions of the EOs (0.06–31.5 μL mL−1) in TSB medium were distributed into each well of ELISA microplates (96 well). A negative control, which contained dimethyl sulfoxide (DMSO) and bacteria), and a positive control, which contained DMSO, bacteria and Norfloxacin (1 mg/mL), were also prepared in the last row. It should be noted that DMSO solution was previously used to increase the solubility of the EOs at the final concentration of 0.1%. In the following, ELISA microplates were aerobically incubated at 37 °C in 5% CO2 for 24 h. MIC was recorded as the lowest concentration of the EOs that no visible growth was detected after overnight incubation (Man et al. 2019). To determine MBC, 5-μL solution from the last three wells that did not show bacterial growth were spot-inoculated on blood agar plates and incubated overnight at 37 °C. The MBC was noted as the lowest concentration at which no bacterial colonies were developed.
Measurement of the synergistic effects
The synergistic effects of A. sativum and C. cyminum EOs were assessed using the broth microdilution checkerboard method. The fractional inhibitory concentration index (FICi) for two combined antimicrobial agents was calculated as follows: . The FIC was interpreted as follows: FICi ≤ 0.5: synergy; FICi > 0.5–1.0: addition; FICi < 4.0: indifference and FICi ≥ 4.0: antagonism (Cetin et al. 2013; de Medeiros et al. 2016).
Effect of the EOs on the biofilm formation
The effects of the EOs on the biofilm formation of S. typhimurium were determined by Microtiter plate (MtP) assay (Thenmozhi et al. 2009). Briefly, 98 μL of Trypticase Soy Broth (TSB) that contained MIC/2, MIC/4, MIC/8 and MIC/16 concentrations of the EOs was allocated in four wells for each concentration and 2 μL of overnight cultures (~ 1.5 × 108 CFU/mL) added to each well. One microplate well in each row was also assigned to the control sample. The applied negative controls contained medium DMSO with S. typhimurium. After incubation without agitation for 24 h at 37 °C, the planktonic cells were removed. The surface-adhered cells were stained with 200 μL of 0.2% crystal violet (Hi Media, India) for 20 min and the excess dye solution was removed and the wells were washed with PBS. In the following, crystal violet in the stained cells was solubilized with 100 μL of 95% ethanol and optical density values (OD) was quantified using UV–Vis spectrophotometer (Bio-Tek, Winooski, USA). Eventually, the percentage of inhibition was obtained by the following formula: percentage of inhibition = 100 − [(OD490 nm of the treated wells)/(mean OD490 nm of the negative control wells contained no antimicrobial agent) × 100)] (Onsare and Arora 2015).
Quantitative real-time PCR
The effect of sub-MIC concentrations (MIC/2) of C. cyminum and A. sativum EOs on the expression of QS (sdiA and luxS) and cellulose synthesis (csgD and adrA) gens were measured in clinical isolates (8 strains) and reference strain (S. typhimurium ST38, Pasteur Institute of Iran). Total RNA was extracted using a RNA Isolation Kit (SinaClon, Iran), quantified by BioDrop (BioDrop, UK) and was stored at − 80 °C until further use. Then, complementary DNA (cDNA) was synthesized from RNA template, using the cDNA Reverse Transcription Kit and random primer oligonucleotides (SinaClon, Iran). The primers, qPCR assays and thermocycling conditions was determined as described previously (Latasa et al. 2005; Halatsi et al. 2006; Karavolos et al. 2008; Lee et al. 2009). The primer sequences used in this study are shown in Table 1. The target genes expression levels in comparison to the internal 16 s rRNA control were evaluated with the 2−ΔΔCt method (Livak and Schmittgen 2001), where ΔΔCt = (Ct target genes-Ct 16 s rRNA) treatment−(Ct target genes-Ct 16 s rRNA) control. Three experimental and three technical replicates were used to determine the relative fold changes. FC is presented as mean–standard deviation (SD) from at least three independent qPCR amplifications from each RNA preparation.
Table 1.
Primers used in real-time PCR assays
| Target gene | Sequence 5ʹ–3ʹ | References |
|---|---|---|
| 16 s rRNA |
F: AGGCCTTCGGGTTGTAAAGT R: GTTAGCCGGTGCTTCTTCTG |
Karavolos et al. (2008) |
| bapA |
F: ATGCGCCCAACATTCCTCT R: TGGATGACTGTGCCTGCG |
|
| adrA |
F: GAAGCTCGTCGCTGGAAGTC R: TTCCGCTTAATTTAATGGCCG |
|
| sdiA |
F: AATATCGCTTCGTACCAC R: GTAGGTAAACGAGGAGCAG |
Onsare and Arora (2015) |
| luxS |
F: ATGCCATTATTAGATAGCTT R:GAGATGGTCGCGCATAAAGCCAGC |
Thenmozhi et al. (2009) |
Electron microscopy (SEM)
SEM was employed for investigating the effect of each EO (MIC/2 concentration) on the biofilm structure of S. typhimurium isolate. A glass slide was placed in TSB medium with each EOs (final concentration of essential oil = MIC/2), and incubated for 24 h at 37 °C. Then, the glass slides were extracted from the medium and transported to the pathology laboratory for fixation (in 2.5% buffered glutaraldehyde) and preparation for observation by scanning electron microscope (JEOL JSM-840) at an accelerating voltage of 15 kV.
Cytotoxicity assay
Cytotoxicity of EOs was evaluated using 3-(4, 5-dimethylthiazol-2-yl)—2, 5-diphenyltetrazolium bromide (MTT) assay through the previously described method. Vero cells were cultured in the RPMI-1640 medium supplemented with 10% fetal calf serum, 1% (w/v) glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin as the growth medium (All materials prepared from Gibco, UK). Then, the EOs were added at different concentrations from 0.156 to 20 μL mL-1 in 96-well plates and distributed by Vero cells (cell density = 2 × 104 to 2 × 105). After incubation of plates (37 °C in humidified air containing 5% CO2 for 24 h) and removing of the MTT dye, fluorescence signals were measured at 490 nm using a microplate reader (ELx808, BioTek, USA).
Statistical analysis
Each experiment was performed at least three times, and the data are expressed as means + SD. Eventually, the results were analyzed by Student’s t test using SPSS software and p < 0.05 was considered statistically significant.
Results
Biofilm assay
According to the previous study by Tendolkar and colleagues, isolates were categorized in four groups: (i) non-biofilm forming (OD ≤ ODc), (ii)weak biofilm forming (ODc < OD ≤ 2 ODc), (iii) moderate biofilm forming (2 OD < OD < 4 ODc) and (iv) strong biofilm forming (OD > 4ODc) bacteria. Overall, a total of 78 Salmonella isolates, 30.76% was non-biofilm producers (9 isolates from human, 15 isolates from chicken), 20.5% weak biofilm producers (5 isolates from human, 11 isolates from chicken), 21.8% moderate biofilm producers (12 isolates from human, 5 isolates from chicken) and 27% (14 isolates from human, 7 isolates from chicken) was strong biofilm producers.
GC/MS analysis of essential oils
The amount of oil obtained from C. cyminum and A. sativum was 2.7% and 1.2%, respectively. The results regarding the GC/MS analysis of essential oils are given in Table 2. In this study, the EO obtained from C. cyminum was mainly composed of cuminaldehyde (34.37%), followed by α,β-dihydroxyethylbenzene (22.25%), 2-caren-10-al (16.11%) and γ-terpinene (8.65%); while, the EO from A. sativum contained diallyl tetrasulfide (29.47%), allyl disulfide (22.49%), nitrosothymol (8.55%) and 1H-1,2,4-triazole, 3-thiol-5-methyl (7.75%).
Table 2.
Chemical compositions of the Cuminum cyminum and Allium sativum essential oils
| No | Cuminum cyminum | Allium sativum | ||||
|---|---|---|---|---|---|---|
| Compound | % | RT | Compound | % | RT | |
| 1 | δ-3-Carene | 0.04 | 9.754 | 1,2-Dithiolane | 0.08 | 4.557 |
| 2 | trans-p-Menth-2-ene | 0.06 | 9.376 | Diallyl sulfide | 0.23 | 13.896 |
| 3 | 1-Phenylethanol | 0.08 | 7.143 | 5-Chloro-beznofurazan oxide | 0.34 | 14.096 |
| 4 | α-Terpinene | 0.08 | 9.478 | Methyl 4-methoxy-4,8,12,16-tetramethylheptadecanoate | 0.42 | 25.843 |
| 5 | trans-Sabinene hydrate | 0.09 | 9.479 | Pyridine, 2-(1,2,4-oxadiazol-3-yl) | 0.56 | 25.408 |
| 6 | α-Terpinene | 0.12 | 14.832 | Allyloxy-butyldimethylsilane | 0.67 | 20.281 |
| 7 | Phellandrene | 0.13 | 8.546 | cis-Propenyl methyl disulfide | 0.85 | 4.77 |
| 8 | α-Thujene | 0.16 | 6.274 | Diallyl hexasulfide | 0.87 | 1.23 |
| 9 | Terpineol, Z-.beta | 0.16 | 13.321 | Benzimidazole, 2-amino-1-methyl- | 0.87 | 27.497 |
| 10 | Sabinene | 0.18 | 8.346 | Diallyl pentasulfide | 0.98 | 1.32 |
| 11 | β-phellandrene | 0.19 | 10.405 | Semioxamazide (Thiourea, N,N′-dimethyl-) | 1.02 | 7.046 |
| 12 | Cumene | 0.24 | 8.232 | 2,3-Dicarboxythiophene | 1.06 | 1.75 |
| 13 | p-Menth-1-en-8-ol | 0.42 | 18.354 | 3-vinyl-[4H]-1,2-dithiin | 1.11 | 12.459 |
| 14 | β-Myrcene | 1.42 | 6.731 | 2(1H)-Quinolinone, 3-fluoro-4-hydroxy- | 1.33 | 30.461 |
| 15 | 1,3-Benzenediol, 4-ethyl | 1.61 | 22.432 | Arachidonic acid, trimethylsilyl ester | 1.34 | 23.915 |
| 17 | 4-Isopropyl-1,3-cyclohexadien-1-yl | 1.76 | 17.556 | Diallyl disulfide | 1.79 | 18.685 |
| 18 | α-Pinene | 2.21 | 6.757 | Silane, butyltrimethoxy- | 2.16 | 25.094 |
| 19 | UNKNOWN FROM LIME OIL | 2.53 | 27.621 | M-Dithiane (Catecholborane) | 2.18 | 6.079 |
| 20 | 4-Terpineol | 3.73 | 13.485 | Thiopropionamide | 2.59 | 18.284 |
| 21 | p-Mentha-1,4-dien-7-ol | 4.21 | 29.226 | 1,3,5-Trithiane | 3.07 | 12.065 |
| 22 | β-Pinene | 5.43 | 8.216 | Allyl sulfide | 5.32 | 4.866 |
| 23 | Benzene, 1-methyl-4-(1-methylethyl) | 6.33 | 8.892 | 1H-1,2,4-Triazole, 3-thiol-5-methyl- | 7.75 | 22.805 |
| 24 | γ-Terpinene | 9.39 | 10.125 | Nitrosothymol | 8.55 | 28.87 |
| 25 | 2-Caren-10-al | 12.14 | 23.793 | Diallyl disulfid | 22.49 | 10.583 |
| 26 | α,β-Dihydroxyethylbenzene | 15.16 | 24.543 | Diallyl tetra sulfide | 29.47 | 16.79 |
| 27 | p-Cumic aldehyde | 28.67 | 21.423 | – | – | – |
MIC and MBC determinations
The Geometric mean of the MIC and MBC values of A. sativum and C. cyminum EOs for all isolates were 0.66, 2.18, 2.62 and 5.24 μL mL−1, respectively. Further details are shown in Table 3.
Table 3.
MIC, MBC and FICi of Allium sativum and Cuminum cyminum EOs against the isolates
| Isolates | Source | EOs (μL mL−1) | ||||
|---|---|---|---|---|---|---|
| Allium sativum | Cuminum cyminum | FICi | ||||
| MIC | MBC | MIC | MBC | EOs | ||
| ST38 | – | 0.98 | 1.96 | 1.96 | 3.93 | 0.77 |
| MDR | Human | 0.98 | 1.96 | 3.93 | 7.86 | 0.64 |
| MDR | Human | 0.98 | 3.93 | 3.93 | 7.86 | 0.64 |
| Non-MDR | Human | 0.5 | 1.96 | 1.96 | 3.93 | 1.26 |
| Non-MDR | Human | 0.5 | 1.96 | 1.96 | 3.93 | 1.26 |
| MDR | Chicken | 0.5 | 1.96 | 3.93 | 7.86 | 1.13 |
| Non-MDR | Chicken | 0.5 | 1.96 | 1.96 | 3.93 | 1.26 |
| Non-MDR | Chicken | 0.5 | 1.96 | 1.96 | 3.93 | 1.26 |
| Non-MDR | Chicken | 0.5 | 1.96 | 1.96 | 3.93 | 1.26 |
| Geometric mean | 0.66 | 2.18 | 2.62 | 5.24 | 1.05 | |
MDR Multidrug resistant
Measurement of the synergistic effect
A synergistic action of A. sativum and C. cyminum EOs against planktonic growth isolates was detected by the checkerboard assay and calculation of the Fractional Inhibitory Concentration (FIC) Index. There were no antagonistic effects among the 8 isolates studied, and in the two isolates an addition effect was observed. The geometric mean of the FICi for all strains for EOs was 1.05 (Table 3). A paired sample t test showed significant difference between ΣFIC results of multi-drug resistant (MDR) and non-MDR isolates (p < 0.05).
Effects of the EOs on the biofilm formations
In Table 4, the biofilm formation of the 9 strains (8 isolates and one standard isolates) in the presence of different concentrations of sub-MICs concentrations of both EOs is presented. In fact, as the concentration of the EOs decreased and the amount of optical absorption increased, indicating an increase in biofilm formation. In MtP assay, sub-MICs concentrations of both EOs (Except MIC/16 EO of C. cyminum) play significant roles in inhibition of biofilm formation by all of the bacteria (p < 0.001).
Table 4.
Effects of the EOs in different concentrations on the biofilm formations
| Isolates | Optical density/Allium sativum | Optical density/Cuminum cyminum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC/2 | MIC/4 | MIC/8 | MIC/16 | Control | MIC/2 | MIC/4 | MIC/8 | MIC/16 | Control | |
| ST38 | 1.52 | 1.73 | 1.82 | 2.13 | 2.83 | 1.48 | 1.65 | 1.91 | 2.32 | 2.74 |
| MDR | 1.74 | 1.92 | 2.03 | 2.52 | 2.71 | 1.76 | 1.94 | 2.41 | 2.73 | 2.77 |
| MDR | 1.67 | 1.84 | 1.91 | 2.37 | 2.67 | 1.78 | 1.95 | 2.48 | 2.78 | 2.85 |
| Non-MDR | 1.58 | 1.81 | 1.89 | 2.34 | 2.7 | 1.63 | 1.82 | 2.39 | 2.64 | 2.71 |
| Non-MDR | 1.53 | 1.75 | 1.84 | 2.25 | 2.55 | 1.71 | 1.87 | 2.34 | 2.66 | 2.68 |
| MDR | 1.67 | 1.81 | 1.86 | 2.26 | 2.68 | 1.69 | 1.82 | 2.29 | 2.58 | 2.63 |
| Non-MDR | 1.55 | 1.77 | 1.82 | 2.28 | 2.55 | 1.65 | 1.85 | 2.44 | 2.5 | 2.57 |
| Non-MDR | 1.46 | 1.63 | 1.72 | 2.23 | 2.63 | 1.68 | 1.87 | 2.23 | 2.49 | 2.58 |
| Non-MDR | 1.51 | 1.72 | 1.81 | 2.31 | 2.73 | 1.55 | 1.71 | 2.17 | 2.55 | 2.65 |
Scanning electron microscopy (SEM)
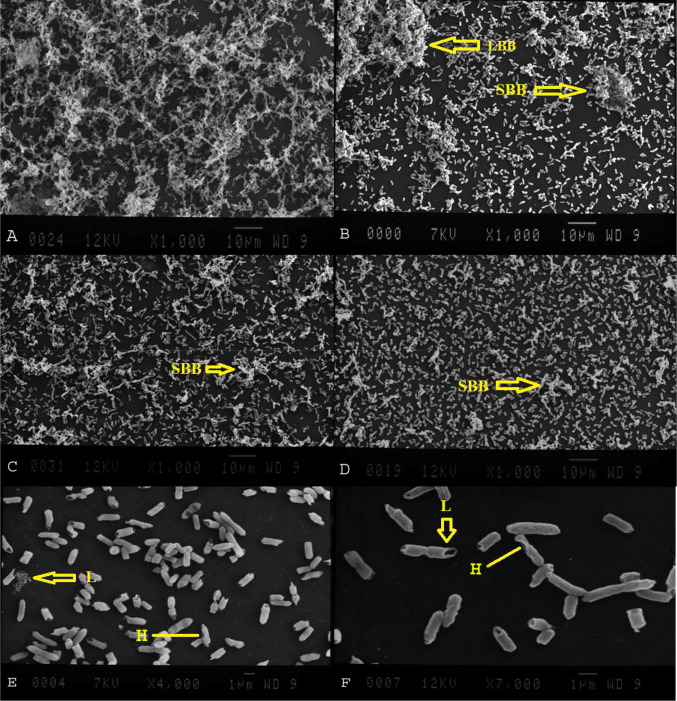
In the control slides, the bacteria were tightly clinging together and encased in a thick matrix, but in bacteria, cells treated with MIC/2 showed the predominant release of cells from biofilms (Fig. 1).
Fig. 1.
Effect of EOs on biofilm of S. typhimurium. a Untreated group. b, eA. sativum at MIC/2, c–fC. cyminum at MIC/2. LBB Large biofilm biomass, SBB small biofilm biomass, H holes, L lysis
Quantification of gene expression by qPCR
The results of qPCR showed that the expression levels of QS (sdiA and luxS) and cellulose synthesis (csgD and adrA) genes were significantly (p < 0.01) reduced in the clinical and reference strains, treated by MIC/2 of Eos, compared to the negative control, non-treated by EOs. As shown in the Table 5, the EO of C. Cuminum has reduced the expression of all genes in all isolates; however, the increased FC was less than 2 in 7 cases, and they were not statistically significant. A. sativum EO caused a significant down-regulation of csgD (6 of 9 strains), adrA (8 of 9 strains) and all of the sdiA and luxS genes in the clinical isolates. In general, the expression of QS genes was further inhibited in comparison with the cellulose genes.
Table 5.
The expression of quorum sensing (QS) and cellulose synthesis gens in the bacteria that were treated with 1/2MIC of Cuminum cyminum and Allium sativum essential oils
| Strains | Cuminum cyminum | Allium sativum | ||||||
|---|---|---|---|---|---|---|---|---|
| sdiA | luxS | csgD | adrA | sdiA | luxS | csgD | adrA | |
| Standard strain (ST38) | − 4.04* | − 3.41* | − 3.83* | − 2.75* | − 3.46* | − 3.31* | − 4.78* | − 3.96* |
| MDR | − 2.82* | − 3.05* | − 2.72* | − 3.56* | − 4.31* | − 2.56* | − 1.73# | − 2.27* |
| MDR | − 1.7# | − 1.56# | − 2.41* | − 2.46* | − 5.63* | − 4.24* | − 2.32* | − 4.63* |
| Non-MDR | − 2.49* | − 5.37* | − 4.14* | − 4.54* | − 3.23* | − 2.58* | − 3.23* | − 3.16* |
| Non-MDR | − 3.27* | − 3.45* | − 1.18# | − 1.83# | − 4.23* | − 3.64* | − 1.43# | − 3.69* |
| MDR | − 2.16* | − 3.83* | − 4.51* | − 2.36* | − 2.24* | − 4.52* | − 1.42# | − 1.74# |
| Non-MDR | − 2.73* | − 3.49* | − 1.07# | − 3.61* | − 2.87* | − 5.72* | − 4.29* | − 3.18* |
| Non-MDR | − 4.16* | − 2.63* | − 3.42* | − 1.29# | − 3.81* | − 3.77* | − 2.86* | − 3.46* |
| Non-MDR | − 4.11* | − 3.42* | − 1.21# | − 4.72* | − 4.13* | − 3.31* | − 2.63* | − 4.14* |
*Significant downregulation
#Non-significant downregulation
Cytotoxicity assay
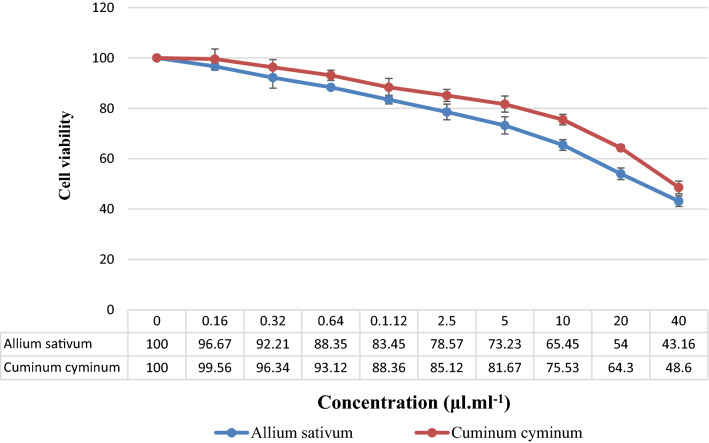
IC50 values for EO C. cyminum and A. sativum were calculated to be 39.5 μL mL−1 and 33.7 μL mL−1, respectively. Detailed data are presented in Fig. 2.
Fig. 2.
Viability percentage of Vero cells after treatment with Cuminum cyminum and Allium sativum essential oil
Discussion
The formation of biofilms is a dynamic and complex process that plays important roles in bacterial resistance to antimicrobial agents. Biofilm formation by S. typhimurium was considered as a chronicity factor for diseases caused by these bacteria. Also, biofilm formation by salmonella SPP is of critical importance in food industries, and may induce massive outbreaks of the infection (Galiè et al. 2018).
In this study, the ability of biofilm formation by S. typhimurium isolated from food and clinical samples was measured using phenotypic and molecular methods. According to our results, 69.2% of Salmonella isolates were capable of forming biofilm while 39% of these isolates formed strong biofilms. However, 67% of strong biofilm producer strains belonged to human isolates. These findings indicated that the human isolates have higher probability of pathogenicity compared to chicken isolates. Sereno et al., (2017) reported that 72% of Salmonella sp. strains isolated from frozen poultry carcasses in Brazil were able to produce biofilm, but none of the isolates showed strong biofilm (Sereno et al. 2017).
Due to the increased resistance to antimicrobial agents, more studies have been performed for controlling biofilm formation by new chemical component. Various natural components such as EOs of C. cyminum and A. sativum have been used to control of bacterial biofilms.
The MIC results demonstrated that the EOs of C. cyminum and A. sativum had strong antimicrobial activities against S. typhimurium isolates. The antibacterial properties of A. sativum are mainly due to the presence of sulfur compounds (Ikram et al. 2019). Also, C. cyminum EO has some compounds, such as pinene, carene, α and β terpineols, Menthol, which have antimicrobial effects. In the present study, the results of the GC analysis showed that the sulfur compounds and phenolic compounds were at an acceptable level in the EOs of A. sativum and C. cyminum, respectively. This is in agreement with the previously published reports, wherein, the EOs containing sulfur and phenolic compounds have strong antimicrobial and antibiofilm activities (Packiavathy et al. 2012; Li et al. 2018).
The synergistic inhibitory effects of EOs on planktonic cell growth were evaluated, using the FICi value with the checkerboard method. We found that the EOs did not have a synergistic effect against S. typhimurium isolates. However, in some of the isolates, the addition effect was observed, which was probably due to the same antimicrobial mechanism.
The anti-biofilm properties of the EOs were examined, using MtP assay. To do this, we used sub-MIC concentrations of the EOs which were insufficient to inhibit the bacterial growth. The results indicated that these EOs can inhibit Salmonella biofilm formation at a sub-MIC concentration. In this regard, Nidadavolu et al. reported that garlic ointment (GarO) could be used as a biofilm inhibitor by wound pathogens including Staphylococcus aureus, Staphylococcus epidermidis, Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae strains (Nidadavolu et al. 2012). Researchers have shown that the garlic component allicin is one of the main factors in inhibiting of biofilm formation by pathogens (Jakobsen et al. 2012, Nidadavolu et al. 2012, Wu et al. 2015). In the present study, allicin was considered as the second most common substance and included 22% of garlic EO, and this can be considered as a reason for controlling Salmonella biofilms. The phenolic compounds in C. cyminum are also known as biofilm inhibitors of bacteria. They interfere with flagella motility and exopolysaccharides (EPS) production by bacteria, and promote the loosening of biofilm architecture (Packiavathy et al. 2012).
SEM is the most commonly used method for analysis of biofilm morphology, which assists in a greater understanding of formation and persistence (Wilson et al. 2017). In the current study, using SEM images demonstrated that MIC/2 concentration of C. cyminum and A. sativum EOs could inhibit formation of biofilm by S. typhimurium. The mechanism of this EOs impact on biofilm formation has not been completely clear though some of the studies have shown that this formation might be connected with inhibition of bacterial adhesion (Nakamoto et al. 2020). Researchers have shown that EO of A. sativum inhibits biofilm formation by reducing metabolic activity (Mohsenipour and Hassanshahian 2015). As presented in the Fig. 1, untreated bacterial cells were well connected to each other and formed microcolonies, but these structures were rarely seen in EOs-treated S. typhimurium cells. Decrease of adhesion and biofilm formation by S. typhimurium cells may be due to anti-adhesive properties of the EOs compounds on production of agents which play essential roles in the biofilm formation (Koo et al. 2013).
Though C. cyminum and A. sativum are well known for their antimicrobial activities against several important pathogens, including enterobacteriaceae, the mechanism of their effects on the biofilm formations has not been fully explained (Jakobsen et al. 2012; Nidadavolu et al. 2012; Packiavathy et al. 2012). In this study, the effect of sub-MIC concentrations of EOs on the expression of biofilm-related genes was measured and the results showed that the MIC/2 concentrations of EOs caused a significant down-regulation of QS and cellulose synthesis gens (p < 0.05) in most of strains, which accounts for the formations of biofilm. Lamas et al. (2016) reported that there was a relationship between the expression of biofilm and QS-related genes in S. typhimurium (Lamas et al. 2016).
Toxicity potential of selected plants in this study has not been demonstrated, but we assessed cytotoxic effects of various concentrations of C. cyminum and A. sativum EOs on the eukaryotic Vero cells using MTT test. Cytotoxicity results demonstrated that C. cyminum and A. sativum EOs at MIC to 4 MIC concentrations had not significant cytotoxic effects.
Conclusion
According to the results, the EOs of C. cyminum and A. sativum are efficient for control and elimination of the planktonic and biofilm forms of S. typhimurium isolates by downregulation of QS (sdiA and luxS) and cellulose synthesis (csgD and adrA) gens. These results show that A. sativum and C. cyminum essences may be suitable as the natural preservatives for controlling the growth of food microorganisms; however, these EOs did not have a synergistic effect against S. typhimurium isolates.
Acknowledgements
The work was funded by AJA University of Medical Sciences in Iran.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
References
- Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol. 2006;105(3):427–435. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Al-Rubaye AF, Hameed IH, Kadhim MJ. A review: uses of gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive natural compounds of some plants. Int J Toxicol Pharmacol Res. 2017;9(8):81–85. [Google Scholar]
- Bai AJ, Rai VR. Bacterial quorum sensing and food industry. Compr Rev Food Sci F. 2011;10(3):183–193. [Google Scholar]
- Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2015;21(1):5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- Burt SA, Ojo-Fakunle VT, Woertman J, Veldhuizen EJ. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE. 2014;9(4):e93414. doi: 10.1371/journal.pone.0093414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraher E, Reynolds G, Murphy P, McClean S, Callaghan M. Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur J Clin Microbiol Infect Dis. 2007;26(3):213–216. doi: 10.1007/s10096-007-0256-x. [DOI] [PubMed] [Google Scholar]
- Carneiro VA, Santos HSd, Arruda FVS, Bandeira PN, Albuquerque MRJR, Pereira MO, et al. Casbane diterpene as a promising natural antimicrobial agent against biofilm-associated infections. Molecules. 2010;16(1):190–201. doi: 10.3390/molecules16010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin ES, Tekeli A, Ozseven AG, Us E, Aridogan BC. Determination of in vitro activities of polymyxin B and rifampin in combination with ampicillin/sulbactam or cefoperazone/sulbactam against multidrug-resistant Acinetobacter baumannii by the E-test and checkerboard methods. Jpn J Infect Dis. 2013;66(6):463–468. doi: 10.7883/yoken.66.463. [DOI] [PubMed] [Google Scholar]
- Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complem Altern Med. 2011;11(1):29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MA, Ahmad SM, Bhat SA, Ahmed R, Urwat U, Mumtaz PT, et al. Salmonella typhimurium in poultry: a review. World Poultry Sci J. 2017;73(2):345–354. [Google Scholar]
- de Medeiros Barbosa I, da Costa Medeiros JA, de Oliveira KÁR, Gomes-Neto NJ, Tavares JF, Magnani M, et al. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis in leafy vegetables. Food Control. 2016;59:468–477. [Google Scholar]
- Ebrahimi A, Hemati M, Dehkordi SH, Bahadoran S, Khoshnood S, Khubani S, et al. Chlorhexidine digluconate effects on planktonic growth and biofilm formation in some field isolates of animal bacterial pathogens. Jundishapur J Nat Pharm Prod. 2014;9(2):1–12. doi: 10.17795/jjnpp-14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi KAA, Habibian DS, Shabanpour Z, Hakimi AR, Hemati M. Effect of benzalkonium chloride on biofilm of bacteria causing nosocomial infections. Med Lab J. 2016;6(10):14–20. [Google Scholar]
- El Gendy AN, Leonardi M, Mugnaini L, Bertelloni F, Ebani VV, Nardoni S, et al. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai. Egypt. Ind Crops Prod. 2015;67(1):201–207. [Google Scholar]
- Galiè S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. Biofilms in the food industry: health aspects and control methods. Front Microbiol. 2018;7(9):898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatsi K, Oikonomou I, Lambiri M, Mandilara G, Vatopoulos A, Kyriacou A. PCR detection of Salmonella spp. using primers targeting the quorum sensing gene sdiA. FEMS Microbiol Lett. 2006;259(2):201–207. doi: 10.1111/j.1574-6968.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Ikram R, Low KH, Hashim NB, Ahmad W, Nasharuddin MNA. Characterization of sulfur-compounds as chemotaxonomic markers in the essential oils of allium species by solvent-free microwave extraction and gas chromatography-mass spectrometry. Anal Lett. 2019;52(4):563–574. [Google Scholar]
- Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother. 2012;56(2):2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolos M, Bulmer D, Winzer K, Wilson M, Mastroeni P, Williams P, et al. LuxS affects flagellar phase variation independently of quorum sensing in Salmonella enterica serovar Typhimurium. J Bacteriol. 2008;190(2):769–771. doi: 10.1128/JB.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92(12):1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas A, Miranda J, Vázquez B, Cepeda A, Franco C. Biofilm formation, phenotypic production of cellulose and gene expression in Salmonella enterica decrease under anaerobic conditions. Int J Food Microbiol. 2016;238(5):63–67. doi: 10.1016/j.ijfoodmicro.2016.08.043. [DOI] [PubMed] [Google Scholar]
- Latasa C, Roux A, ToledoArana A, Ghigo JM, Gamazo C, Penadés JR, et al. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol. 2005;58(5):1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jung BY, Rayamahji N, Lee HS, Jeon WJ, Choi KS, et al. A multiplex real-time PCR for differential detection and quantification of Salmonella spp., Salmonella enterica serovar Typhimurium and Enteritidis in meats. J Vet Sci. 2009;10(1):43–51. doi: 10.4142/jvs.2009.10.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-R, Ma Y-K, Xie X-B, Shi Q-S, Wen X, Sun T-L, et al. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa quorum sensing systems and corresponding virulence factors. Front Microbiol. 2018;7(9):23333. doi: 10.3389/fmicb.2018.03222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Man A, Santacroce L, Jacob R, Mare A, Man L. Antimicrobial activity of six essential oils against a group of human pathogens: a comparative study. Pathogens. 2019;8(1):15. doi: 10.3390/pathogens8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenipour Z, Hassanshahian M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015;8(8):e18971. doi: 10.5812/jjm.18971v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto M, Kunimura K, Suzuki JI, Kodera Y. Antimicrobial properties of hydrophobic compounds in garlic: allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp Ther Med. 2020;19(2):1550–1553. doi: 10.3892/etm.2019.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidadavolu P, Amor W, Tran PL, Dertien J, Colmer-Hamood JA, Hamood AN. Garlic ointment inhibits biofilm formation by bacterial pathogens from burn wounds. J Med Microbiol. 2012;61(5):662–671. doi: 10.1099/jmm.0.038638-0. [DOI] [PubMed] [Google Scholar]
- Onsare J, Arora D. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J Appl Microbiol. 2015;118(2):313–325. doi: 10.1111/jam.12701. [DOI] [PubMed] [Google Scholar]
- Packiavathy IASV, Agilandeswari P, Musthafa KS, Pandian SK, Ravi AV. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res Int. 2012;45(1):85–92. [Google Scholar]
- Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complem Alternat Med. 2011;10:1–15. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int. 2015;10(1):1–17. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno M, Ziech R, Druziani J, Pereira J, Bersot L. Antimicrobial susceptibility and biofilm production by Salmonella sp strains isolated from frozen poultry carcasses. Rev Bras Cienc Avic. 2017;19(1):103–108. [Google Scholar]
- Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Girón JA, Kaper JB. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157: H7. J Bacteriol. 2011;183(17):5187–5197. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun. 2004;72(10):6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenmozhi R, Nithyanand P, Rathna J, Karutha Pandian S. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol. 2009;57(3):284–294. doi: 10.1111/j.1574-695X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182(10):2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, et al. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. J Eng Technol Res. 2017;6(4):1–42. [PMC free article] [PubMed] [Google Scholar]
- Wu X, Santos RR, Fink-Gremmels J. Analyzing the antibacterial effects of food ingredients: model experiments with allicinand garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci Nutr. 2015;3(2):158–168. doi: 10.1002/fsn3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]