Abstract
Background
Left atrial appendage (LAA) closure has been well evaluated in the prevention of stroke in patients with atrial fibrillation. Device embolization remains one of the most common complications. To the best of our knowledge, there have been no reports of late discovery of LAA occluder device embolization at 1.5 years after implantation.
Case presentation
We describe the case of a 77-year-old man who underwent uneventful LAA closure. Echocardiography performed the next day showed the device in place. The patient was discharged but was then lost to follow-up. 1.5 years later, he was admitted for ischemic stroke. Transesophageal echocardiography showed the absence of the occluder device in the LAA. Computed tomography scan of the abdomen showed the device in the abdominal aorta. Due to the high cardiovascular risk, the device was kept in place and the patient was treated medically.
Conclusions
Per-procedural and late device embolization are not uncommon. Review of the literature however showed no report of late discovery of device embolization at 1.5 years. Follow-up echocardiography is mandatory for the detection of endothelialization or embolization.
Keywords: Left atrial appendage closure, Watchman device, Atrial fibrillation, Stroke, Echocardiography, Case report
Background
Several studies have evaluated different left atrial appendage (LAA) occluder devices and demonstrated non-inferiority in stroke prevention compared to warfarin in patients with atrial fibrillation (AF) [1, 2]. Early device embolization remains one of the most common complications, which requires urgent extraction. We herein describe a case of late discovery of an occluder device embolization that was not extracted but rather medically managed.
Case presentation
A 77-year-old male patient with a medical history significant for permanent AF with a CHA2DS2-VASC score of 6, ischemic stroke with residual seizure and two hemorrhagic strokes, was referred for LAA closure using a Watchman device (Boston Scientific, Inc., Natick, Massachusetts). LAA morphology and measurements were obtained from cardiac computed tomography (CT) angiography and transesophageal echocardiography (TEE). LAA was bilobed. The maximum width of the ostium was measured at 20 mm. Hence, a 24 mm device was successfully implanted. The device was well aligned with the axis of the LAA. A gentle tug test did not change the device position. The patient remained stable and there were no complications noted during or after the procedure. Transthoracic echocardiography (TTE) performed the next day showed the device in place. The patient was discharged with a scheduled TEE six weeks after the procedure but was lost to follow-up.
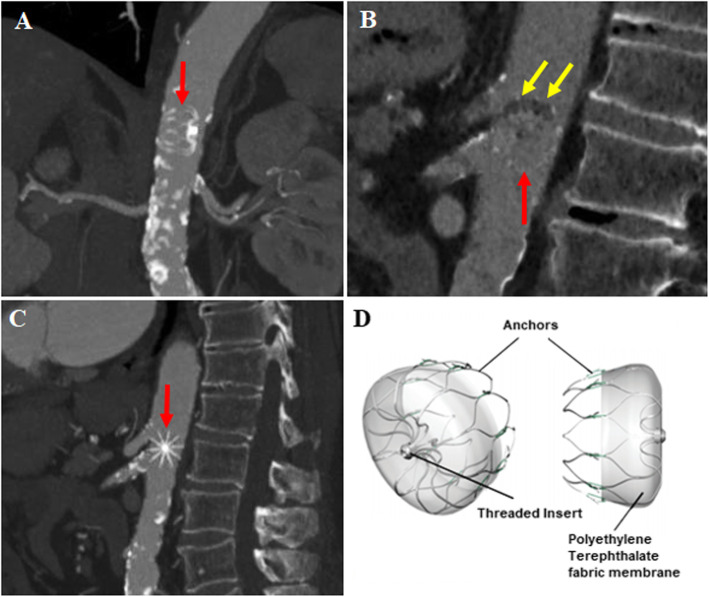
1.5 years later, he presented with two new ischemic strokes and unexplained left foot pain. Repeat TTE/TEE showed the absence of the occluder device in the LAA. CT scan of the chest and abdomen showed the device in the abdominal aorta between the ostium of the celiac trunk and the superior mesenteric artery (Fig. 1, Panels A-C). Mild thrombosis was seen in the device at the level of the fabric membrane (Panels B and D). The abdominal aorta was severely calcified (Panels A and C). Due to the high cardiovascular risk, surgical or percutaneous extraction were not done and the device was kept in place. Low dose aspirin was added to his medical treatment. The patient died 3 months later from seizure.
Fig. 1.
Watchman device (red arrows) located in the abdominal aorta in coronal (a), sagittal right (b) and sagittal left (c) views. Note the mild thrombus formation in the device in panel B (yellow arrows). Panel d illustrates the general structure of the Watchman device
Discussion and conclusions
Complications of Watchman device implantation are rare, with device embolization rates of 0.6 and 0.7% [1, 2]. Device extraction can be performed either percutaneously via a snare introduced in the femoral artery sheath (e.g., for Watchman device), or surgically (for larger devices) [3]. Percutaneous removal remains the treatment of choice for vascular embolization, particularly in patients with multiple comorbidities and the elderly population. Device embolization risk depends on the operator’s experience, the choice of device size and the final position. Patient related characteristics such as LAA morphology and length, ostium size or unusual morphologies are also important criteria. Per procedural TEE guidance is mandatory, thereby avoiding vigorous tug testing (usually performed for proof of device stability). Nevertheless, aggressive physical movements are not advised before endothelialization [4].
Published articles retrieved from PubMed database included single center/multicenter registries, randomized controlled trials, observational studies, case reports and a systematic review [3–24] (Table 1). Device embolization occurred mostly during the procedure and within 7 days in the described cases. Some cases reported embolization at 45 and 48 days [3, 16, 19]. A study published by Swaans et al. [5] described device embolization 3 months following the procedure. Another case described percutaneous retrieval of an AMPLATZER cardiac plug 6 months after embolization [23]. In a systematic review, Aminian et al. [24] concluded that embolization occurred mostly in the periprocedural period but late embolization was not uncommon. Review of the literature however showed no report of late discovery of device embolization at 1.5 years. Since in the majority of cases device embolization is asymptomatic, patient education for short and long term follow-up is extremely important as there is no way to know the exact timing of device embolization. Hence, in our case, embolization could have occurred earlier but was lately picked up due to loss of follow-up.
Table 1.
Summary of published data on Watchman device embolization
| Reference | Study Design | Number of device embolization | Device size | Device location | Timing | Retrieval Approach |
|---|---|---|---|---|---|---|
| Holmes et al. [2] | Randomized controlled trial (N = 269) | 2 | 27 mm | LV | Post procedure day 1 | Surgery |
| Holmes et al. [3] | Randomized non-inferiority trial (N = 463) | 3 | 30 mm |
LV Thoracic Aorta AA |
Intraprocedural 45 days 45 days |
Surgery Percutaneous (femoral – snare) Surgery |
| Sick et al. [4] | Multicenter registry (N = 66) | 2 | NA | NA | Intraprocedural | Percutaneous (femoral – snare) |
| Swaans et al. [5] | Single center registry (N = 30) | 1 | NA | AA | 3 months | Surgery |
| Reddy et al. [6] | Multicenter registry (N = 150) | 2 | NA | Descending Aorta | Intraprocedural | Percutaneous (femoral – snare) |
| Matsuo et al. [7] | Single center registry (N = 179) | 2 | NA | AA | Post procedure within 12 h | Percutaneous (femoral – snare) |
| Pérez Matos et al. [8] | Case report | 1 | 27 mm | LV | Post procedure day 1 | Transapical access and pulling catheter |
| Chopra et al. [9] | Case report | 1 | 34 mm | LA | Post procedure day 1 | Transseptal |
| Vivek et al. [10] |
Registry (N = 3822) |
9 | NA | NA | NA |
6 surgery 3 Percutaneous |
| Boersma et al. [11] | Cohort (N = 1025) | 2 | NA | NA | Within 7 days |
1 surgery 1 percutaneous |
| Vivek et al. [12] | RCT (N = 707) | 3 | NA | NA | Early | NA |
| Pillarisseti et al. [13] | Multicenter observational study (N = 478) | 1 | NA | NA | NA | Surgery |
| Betts et al. [14] | Multicenter retrospective registry (N = 371) | 1 | NA | NA | Per procedure | NA |
| Saw et al. [15] | Multicenter experience | 1 | NA | NA | Early | Percutaneous –Snared |
| Fanari et al. [16] | Case report | 1 | 21 mm | AA | 48 days | Percutaneous |
| Gabriels et al. [17] | Case report | 1 | 24 mm | LA | Intraprocedural | Percutaneous – transseptal |
| Fastner et al. [18] | Case report | 1 | NA | LA | Intraprocedural | Percutaneous |
| Hai Deng et al. [19] | Case report | 1 | 30 mm | Aortic arch | 45 days | Percutaneous – snared |
| Stollberger et al. [20] | Case report | 1 | 30 mm | LV | Periprocedural | Surgery |
| Barth et al. [21] | Case Report | 2 |
24 mm 21 mm |
LA Descending Aorta |
Periprocedural |
Percutaneous – transseptal Percutaneous –Snared |
| Bôsche et al. [22] | Single center prospective study | 1 | NA | NA | Within 7 days | Percutaneous |
| Obeid et al. [23] | Case report | 1 | 24 mm | LA | 6 months | Percutaneous |
| Aminian et al. [24] | Systematic Review | 21 | NA |
9 Aorta 9 LV 3 LA |
Until 90 days |
Surgical Percutaneous |
AA abdominal aorta, LA left atrium, LV left ventricle, NA not applicable
We report a unique case of late discovery of LAA occluder device embolization in the abdominal aorta. Per procedural and follow-up echocardiography is crucial for the detection of device endothelialization or embolization.
Acknowledgments
This article was also published as an abstract in EP Europace [25].
Abbreviations
- AF
Atrial fibrillation
- CT
Computed tomography
- LAA
Left atrial appendage
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
Authors’ contributions
MJM collected the data and drafted the manuscript, CB reviewed the literature and contributed in data interpretation, RD and MG revised the manuscript, AN took care of the patient and contributed in the conception and design of the manuscript, and JM extensively revised the manuscript and was the main investigator. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Patient’s next-of-kin gave written consent for their relatives personal or clinical details along with any identifying images to be published in this manuscript.
Competing interests
Professor Jacques Mansourati is consultant and receives research fees from Boston Scientific and Abbott Laboratories. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamad Jihad Mansour, Email: mohamad_J_mansour@hotmail.com.
Clément Bénic, Email: clementbenic@gmail.com.
Romain Didier, Email: romain.didier@gmail.com.
Martine Gilard, Email: martine.gilard@gmail.com.
Jacques Mansourati, Email: jacques.mansourati@chu-brest.fr.
References
- 1.Fountain RB, Holmes DR, Chandrasekaran K, Packer D, Asirvatham S, Van Tassel R, et al. The PROTECT AF (WATCHMAN left atrial appendage system for embolic PROTECTion in patients with atrial fibrillation) trial. Am Heart J. 2006;151(5):956–961. doi: 10.1016/j.ahj.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet Lond Engl. 2009;374(9689):534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 4.Sick PB, Schuler G, Hauptmann KE, Grube E, Yakubov S, Turi ZG, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49(13):1490–1495. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Swaans MJ, Post MC, Rensing BJWM, Boersma LVA. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J Am Heart Assoc. 2012;1(5):e002212. doi: 10.1161/JAHA.112.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, et al. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix feasibility study with watchman left atrial appendage closure technology) J Am Coll Cardiol. 2013;61(25):2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo Y, Sandri M, Mangner N, Majunke N, Dähnert I, Schuler G, et al. Interventional closure of the left atrial appendage for stroke prevention. Circ J. 2014;78(3):619–624. doi: 10.1253/circj.CJ-13-0828. [DOI] [PubMed] [Google Scholar]
- 8.Pérez Matos AJ, Swaans MJ, Rensing BJWM, Heijmen RH, Mast EG, Boersma LVA, et al. Embolization of a left atrial appendage closure device unmasked by intermittent left bundle branch block. JACC Cardiovasc Interv. 2014;7(9):e115–e117. doi: 10.1016/j.jcin.2014.01.177. [DOI] [PubMed] [Google Scholar]
- 9.Chopra M, Wong YH, Sondergaard L, De Backer O. Percutaneous retrieval of an embolized left atrial appendage closure device from the left atrium in a patient with previous MitraClip. BMC Cardiovasc Disord. 2019;19:196. doi: 10.1186/s12872-019-1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy VY, Gibson DN, Kar S, O’Neill W, Doshi SK, Horton RP, et al. Post-approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69(3):253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Boersma LVA, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 13.Pillarisetti J, Reddy YM, Gunda S, Swarup V, Lee R, Rasekh A, et al. Endocardial (watchman) vs epicardial (lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm. 2015;12(7):1501–1507. doi: 10.1016/j.hrthm.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Betts TR, Leo M, Panikker S, Kanagaratnam P, Koa-Wing M, Davies DW, et al. Percutaneous left atrial appendage occlusion using different technologies in the United Kingdom: a multicenter registry. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2017;89(3):484–492. doi: 10.1002/ccd.26782. [DOI] [PubMed] [Google Scholar]
- 15.Saw J, Fahmy P, Azzalini L, Marquis J-F, Hibbert B, Morillo C, et al. Early Canadian multicenter experience with WATCHMAN for percutaneous left atrial appendage closure. J Cardiovasc Electrophysiol. 2017;28(4):396–401. doi: 10.1111/jce.13168. [DOI] [PubMed] [Google Scholar]
- 16.Fanari Z, Goel S, Goldstein JA. Successful percutaneous retrieval of embolized transcatheter left atrial appendage closure device (watchman) using a modified vascular retrieval forceps. Cardiovasc Revasc Med. 2017;18(8):616-18. [DOI] [PubMed]
- 17.Gabriels J, Beldner S, Khan M, Zeitlin J, Jadonath R, Patel A. Embolization of watchman device following a hybrid radiofrequency ablation of atrial fibrillation and watchman implantation procedure. J Cardiovasc Electrophysiol. 2017;28(7):835–836. doi: 10.1111/jce.13201. [DOI] [PubMed] [Google Scholar]
- 18.Fastner C, Lehmann R, Behnes M, Sartorius B, Borggrefe M, Akin I. Veno-venous double lasso pull-and-push technique for transseptal retrieval of an embolized watchman occluder. Cardiovasc Revascularization Med Mol Interv. 2016;17(3):206–208. doi: 10.1016/j.carrev.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Deng H, Liao H, Liu Y, Chen S, Xue Y, Zhan X, et al. Acute heart failure caused by dislocation of a WATCHMAN left atrial appendage Occluder. JACC Cardiovasc Interv. 2016;9(10):e97–e99. doi: 10.1016/j.jcin.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Stöllberger C, Schneider B, Finsterer J. Serious complications from dislocation of a watchman left atrial appendage occluder. J Cardiovasc Electrophysiol. 2007;18(8):880–881. doi: 10.1111/j.1540-8167.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 21.Barth C, Behnes M, Borgrefe M, Akin I. Peri-interventional embolization of left atrial appendage occlusion devices: two manoeuvers of successful retrieval. Eur Heart J Case Rep. 2018;2(1):yty001. doi: 10.1093/ehjcr/yty001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bösche LI, Afshari F, Schöne D, Ewers A, Mügge A, Gotzmann M. Initial experience with novel Oral anticoagulants during the first 45 days after left atrial appendage closure with the watchman device. Clin Cardiol. 2015;38(12):720–724. doi: 10.1002/clc.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obeid S, Nietlispach F, Luscher TF, Alibegovic J. Percutaneous retrieval of an endothelialized AMPLATZER cardiac plug from the abdominal aorta 6 months after embolization. Eur Heart J. 2014;35(47):3387. doi: 10.1093/eurheartj/ehu361. [DOI] [PubMed] [Google Scholar]
- 24.Aminian A, Lalmand J, Tzikas A, Budts W, Benit E, Kefer J. Embolization of left atrial appendage closure devices: a systematic review of cases reported with the watchman device and the amplatzer cardiac plug. Catheter Cardiovasc Interv. 2015;86(1):128–135. doi: 10.1002/ccd.25891. [DOI] [PubMed] [Google Scholar]
- 25.Benic C, Noel A, Fofana A, Pouliquen MC, Didier R, Fatemi M, Mansourati J. P474 Late discovery of a left atrial appendage occluder embolization. EP Europace. 2018;20(suppl_1):i97. doi: 10.1093/europace/euy015.283. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.