Abstract
Objective
To identify different trajectories of disease activity in patients with RA following initiation of a first anti-TNF.
Methods
Patients with RA starting their first anti-TNF between 2001 and 2013 were selected from the British Society for Rheumatology Biologics Register for RA. Six-monthly DAS28-ESR scores were used to identify trajectories of disease activity using latent class modelling. Data were included for six follow-ups after registration (approximately 3 years). Subgroup analysis examined changes in disease activity profiles over time.
Results
A total of 14 436 patients with RA starting their first anti-TNF were enrolled between 2001 and 2013 (13 115 between 2001 and 2008, 1321 between 2010 and 2013). The mean number of DAS28-ESR scores was 3.5/patient (s.d. 2.1), with a mean of 184.9 days (s.d. 69.9) between scores. The DAS28-ESR nadir was achieved within 250 days of commencing anti-TNF, although apparent trajectory divergence emerged by first 6-monthly follow-up at 180 days. Four distinct response trajectories comprised the most stable model. Most patients fitted into ‘modest’ (7986 patients; 55.3%) or ‘substantial’ (4676 patients; 32.4%) response trajectories. Of the remainder, 1254 (8.7%) and 520 (3.6%) fitted ‘maximal’ and ‘minimal’ response trajectories, respectively. There was a significant (P < 0.01) increase in proportion achieving ‘maximal’ response between 2001–2008 and 2010–2013.
Conclusion
This is the largest study to identify long-term response trajectories with anti-TNF. By 6 months, longer-term trajectory profiles of DAS28 could already be identified, with many patients identified earlier. The majority of patients had persistent moderate response, equivalent to maintained DAS28-ESR moderate disease activity. The maximal response trajectory (equivalent to sustained DAS2-ESR remission) was only achieved by approximately one-third of patients.
Keywords: rheumatoid arthritis, biologic therapies, outcome measures, epidemiology, DMARDS
Rheumatology key messages
Sustained remission is uncommon in biologic-naïve patients taking anti-TNF for RA.
Six-month DAS28-ESR scores are representative of long-term outcomes in biologic-naïve RA patients starting anti-TNF.
Introduction
Over the past 20 years, anti-TNF medications have radically changed the therapeutic landscape of RA. Treat-to-target (T2T) approaches aim to rapidly achieve a state of remission with regular reviews and medication changes and are associated with improved outcomes and reduced radiographic damage [1]. Because RA is a chronic disease, remission should be maintained over time (sustained remission) and is associated with improved outcomes and reduced mortality in early arthritis cohorts [2, 3]. While extensive evidence supports anti-TNF use, most studies report single time point outcomes and studies of sustained response to anti-TNF are sparse [4]. Contributing to challenges in establishing an evidence base for sustained remission is the difficulty in achieving a standardized definition of the outcome, in terms of both which outcome measures to use, and the minimum duration required to be classed as ‘sustained’ [5].
T2T approaches use a threshold (remission) as the ‘target’ of treatment. However, most clinical outcome measures have a continuous scale, and in a clinical setting the use of pre-defined thresholds to define sustained remission can pose challenges for patients straddling remission thresholds. For these patients, evidence would suggest further intensification of treatment. However, they may feel well, and potential additional clinical improvements in symptoms may only be slight [6].
Latent class modelling methods are an alternative way of assessing longitudinal disease activity and identifying different trajectories of disease activity within these data without requiring a pre-set threshold. Such methodology uses multiple longitudinal measures of disease activity to identify mean trajectories [7]. This allows accurate representation of longitudinal outcomes of a cohort of patients, without artificial dichotomization of data, and could predict future outcomes given a patient’s prior trajectory. Such modelling techniques have previously been used to identify different classes of response to T2T strategies in an early arthritis cohort and in RA patients taking abatacept [8–10].
This study aims to identify different trajectories of disease activity in patients with RA following initiation of a first anti-TNF.
Methods
This is an analysis of patients receiving anti-TNF who are part of the British Society for Rheumatology Biologics Register for RA (BSRBR-RA). The BSRBR-RA, launched in 2001, is a national, prospective, longitudinal, observational study examining long-term effectiveness and safety of biologic agents in patients with RA in the UK. Ethical approval was obtained from the Multicentre Research Ethics Committee for the North-West of England. All patients enrolled provided written informed consent. The methods of the BSRBR-RA have been described previously [11]. Biologic use in England and Wales is directed by NICE guidance, which requires patients to have persistent high disease activity (DAS28 score of >5.1) despite treatment with at least two conventional synthetic DMARDs (csDMARDs), one of which should be methotrexate (unless contraindicated) [12]. Therefore, the BSRBR-RA is a cohort enriched for high baseline disease activity, and does not include early arthritis patients.
Patient data are collected 6-monthly for the first 3 years following enrolment. Thereafter data collection occurs annually. Only the first 3 years of data were included for each patient to ensure sufficient data granularity to identify temporal changes in disease activity. All patients starting their first anti-TNF between 2001 and 2013 were included, to allow 3 years of data collection up to a data censor date of 30 September 2016. There was a pause in new enrolments for anti-TNF in the BSRBR-RA between 2008 and 2010, so this was chosen as the point to split the dataset to allow evaluation of changes in responses to anti-TNF treatment over time (enrolments between 2001 and 2008 were included in one subgroup, and enrolments between 2010 and 2013 included in the second subgroup). This also coincided with the publication of the first EULAR recommendations for the management of RA that incorporated T2T recommendations, which is likely to have influenced clinical practice subsequently [6]. Elapsed time was calculated in days from the date on the baseline enrolment form and subsequent DAS28-ESR measurements. Data were censored at time of switching to another anti-TNF or biologic agent, or discontinuation of anti-TNF.
Data were examined to ensure ‘missingness’ occurred at random and bootstrapped multiple imputation (using five datasets) was used for missing data. Latent class mixed modelling (LCMM; R package) was used to model DAS28-ESR scores over time and identify different trajectories [7]. The lcmm function was used and a linear link function was selected, which deployed a standard linear mixed model (using the same estimation as the hlme function within the lcmm package). DAS28-ESR scores were modelled over time and grouped by patient. The lowest Bayesian Information criterion (BIC) was used to identified the model with the number of trajectories that best fit the data of the whole cohort [13]. Because of the difference in subgroup sizes, BIC values were used to identify the most stable model in the smaller subgroup (2010–2013) and this model was then applied to the larger subgroup (2001–2008) to allow subgroup comparisons. Posterior probabilities were examined to investigate the reliability of the selected model [14]. The graphical representations of all the trajectories inferred by the LCMM for all the imputed datasets were manually checked (by P.H.) to ensure trajectories identified were similar between imputations.
Results
A total of 14 436 patients with RA starting their first anti-TNF were enrolled between 2001 and 2013. Of these, 13 115 were enrolled between 2001 and 2008, and 1321 between 2010 and 2013. The baseline characteristics of the patients are outlined in Table 1. The mean (s.d.) number of DAS28-ESR scores per patients was 3.5 (2.1), with a mean (s.d.) of 184.9 (69.9) days between scores, and 198.2 (75.3) days between enrolment and first recorded DAS28-ESR.
Table 1.
Baseline characteristics and change in patient characteristics at start of first anti-TNF recorded in the BSRBR-RA over-time
| Variable | Whole cohort (2001–2013) | 2001-2008 subgroup | 2010-2013 subgroup | P-valuea |
|---|---|---|---|---|
| Number | 14 436 | 13 115 | 1321 | NA |
| Female, % | 76.3 | 76.3 | 75.7 | 0.6 |
| Age, (s.d.), years | 56.0 (12.3) | 56.0 (12.2) | 56.3 (12.7) | 0.4 |
| DAS28-ESR, range 0–10 (s.d.) | 6.5 (1.0) | 6.6 (1.0) | 6.0 (1.0) | <0.01 |
| Swollen joint count, range 0–28 (s.d.) | 11.1 (6.2) | 11.4 (6.2) | 8.7 (5.2) | <0.01 |
| Tender joint count, range 0–28 (s.d.) | 15.5 (7.4) | 15.6 (7.4) | 14.6 (7.5) | <0.01 |
| Patient global assessment, range 0–100 mm (s.d.) | 72.5 (19.8) | 72.5 (19.8) | 72.2 (19.5) | 0.6 |
| ESR, (s.d.), mm/h | 44.7(28.2) | 46.0 (28.3) | 29.6 (22.8) | <0.01 |
| HAQ, range 0–3 (s.d.) | 2.0 (0.6) | 2.0 (0.6) | 1.6 (0.7) | <0.01 |
| BMI, (s.d.), kg/m2 | 27.2 (8.1) | 27.0 (6.8) | 29.6 (17.1) | <0.01 |
| Disease duration, mean, median (s.d.), years | 12.7, 11.0 (9.6) | 13.0, 11.0 (9.6) | 9.6, 6.0 (9.5) | <0.01 |
| Time from first rheumatology consult to biologics, mean, median (s.d.), years | 12.0, 10.0 (9.0) | 12.2, 10.0 (8.9) | 9.5, 6.0 (9.0) | <0.01 |
| Baseline MTX, n (%) | 8176 (56.6) | 7332 (55.9) | 844 (63.9) | <0.01 |
| Current smoker, n (%) | 3108 (21.8) | 2861 (22.0) | 247 (19.9) | 0.03a |
| Ever smoked, n (%) | 5368 (37.7) | 4922 (37.8) | 446 (36.0) | |
| Never smoked, n (%) | 5778 (40.5) | 5232 (40.2) | 546 (44.1) |
Unless otherwise specified, numbers shown are mean values. aComparing subgroups using unpaired t test, except gender and smoking data, which used χ2. BSRBR-RA: British Society for Rheumatology Biologics Register for RA; NA: not applicable.
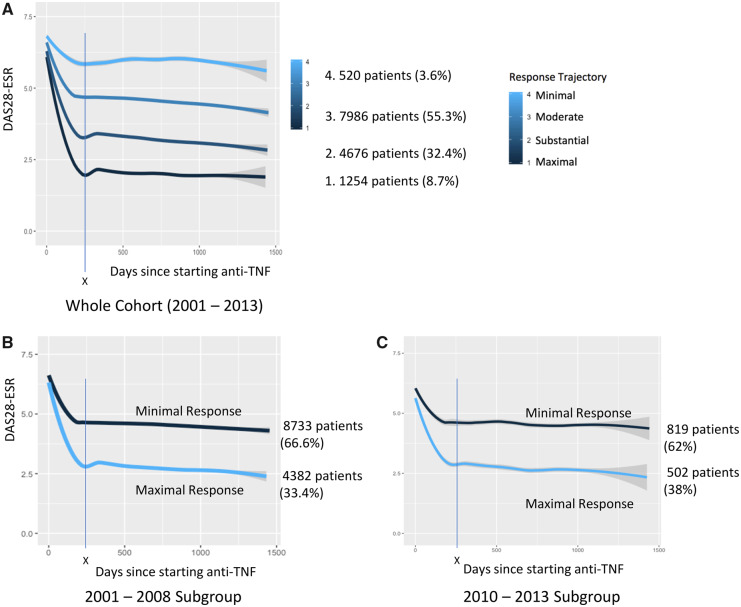
Mean BIC was calculated for two- (219 136), three- (219 063), four- (219 032) and five-class (219 038) LCMM models in the whole cohort (2001–2013). The four-class model had the lowest mean BIC representing the most stable model. The most stable LCMM model for the 2010–2013 subgroup was the two-class model (BIC values: two-class: 18 585; three-class: 18 600; four-class: 18 617). Because LCMM does not adhere to predefined DAS28-ESR disease activity thresholds, the trajectories for the four-class model were labelled maximal/substantial/modest/minimal, and maximal and minimal for the two-class model, to respectively represent the greatest to least reduction in DAS28-ESR from baseline. Graphical representation showed four distinct trajectories of response for the whole cohort, and two trajectories for the 2001–2008 and 2010–2013 subgroups, with the greatest reduction in DAS28-ESR achieved by 250 days after starting anti-TNF in all groups (Fig. 1).
Fig. 1.
Illustrative trajectory plots of response to anti-TNF over time for two- and four-class trajectory models
(A) The four-class model had the lowest BIC for the overall dataset and is shown here. The two-class model was most stable for the 2010–2013 subgroup. To allow comparison between 2001–2008 and 2010–2013 subgroups, the two-class graphical illustrations are shown in (B) and (C). X: maximal response is seen in all trajectories by 250 days. Grey shading represents 95% CI.
Posterior probabilities for the two-class model show a high likelihood of correct trajectory classification (Supplementary Table S1, available at Rheumatology online). For the four-class model, posterior probabilities for the maximal and minimal trajectory classification were high (>0.7) [7]. However, the posterior probabilities for the substantial and modest response trajectories were more ambiguous, with values of 0.62 and 0.64, respectively. The maximal response trajectory was uncommon with only 1254 patients (8.7%) achieving this outcome. The majority of patients achieved either a modest (7986 patients; 55.3%) or a substantial response (4676 patients; 32.4%). Only 520 patients (3.6%) were classified as minimal responders. There was a significant (χ2P < 0.01) improvement in maximal response in the two-class model from 4382 patients (33%) in the 2001–2008 subgroup to 502 patients (38%) in the 2010–2013 subgroups (Fig. 1).
Discussion
This study is the first to apply LCMM to evaluate patterns of treatment response in RA patients with high disease activity commencing their first anti-TNF therapy. Different trajectory patterns are identifiable and emerge within a few months of treatment instigation. These data support current NICE guidance for assessing response to anti-TNF at 6 months (180 days), by which point different trajectories of response are clearly defined [12]. All trajectories follow a similar profile, with an initial rapid change in DAS28-ESR followed by a plateau phase where disease activity scores achieved by 250 days remain stable. Our results show that responses seen at 6 months are representative of long-term outcomes and that it may be possible to identify long-term responses to anti-TNF earlier than 6 months.
Other studies have shown that responses to anti-TNF can be seen earlier than 6 months [15, 16]. The graphical representation of the trajectories from this study (Fig. 1) suggest that this may be the case in these data as well, although confirmatory DAS28-ESR data are sparse before 6 months (Supplementary Fig. S1, available a Rheumatology online).
Unlike other studies we did not observe differences in rate of response (such as a rapid and gradual response curve), or a ‘U-shaped’ response trajectory representing a secondary non-response [8, 10]. The lack of a U-shaped secondary non-response trajectory may be due to the infrequency of secondary non-response in the BSRBR-RA dataset, or that a 6-month data collection is insufficiently frequent to identify secondary non-response before switching occurs (and hence censoring from the dataset). Examination of the trajectory plots (Fig. 1) shows a small rebound at approximately 6 months in maximal and substantial response trajectories, which may be evidence of early secondary non-responders, who subsequently switch drug before the next data collection point. The absence of ‘rapid’ or ‘gradual’ responder curves may be because these trajectories truly do not exist in the dataset, or that an insufficient frequency of assessments misses this response trajectory.
The minimal response trajectory in the four-class model is likely to comprise patients from the earlier years of the BSRBR-RA when there were fewer options for switching biologic, and/or patients who may have refractory disease in which clinician and patient may have decided further improvements in disease activity are unlikely with alternative disease modifying agents [17]. The minimal response trajectory could be explained by the fact that trajectories are smoothed group-level trajectories and do not show all the linked individual data points for each patient. An individual patient’s disease activity will fluctuate around the smoothed mean trajectory over time and temporary improvements may encourage a clinician and patient to continue the anti-TNF, which with hindsight can be seen to be ineffective on average. There may also be other undocumented factors that may make a clinician and patient opt to remain on a treatment despite ongoing disease activity. Analyses of factors associated with the attainment of different levels of disease activity were not undertaken, but they have been examined in depth in other work by our group and others [4].
The number of patients achieving the maximal or substantial response trajectory in the four-class model is similar to that of other studies investigating sustained remission, and highlights that the majority of patients taking anti-TNFs agents in this cohort (and others) have persistent moderate disease activity [4]. Even when examining outcomes from patients commenced on anti-TNF after 2010, the majority of patients do not achieve sustained responses in the remission or low disease activity range. These data demonstrate that despite changes in clinical practice and widely accepted T2T recommendations, the clinical reality of outcomes of RA patients taking anti-TNF is more modest than may be perceived [18]. EULAR recommendations suggest a target of remission or low disease activity should be a realistic goal for the majority of RA patients, and advise that if these targets are not obtained, treatment should be intensified or switched as soon as is possible. Furthermore, these recommendations are based upon the EULAR and ACR agreed Boolean, or simplified or clinical DAI definition of remission, which is even more stringent than the DAS28-ESR definition of remission used in this study [19, 20]. The results of this study show that while the EULAR recommendations are sound and robustly evidence-based, achieving the target of sustained remission or low disease activity may remain aspirational for the majority of patients who require anti-TNF in the UK and elsewhere, most of whom have already shown resistance to csDMARDs.
This study has limitations. It is an observational study, and as such, causality cannot be inferred. While data examined are from a cross-section of clinical sites across the UK and the BSRBR-RA has broad inclusion criteria, it is possible recruitment bias may have occurred. Data collection occurred 6-monthly. While separation of trajectories appears to occur early after anti-TNF commencement and there are data for this period (Supplementary Fig. S1, available at Rheumatology online), it is limited, and there is no formal data collection before 6 months. Therefore, it remains possible the earlier separation seen is an artefact of trajectory smoothing within the statistical software. It should also be noted that the BSRBR-RA only collects data at 6-monthly intervals meaning that any additional clinic visit data between these 6-monthly intervals is not captured. While the four-class trajectory model is the most stable model identified for the cohort as a whole, there is still a degree of uncertainty about the class assignment relating to the substantial and modest trajectories.
This is the largest study of response trajectories to anti-TNF in patients with RA and shows that the goal of achieving sustained remission or low disease activity (maximal and substantial response trajectories) is seldom achieved, with the majority of patients only achieving a sustained moderate disease activity (modest response trajectory).
Different trajectories of response identified at 6 months are indicative of future outcomes, with maximal therapeutic benefit identified ∼250 days after starting anti-TNF. Early divergence of trajectories suggests assessment of anti-TNF efficacy may be possible before the currently recommended 6-month review, which would enable patients with suboptimal response to switch treatments earlier, improving outcomes and minimizing cost of inefficacious treatments. Further work is necessary to identify if the results identified for anti-TNF agents are common across all DMARDs, and for patients who have switched to second- or third-line biologic agents.
Funding: This work was supported by the British Society for Rheumatology (BSR). The BSR commissioned the BSR Biologics Register in rheumatoid arthritis (BSRBR-RA) as a UK wide national project to investigate the safety of biologic agents in routine medical practice. K.H. is the principal investigator. BSR receives restricted income from UK pharmaceutical companies, including Abbvie, Celltrion, Hospira, MSD, Pfizer, SOBI, Samsung, UCB and Roche. This income finances a wholly separate contract between the BSR and the University of Manchester. The principal investigator and the BSRBR-RA team at the University of Manchester have full academic freedom and are able to work independently of pharmaceutical industry influence. All decisions concerning analyses, interpretation and publication are made autonomously of any industrial contribution. Members of the BSRBR-RA University of Manchester team, BSR trustees, committee members and staff complete an annual declaration in relation to conflicts of interest. All relevant information regarding serious adverse events outlined in the manuscript have been reported to the appropriate pharmaceutical company as per the contractual agreements/standard operating procedures.
Disclosure statement: P.H. has received honoraria of less than $10 000 from Decision Resources Group Ltd and provided consultancy for, and has an options and limited royalty agreement with, Living With Ltd software company for development of a health-related mobile phone application that is unrelated to the work in this manuscript; K.H. has received grant income (paid to her institute) from BMS, Pfizer and UCB for work unrelated to this manuscript. K.L.H. has received honoraria/speakers fees (paid to her institute) from Abbvie and BMS; N.M. has received grant income from Eli Lilly and Celgene for work unrelated to this manuscript; the other authors have declared no conflicts of interest.
Supplementary Material
Acknowledgements
The authors acknowledge the enthusiastic collaboration of all consultant rheumatologists and their specialist nurses in the UK in providing the data (visit www.bsrbr.org for a full list of contributors). The authors would like to gratefully acknowledge the support of the National Institute for Health Research, through the Comprehensive Local Research Networks at participating centres. In addition, the authors acknowledge support from the BSR Executive, the members of the BSRBR Registers Committee and the BSRBR Project Team in London for their active role in enabling the register to undertake its tasks. The authors also acknowledge the seminal role of the BSR Clinical Affairs Committee for establishing national biologic guidelines and recommendations for such a register. This work was conducted on behalf of BSR, and P.H. would like to thank BSR for its help and support. Infrastructure, database and statistical support for the BSRBR-RA was also received from the Arthritis Research UK Centre for Epidemiology (Arthritis Research UK Grant ID 21755). P.H.’s research was funded by the BSR through the Clinical Research Fellowship in Musculoskeletal Epidemiology.
References
- 1. Stoffer MA, Schoels MM, Smolen JS. et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scirè CA, Lunt M, Marshall T, Symmons DPM, Verstappen S.. Early remission is associated with improved survival in patients with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis 2014;73:1677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermeer M, Kuper HH, Moens HJB. et al. Sustained beneficial effects of a protocolized treat-to-target strategy in very early rheumatoid arthritis: three-year results of the Dutch Rheumatoid Arthritis Monitoring remission induction cohort. Arthritis Care Res 2013;65:1219–26. [DOI] [PubMed] [Google Scholar]
- 4. Hamann P, Holland R, Hyrich K. et al. Factors associated with sustained remission in rheumatoid arthritis in patients treated with anti-tumor necrosis factor. Arthritis Care Res 2017;69:783–93. [DOI] [PubMed] [Google Scholar]
- 5. Ajeganova S, Huizinga T.. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis 2017;9:249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Proust-Lima C, Philipps V, Liquet B.. Estimation of extended mixed models using latent classes and latent processes: the R Package lcmm. J Stat Softw 2017;78:1–56. [Google Scholar]
- 8. Siemons L, Klooster ten PM, Vonkeman HE, Glas CAW, van de Laar M.. Distinct trajectories of disease activity over the first year in early rheumatoid arthritis patients following a treat-to-target strategy. Arthritis Care Res 2014;66:625–30. [DOI] [PubMed] [Google Scholar]
- 9. Barnabe C, Sun Y, Boire G. et al. Heterogeneous disease trajectories explain variable radiographic, function and quality of life outcomes in the Canadian early arthritis cohort (CATCH). PLoS One 2015;10:e0135327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Courvoisier DS, Alpizar-Rodriguez D, Gottenberg JE. et al. Rheumatoid arthritis patients after initiation of a new biologic agent: trajectories of disease activity in a large multinational cohort study. EBioMedicine 2016;11:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watson K, Symmons D, Griffiths I, Silman A.. The British Society for Rheumatology biologics register. Ann Rheum Dis 2005. Nov;64(Suppl 4):iv42–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NICE. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. NICE Technology appraisal guidance, TA 375. 2016. https://www.nice.org.uk/guidance/ta375 (1 November 2019, date last accessed).
- 13. Schwarz G. Estimating the dimension of a model. Ann Stat 1978;6:461–4. [Google Scholar]
- 14.Kelly D, Smith C.. More complex models for random durations In: Bayesian Inference for Probabilistic Risk Assessment. London: Springer, 2011: 39–50. [Google Scholar]
- 15. Singh JA, Hossain A, Tanjong Ghogomu E. et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2016;(5):CD012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis JR, Yang S, Chen L. et al. Predicting low disease activity and remission using early treatment response to anti-tumour necrosis factor therapy in patients with rheumatoid arthritis: exploratory analyses from the TEMPO trial. Ann Rheum Dis 2012;71:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kearsley-Fleet L, Davies R, De Cock D. et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018;77:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 19. Felson DT, Smolen JS, Wells G. et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felson DT, Smolen JS, Wells G. et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.