Abstract
Topical anesthetics are widely used in dental procedures. However, most commercially available medications are in the form of liquid or semisolid, which cannot provide prolonged effect intraorally. To address this issue, we proposed the use of three-dimensional printing (3DP) to fabricate a customizable dental anesthetic patch loaded with lidocaine that can be fitted perfectly onto the affected tooth. It has been shown that that patch can adhere on the tooth for more than 1 h, while releasing lidocaine from the patch made of hydrogels. In addition, the results illustrated the possibility of controlling the drug release profile by altering the shape of the patch, as well the use of a 3DP tooth model as the drug testing platform. Taken together, these data further reinforce the vast potential of the application of 3DP technology in personalized medicine.
Keywords: Dental patch, Topical anesthetics, Three-dimensional printing, Personalized medicine, Adhesive, Drug release
1. Introduction
For many dental surgical procedures, topical anesthetics are needed to anesthetize the gum before the subsequent treatments. They are widely used in various applications including removal of deciduous teeth, debriding/suturing oral soft tissue, and treatment of children’s pulpitis. In addition, they are also useful in alleviating anxiety and fear of needle insertion triggered by pain during procedures[1,2] such as tooth extraction, root canal, and other dental surgeries.
Lidocaine or lidocaine-based medications are one of the most commonly used anesthetics in the dentistry[2]. Lidocaine, an amide derivative, is a relatively safer anesthetic drug[3]. At present, lidocaine is often combined with prilocaine, to form a eutectic mixture, known as eutectic mixture of local anesthetics (EMLA™), to serve this purpose[4].
However, most commercially available medications, such as EMLA™, are usually formulated as foams, ointments, pastes, creams, and gels[5] to be applied onto the affected area intraorally. These topical medications generally do not provide sustained action (short retention time)[6] and they usually act on other non-targeted parts of the oral cavity as well, which can cause numbness of the mouth and throat, leading to trouble swallowing and even choking, especially in the pediatric patients[7,8].
To this end, we hypothesized that a dental patch containing anesthetics can be fabricated, based on the tooth model specific to the patient. The patch can be fitted perfectly onto the target tooth or affected areas, where sustained drug release can occur from it for prolonged local action.
The active ingredient to be formulated into the adhesive patch is lidocaine hydrochloride, which is a widely used anesthetic. It is commercially available as dermal formulation, intravenous injection, oral gel and topical gel, or cream. Lidocaine has been commonly used as anesthetic agent in dental procedures such as dental scaling, root planing, and early wound healing following non-surgical periodontal therapy.
To optimize drug delivery and therapeutic outcomes, three-dimensional printing (3DP) technology was utilized to achieve personalization in the fabrication of patches. It involves manufacturing process of fusing or depositing various materials such as plastic, metal, ceramics, powders, liquids, or even living cells[9]. Computer-aided design (CAD) and computer-aided manufacturing systems are crucial part of 3DP, it helps in acquisition and digitization of the data, facilitate the design, and verification of the design before the production (through stimulation), as such that any wearable device can be tailored based on the patient-specific anatomical features[10,11].
Recently, 3DP emerged as a powerful technology for pharmaceutical applications[12], the unprecedented U.S. Food and Drug Administration approval of the 3D-printed rapid orodispersible antiepileptic tablet (SPRITAM) in 2015[13] has further spurred the interest of using 3DP in drug delivery. Different types of 3DP techniques were applied and currently under investigation in the field of pharmaceutical drug delivery such as inkjet printing where different combinations of active ingredients and excipients are sprayed and printed in specific quantities, and fused deposition modeling where the active ingredients are mixed with polymers and extruded to create a specific shape and size[14-16]. Please refer to our recent review on this topic for detailed discussions[12].
The sophistication of 3DP enabled the possibility of producing drug delivery devices with high spatial complexities and controllable release patterns[17], which are otherwise not possible or merely too tedious to accomplish. Using 3DP, patches can be easily customized to fit onto the teeth of a patient and be produced with precision and ease. The perfectly fitted patches will be able to “target” the affected area(s) and thereby optimizing drug delivery.
In this study, we develop a personalized dental patch with the help of 3DP as a novel way of drug delivery onto targeted area in the oral cavity with prolonged activity and improved patient compliance. Moreover, the tooth model made from 3DP is used as a novel testing platform, better mimicking the patient’s oral condition, thereby improving the accuracy and precision of the testing (Figure 1).
Figure 1.
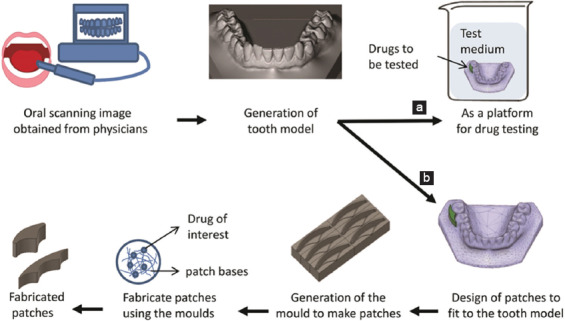
Schematic representation of the generation of the three-dimensional (3D)-printed tooth model. Use of the 3D-printed tooth model as (a) drug testing platform and (b) for generation of personalized patches which can be loaded with lidocaine for anesthesia.
2. Materials and Methods
2.1 Materials
Polyethylene glycol (PEG) 1500 and lidocaine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Xylitol was obtained from Roquette Frères (Lestrem, France). Hydroxypropyl methylcellulose (HPMC) E3 Premium LV was obtained from Colorcon (Harleysville, PA, USA). The patch molds were made of using 1.75 mm acrylonitrile-butadiene-styrene (ABS) filament purchased from XYZprinting (XYZprinting, San Diego, USA). All materials are used as supplied without further purification.
2.2 Methods
2.2.1 Preparation of 3D Design and Model
The tooth model was obtained from a public database[18], the owner of the model used the intraoral 3D scanner (3M True Definition Scanner[19]). Subsequently, Fusion 360 (Autodesk version 2.03257, San Rafael, CA) was used for converting the 3D model into a CAD file (Figure 2). The two different patches were designed with reference to the tooth model. The patch molds were then modeled using Fusion 360 and sliced into two parts to facilitate the demolding process of the patches. The final mold models were exported as a stereolithography file (.stl) into the XYZware (XYZ printing, San Diego, USA) for printing.
Figure 2.
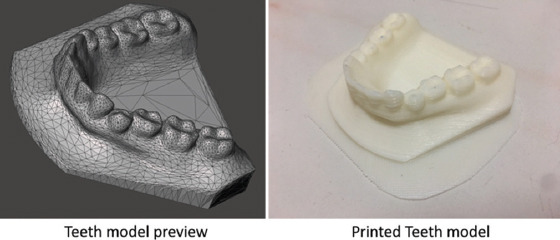
The computer-aided design file of the tooth model for three-dimensional printing and the final printed tooth model.
2.2.2 Printing of the Molds
The patch molds and tooth model were printed using an XYZ Da Vinci 1.0A 3D printer (XYZprinting, San Diego, USA) with ABS filaments. The. stl files only encoded the surface data of the molds, to print out the final objects, other parameters were defined as the following: Infill was set at 30% with 0.1 mm layer resolution, extrusion temperature and platform temperature were set at 230°C and 110°C, respectively, wherein higher than the transition temperature of ABS filament (~105°C) with former that melt the filament and the later ensures extruded plastic remained warm, thus preventing warping.
2.2.3 Preparation of Lidocaine-loaded Patches
The lidocaine-loaded patches were prepared using the method as reported[20]. Briefly, 14.6 g PEG was first melted on water bath, and then, 4 g xylitol, 1.4 g HPMC, and lidocaine (1% w/w) were added to form a homogeneous mixture. Subsequently, the mixture was poured into the 3D-printed molds as shown in Figure 3. The polymer mixture was allowed to cool and harden, which facilitated the separation of the patches and the molds. The detailed dimension of the patches is shown in Table 1.
Figure 3.

The computer-aided design of patch molds for (A) 2-tooth model and (B) 3-tooth model for three-dimensional printing and their respective final products.
Table 1.
Physical characteristics of 3DP patches.
| Model | 2-tooth model | 3-tooth model |
|---|---|---|
| Surface area (mm2) | 988.14 | 1233.39 |
| Volume (mm3) | 1760 | 1760 |
| SA/V ratio | 0.56 | 0.70 |
| Weight (mg) | 1167 ± 88 | 1168 ± 66 |
| Density (mg/mm3) | 0.66 ± 0.50 | 0.66 ± 0.38 |
| Drug encapsulation (mg) | 11.90 ± 1.43 | 13.00 ± 0.87 |
3DP: Three-dimensional printing, SA/V: Surface area-to-volume ratio
2.2.4 Adhesion and Fitting Test
The soft patches were adhered and fitted onto the printed tooth model as shown in Figure 4, and the dissolution of the patches was observed until the patches were fully dissolved.
Figure 4.

Adhesion and fitting test of the respective patches, (A) 2-tooth model and (B) 3-tooth model (Time [T] is in min). (The yellow color rod was used to keep the printed tooth model inside the liquid).
2.2.5 In Vitro Drug Release Study
Drug release profiles are shown in Figure 5. The patches were placed in a tube containing 15 mL of phosphate buffer saline (PBS) (pH 7.4) and incubated at 37°C in a water bath. At the designated time intervals, the patches were removed from the tube and entire release medium was replaced with 15 mL of fresh PBS to maintain the sink condition. The release process continued until the patches were dissolved fully. The accumulative release profile was determined using high-performance liquid chromatography method described by Liawruangrath et al.[21] All release studies were done in triplicates.
Figure 5.
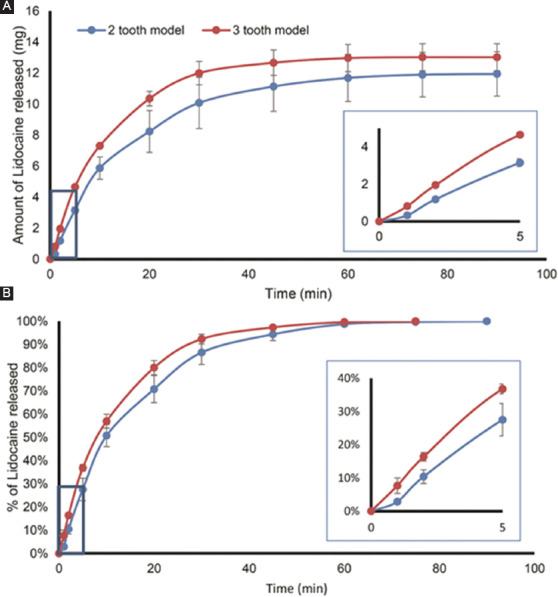
In vitro drug release profile: (A) The cumulative amount and (B) percentage release, for the 2-tooth model (blue) and 3-tooth model (red), respectively. The insets show the first 5 min of the release study; the difference in the rate of dissolution between the two patches was significant (P<0.01).
2.2.6 Statistical Analysis
All the data obtained were tabulated and computed using Microsoft Office Excel 2016 (Microsoft, Redmond, USA). The data were compared using one-way analysis of variance and P<0.05 was considered statistically significant.
3. Results
3.1 Physical Characteristics of the Lidocaine-loaded Patches
Table 1 shows the physical property of the printed patches. The surface area and volume of two different types of patches were calculated using the 360 Autodesk software and surface area-to-volume ratio (SA/V) was then calculated. The volume of the patches was kept constant (1760 mm3), while the size and shape of patches were varied, to obtain two different designs, namely the 2-tooth model and 3-tooth model. The weights of the two different types of patches were about 1168 mg. The total drug encapsulated is similar (P=0.24), 11.90 ± 1.43 mg and 13.00 ± 0.87 mg in 2-tooth and 3-tooth patches, respectively (n = 3).
3.2 Adhesion/Fitting Test
Figure 4 shows the fit of the 2-tooth and 3-tooth patches onto the 3D-printed tooth model. The patches fitted perfectly onto the tooth model without the use of any adhesives. The size of the patches decreased with time, due to the erosion of the hydrogel patches.
3.3 In vitro Drug Release Study
The release profile of the patches is shown in Figure 5. The 3-tooth patch showed a faster release profile as compared to 2-tooth patch in the first 30 min; eventually as both patches were fully dissolved, the curves converged. The 3-tooth patches also released larger amount of lidocaine overall as compared to the 2-tooth patches.
Drug release rate from water-soluble and water-swellable polymers is governed by the relative contribution of two mechanisms, drug diffusion and polymer dissolution (surface erosion)[22]. The predominance of one or other mechanism depends on different factors such as drug solubility and nature of the excipients. Some previous work on the influence of tablet shape using HPMC and several models accounting for geometry were developed, but, generally, these are applicable only to specific formulations[23,24].
4. Discussion
Local anesthetics, including lidocaine (Xylocaine), procaine (Novocaine), prilocaine (Citanest), propoxycaine (Ravocaine), mepivacaine (Carbocaine), benzocaine (Monocaine), bupivacaine (Marcaine), tetracaine (Pontocaine), and articaine (Septocaine), are the most commonly used medications in dentistry. Before any dental procedures, it is usually favorable to anesthetize the gum to prevent any severe pain that might interrupt the procedure or cause immense discomfort to the patient. This is mostly done by injection, which, unfortunately, is painful by itself. To alleviate the injection pain, topical anesthetics can be used.
However, the commercially available dosage forms such as oral gels, solutions, or pills usually have rather a short effect as these medications can easily get washed off by saliva before it can exert any anesthetizing impact[25], thereby undermining the effect of the painkillers, especially in the case of chronic toothache. Mucoadhesive polymers, such as HPMC, were commonly used in the fabrication of mucoadhesive formulations such as buccal tablets[26]. While these formulations were generally found to improve the retention time of the medication in the oral cavity, their applications were usually not directly targeted on the affected area. Another disadvantage of the conventional mucoadhesive formulations was the potential uncomfortable mouthfeel due to the incompatible shapes. Such discomfort may potentially hinder the patients’ acceptability or cause premature detachment and swallowing[27].
Therefore, in this research, we aimed to use 3DP technology as a novel way to develop dental patches that can fit perfectly onto the patient’s teeth to improve the patients’ experience and optimize its therapeutic outcome. We demonstrated this through designing the patches which were complementary to the molar tooth region of the model. Manufacturing of medications, particularly patches in complex shape, is known to be a laborious and difficult task. However, with 3DP, we were able to fabricate complex scaffolds to fit body contours[11].
Acquiring a digital oral 3D model using the intraoral scanner has been applied in various applications in dentistry for diagnosis and fabrication of implants[28]. In this work, we proposed that the 3D-printed tooth model made can be potentially used as a platform for drug testing to improve the accuracy of the tests to predict the drug performance in vivo. With the help of the tooth model, the surface area of the adhesion side of the patches, or potentially other forms, such as gels or ointments, may be taken into consideration and thereby reducing the possibility of overestimating the rate of drug release.
From the dissolution profiles, the 3-tooth model with bigger SA/V was able to achieve faster rate of drug release as compared to the 2-tooth model. The in vitro drug release profile is likely to be independent of the patch shape and directly related to the SA/V ratio as demonstrated by Kimber et al.[29] using the swelling tablet. The study done by Siepmann et al., in 1999,[23] also demonstrated that tablets with higher SA/V ratio would display faster dissolution rates. All these suggested, by manipulating SA/V ratio parameters, one can design a patch with desirable release kinetics, contributing to personalized medicine. At the same time, knowledge on how release kinetics related to the SA/V can also be used to help in the prediction of the release profile[17].
Here, we conducted the drug release in PBS at pH 7.4, to demonstrate the possibility of altering the release profile by changing the geometry of the patches. To better mimic the in vivo condition, further studies can be done using the artificial saliva since pH and viscosity of the saliva may play a role in the dissolution of polymer patches[30]. Yet, the sophistication of the human oral cavity is challenging to simulate[31]. The anatomical and physiological complexities of our oral cavity, such as the varying degree of keratinization found at the different region of oral mucosa[32] and our body produced saliva with different characteristics, such as different pH, volume, and flow rate[33], have proven to be difficult to mimic in vitro.
It was noted that the drug release of our patches lasted about 60 min, and studies have shown the onset of the anesthetic effect within 5 min[2,25]. Thus, this indicated that our patches were able to improve and prolong the local anesthetic effect. As the duration of the action of lidocaine ranged between 15 and 18 min[25], prolonging the residence time of the drug would potentially be beneficial for local pain relief (e.g., ulcer[34]) or anesthesia for longer dental procedures (e.g., periodontal manipulation)[35].
5. Conclusion
With 3DP, it is now possible to personalize the design of specially shaped patches that can perfectly fit between the teeth. Moreover, controlled release of drugs can also be achieved by careful design of the shape and thereby overcoming the problem of the current dosage forms which are short acting. In this work, we have demonstrated the possibility of personalizing the anesthetic patches with tunable geometry and prolonged drug release. This can be of great importance to dental applications, in which pain has always been a problem, especially for the pediatric population.
Authors’ Contributions
Yi-Hsuan Ou and L.K conceptualized the whole project and designed all the experiments. Yi-Hsuan Ou and Yi-Hui Ou performed the experiments. Yi-Hsuan Oh, Yi-Hui Oh, J.G., and L.K. wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Armfield JM, Milgrom P. 2011, A Clinician Guide to Patients Afraid of Dental Injections and Numbness. SAAD Dig. 27:33–9. [PubMed] [Google Scholar]
- 2.Lee HS. 2016, Recent Advances in Topical Anesthesia. J Dent Anesth Pain Med. 16(4):237. doi: 10.17245/jdapm.2016.16.4.237. DOI 10.17245/jdapm.2016.16.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oni G, Brown S, Kenkel J. 2012, Comparison of Five Commonly-available, Lidocaine-containing Topical Anesthetics and their Effect on Serum Levels of Lidocaine and Its Metabolite Monoethylglycinexylidide (MEGX) Aesthet Surg J. 32(4):495–503. doi: 10.1177/1090820X12442672. DOI 10.1177/1090820X12442672. [DOI] [PubMed] [Google Scholar]
- 4.Muniz BV, Baratelli D, Carla SD, et al. 2018, Hybrid Hydrogel Composed of Polymeric Nanocapsules Co-loading Lidocaine and Prilocaine for Topical Intraoral Anesthesia. Sci Rep. 8(1):17972. doi: 10.1038/s41598-018-36382-4. DOI 10.1038/s41598-018-36382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda HMC, Shah VP, Derdzinski K, et al. 2009, Topical and Transdermal Drug Products. U S Pharmacopeial Natl Formul. 35(3):750–64. [Google Scholar]
- 6.Nguyen S, Hiorth M. 2015, Advanced Drug Delivery Systems for Local Treatment of the Oral Cavity. Ther Deliv. 6(5):595–608. doi: 10.4155/tde.15.5. DOI 10.4155/tde.15.5. [DOI] [PubMed] [Google Scholar]
- 7.Silvers SL. 2014, Practical Techniques in Office-based Balloon Sinus Dilation. Oper Tech Otolaryngol Neck Surg. 25(2):206–12. DOI 10.1016/j.otot.2014.02.012. [Google Scholar]
- 8.Soweid AM, Yaghi SR, Jamali FR, et al. 2011, Posterior Lingual Lidocaine:A Novel Method to Improve Tolerance in upper Gastrointestinal Endoscopy. World J. Gastroenterol. 17(47):5191. doi: 10.3748/wjg.v17.i47.5191. DOI 10.3748/wjg.v17.i47.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert C, van Langeveld MC, Donoso LA. 2014, Innovations in 3D Printing:A 3D Overview from Optics to Organs. Br J. Ophthalmol. 98(2):159–61. doi: 10.1136/bjophthalmol-2013-304446. DOI 10.1136/bjophthalmol-2013-304446. [DOI] [PubMed] [Google Scholar]
- 10.Goyanes A, Det-Amornrat U, Wang J, et al. 2016, 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J Control Release. 234:41–8. doi: 10.1016/j.jconrel.2016.05.034. DOI 10.1016/j.jconrel.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Liang K, Carmone S, Brambilla D, et al. 2018, 3D Printing of a Wearable Personalized Oral Delivery Device:A First-in-Human study. Sci Adv. 4(5):2544. doi: 10.1126/sciadv.aat2544. DOI 10.1126/sciadv.aat2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SH, Kathuria H, Tan JJY, et al. 2018, 3D Printed Drug Delivery and Testing Systems a Passing Fad or the Future? Adv Drug Deliv Rev. 132:139–68. doi: 10.1016/j.addr.2018.05.006. DOI 10.1016/j.addr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Prakash A, Sarma P, et al. Three-dimensional Drugs:A New Era in the Pharmaceutical Development. Indian J Pharmacol. 49(6):417–8. doi: 10.4103/ijp.IJP_119_18. DOI 10.4103/ijp.IJP_119_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jassim-Jaboori AH, Oyewumi MO. 2015, 3D Printing Technology in Pharmaceutical Drug Delivery:Prospects and Challenges. J Biomol Res Ther. 4:4. DOI 10.4172/2167-7956.1000e141. [Google Scholar]
- 15.Palo M, Holländer J, Suominen J, et al. 3D Printed Drug Delivery Devices:Perspectives and Technical Challenges 3D Printed Drug Delivery Devices:Perspectives and Technical Challenges. Expert Rev Med Devices. 14(9):685–96. doi: 10.1080/17434440.2017.1363647. DOI 10.1080/17434440.2017.1363647. [DOI] [PubMed] [Google Scholar]
- 16.Jamróz W, Szafraniec J, Kurek M, et al. 2018, 3D Printing in Pharmaceutical and Medical Applications Recent Achievements and Challenges. Pharm Res. 35(9):176. doi: 10.1007/s11095-018-2454-x. DOI 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyanes A, Martinez PR, Basit AW. 2015, Effect of Geometry on Drug Release from 3D Printed Tablets. Int J Pharm. 494(2):657–63. doi: 10.1016/j.ijpharm.2015.04.069. DOI 10.1016/j.ijpharm.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 18.Scherer MD. 2019, Lower Dental Tooth Model. [[Last accessed on 2019 Jan 24]];NIH 3D Print Exchange. Available from: https://www.3dprint.nih.gov/discover/3dpx-003003 .
- 19.Scherer MD. 2015, Implementing a Contemporary, In-office Fully Digital Workflow in a Clinical Implant Practice:Intraoral Scanning, Virtual Design, and 3D printing. Implant Pract US. 8(2):1–21. [Google Scholar]
- 20.Mesnukul A, Yodkhum K, Phaechamud T. 2009, Solid Dispersion Matrix Tablet Comprising Indomethacin-PEG-HPMC Fabricated with Fusion and Mold Technique. Indian J Pharm Sci. 71(4):413–20. doi: 10.4103/0250-474X.57290. DOI 10.4103/0250-474X.57290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liawruangrath S, Liawruangrath B, Pibool P. 2001, Simultaneous Determination of Tolperisone and Lidocaine by High Performance Liquid Chromatography. J Pharm Biomed Anal. 26(5-6):865–72. doi: 10.1016/s0731-7085(01)00462-9. DOI 10.1016/S0731-7085(01)00462-9. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds TD, Mitchell SA, Balwinski KM. 2002, Investigation of the Effect of Tablet Surface Area/Volume on Drug Release from Hydroxypropylmethylcellulose Controlled-release Matrix Tablets. Drug Dev Ind Pharm. 28(4):457–66. doi: 10.1081/ddc-120003007. DOI 10.1081/DDC-120003007. [DOI] [PubMed] [Google Scholar]
- 23.Siepmann J, Kranz H, Bodmeier R, et al. 1999, HPMC-Matrices for Controlled Drug Delivery:A New Model Combining Diffusion, Swelling, and Dissolution Mechanisms and Predicting the Release Kinetics. Pharm Res. 16(11):1748–56. doi: 10.1023/a:1018914301328. DOI 10.1023/A:1018914301328. [DOI] [PubMed] [Google Scholar]
- 24.Siepmann J, Kranz H, Peppas NA, et al. 2000, Calculation of the Required Size and Shape of Hydroxypropyl Methylcellulose Matrices to Achieve Desired Drug Release Profiles. Int J Pharm. 201(2):151–64. doi: 10.1016/s0378-5173(00)00390-2. DOI 10.1016/S0378-5173(00)00390-2. [DOI] [PubMed] [Google Scholar]
- 25.Bhushan NVV, Nayak RN. 2010, A Comparison of the Efficacy of Topical Application of Lignocaine Hydrochloride 5% gel and Bupivacaine Hydrochloride 5% gel for Extraction of Teeth. J Maxillofac Oral Surg. DOI 10.1007/s12663-010-0038-3. 9(2):119–26. doi: 10.1007/s12663-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosal K, Chakrabarty S, Nanda A. 2011, Hydroxypropyl Methylcellulose in Drug Delivery. Pharm Sin. 2(2):152–68. [Google Scholar]
- 27.Reddy PC, Chaitanya KSC, Rao YM. 2011, A Review on Bioadhesive Buccal Drug Delivery Systems:Current Status of Formulation and Evaluation Methods. Daru. 19(6):385–403. [PMC free article] [PubMed] [Google Scholar]
- 28.Mangano F, Gandolfi A, Luongo G, et al. 2017, Intraoral Scanners in Dentistry:A Review of the Current Literature. BMC Oral Health. 17(1):149. doi: 10.1186/s12903-017-0442-x. DOI 10.1186/s12903-017-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimber JA, Kazarian SG, Štěpánek F. 2013, DEM Simulation of Drug Release from Structurally Heterogeneous Swelling Tablets. Powder Technol. 248:68–76. DOI 10.1016/j.powtec.2012.12.039. [Google Scholar]
- 30.Gittings S, Turnbull N, Henry B, et al. 2015, Characterisation of Human Saliva as a Platform for Oral Dissolution Medium Development. Eur J Pharm Biopharm. 91:16–24. doi: 10.1016/j.ejpb.2015.01.007. DOI 10.1016/j.ejpb.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Gittings S, Turnbull N, Roberts CJ, et al. 2014, Dissolution Methodology for Taste Masked Oral Dosage Forms. J Control Release. 173:32–42. doi: 10.1016/j.jconrel.2013.10.030. DOI 10.1016/j.jconrel.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Sohi H, Ahuja A, Ahmad FJ, et al. 2010, Critical Evaluation of Permeation Enhancers for Oral Mucosal Drug Delivery Permeation Enhancers for Oral Mucosal Drug Delivery. Drug Dev Ind Pharm. 36(3):254–82. doi: 10.1080/03639040903117348. DOI 10.3109/03639040903117348. [DOI] [PubMed] [Google Scholar]
- 33.Fenoll-Palomares C, Muñoz Montagud JV, Sanchiz V, et al. 2004, Unstimulated Salivary Flow Rate, pH and buffer Capacity of Saliva in Healthy Volunteers. Rev Esp Enferm Dig. 96(11):773–83. doi: 10.4321/s1130-01082004001100005. DOI 10.4321/S1130-01082004001100005. [DOI] [PubMed] [Google Scholar]
- 34.Young JL, Bogner RH. 2009, Case Report:Lidocaine Mucoadhesive Buccal Tablets for Local Relief of Mouth Ulcers. Int J Pharm Compd. 13(3):214–7. [PubMed] [Google Scholar]
- 35.Okamoto H, Nakamori T, Arakawa Y, et al. 2002, Development of Polymer Film Dosage Forms of Lidocaine for Buccal Administration:II Comparison of Preparation Methods. J Pharm Sci. 91(11):2424–32. doi: 10.1002/jps.10228. DOI 10.1002/jps.10228. [DOI] [PubMed] [Google Scholar]


