Abstract
The incidence of acute myeloid leukemia (AML) is rising and the outcome of current therapy, which has not changed significantly in the last 40 years, is suboptimal. Cellular oxidative state is a credible target to selectively eradicate AML cells, because it is a fundamental property of each cell that is sufficiently different between leukemic and normal cells, yet its aberrancy shared among different AML cells. To this end, we tested whether a short-time treatment of AML cells, including cells with FLT3-ITD mutation, with sub-lethal dose of dichloroacetate (DCA) (priming) followed by pharmacologic dose of arsenic trioxide (ATO) in presence of low-dose DCA could produce insurmountable level of oxidative damage that kill AML cells. Using cellular cytotoxicity, apoptotic and metabolic assays with both established AML cell lines and primary AML cells, we found that priming with DCA significantly potentiated the cytotoxicity of ATO in AML cells in a synergistic manner. The combination decreased the mitochondrial membrane potential as well as expression of Mcl-1 and GPx in primary AML cells more than either drug alone. One patient with AML whose disease was refractory to several lines of prior treatments was treated with this combination, and tolerated it well. These data suggest that targeting cellular redox balance in leukemia may provide a therapeutic option for AML patients with relapsed/refractory disease.
Keywords: AML, Oxidative Stress, Glycolysis, Mitochondria, Antioxidants
Introduction
Arsenic trioxide (ATO) is approved by the U.S. Food and Drug Administration (FDA) for treatment of patients with acute promyelocytic leukemia (APL) whose disease failed to respond to or relapsed following all-trans retinoic acid/anthracycline therapy1. In APL, ATO induces both differentiation and apoptosis of leukemic cells2. It is reported that ATO can open the permeability transition pore complex (PTPC) in mitochondrial membranes by directly binding to thiol groups in the PTPC. This decreases mitochondrial membrane potential (ΔΨm), which activates caspase pathways, and results in induction of apoptosis3. Additionally, inhibition of the reactive oxygen species (ROS)-related enzymes glutathione peroxidase and glutathione reductase by ATO may potentiate intracellular ROS accumulation and enhance apoptosis4,5. ATO has shown promising clinical activity when used for treatment of non-APL acute myeloid leukemia (AML) combined with low-dose cytarabine in patients aged ≥ 60 years6 or as a priming agent before use of cytarabine and idarubicin in patients aged < 60 years7. Higher concentrations of ATO (0.5 to 2.0 μM) is required to induce apoptosis in leukemia cells in vivo compared to lower concentrations (0.1 to 0.5 μM) to induce differentiation8,9. This appears to be a limiting factor for clinical use of ATO for treatment of patients with non-APL AML, because increasing the dose of ATO to achieve plasma concentration of > 0.5 μM will cause severe treatment-related adverse events.
In both normal and neoplastic cells, glucose, via glycolysis, is converted to pyruvate. When oxygen is available, pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase (PDH) in the mitochondrion and further metabolized through the tricarboxylic acid cycle. When oxygen is limited, for example in bone marrow microenvironment, transcription factor hypoxia inducible factor-1α (HIF-1α) induces pyruvate dehydrogenase kinase (PDK), which phosphorylates and inactivates PDH10. Dichloroacetate (DCA) binds to the active site of PDK and inactivates its kinase activity11, hence, shunts more pyruvate into mitochondrion and away from conversion to lactate. DCA, administered at 12.5–50 mg/Kg body weight, has been tested clinically for the treatment of lactic acidosis in children and adults12–16. Inhibition of PDK also can decrease ΔΨm of hyperpolarized mitochondria in cancer cells resulting in augmented ROS production and induction of apoptosis17,18. Several in vitro studies report on anti-neoplastic activity of single agent DCA in a variety of cancer cell lines with IC50 values ranging from 17 to 40 mM after 48 hours exposure19–22. Orally administered DCA has shown anti-tumor effects in a xenograft model of human non-small cell lung carcinoma in rats23. A few case reports have shown DCA activity in refractory non-Hodgkin’s lymphoma and hepatocellular carcinoma24,25. DCA also has shown promising anti-neoplastic activity in a clinical trial involving patients with glioblastoma multiforme26. High dose DCA can be toxic to neuromuscular cells that are heavily dependent on glycolysis; however, at a dose of 6.25 mg/kg orally twice daily which anti-neoplastic activity still can be exerted, no patients with brain tumor experienced clinically significant peripheral neuropathy26.
The combination of DCA and ATO has been tested against breast cancer cells and has shown to be more detrimental to cell survival than either drug alone27. AML is genetically very heterogeneous which has contributed to many failed attempts for targeting any particular mutation28; hence, targeting fundamental cellular property such as reductive-oxidative (RedOx) state independent of any specific genetic aberrancy appears to be a reasonable hypothesis to test. In this study, we attempted to take advantage of metabolic dysregulation of leukemia cells to kill them more effectively with less required dose of ATO and DCA. We hypothesized that “priming” mitochondria by directing more pyruvate into oxidative phosphorylation and away from glycolysis, with DCA, followed by addition of ATO would have synergistic anti-leukemic effect.
Materials and Methods
AML cell lines and primary AML cells from patients
AML cell lines MOLM-14, MV4–11 (with FLT3-ITD mutation), MonoMac 6 (with activating FLT3-V592A point mutation), and THP-1 (FLT3 wild type) were purchased from the American Type Culture Collection (ATCC). The short tandem repeat (STR) analysis was not done on leukemia cell lines. The primary leukemia cells were isolated from bone marrow or peripheral blood of AML patients under the auspices of the institutional (IRB approved) Tumor and Cell Procurement Bank at University of Maryland and Johns Hopkins University with informed patient consent in accordance with the Declaration of Helsinki. The mononuclear cells were isolated using Lymphocyte Separation Medium (Cellgro, Mediatech) according to manufacturer’s protocol. Primary cells were used either fresh or after viable freezing in FBS/5% DMSO. AML16, AML17, and AML18 cells were used after thawing. AML20 cells were used freshly collected without cryopreservation. All cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS (HyClone, Thermo Scientific) and 200 mM L-Glutamine (Life Technologies).
Reagents
Arsenic trioxide (ATO), an injectable solution at 5.055 mM (Trisenox®, Cephalon, Inc.) was used in vitro according to the manufacturer’s recommendation. For clinical use, ATO was given intravenously according to the U.S. FDA Prescribing Information1. Sodium dichloroacetate salt (DCA) was purchased from Sigma for in vitro studies, 2M stock solution prepared in phosphate buffered saline (PBS), and was filtered, aliquoted and stored at +4°C. For patient use, clinical grade sodium dichloroacetate (Catalogue number Z2301) were purchased from TCI America (Portland, OR). DCA was weighed by a pharmacist based on mg/Kg body weight and given to the patient after dissolving it in 20–30 mL water. DCA was taken on an empty stomach every 12 hours.
Cell Viability and Cytotoxicity
Decreased cell viability induced by ATO, DCA or their combination was tested in AML cell lines and primary cells using metabolic alterations measured by WST-1 reagent (Roche) and by measuring the frequency of cells with non-damaged plasma membrane by trypan blue exclusion. Cells were seeded in 96-well plates the day before treatment. DCA and/or ATO were added at different concentrations (0.08–100 mM and 0.0045–5.055 μM, respectively). Control cells were treated with equivalent volume of vehicle (PBS) for each drug. Four to eight replicates were performed for each drug dilution. Every experiment was repeated at least twice. Cells were cultured in presence of drugs for 72 h (48 h for primary cells) and terminated by addition of WST-1 reagent. Colorimetric readouts were performed using Synergy HT, Multidetection Microplate Reader (BioTek). IC50 and IC30 values were calculated using GraphPad Prism v.5 software. After the treatment with ATO, DCA, or their combination, cell viability was also determined using trypan blue exclusion. Cells were counted using Countess® Automated Cell Counter (Invitrogen, Life Technologies).
Potentiation and Drug Synergism (Isobologram) Assays
The effect of DCA presence on potentiating ATO cytotoxicity (i.e., decreasing IC50) was investigated by combining the two agents sequentially. Cells were seeded for 24 h, then exposed (primed) to low dose DCA (IC30 for each cell line) for 24 h, then treated with ATO in the presence of freshly added DCA at IC30 daily for additional 24–48 hours.
For determination of synergism, the results of the WST-1 assay were analyzed by median effect analysis using Calcusyn v.2.11 software (Biosoft, Ferguson, MO, USA), which uses the principles developed by Chou and Talalay29 to determine whether combinations of DCA and ATO were synergistic, additive or antagonistic. Briefly, cells were treated with DCA, ATO, or their combination at equal ratios of 0.125–2X of IC50 for each drug and dose-response curves were generated for each drug individually and combined. Combination indices (CI) were calculated by the software Calcusyn. The software generates the effective doses that kill a specific percentage of the cells at IC10, IC25, IC50, and IC75 by each drug alone or in combination. Synergism is defined when the CI values are less than 0.9. Additive combinations would result in a CI between 0.9 and 1.1 and antagonistic combinations give a CI of greater than 1.1.
Detection of Cell Apoptosis
Leukemia cells were primed with DCA at their corresponding IC30 or vehicle (PBS) for 24 hours. In control group, cells continued to be exposed only to vehicle. For treatment groups, the cells were treated with DCA at IC30 alone or in combination with ATO (IC50) plus DCA (IC30) for 24 hours for primary cells or 48 hours for cell lines. At planned time points cells were harvested, washed and stained using FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen) and acquired within one hour on FACScan (BD Bioscience). The amount of apoptosis was analyzed using FlowJo software (Tree Star).
Detection of changes in Mitochondrial Membrane Potential
Alteration of mitochondrial membrane potential upon treatment with DCA, ATO or their combination as described above was tested using MitoPotential Red (Millipore). Cells were first primed with DCA at their corresponding IC30 or vehicle for 24 hours and then treated with DCA alone (IC30) or ATO (IC50) plus DCA (IC30) for 24 hours for primary cells and 48 hours for cell lines. Cells were stained using MitoPotential Red and acquired within one hour on LSRII (BD Bioscience). The change in mitochondrial membrane potential was analyzed using FlowJo software (Tree Star).
Intracellular ROS assay
Induction of intracellular ROS was measured using a 2′,7′-dichlorodihidro-fluorescein diacetate, CM-H2DCFDA, probe (Life Technologies) according to manufacturer’s recommendation. Approximately 2×106 of MOLM-14 or THP-1 cells were stained with 5 μM solution of CM-H2DCFDA for 30 minutes at 37°C. Next, the staining solution was removed by centrifugation; cells were added to 96-well plate at 20,000 cells/well and treated with DCA, ATO or their combination as described above at 37°C for additional time (up to 48 hours). The cell fluorescence was measured using Synergy HT, Multidetection Microplate Reader (BioTek) at 480/528 nm. Hydrogen peroxide (H2O2, 100 μM) (Sigma) solution was used as a positive control for ROS assay. In parallel wells, 4 mM N-acetylcysteine (Sigma) was added to scavenge the produced ROS.
Western blot analysis and quantitation
Effect of DCA, ATO and their combination on different protein expression was measured in AML cell lines and primary AML cells. Total protein extracts were prepared using RIPA buffer (SIGMA) supplemented with Complete Mini™ protease inhibitor and PHOStop™ phosphatase inhibitors (Roche). Equal amounts of proteins (up to 25 μg) were separated on 4–12% NuPAGE gels in 1X MOPS or 1X MES buffer (Invitrogen) and transferred onto PVDF membranes (Millipore). The membrane was blocked with 5% dry milk in 1X TBS/0.1% Tween 20 (TBST) for at least one hour at room temperature, and incubated with human specific primary antibodies: GPx (Cell Signaling Technologies), and Mcl-1 (Santa Cruz) or mouse anti-β-actin (SIGMA) overnight at 4°C. Next day membrane was washed 3 times in TBST and incubated with a horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody (Cell Signaling Technologies) for one hour at room temperature. Blots were again washed and the signal was detected with SuperSignal West Femto Chemiluminescent Substrate (Pierce) and exposed to HyBlot CL® autoradiography film (Denville). The signals were quantified using the Image J v.1.48s software (NIH). The signal for each antibody was normalized to β-actin and then relative difference was calculated against vehicle control at each time point (numbers below each Western blot strip).
GSH Measurement
Levels of total reduced glutathione (GSH) were measured using a Glutathione (GSSH/GSH) detection kit from Enzo Life Sciences (Farmingdale, NY). The enzymatic assay is based on the interaction of the sulfhydryl group of GSH on DTNB (5,5′-dithiobis-2-nitrobenzoic acid, Ellman’s reagent) producing a yellow colored 5-thio-2-nitrobenzoic acid (TNB) that absorbs at 405 nm. The rate of TNB production directly correlates to the concentration of glutathione. Approximately 5×106 MOLM-14, THP-1 and AML20 cells were exposed to vehicle, ATO (IC50), DCA (IC30) or their combination for 24 hours. As per the manual, after cells were washed in PBS, they were homogenized in 5% (w/v) metaphosphoric acid and frozen/thawed twice. The suspension was spun at 12,000 g for 5 minutes at +4oC and the supernatant was frozen at −80oC. After running the assay as directed by the kit manual, the absorbance was measured using Synergy HT, Multidetection Microplate Reader (BioTek) at 405 nm. GSH were measured using Graphpad Synergy software and its concentration was calculated in micromole per liter in cell lysate.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Software. All p-values are two-sided, unpaired and calculated by Student t-test and those <0.05 were considered as statistically significant.
Results
DCA and ATO are toxic to AML cells; both to cells with FLT3-ITD mutation and FLT3-WT
Fms-like tyrosine kinase receptor 3 internal tandem duplication (FLT3-ITD) mutation is one of the most clinically relevant mutations in AML that dictates significant poor outcome even after hematopoietic stem cell transplantation (HSCT)30. Since it has been reported that ITD mutation of FLT3 increases ROS production in AML cells31, we elected to measure the cytotoxic effect of ATO or DCA in both FLT3 mutant and FLT3 wild type cells. We used MOLM-14 and MV4–11 cells, which carry FLT3-ITD mutation, MonoMac6 with FLT3-V592A activating single point mutation and THP-1 cells, which are FLT3 wild type (FLT3-WT). Similarly, we tested primary leukemia blasts isolated from AML patients with or without FLT3-ITD mutation.
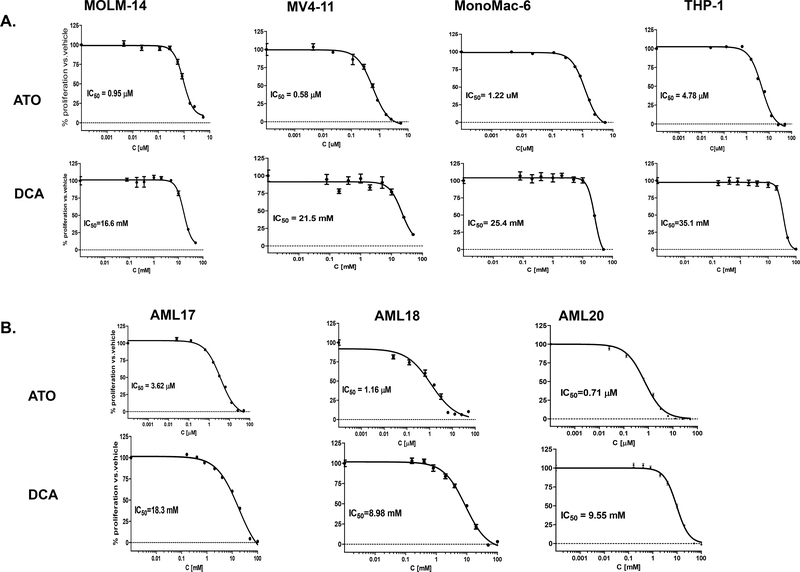
Cell lines carrying FLT3-ITD mutation as well as FLT3-WT were sensitive to ATO and DCA. Representative growth inhibition curves for ATO and DCA for each cell are shown in Figure 1A. The mean of IC50s and IC30s (for DCA) are listed in Table 1. For primary leukemia cells (AML17, AML18, and AML20), representative graphs are shown in Figure 1B and IC50s are listed in Table 1.
Figure 1. AML cell lines and primary cells with or without FLT3-ITD mutation are sensitive to ATO and DCA treatment.
MOLM-14, MV4–11, MonoMac6 and THP-1 cells as well as AML17, AML18, AML20 cells were exposed to a range of ATO and DCA concentrations as described in Methods and Materials with approximate IC50 range of 0.5–5 μM for ATO and 9–35 mM for DCA. Cells were cultured in presence of drugs for 72 h (48 h for primary cells) and terminated by addition of WST-1 reagent. The representative growth inhibition curves for each cell are shown in this Figure. Error bars represent standard error of mean of triplicate wells. The mean ± standard deviation of IC50 values are shown in Table 1.
Table 1. Cytotoxic effects of DCA, ATO or their combination on AML cell lines and primary AML cells.
Leukemia cells showed sensitivity to DCA, ATO and sequential combination of both drugs. IC50 values were calculated as mean ± standard deviation from at least two independent experiments (48 h exposure for primary cells and 72 h exposure for cell lines). If the availability of the primary blasts was limited, the IC50 was obtained from a single experiment. IC50: inhibitory concentration that inhibits proliferation of 50% cells; IC30: inhibitory concentration that inhibits proliferation of 30% cells; PF = potentiation factor (the reverse ratio of IC50 when cells were treated with DCA+ATO compared to vehicle+ATO for 24 h exposure for primary cells and 48 h exposure for cell lines); Decreased Cell Viability: Percentage of increased cell kill when cells were sequentially treated with IC30 of DCA versus vehicle for 48 h and ATO for 48 h; CI = combination index; FLT3: Fms-like tyrosine kinase 3; ITD = internal tandem duplication, V592A mutation = activating point mutation in FLT3 gene.
| Cell | FLT3 Status | IC50 | IC30 (DCA) | Potentiation Factor (PF); IC50 of ATO primed with DCA vs IC50 of ATO alone | Decreased Cell Viability | CI | |
|---|---|---|---|---|---|---|---|
| ATO (μM) | DCA (mM) | ||||||
| MOLM-14 | ITD | 0.88 ± 0.04 | 15.9 ± 1.2 | 11.8 ± 2.5 | 2.0 (0.78 vs 1.60) | 44% | 0.80 |
| MV4–11 | ITD | 0.53 ± 0.03 | 19.1 ± 2.3 | 11.3 ± 0.13 | 1.8 (1.13 vs 2.06) | 93% | - |
| MonoMac6 | V592A | 1.08 ± 0.1 | 25.1 ± 2.0 | 23.7 ± 2.1 | 2.2 (0.88 vs 1.9) | 75% | 0.81 |
| THP-1 | WT | 4.68 ± 0.13 | 35.5 ± 0.3 | 29.5 ± 1.1 | 0.9 (5.25 vs 4.55) | 20% | 1.1 |
| AML17 | WT | 4.35 ± 1.3 | 19.4 ± 1.6 | 8.6 ± 1.1 | 1.8 (1.53 vs 2.75) | NMa | 0.71 |
| AML18 | ITD | 1.22 ± 0.08 | 9.9 ± 1.3 | 5.3 ± 0.0 | 1.3 (1.41 vs 1.87) | NM | 0.62 |
| AML20 | WT | 0.7 | 9.6 | 5.8 | NM | NM | NM |
NM=not measured
Combination of DCA and ATO demonstrates synergistic effect in killing leukemia cells
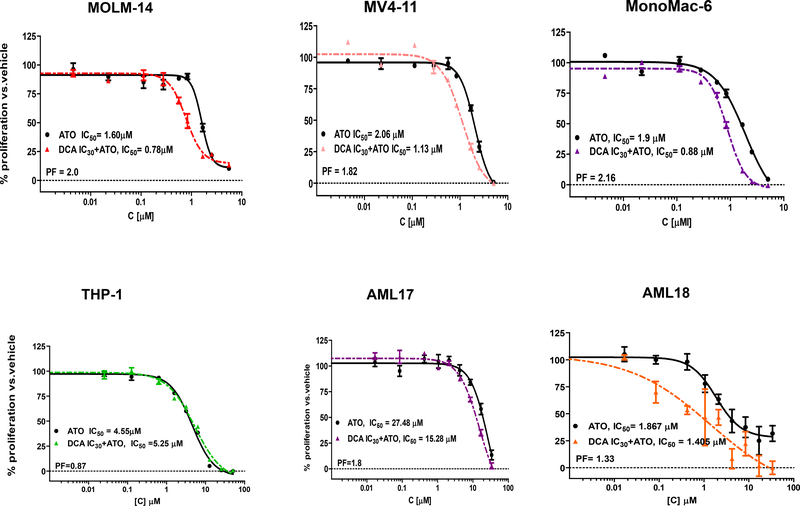
Next, we investigated whether priming AML cells with low dose DCA could potentiate the cytotoxic effect of ATO in vitro. We defined “priming” as treatment of leukemia cells for 24–48 hours with IC30 of DCA. This dose is considered non-cytotoxic since the reported in vivo half-life is less than one hour and we used a very low concentration dependent on the sensitivity of each individual cell line. Leukemia cells were treated either with ATO alone (at IC50) or treated sequentially meaning the cells were first primed with DCA then exposed to a range of ATO concentrations in the presence of freshly added DCA daily or vehicle (Figure 2). In MOLM-14 cells, pretreatment with DCA (IC30) increased the cytotoxicity of stand-alone ATO by 2-fold (Figure 2; Table 1). The potentiation factor (PF) is defined as the quotient of IC50 of ATO alone divided by the IC50 for the combination. In MV4–11 and MonoMac-6 cells the effect of the sequential combination treatment was similar (Figure 2; Table 1). Interestingly, in the FLT3-WT THP-1 cells, DCA priming did not increase the ATO cytotoxicity (PF = 0.87, Figure 2; Table 1). Also, after priming primary AML cells with DCA, ATO cytotoxicity was increased by 1.3 to 1.8-fold (Table 1). To confirm decreased cell viability by another method, trypan blue exclusion was used to compare cell viability after exposure to vehicle plus ATO (IC50) with DCA (IC30) plus ATO (IC50). Cell viability decreased by 20–93% in both FLT3-ITD and FLT3-WT cell lines when cells were treated with the sequential combination regimen (Table 1).
Figure 2. Enhancing ATO cytotoxicity with priming cells with DCA at IC30.
AML cell lines were exposed first to DCA at IC30 or vehicle control for 48 hours. The cells were then treated with a range of ATO concentrations for an additional 48 hour in presence of fresh DCA (IC30) or vehicle. Primary leukemia cells, AML17 and AML18, were treated with DCA (IC30) or vehicle control for 24 hours and then treated with a range of ATO concentrations for an additional 24 hours in presence of fresh DCA (IC30) or vehicle. The cell viability was measured by WST-1 assay. Each experiment was repeated at least two times and representational graph is presented. PF = potentiation factor (the reverse ratio of IC50 when cells were treated with DCA+ATO compared to vehicle+ATO).
To determine if the two agents act synergistically when sequentially combined in leukemia cells, Isobolic analysis was performed based on WST-1 cell proliferation data and combination indices (CIs) were calculated. Combination of DCA and ATO demonstrated a synergistic anti-leukemic effect (CI < 0.9) in MOLM-14, MonoMac6, AML17, and AML18 (Table 1). The combination showed an additive effect on THP-1 cells (Table 1).
Sequential administration of DCA (priming) and ATO significantly induces more apoptosis and decreases mitochondrial membrane potential in leukemia cells compared to either drug alone or their combination without priming
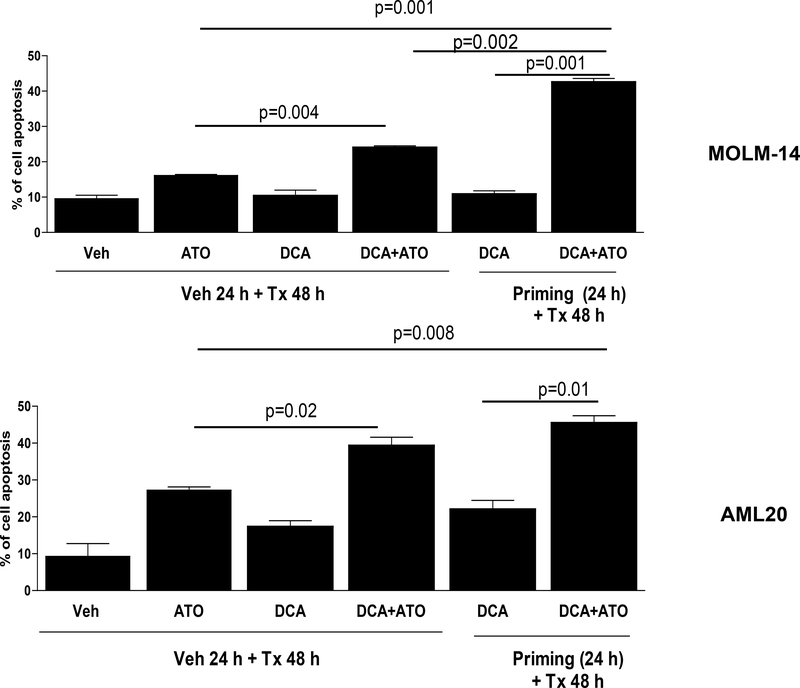
Next, we sought to assess apoptosis after exposure of AML cells to ATO, DCA, or their combination (priming vs no priming) using flow cytometric assay to measure apoptosis. Leukemia cells were first primed with DCA or vehicle control (PBS) for 24 hours, and then treated with fresh DCA at IC30, ATO at IC50 or their combination for 24 hours in primary cells or 48 hours in cell lines (Figure 3). Treatment of MOLM-14 cells with combination of DCA+ATO increased the cells apoptosis by 50% when compared to treatment with ATO alone (p=0.004). However, priming of cells with DCA followed by treatment with DCA+ATO, significantly increased the apoptosis by 165% vs. ATO alone (p=0.001). In addition, cells primed with DCA and treated with DCA+ATO showed increase of apoptosis by 296% when compared to cells treated only with DCA (p=0.001). The DCA priming increased cell apoptosis by 77.3% when compared to cells treated with DCA+ATO without priming (p=0.002). In THP-1 cells, a significant increase in apoptosis was detected by flow cytometry after treatment with DCA+ATO compared to ATO alone (data not shown). However, assessing the effect of priming THP-1 cells with DCA was difficult due to significant number of apoptotic cells detected by flow after exposure to DCA alone. In the primary cells AML20, combination of DCA+ATO increased cell apoptosis by 43.8% vs. ATO alone (p=0.02). With the DCA priming, apoptosis rate was increased 66.4% when compared to cells treated with ATO alone (p=0.008). Cells primed with DCA and treated with DCA+ATO has an increased rate of apoptosis by 107% when compared to DCA alone (p=0.01). The apoptotic effects of DCA+ATO against AML20 were not statistically different in presence or absence of DCA priming (Figure 3).
Figure 3. Comparison of apoptosis in AML cells with different treatment strategies.
Treatment of MOLM-14 and AML20 cells with combination of DCA at their corresponding IC30s and ATO at their corresponding IC50s with priming strategy as described in Methods and Materials significantly (p<0.05) increased apoptosis compared to ATO alone or DCA alone or their combination without priming.
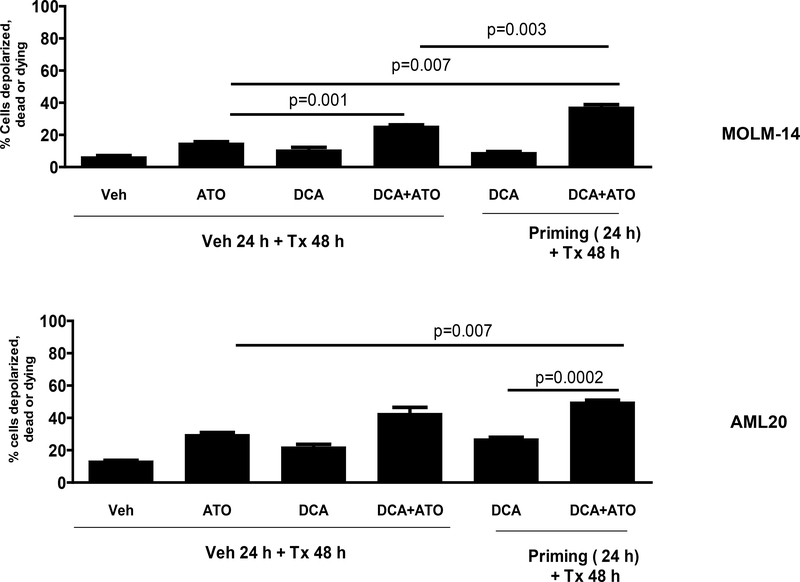
To investigate the mechanism of apoptosis, we used flow cytometry with MitoPotential Red stain to test whether the treatment with DCA, ATO or sequential combination could significantly induce depolarization of the transmembrane potential (ΔΨm) resulting in releasing apoptogenic factors. Forty-eight hours concurrent treatment of MOLM-14 cells with DCA at IC30 (11 mM) and ATO at IC50 (0.9 μM) decreased mitochondrial membrane potential by 79% (p=0.001) compared to ATO alone (Figure 4). On the other hand, priming of MOLM-14 cells followed by treatment with ATO at similar doses for 48 hours resulted in a 149% decrease in mitochondrial ΔΨm compared to ATO alone (p < 0.007). ΔΨm was decreased by 46% (p=0.003) when priming occurred compared to concurrent presence of the two agents (Figure 4). In AML20, priming the cells with DCA at IC30 (5.8 mM) for 24 hours followed by treatment with ATO at IC50 (0.7 μM) for 48 hours resulted in a 68% (p < 0.007) and 84% (p=0.0002) decrease in mitochondrial ΔΨm compared to ATO alone and DCA alone, respectively (Figure 4). Compared to the concurrent use, sequential treatment with DCA (priming) and ATO increased the loss of ΔΨm by 16%, but the difference was not statistically significant.
Figure 4. Comparison of induction of depolarization of the mitochondrial transmembrane potential (Δψm) in AML cells with different treatment strategies.
Treatment of MOLM-14 and AML20 cells with combination of DCA at their corresponding IC30s and ATO at their corresponding IC50s with priming strategy as described in Methods and Materials significantly (p<0.05) decreased mitochondrial membrane potential compared to ATO alone or DCA alone or their combination without priming.
DCA increases the production of ROS in AML cells
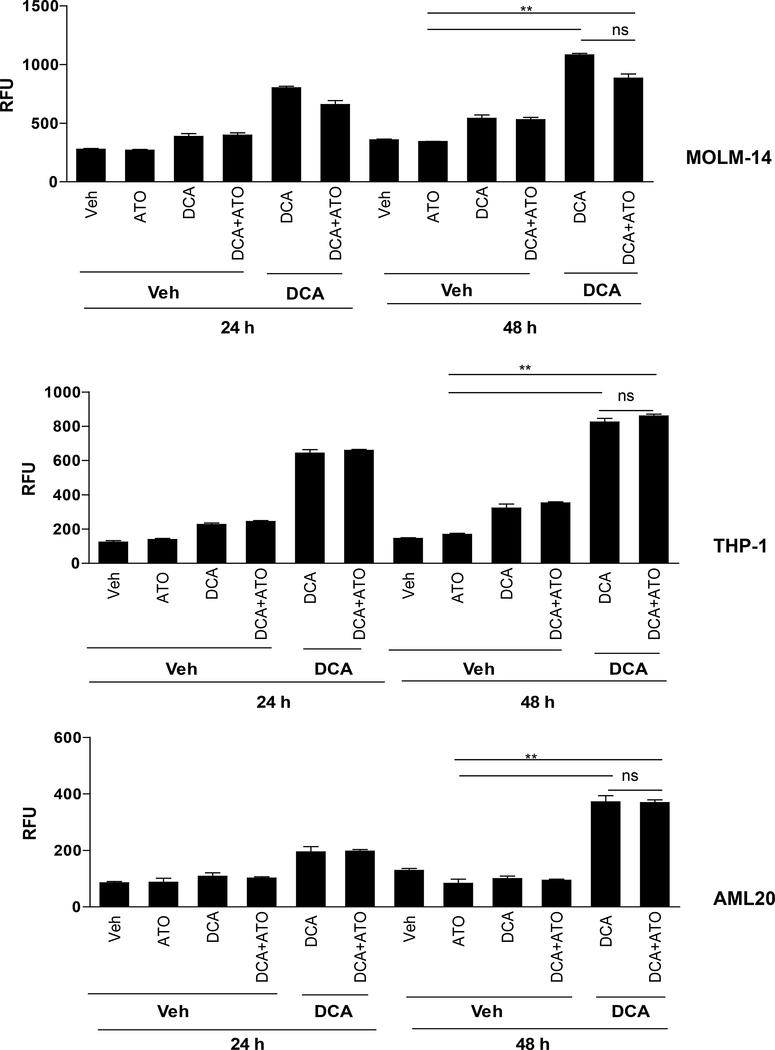
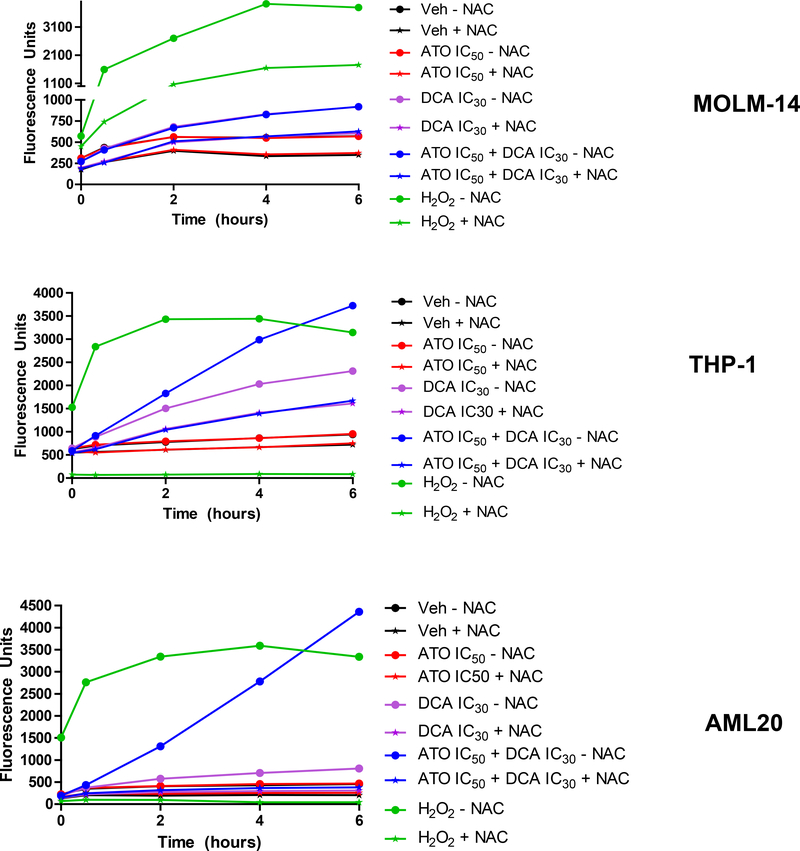
To investigate whether change in mitochondrial ΔΨm, induced by DCA ± ATO resulted in increasing intracellular ROS in leukemia cells, we stained cells with the CM-H2DCFDA fluorescent probe and treated them with different concentrations of DCA and ATO for 24 and 48 hours. In both MOLM-14 and THP-1 cells, a significant increase in ROS production was observed after 24 hours exposure to DCA (Figure 5). With the sequential treatment, 24 hours priming of MOLM-14 cells with IC30 of DCA increased ROS production by 167% when compared to PBS control (Figure 5). Similar results were observed in THP-1 cells and primary AML20 cells. Cellular ROS was elevated by almost 2-fold higher levels in MOLM-14 cells with FLT3-ITD mutation compared with FLT3-WT THP-1 cells (Figure 5). Surprisingly, there was no additional increase in intracellular ROS after addition of ATO to DCA, as tested by this assay. ATO was used at IC50 concentration for each individual cell, i.e., approximately 1 μM for MOLM-14 and AML20 and approximately 5 μM for THP-1. Increasing the concentration of ATO to 10 μM and higher increased the level of intracellular ROS (data not shown). To confirm these findings, in both cell lines and the primary cells AML20, we tested the effect of the radical scavenger, N-acetyl-cysteine (NAC) when adding vehicle, ATO at IC50, DCA at IC30, their combination and the positive control hydrogen peroxide (H2O2) for up to 6 hours (Figure 6). We observed a very little ROS production in 6 hours with ATO alone but a steady increase with IC30 DCA and an even more ROS when ATO and DCA were combined. The combination of DCA+ATO considerably induced ROS production in AML20. As expected, NAC scavenged approximately 50% of the produced ROS. The positive control, H2O2 was included. These data indicate that in AML cell lines ROS production mainly was driven by DCA.
Figure 5. DCA augments ROS production in AML cell lines.
MOLM-14, THP-1, and AML20 cells were first exposed to DCA at IC30 or vehicle control for 24 hours. Then the cells treated with vehicle, ATO (IC50) alone, DCA (IC30) alone or DCA(IC30)+ATO(IC50) combined for additional 24 and 48 hours. DCA caused negligible ROS over-production without priming (Veh-DCA), but ROS production was high in cases of priming (DCA-DCA and DCA-ATO) for 24 and 48 hours. Up to 4-fold increase in the ROS production was detected in leukemia cells after 24 hours of the DCA treatment when compared to vehicle control. Hence, it appears that the difference resides in the time of exposure to DCA. *P < 0.05, ns: non-significant.
Figure 6. ROS production in AML cell lines is mainly induced by DCA.
MOLM-14 and THP-1 cells were treated with ATO (IC50), DCA (IC30) and their combination at similar concentrations in presence or absence of N-acetyl cysteine (NAC) for 6 hours. H2O2 (100 M) was used as the positive control for 6 hours. ROS production was measured according to Material and Methods, and it mainly was driven by DCA and additional ATO had little extra effect on ROS generation.
Treatment with DCA and ATO decreased the expression of Mcl-1 and GPx in primary AML cells
Considering the clear synergism between ATO and DCA in killing AML cells and no observed additional increase in ROS level by ATO, we turned our attention to antioxidant and antiapoptotic proteins as alternative mechanisms that could be negatively influenced by ATO in promoting apoptosis in AML cells treated with sequential DCA+ATO combination.
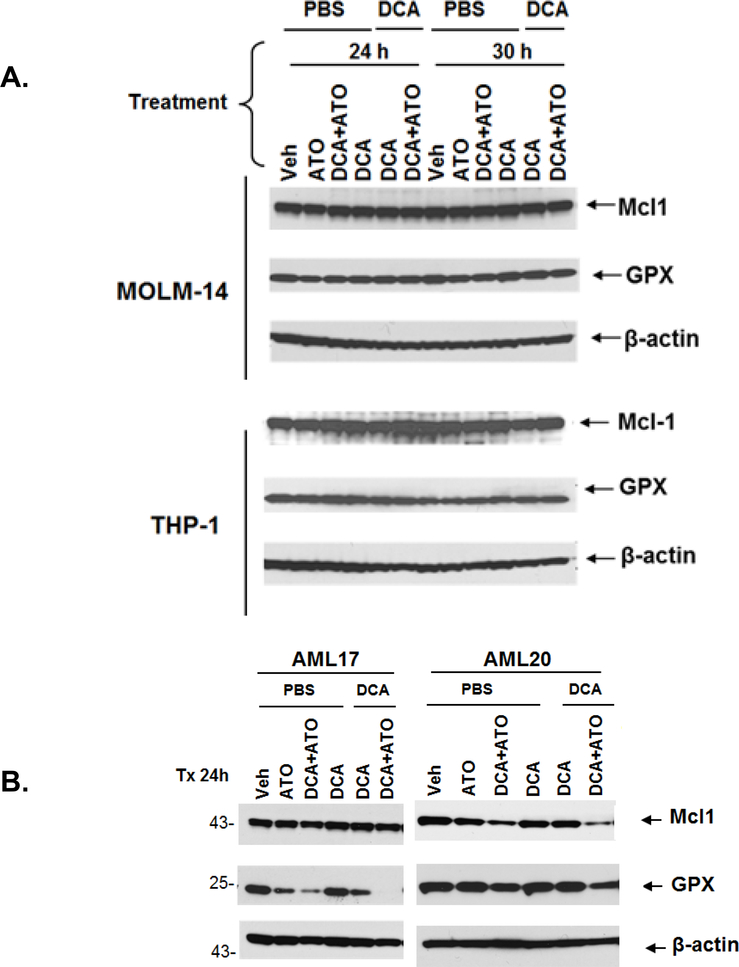
Mcl-1 is one of the major anti-apoptotic proteins in leukemia cells and its presence is critical for survival of human AML cells32. It has been reported that ATO can induce Mcl-1 degradation through activation of glycogen synthase kinase-3β33. Mcl-1 can bind to mitochondrial outer membrane-localized voltage-dependent anion channel and limit calcium uptake into the mitochondrial matrix34. We sought to investigate that by decreasing the level of Mcl-1, ATO may indirectly affect the intracellular ROS level induced by DCA. To test this, we measured Mcl-1 levels in two AML cell lines and two AML cells derived from patients by Western Blot after 24–30 hours treatment with ATO alone or after priming with DCA (Figure 7). No significant changes in Mcl-1 expression were observed in cryopreserved MOLM-14, THP-1 and AML17 cells. However, in freshly used AML20 blasts, a significant reduction in Mcl-1 expression in cells treated with ATO, particularly when primed with DCA, was observed (Figure 7). The DCA-provoked ROS over-production was also significantly increased by co-treatment with ATO in AML20 as shown by a sensible higher ROS production in cells treated with ATO IC50 + DCA IC30 compared to cells treated with IC30 DCA alone in the absence of NAC (i.e. -NAC), (Figure 6)..
Figure 7. Treatment with the combination of low dose DCA and ATO at IC50 decreased the levels of Mcl-1 and GPx in primary AML cells from patients.
With the combination of DCA (IC30) and ATO (IC50) no significant changes in Mcl-1 or GPx were observed in MOLM-14 and THP-1 cells. In AML20 blasts, there was over 60% reduction in Mcl-1 expression in cells treated with the combination of DCA and ATO was observed. In AML17 and AML20, GPx were decreased in cells primed with DCA and treated with DCA and ATO when compared to cells treated without priming. Each Western blot was quantified and densitometry was performed. The expression of each protein was normalized to beta-actin and the relative differences were calculated by comparing the treatments at each time point to its vehicle control (numbers under the strips).
Glutathione is a tripeptide molecule, which in the reduced monomeric form (GSH) is one of the most important cellular antioxidants. As a reductive agent, GSH donates electrons to different molecules and in return becomes oxidized to glutathione disulfide (GSSG) in a process catalyzed by glutathione peroxidase (GPX). Inactivation of GPx results in imbalance in ratio between GSH and GSSG as an indicator of cellular oxidative stress35. Due to the presence of vicinal thiol groups on GPx isoforms36 and the potential for interaction with ATO37, we measured the GPx level by Western Blot. Similar to Mcl-1, GPx levels did not change in AML cell lines MOLM-14 and THP-1. However, in AML17, GPx was significantly decreased with the combination of DCA and ATO. In AML20, GPx level was decreased more after treatment with combination of DCA+ATO compared to either drug alone (Figure 7).
Effect of ATO and DCA on intracellular GSH levels
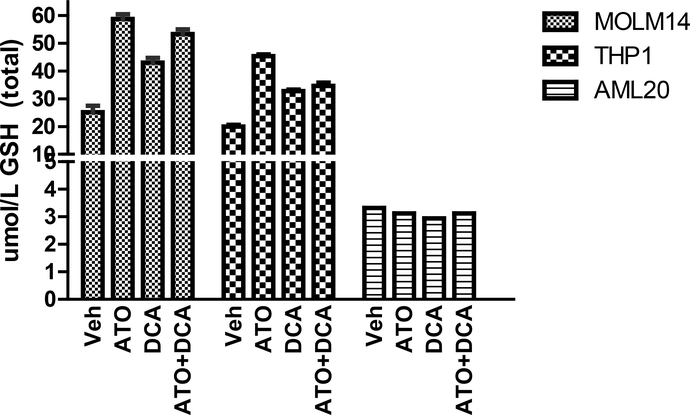
Alterations in GPx might alter the intracellular GSH levels. To this end, we measured the total cellular GSH levels in AML cells. The intracellular levels of GSH increased in MOLM-14 cells by 2.4x, 1.7x and 2.1x after 24 hour exposure to ATO (IC50), DCA (IC30) and their combination, respectively. Similar effects were observed in THP-1 cells: GSH content increased 2.2x, 1.6x and 1.7x when exposed to ATO (IC50), DCA (IC30) and their combination, respectively. Hence, the combination of the two agents did not lead to a significantly higher increase in GSH level. This effect was not observed in the AML20 cells. These data are consistent with the previous report that ATO does not significantly increase the GSH level in AML cells unless is used in concentrations greater than 10 μM38.
The clinical use of the combination of ATO and DCA in treating a patient with refractory AML
A 23 years old patient with refractory extramedullary AML who received a total of 8 prior lines of chemotherapy (see Supplementary Data for details of clinical course and treatments) was treated with the combination of DCA and ATO. The drugs were administered after discussing the case with the patient and his parents, obtaining written informed consent and Institutional Review Board (IRB) approval as well as permission from the FDA (single patient investigational new drug (IND) 121661).
The patient was treated with DCA 12.5 mg/kg orally twice a day. This dose was chosen based on previous clinical use of DCA in patients with brain tumors26,39. ATO (0.15 mg/Kg intravenously daily) was started after the 4th dose of DCA (48 h after starting DCA). The patient received DCA for 11 days and ATO for 9 days. The patient was closely monitored for tumor lysis syndrome; no evidence of lysis requiring intervention was observed. EKG was regularly monitored per institutional guidelines and remained normal. The patient tolerated the combination of DCA+ATO well without experiencing serious treatment related adverse events. He had mild peripheral neuropathy in hands and feet, mild sore throat possibly related to mucositis, and mild confusion while was he was febrile. Due to initial leukocytosis he remained on hydroxyurea. His total leukocyte counts and blast percentage initially increased but started to steadily decreasing 7 days after the initiation of DCA. The treatment was discontinued due to a transfer to another facility for potential enrolment in an AML specific clinical trial.
Discussion
In a heterogeneous disease such as AML where unfortunately many “targeted” agents have not shown great promise thus far, focusing on cellular redox state and mitochondria as fundamental physiological basis for direct cytotoxicity as well as modulating sensitivity to “classical” cytotoxic agents may provide a basis for novel salvage strategies in patients refractory to standard approaches with an acceptable therapeutic index. Increasing evidence suggests that in contrast to normal cells, in many types of cancer cells including AML, redox balance is altered40,41. Since leukemic cells have already upregulated their antioxidant proteins to compensate for oxygen radical upsurge42, they may not be able to tolerate extra oxidative stress, which can be exploited as a important therapeutic strategy.
Previous attempts of using glycolysis modulators such as DCA, 3-bromopyruvate, and 2-deoxy-D-glucose (2-DG) as monotherapy did not demonstrate significant anti-tumor effect, largely because the required high doses of these agents to show clinical activity causes significant treatment-related adverse events that results in discontinuation of the agent by patients43. One strategy to overcome this limitation would be to combine lower and less toxic, hence clinically more tolerable, dose of glycolysis modulators with agents that can either hinder cellular antioxidant system or promote even more ROS generation in cancer cells44,45. The cooperative anti-neoplastic effect of lonidamine, an inhibitor of mitochondria-bound hexokinase, with ATO against human leukemia cell lines has been reported46. Induction of apoptosis by combination of lonidamine+ATO was reported to be due to mitochondrial dysfunction and activation of the intrinsic apoptotic pathway46. The combination of ATO and 3-bromopyruvate also enhanced the apoptotic rate in HL-60 AML cell lines compared to either drug alone47. Recently, DCA was reported to increase sensitivity of multiple myeloma cell lines to bortezomib, and their combination improved the survival of mice with myeloma48. DCA also was shown to enhance cytotoxic effect of adriamycin against hepatoma cell lines, and hepatoma in mice49. The concurrent combination of DCA (5 mM) and high dose ATO (5–20 μM) was reported to be more toxic for breast cancer cell lines than either agent alone27.
ATO at a dose of 0.15 mg/Kg, which results in the serum concentration level of 0.5–2 μM is relatively well tolerated by patients with leukemia, and it is reported to increase intracellular levels of hydrogen peroxide50, and to inhibit the glutathione antioxidant system9,45. To test a combination chemotherapy that can relatively safely be used clinically, in this study we used low (IC30) concentration of DCA as chemosensitizing agent followed by ATO with concentrations (~ 1 μM) compatible with clinical experience. We avoid using high (i.e. > 10 μM) doses of ATO in order to remain pharmacologically relevant. Indeed, DCA at a low concentration significantly potentiated the cytotoxic effect of ATO against a wide range of AML cells including cells with FLT3-ITD mutation. Isobologram analysis of cell viability showed that the combination of DCA+ATO are synergistic with CI values <0.9. The underlying cellular mechanisms involved in the observed synergistic effect included a significantly greater decrease in mitochondrial membrane potential compared to either drug alone. Mitochondrial ΔΨm is necessary for cellular metabolic homoeostasis and disintegrated ΔΨm results in far more negative consequences than disruption of electron transport chain alone. In primary AML cells, other mechanisms may include more inhibition of GPx and Mcl-1 by the combination of ATO+DCA compared to ATO as a single agent. Mcl-1 is a short-lived anti-apoptotic protein that is rapidly degraded after post-transcriptional phosphorylation by kinases51. We observed Mcl-1 level decreased after exposure to the agents only in cells that were not cryopreserved, which might be related to the labile nature of Mcl-1 in cells undergoing freezing and thawing process.
Although ATO has been reported to induce apoptosis in different epithelial and hematologic malignant cells through increasing intracellular content of ROS52, even after several attempts with different assays, we did not observe such phenomenon when ATO was used at concentrations less than 5 μM. This phenomenon might be explained by competing effects of DCA and ATO on PDH activity; with DCA increasing PDH activity, whereas ATO may directly and indirectly inhibit PDH activity53. It is also possible that after 24–48 hours continuous exposure to DCA, cells have already generated ROS near to their maximum capacity that addition of ATO does not alter it to a significant extent. Considering short half-life of DCA in patient, the latter is less clinically important.
Finally, after obtaining all necessary regulatory documents, for the first time we treated a patient with refractory AML with the combination of DCA and ATO per preclinical design. The patient had no other option available. The patient received DCA as twice daily 48 hours before starting ATO and continued the combination for approximately 10 days. The combination was well tolerated without causing severe adverse events.
In conclusion, combination of DCA and ATO at concentrations compatible with clinical experience appears to be more effective in killing leukemia cells than either drug alone. Considering the pharmacology of DCA and ATO and previous clinical experience with each agent alone, the combination may provide an option for patients with relapsed or refractory AML who are not candidate for cytotoxic chemotherapy.
Supplementary Material
Figure 8. ATO at IC50, DCA at IC30, or their combination did not decrease GSH level.
Cells were treated with low dose ATO (< 5 μM) or low dose DCA (IC30) or their combination for 24 hours and reduced GSH level was measured by spectrophotometry according to Materials and Methods. GSH was expressed in nicromole GSH/L. Determination of total GSH content was repeated twice and representative data from one experiment is shown.
Highlights.
As2O3 and dichloroacetate at pharmacologic doses synergistically kill AML cells.
Priming AML cells with low dose DCA significantly potentiates cytotoxicity of ATO.
ATO+DCA significantly perturbs cellular oxidative state and induces apoptosis.
ATO+DCA combination was tolerated well by a patient with refractory AML.
Acknowledgments
We thank the University of Maryland Marlene and Stewart Greenebaum Cancer Center Core Flow Cytometry Facility for assistance in performing flow cytometric data acquisition and analyses, and for their financial support through the NCI P30 Cancer Center Support Grant. This research was partially supported by Emadi Research Foundation Account at University of Maryland Greenebaum Cancer Center (UMGCC) to A.E.
Footnotes
Conflict of interest: AE, EAS, RGL, BC and MS are inventors on the provisional patent “Combination therapy for the treatment of acute myeloid leukemia” assigned to the University of Maryland. MJL and VB report no relevant conflict of interest.
References
- 1.Food and Drug Administration. Trisenox (Arsenic Trioxide) Label. http://wwwaccessdatafdagov/drugsatfda_docs/label/2010/021248s008s009lblpdf 2010.
- 2.Emadi A, Gore SD. Arsenic trioxide - An old drug rediscovered. Blood reviews 2010;24:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kritharis A, Bradley TP, Budman DR. The evolving use of arsenic in pharmacotherapy of malignant disease. Annals of hematology 2013;92:719–30. [DOI] [PubMed] [Google Scholar]
- 4.Li JJ, Tang Q, Li Y, et al. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta pharmacologica Sinica 2006;27:1078–84. [DOI] [PubMed] [Google Scholar]
- 5.Ray A, Chatterjee S, Mukherjee S, Bhattacharya S. Arsenic trioxide induced indirect and direct inhibition of glutathione reductase leads to apoptosis in rat hepatocytes. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 2014. [DOI] [PubMed] [Google Scholar]
- 6.Roboz GJ, Ritchie EK, Curcio T, et al. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer 2008;113:2504–11. [DOI] [PubMed] [Google Scholar]
- 7.Wetzler M, Andrews C, Ford LA, et al. Phase 1 study of arsenic trioxide, high-dose cytarabine, and idarubicin to down-regulate constitutive signal transducer and activator of transcription 3 activity in patients aged <60 years with acute myeloid leukemia. Cancer 2011;117:4861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 1997;89:3345–53. [PubMed] [Google Scholar]
- 9.Miller WH Jr., Schipper HM, Lee JS, Singer J, Waxman S Mechanisms of action of arsenic trioxide. Cancer research 2002;62:3893–903. [PubMed] [Google Scholar]
- 10.Kirito K, Hu Y, Komatsu N. HIF-1 prevents the overproduction of mitochondrial ROS after cytokine stimulation through induction of PDK-1. Cell cycle 2009;8:2844–9. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure 2007;15:992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacpoole PW, Lorenz AC, Thomas RG, Harman EM. Dichloroacetate in the treatment of lactic acidosis. Annals of internal medicine 1988;108:58–63. [DOI] [PubMed] [Google Scholar]
- 13.Stacpoole PW, Wright EC, Baumgartner TG, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. The New England journal of medicine 1992;327:1564–9. [DOI] [PubMed] [Google Scholar]
- 14.Stacpoole PW, Barnes CL, Hurbanis MD, Cannon SL, Kerr DS. Treatment of congenital lactic acidosis with dichloroacetate. Archives of disease in childhood 1997;77:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacpoole PW, Kerr DS, Barnes C, et al. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics 2006;117:1519–31. [DOI] [PubMed] [Google Scholar]
- 16.Parolin ML, Spriet LL, Hultman E, et al. Effects of PDH activation by dichloroacetate in human skeletal muscle during exercise in hypoxia. American journal of physiology Endocrinology and metabolism 2000;279:E752–61. [DOI] [PubMed] [Google Scholar]
- 17.Sutendra G, Michelakis ED. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Frontiers in oncology 2013;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LB. Mitochondrial membrane potential in living cells. Annual review of cell biology 1988;4:155–81. [DOI] [PubMed] [Google Scholar]
- 19.Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecologic oncology 2008;109:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W, Yacoub S, Shiverick KT, et al. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. The Prostate 2008;68:1223–31. [DOI] [PubMed] [Google Scholar]
- 21.Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast cancer research and treatment 2010;120:253–60. [DOI] [PubMed] [Google Scholar]
- 22.Stockwin LH, Yu SX, Borgel S, et al. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. International journal of cancer Journal international du cancer 2010;127:2510–9. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer cell 2007;11:37–51. [DOI] [PubMed] [Google Scholar]
- 24.Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the “Warburg Effect” in a human cancer patient: complete response in non-Hodgkin’s lymphoma after disease progression with rituximab-CHOP. Journal of bioenergetics and biomembranes 2013;45:307–15. [DOI] [PubMed] [Google Scholar]
- 25.Shen YC, Ou DL, Hsu C, et al. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. British journal of cancer 2013;108:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Science translational medicine 2010;2:31ra4. [DOI] [PubMed] [Google Scholar]
- 27.Sun RC, Board PG, Blackburn AC. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Molecular cancer 2011;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekeres MA, Steensma DP. Boulevard of broken dreams: drug approval for older adults with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30:4061–3. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 30.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia 2003;17:1738–52. [DOI] [PubMed] [Google Scholar]
- 31.Sallmyr A, Fan J, Datta K, et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood 2008;111:3173–82. [DOI] [PubMed] [Google Scholar]
- 32.Glaser SP, Lee EF, Trounson E, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes & development 2012;26:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Xia L, Gabrilove J, Waxman S, Jing Y. Downregulation of Mcl-1 through GSK-3beta activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia 2013;27:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Shah K, Bradbury NA, Li C, White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 2014;5:e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto Y, Koh YH, Park YS, et al. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biological chemistry 2003;384:567–74. [DOI] [PubMed] [Google Scholar]
- 36.Tappel AL. Selenium--glutathione peroxidase: properties and synthesis. Current topics in cellular regulation 1984;24:87–97. [PubMed] [Google Scholar]
- 37.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 1999;94:2102–11. [PubMed] [Google Scholar]
- 38.Kumar S, Yedjou CG, Tchounwou PB. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res 2014;33:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunbar EM, Coats BS, Shroads AL, et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Investigational new drugs 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey MR, Sharpless NE. ROS as a tumour suppressor? Nature cell biology 2006;8:1213–5. [DOI] [PubMed] [Google Scholar]
- 41.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Molecular and cellular biology 2005;25:6391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pei S, Minhajuddin M, Callahan KP, et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. The Journal of biological chemistry 2013;288:33542–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006;25:4633–46. [DOI] [PubMed] [Google Scholar]
- 44.Alexandre J, Nicco C, Chereau C, et al. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. Journal of the National Cancer Institute 2006;98:236–44. [DOI] [PubMed] [Google Scholar]
- 45.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 1999;93:268–77. [PubMed] [Google Scholar]
- 46.Calvino E, Estan MC, Simon GP, et al. Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells. Reactive oxygen species generation and defensive protein kinase (MEK/ERK, Akt/mTOR) modulation. Biochem Pharmacol 2011;82:1619–29. [DOI] [PubMed] [Google Scholar]
- 47.Calvino E, Estan MC, Sanchez-Martin C, et al. Regulation of death induction and chemosensitizing action of 3-bromopyruvate in myeloid leukemia cells: energy depletion, oxidative stress, and protein kinase activity modulation. J Pharmacol Exp Ther 2014;348:324–35. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez WY, McGee SL, Connor T, et al. Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. British journal of cancer 2013;108:1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Y, Xiong X, Huang G, et al. Dichloroacetate enhances adriamycin-induced hepatoma cell toxicity in vitro and in vivo by increasing reactive oxygen species levels. PloS one 2014;9:e92962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. Journal of cellular physiology 1998;177:324–33. [DOI] [PubMed] [Google Scholar]
- 51.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS letters 2010;584:2981–9. [DOI] [PubMed] [Google Scholar]
- 52.Woo SH, Park IC, Park MJ, et al. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. International journal of oncology 2002;21:57–63. [PubMed] [Google Scholar]
- 53.Samikkannu T, Chen CH, Yih LH, et al. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chemical research in toxicology 2003;16:409–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.