Abstract
Bioprinting, the printing of living cells using polymeric matrixes (mainly hydrogels), has attracted great attention among science and technology circles. North America has been one of the sources of bioprinting-related technology in recent years. As a natural consequence of geography, high-quality research in the area of bioprinting has started to permeate Latin America. Here, we describe and analyze the knowledge landscape of bioprinting in Latin America using a competitive technology intelligence methodology. Our analysis provides relevant information, such as the scientific publication trends in Latin America and the scientific networks among research groups in Latin America and the world.
Keywords: Three-dimensional bioprinting, Competitive technology intelligence, Latin America, Scientometrics, Patentometrics
1. Introduction
Keeping abreast of novel and emerging technologies is crucial for the research and development (R&D) of any entity, from universities and small firms to transnational companies and governments. This activity is essential for evaluating and deciding on the focus of present and future research efforts[1,2]. In this contribution, we analyze the current knowledge landscape of bioprinting in Latin America, using the well-established competitive technology intelligence (CTI) methodology. Our aim was to provide useful information to foster the transition of talent and resources into the emerging field of bioprinting within Latin America, a huge emerging market of more than 650 million consumers[3].
In general terms, bioprinting can be described as an additive manufacturing technology; it is analogous to three-dimensional (3D) printing but dispenses a bioink in a controlled fashion to fabricate a living construct[4,5]. The bioink contains cells suspended in a viscous matrix (most commonly a hydrogel)[6]. Therefore, the characterization of the performance of a bioprinting process also requires consideration of relevant post-printing biological indicators such as viability, cell proliferation rates, and proper cell differentiation and/or maturation[7].
Although extrusion-based printing is the most widely established methodology[7,8], bioprinting is frankly an evolving field, and many other bioprinting techniques currently under development should be considered in any analysis of the state of the art; these include laser-assisted bioprinting, droplet-bioprinting, spheroid-fusing bioprinting[9], and flow-assisted bioprinting[10-12].
Bioprinting promises to be instrumental in many biomedical engineering areas, including tissue engineering[13-15], pharmaceutical screening and development[5,16], biomedical microbiology[10,17-19], as well as synthetic biology[17,20]. The basic aim of bioprinting is to accommodate biomaterials and living cells in a 3D architecture that will support the proper function of tissue constructs. The bioprinting field is evolving exponentially, and its current outcomes clearly forecast its potential to revolutionize the medical sector. Bioprinting applications are progressing from the fabrication of small versions of organs for drug testing[5,16] to the manufacture of functional tissues[21,22] and complete organs amenable to transplantation[13,15].
Today, bioprinting is one of the leading fronts of research and patent filing worldwide. However, within the field, different degrees of involvement can be perceived among different world regions. In a previous study, Rodríguez-Salvador et al.[23] applied a CTI methodology to study the development of additive manufacturing technology in Latin America. They compared the state of the technology at regional (Latin America) and global levels and discussed its importance and the main challenges it brought to Latin American countries. However, no specific study has yet assessed the development of the scientific and technological production of 3D bioprinting technology in this region.
Establishing the knowledge landscape of a particular technology is not a simple task; moreover, studies that analyze revolutionary technologies, such as 3D bioprinting, are scarce. The recent developments of CTI by Rodríguez-Salvador et al. to analyze the scientific and technological production on the field have contributed to determine trends in this domain[23-27]. Competitive intelligence (CI) processes comprise the systematic, ethical, and legal collection and analysis of data to obtain valuable intelligence that can support the decision-making process of an organization. CI plays a key role in reducing risks and increasing the chances of making successful decisions that will provide a competitive advantage to help enterprises stay ahead of their competitors. The CTI methodology involves a CI process with technology as its main concern. Intelligence obtained serves as an early warning of threats and opportunities possibly presented to an organization by the competitive and technological environment[28]. Scientific articles and patents, as public documents, represent valuable information sources for the CTI methodology[29].
While scientific publications help researchers to communicate and disseminate the knowledge produced from scientific research advances, patents represent a record of the technological novelty and advancement[30]. Applying scientometric and patentometric methods to the analyses of these documents can provide determinant value to the technology assessment[29,31,32]. These methods make use of sophisticated tools to process large amounts of data found in scientific articles and patent filings[29,33]. Analyses made with these methods are useful for monitoring scientific and technological trends and for identifying metrics such as the most prolific countries, researchers, and institutions working in a particular field[34].
This paper presents an analysis of the scientific literature and patents published in Latin America to identify the current trends in 3D bioprinting, as well as the countries, researchers, and institutions that are leading the research efforts.
2. Methodology
The CTI methodology applied in this research was based on two previous studies by Rodríguez-Salvador et al.[23,24]. This methodology involves an iterative process divided into a six-stages cycle (Figure 1) that is enriched with experts’ feedback to validate the most critical steps of the study, to deliver the most reliable knowledge to the end users.
Figure 1.
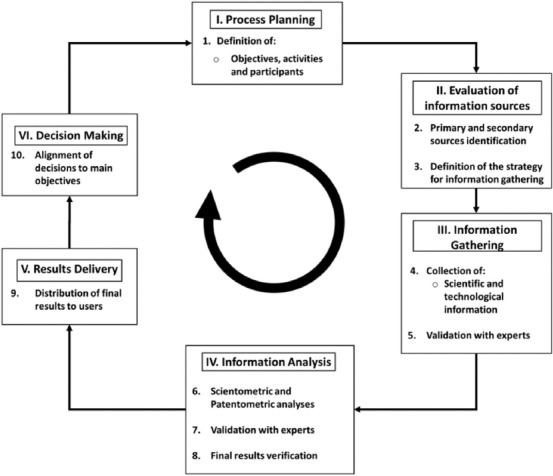
Competitive technology intelligence methodology (based on previous studies by Rodríguez-Salvador et al.)[23,24].
2.1 Process Planning
This stage is the foundation of the entire CTI methodology. Here, the main objectives, activities, and participants of the project are defined to align the process to the needs of the end users. This research was conducted to determine the overall landscape of the development of 3D bioprinting technology in Latin America. Scientific publications and patents were collected and thoroughly analyzed.
2.2 Evaluation of Information Sources
This second stage involves the identification and assessment of primary and secondary information sources and the definition of the data collection strategy to be followed throughout the study.
In this case, primary information was obtained from multiple interviews conducted with top researchers in the field of 3D bioprinting. The interviewees were identified and selected based on several criteria, such as their career trajectory, expertise, and general presence in the field. These experts, who together had over 11,000 Scopus citations, were consulted during the development of the study to obtain valuable insights, to construct the search query, to receive feedback on the analyses, and to validate the final results.
Scientific production was assessed using the Scopus database to retrieve all the scientific documents concerning 3D bioprinting published in Latin America. Scopus is one of the largest abstract and citation databases of peer-reviewed literature; it contains approximately 70 million indexed items and more than 5,000 publishers of different scientific and technological areas, making it a valuable tool on the subject of 3D bioprinting[35]. Technological information was retrieved from the PatSeer software database to collect patents on 3D bioprinting from Latin American organizations. This database contains nearly 120 million records from the most important patent offices worldwide, covering as many as 104 countries[36].
Data were collected by building search queries for each database with specific terms related to 3D bioprinting and using the queries and the advanced search tools from each database. The results were then filtered to include only Latin American countries and inspected to make the necessary adjustments to the search queries.
2.3 Information Gathering
The third stage comprises the gathering of information and its validation by the experts before its analysis.
This step covers the retrieval of scientific and technological information regarding 3D bioprinting from the previously selected databases. Data from global and regional (Latin America) scenarios were collected, the latter being a subset of the first.
Scientific publications published between 2000 (the date of the first reports of 3D bioprinting[37]) and March 12, 2019 (the end date of information collection stage) were identified. Then, the Scopus filtering options were used to select only scientific production from Latin American countries. Scopus assigns a paper to a country based on the affiliations included in each document.
For patents, a similar procedure was used with the PatSeer software for the same time period as the Scopus search which comprised from 2000 to March 12, 2019. The results were filtered to only include patents from Latin American countries.
The queries for this research were based on the ones previously used by Rodríguez-Salvador et al. in their study of 3D bioprinting on a global scale[23]. The queries were constructed with specific keywords that characterized the 3D printing process, the 3D bioprinting process, and the biological terms that denote the principal applications of 3D bioprinting. Each search query was adapted to meet the syntax specifications for its corresponding database. The specific queries used in each database were as follows:
Scientific information (Scopus)
((((TITLE-ABS-KEY((cell OR bone OR cartilage OR tissue OR organ OR scaffold* OR bioscaffold* OR “bio scaffold” OR bio-scaffold OR biomimetic* OR skin) PRE/1 (print*))) OR (TITLE-ABS-KEY((tissue PRE/0 engineer*) OR “regenerative medicine” OR biomedic* OR (cancer PRE/0 model*) OR biomanufactur*))) AND (TITLE-ABS-KEY((((“3D” OR 3d OR 3-d OR “3 d” OR three-d OR “three d” OR additive OR freeform OR desktop) PRE/1 (print* OR manufactur* OR fabricat*)) OR ((rapid PRE/0 prototyp*) OR “layer by layer” OR layer-by-layer))))) OR (TITLE-ABS-KEY(bio-fabricat* OR (bio PRE/0 fabricat*) OR biofabricat* OR bioprint* OR (bio PRE/0 print*) OR bio-print* OR bioink OR bio-ink OR “bio ink”))) AND (LIMIT-TO(PUBYEAR,2019) OR LIMIT-TO(PUBYEAR,2018) OR LIMIT-TO(PUBYEAR,2017) OR LIMIT-TO(PUBYEAR,2016) OR LIMIT-TO(PUBYEAR,2015) OR LIMIT-TO(PUBYEAR,2014) OR LIMIT-TO(PUBYEAR,2013) OR LIMIT-TO(PUBYEAR,2012) OR LIMIT-TO(PUBYEAR,2011) OR LIMIT-TO(PUBYEAR,2010) OR LIMIT-TO(PUBYEAR,2009) OR LIMIT-TO(PUBYEAR,2008) OR LIMIT-TO(PUBYEAR,2007) OR LIMIT-TO(PUBYEAR,2006) OR LIMIT-TO(PUBYEAR,2005) OR LIMIT-TO(PUBYEAR,2004) OR LIMIT-TO(PUBYEAR,2003) OR LIMIT-TO(PUBYEAR,2002) OR LIMIT-TO(PUBYEAR,2001) OR LIMIT-TO(PUBYEAR,2000)) AND (LIMIT-TO(DOCTYPE,”ar”) OR LIMIT-TO(DOCTYPE,”cp”)).
Technological information (PatSeer)
TAC: (((bio-fabricat* OR biofabricat* OR bioprint* OR bio-print* OR bioink OR bio-ink) OR (((3D OR “3d” OR 3-d OR three-d OR additive OR freeform OR desktop) wd1 (print* OR manufactur* OR fabricat*) OR rapid prototyp* OR layer-by-layer) AND (((cell OR bone OR tissue OR organ OR bioscaffold* OR bio-scaffold OR biomimetic* OR bio-mimetic* OR skin OR cartilage OR scaffold*) wd1 (print*)) OR (tissue engineer* OR regenerative medicine OR biomedic* OR cancer model* OR biomanufactur*))))) AND PBD: [2000 to 2019-03-12].
2.4 Information Analysis
The fourth stage includes the analysis of the scientific literature and patent activity, its validation by the experts, and the verification of the final results.
The scientific information was analyzed by performing a data mining process on the collected data to refine the results obtained from Scopus. This process was conducted using the Patent iNSIGHT Pro™ software, which is a comprehensive data analysis platform that identifies relationships within the scientific literature and/or patents using advanced text mining algorithms[38]. In the present study, these algorithms were used to allow an efficient elimination of any duplicate scientific documents. A manual inspection was also conducted to validate this process and make any necessary adjustments to the remaining records. Thereafter, a scientometric analysis was conducted to identify the most productive nations, authors, and organizations in the field using Scopus and Patent iNSIGHT Pro™. A network analysis of the Latin American organizations was made by applying the Gephi software, which is designed for visualization and exploration of graphs and networks[39].
No patentometrics analysis was conducted, as no patents were found. This will be discussed in the following sections.
2.5 Results Delivery
The fifth stage corresponds to the results delivery step, where the analyzed information, known as intelligence, is distributed to the final user in an appropriate way that allows decision-makers to make strategic decisions.
2.6 Decision-making
In the final stage, the end users make strategic decisions based on the intelligence obtained.
3. Results
3.1 Scientometric Analysis
The results from this study indicate that the incursion into scientific research on 3D bioprinting has been slow-paced in Latin America so that when Latin American countries are compared with global leaders, this research area is still in its infancy. First, in the collection stage, 7073 papers related to 3D bioprinting were retrieved from Scopus, but only 213 of those were from the Latin American region. Next, data from both sets of documents (worldwide and regional) were downloaded and subjected to a deduplication process with patent iNSIGHT Pro™, followed by manual inspection of the results. This process decreased the number of records in the global and Latin American sets to 7027 and 202, respectively. Therefore, the Latin American region accounts for only 2.87% of the global scientific production on the subject. The results from the analysis of these documents are presented in Table 1.
Table 1.
Three-dimensional bioprinting scientific research published in Scopus by Latin American individuals or organizations.
| Latin American countries | ||||||
|---|---|---|---|---|---|---|
| 3D bioprinting | No. of papers | |||||
| Global | Latam | Latam (%) | Brazil | Mexico | Other Latam countries | |
| 7072 | 202 | 2.87 | 123 | 37 | 48 | |
*The sum of the number of papers from Brazil, Mexico, and other Latam countries exceeds 202 because six papers were coauthored by two Latin American countries. 3D: Three-dimensional
The results of detailed analyses of the 202 Latin American publications are presented in Figure 2A-D. Records from the year 2019 (11 documents) were excluded from the analysis in Figure 2A as they only represent a fraction of the year (January 1-March 12).
Figure 2.
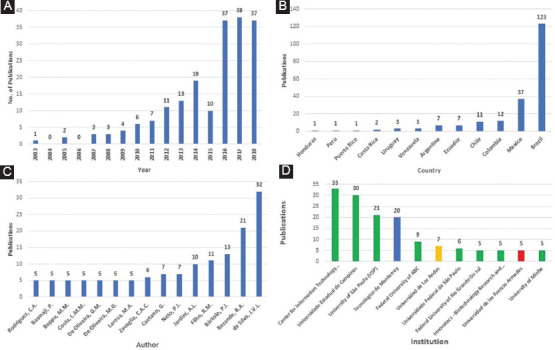
Latin American scientific publishing trends in three-dimensional bioprinting. (A) Yearly publishing dynamics, from 2003 to 2018. (B) Publications by Latin American country. (C) Top 10 publishing authors (15 authors are reported due to a tie for the 9th and 10th positions), and (D) Top 10 publishing institutions; Brazil is represented in green, Mexico in blue, Colombia in yellow, and Ecuador in red (11 affiliations are presented due to a tie for the 8th, 9th, and 10th positions).
Results of the yearly publishing dynamic are shown in Figure 2A. Curve fitting was used to determine the growth kinetics and trends of the number of publications per year on 3D bioprinting in Latin America. An exponential regression of the data from 2007 (when the publication trend shows a continuous growth) through 2018 was used to build a mathematical function that models this behavior (3.1). This equation has a coefficient of determination of R2 = 0.9214. If scientific production in Latin America continues to follow this growth rate, then 61 articles will have been published by 2019, and this number would increase to 79 by 2020.
y = (5x10−219)e0.251x (1)
As shown in Figure 2B, Brazil is the leading Latin American country publishing scientific knowledge on 3D bioprinting, accounting for 59.13% of all published documents. Brazil is followed by Mexico (17.78%), while the remaining countries each contribute 5.769% or less. This is no surprise, as Brazil, Mexico, Argentina, and Chile are considered the leading scientific systems in Latin America, as measured by the overall number of scientific publications produced[40]. Even though Brazil and Mexico have made valuable research efforts, a comparison of their research productivity with that of the global leaders (the USA with 2227 documents and China with 1368) shows that Latin American countries clearly have lagged in investing research efforts on this topic. For this reason, they have fallen behind in the global research scenario.
Regarding authors, the most productive ones publishing on 3D bioprinting in Latin America are Brazilian, as shown in Figure 2C. The first eight places on this list are occupied by Brazilians, while the 9th and 10th positions are shared equally by six Brazilian researchers and a Mexican one. The authors with the most publications, namely, Jorge Vicente Lopes Da Silva (32 documents) and Rodrigo Alvarenga Rezende (21 documents), both have published within the Center for Information Technology Renato Archer in Campinas, Brazil.
When focusing on Latin American institutions that have published the most on the subject, our results were congruent with previous findings. As shown in Figure 2D, the majority of the organizations (8 of 11) are Brazilian, including the top three: The Center for Information Technology Renato Archer (33 documents), the Universidade Estadual de Campinas (30 documents), and the University of São Paulo (21 documents). A Mexican, a Colombian, and an Ecuadorian University are also present in this ranking as well, with the Tecnologico de Monterrey in Mexico (20 documents) being the closest to the Brazilian leaders.
Two separate groups of institutions were identified: Those who have published a large number of papers (1st through 4th positions) and those who have published modestly (5th through 10th positions). On average, the first group has produced 4.3 times more scientific knowledge than the second, showing that a significant difference exists between them in this regard. However, the members of each group are separated by a minimal difference in published papers, so their places in the ranking may change in the upcoming years.
The collaboration dynamics and main research partners were determined for the four most prolific Latin American institutions. For this aim, collaboration was estimated based on the number of coauthored scientific papers on 3D bioprinting by each institution. Joint efforts of these institutions are presented in a network map in Figure 3. Figure 3A encompasses the dynamics of three Brazilian organizations, and Figure 3B portrays the collaborative network of the leading Mexican institution in this field. The main research partners of the four institutions are presented next:
Figure 3.
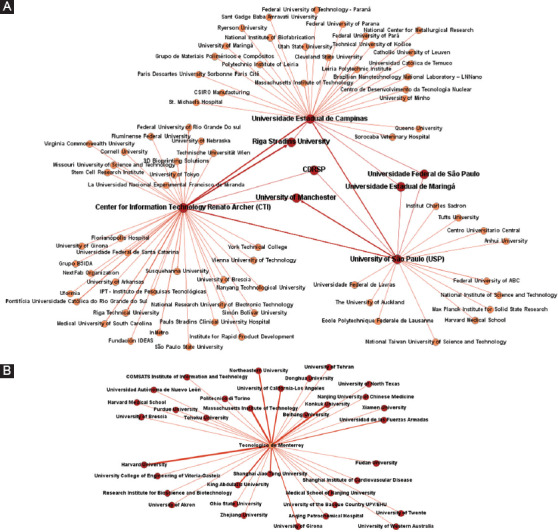
Research networks of the four most prolific Latin American institutions. (A) Collaboration dynamics of the Brazilian institutions. (B) Collaboration dynamics of the Mexican institution. Thickness and color intensity in the lines and nodes show the strengths of the relationships between each organization.
Center for Information Technology Renato Archer has developed the strongest research efforts with the Riga Stradins University (eight papers), followed by Riga Technical University (four papers) and Universidade Estadual de Campinas, University of São Paulo and Pontificia Universidade Católica do Rio Grande do Sul (three papers each)
Universidade Estadual de Campinas has worked the most with the Center for Information Technology Renato Archer (three papers) and strongly with Federal University of Pará, Leiria Polytechnic Institute, Massachusetts Institute of Technology, and University of São Paulo (two papers each)
The most important collaborator of the University of São Paulo is the University of Manchester (six papers), followed by the Center for Information Technology Renato Archer (three papers) and by Universidade Estadual de Campinas and Federal University of ABC (two papers each)
The major partners of Tecnologico de Monterrey are Harvard University, Konkuk University, and the Massachusetts Institute of Technology (four papers each), as well as King Abdulaziz University, Northeastern University, Shanghai Jiao Tong University, and University of California-Los Angeles (three papers each).
3.2 Patenting Activity in 3D Bioprinting
Even though the increasing interest that Latin American organizations and individuals have in 3D bioprinting, no patents concerning this subject were found through the PatSeer software analysis for the time period 2000 to March 12, 2019. As already mentioned in section 2.2 on the evaluation of information sources, this software was selected due to its broad extension (approximately 120 million records derived from the main patent authorities worldwide, covering as many as 104 countries). Considering that 3D bioprinting is a relatively new research topic in Latin America and that patenting in most cases is a slow-paced process, research on this topic has not yet reached the patenting stage for Latin America. However, this condition is expected to change in the upcoming years, as research is growing steadily, and this technology is becoming more affordable.
4. Discussion
The results obtained from the scientometric analysis depict the following overall scientific landscape for 3D bioprinting in Latin America: Research on this topic began in 2003, with intermittent activity until 2007, when it started to increase steadily to reach its current state; it has remained stable for the past 3 years (2016-2018).
Since 2003, Brazil has pioneered the development of scientific research on 3D bioprinting and has been the most extensive producer of scientific knowledge in Latin America, developing collaborations with several countries, including Latvia, the USA, and the UK, through the years. However, almost half of its research production (47.15%) has been made solely by Brazilian organizations. Mexico, as the second most prolific Latin American country in terms of publishing activity, also has held a prominent position since 2009 and has collaborated with countries such as the USA, South Korea, Saudi Arabia, and China. Likewise, the most productive research authors and institutions are Brazilian, followed by Mexicans. Finally, according to the Scopus domain classification, most research efforts in Latin America have been oriented toward engineering, material science, and chemical science fields.
Despite their still modest productivity, Latin American countries appear to have a high potential to become important players in bioprinting. Some of their scientists have been trained at leading institutions in the field (i.e., Massachusetts Institute of Technology, Harvard University, Nanyang Technological University), and a well-established collaborative network exists between some Latin American universities and these leading institutions. In addition, several groups in Latin America[41-44] have strong expertise in mammalian cell culture, which is highly advantageous for the effective establishment of a bioprinting cell line. In addition, the arrival of relatively affordable commercial bioprinters[45] (~10,000 USD) will further foster the interest in bioprinting applications among Latin American engineers. The development of in house bioprinting units is also feasible and publishable[18,46].
Results from the research on the patenting activity concerning 3D bioprinting in Latin America give valuable insights into the current state of development of this technology in this region. The lack of patents within this subject indicates that Latin American individuals and organizations have fallen behind in the translation of their scientific research into technological inventions. Therefore, this condition represents an opportunity for Latin American entities to capitalize on their investigations with formal protections through legal instruments, such as patents.
5. Conclusions and Perspective
In this study, a CTI methodology was applied to determine the current knowledge landscape of 3D bioprinting technology in Latin America. A scientometric analysis of scientific literature was conducted to reveal insights into the research efforts being made by individuals and institutions from this region and to identify relevant trends and the most prolific countries, authors, and institutions. Patenting by Latin American entities was studied to determine the current state of patents in the region.
Bioprinting is now receiving attention in Latin American universities. Brazil leads the publishing efforts, followed by institutions in Mexico, Colombia, and Chile. However, the amount of Latin American scientific production on the topic of bioprinting is still very modest, accounting for <3% of the worldwide production in a region containing nearly 10% of the world population. Patenting in the area of bioprinting has not yet begun. This may certainly change in the years to come, as some start-ups and universities begin to generate an intellectual property in the particular subjects of novel bioprinting equipment and bioinks.
Establishing the reasons for the gap in scientific production in bioprinting between Latin America and other regions (i.e., the USA, Europe, and Asia[47]) are beyond the scope of our study. However, a potential clearly exists for important growth. Several top Latin American universities are already players in the area of bioprinting and are collaborating with the leading institutions in the field. A natural commercial interest also exists for developing a network of users of commercial bioprinters in Latin America, since several top executives of leading bioprinting companies (i.e., Cellink and Allevi [formerly BioBots]) have Latin American roots. In addition, Latin America has a strong tradition in mammalian cell culture, with strong groups in Brazil, Argentina, Mexico, Chile, and Colombia.
Investment, both from the private sector and the government, will be highly beneficial at the stage where bioprinting is still an emerging field globally, and the clear opportunity exists for significant contributions. Local governmental funding agencies, such as the National Council for Scientific and Technological Development (CNPq) in Brazil and the National Council of Science and Technology (CONACYT) in Mexico, have encouraged research activities in 3D bioprinting in their respective scientific communities. Their involvement in upcoming years will be essential to further promote these activities in the region.
The next 5 years are decisive for establishing if bioprinting will become an important niche of activity in the scientific and technological community in Latin America. The combination of funding from private and public sectors, in addition to strategic international collaborations, specialized expertise in areas as mammalian cell culture, and availability of commercial bioprinters will drive the growth of Latin American research on 3D bioprinting in the near future. In the years to come, groups that are currently active in the field in Latin America will certainly incubate a sizeable generation of young scientists trained to understand bioprinting as a flexible and powerful tool for tissue engineering, medicine, pharmacological testing, microbiology, and food technology.
This study presents relevant trends of the current condition of the scientific and technological production of 3D bioprinting in Latin America. These insights can be useful in assessing and deciding on the present and future research efforts of R&D groups at universities and companies interested in the 3D bioprinting field.
Acknowledgments
The authors acknowledge institutional funding received from Tecnológico de Monterrey and Consejo Nacional de Ciencia y Tecnología (CONACyT). G.T.dS. acknowledges funding received from L’Oréal-UNESCO-AMC-CONACyT.
Conflicts of Interest
No conflicts of interest are reported by the authors.
References
- 1.Kerr C, Phaal R. Directing the Technology Intelligence Activity:An Information Needs Template for Initiating the Search. Technol Forecast Soc Change. 2018;134:265–76. DOI 10.1016/j.techfore.2018.06.033. [Google Scholar]
- 2.Rodriguez-Salvador M, Garcia-Garcia LA. Additive Manufacturing in Healthcare. Foresight STI Gov. 2018;12:47–55. [Google Scholar]
- 3. [[Last accessed on 2019 Mar 06]];Comisión Económica Para America Latina y el Caribe. 2019 Available from: https://www.cepal.org . [Google Scholar]
- 4.Tasoglu S, Demirci U. Bioprinting for Stem Cell Research. Trends Biotechnol. 2013;31:10–9. doi: 10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng W, Unutmaz D, Ozbolat IT. Bioprinting Towards Physiologically Relevant Tissue Models for Pharmaceutics. Trends Biotechnol. 2016;34:722–32. doi: 10.1016/j.tibtech.2016.05.013. DOI 10.1016/j.tibtech.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D Bioprinting:An Overview. Biomater Sci. 2018;6:915–46. doi: 10.1039/c7bm00765e. DOI 10.1039/c7bm00765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byambaa B, Annabi N, Yue K, et al. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue. Adv Healthc Mater. 2017;6:1700015. doi: 10.1002/adhm.201700015. DOI 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Zhang YS, Heinrich MA, et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater. 2017;29(3) doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurie H, Ikeguchi R, Aoyama T, et al. The Efficacy of a Scaffold-free Bio 3D Conduit Developed from Human Fibroblasts on Peripheral Nerve Regeneration in a Rat Sciatic Nerve Model. PLoS One. 2017;12:ne171448. doi: 10.1371/journal.pone.0171448. DOI 10.1371/journal.pone.0171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trujillo-de Santiago G, Alvarez MM, Samandari M, et al. Chaotic Printing:Using Chaos to Fabricate Densely Packed Micro and Nanostructures at High Resolution and Speed. Mater Horizons. 2018;5:813–22. DOI 10.1039/c8mh00344k. [Google Scholar]
- 11.Guillotin B, Souquet A, Catros S, et al. Laser Assisted Bioprinting of Engineered Tissue with High Cell Density and Microscale Organization. Biomaterials. 2010;31:7250–6. doi: 10.1016/j.biomaterials.2010.05.055. DOI 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 12.Gudapati H, Dey M, Ozbolat I. A Comprehensive Review on Droplet-based Bioprinting:Past, Present and Future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. DOI 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SV, Atala A. 3D Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. DOI 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YS, Yue K, Aleman J, et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng. 2017;45:148–63. doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang HW, Lee SJ, Ko IK, et al. A 3D Bioprinting System to Produce Human-scale Tissue Constructs with Structural Integrity. Nat Biotachno. 2016;l34(3):312–9. doi: 10.1038/nbt.3413. DOI 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 16.Bhise NS, Manoharan V, Massa S, et al. A Liver-on-a-chip Platform with Bioprinted Hepatic Spheroids. Biofabrication. 2016;8:14101. doi: 10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 17.Lehner BA, Schmieden DT, Meyer AS. A Straightforward Approach for 3D Bacterial Printing. ACS Synth Biol. 2017;6:1124–30. doi: 10.1021/acssynbio.6b00395. DOI 10.1021/acssynbio.6b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiesz EM, Yu K, Lehner BA, et al. Three-dimensional Patterning of Engineered Biofilms with a Do-it-yourself Bioprinter. J Vis Exp. 2019;147:ne59477. doi: 10.3791/59477. DOI 10.3791/59477. [DOI] [PubMed] [Google Scholar]
- 19.Hynes WF, Chacón J, Segrè D, et al. Bioprinting Microbial Communities to Examine Interspecies Interactions in Time and Space. Biomed Phys Eng Express. 2018;4:55010. DOI 10.1088/2057-1976/aad544. [Google Scholar]
- 20.Rothschild LJ. Synthetic Biology Meets Bioprinting:Enabling Technologies for Humans on Mars (and Earth) Biochem Soc Trans. 2016;44:1158–64. doi: 10.1042/BST20160067. DOI 10.1042/bst20160067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional Bioprinting of Thick Vascularized Tissues. Proc Natl Acad Sci U S A. 2016;113:3179–84. doi: 10.1073/pnas.1521342113. DOI 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X, Breitenkamp K, Finn MG, et al. Direct Human Cartilage Repair Using Three-dimensional Bioprinting Technology. Tissue Eng Part A. 2012;18:1304–12. doi: 10.1089/ten.tea.2011.0543. DOI 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Salvador M, Hernandez-de Menendez AM, Arcos-Novillo DA, et al. Additive Manufacturing:Importance and Challenges for Latin America. Cham:Springer. 20162016:249–71. DOI 10.1007/978-3-319-39056-7_14. [Google Scholar]
- 24.Rodriguez-Salvador M, Rio-Belver RM, Garechana-Anacabe G. Scientometric and Patentometric Analyses to Determine the Knowledge Landscape in Innovative Technologies:The Case of 3D Bioprinting. PLoS One. 2017;12:e180375. doi: 10.1371/journal.pone.0180375. DOI 10.1371/journal.pone.0180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Quintanar L, Rodriguez-Salvador M. Discovering new 3D Bioprinting Applications:Analyzing the Case of Optical Tissue Phantoms. Int J Bioprint. 2019;5(1):178. doi: 10.18063/IJB.v5i1.178. DOI 10.18063/ijb.v5i1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Salvador M, Ruiz-Cantu L. Revealing Emerging Science and Technology Research for Dentistry Applications of 3D Bioprinting. Int J Bioprint. 2019;5(1):170. doi: 10.18063/ijb.v5i1.170. DOI 10.18063/ijb.v5i1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Garcia LA, Rodriguez-Salvador M. Uncovering 3D Bioprinting Research Trends:A Keyword Network Mapping Analysis. Int J Bioprint. 2018;4(2):147. doi: 10.18063/IJB.v4i2.147. DOI 10.18063/ijb.v4i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashton WB, Stacey GS. Technical Intelligence in Business:Understanding Technology Threats and Opportunities. [[Last accessed on 2019 May 20]];Int J Technol Manage. 1995 10(1):79–103. Available from: http://www.paper.shiftit.ir/sites/default/files/article/02M-Ashton and Stacey-1995.pdf . [Google Scholar]
- 29.Garcia-Garcia LA, Rodriguez-Salvador M. Competitive and Technology Intelligence to Reveal the Most Influential Authors and Inter-institutional Collaborations on Additive Manufacturing for Hand Orthoses. J Intell Stud Bus. 2018;8(3):32–44. [Google Scholar]
- 30.Huang C, Notten A, Rasters N. Nanoscience and Technology Publications and Patents:A Review of Social Science Studies and Search Strategies. J Technol Transf. 2011;36:145–72. DOI 10.1007/s10961-009-9149-8. [Google Scholar]
- 31.Mingers J, Leydesdorff L. A Review of Theory and Practice in Scientometrics. Eur J Oper Res. 2015;246:1–19. [Google Scholar]
- 32.Garcia-Garcia LA, Rodriguez-Salvador M. Additive Manufacturing Knowledge Incursion on Orthopaedic Device:The Case of Hand Orthoses. Proceedings of the 3rd International Conference on Progress in Additive Manufacturing, (Pro-AM 2018) 2018:571–6. [Google Scholar]
- 33.Rotolo D, Rafols I, Hopkins MM, et al. Strategic Intelligence on Emerging Technologies:Scientometric Overlay Mapping. J Assoc Inf Sci Technol. 2017;68:214–33. DOI 10.1002/asi.23631. [Google Scholar]
- 34.Konur O. The Scientometric Evaluation of the Research on the Production of Bioenergy from Biomass. Biomass Bioenergy. 2012;47:504–15. DOI 10.1016/j.biombioe.2012.09.047. [Google Scholar]
- 35. [[Last accessed on 2019 Mar 06]];Elsevier 2019, About Scopus. Available from: http://www.elsevier.com/solutions/scopus .
- 36.Gridlogics 2019, Pat Seer. [Last accessed on 2019 Mar 06] Available from: https://www.patseer.com/patseer-content . [Google Scholar]
- 37.Melchels FP, Domingos MA, Klein TJ, et al. Additive Manufacturing of Tissues and Organs. Prog Polym Sci. 2012;37:1079–104. [Google Scholar]
- 38.Gridlogics 2019, Patent iNSIGHT Pro. [Last accessed on 2019 Mar 06] Available from: https://www.patentinsightpro.com/product.html .
- 39.Gephi 2019, Gephi. Available from: https://www.gephi.org . [Last accessed on 2019 Apr 12]
- 40.Confraria H, Vargas F. Scientific Systems in Latin America:Performance, Networks, and Collaborations with Industry. J Technol Transf. 2019;44:874–915. DOI 10.1007/s10961-017-9631-7. [Google Scholar]
- 41.Garza-García LD, Carrillo-Cocom LM, Araiz-Hernández D, et al. A Biopharmaceutical Plant on a Chip:Continuous Micro-devices for the Production of Monoclonal Antibodies. Lab Chip. 2013;13:1243–6. doi: 10.1039/c3lc50104c. DOI 10.1039/c3lc50104c. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza-Pérez E, Hernández V, Palomares LA, et al. An Integrated System for Synchronous Culture of Animal Cells Under Controlled Conditions. Biotechniques. 2016;61(3):129–36. doi: 10.2144/000114451. DOI 10.2144/000114451. [DOI] [PubMed] [Google Scholar]
- 43.Tossolini I, López-Díaz FJ, Kratje R, et al. Characterization of Cellular States of CHO-K1 Suspension Cell Culture Through Cell Cycle and RNA-sequencing Profiling. J Biotechnol. 2018;286:56–67. doi: 10.1016/j.jbiotec.2018.09.007. DOI 10.1016/j.jbiotec.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Luchese MD, dos Santos ML, Garbuio A, et al. A New CHO (Chinese Hamster Ovary)-Derived Cell Line Expressing Anti-TNFαMonoclonal Antibody with Biosimilar Potential. Immunol Res. 2018;66:392–405. doi: 10.1007/s12026-018-8997-4. DOI 10.1007/s12026-018-8997-4. [DOI] [PubMed] [Google Scholar]
- 45.Choudhury D, Anand S, Naing MW. The Arrival of Commercial Bioprinters-Towards 3D Bioprinting Revolution! Int J Bioprint. 2018;4(2):139. doi: 10.18063/IJB.v4i2.139. DOI 10.18063/ijb.v4i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McElheny C, Hayes D, Devireddy R. Design and Fabrication of a Low-Cost Three-Dimensional Bioprinter. J Med Device. 2017;11:41001. doi: 10.1115/1.4037259. DOI 10.1115/1.4037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cimoli M, Pereima JB, Porcile G. A Technology Gap Interpretation of Growth Paths in Asia and Latin America. Res Policy. 2019;48:125–36. DOI 10.1016/j.respol.2018.08.002. [Google Scholar]


