Supplemental Digital Content is available in the text.
Keywords: cardiovascular disease, cytokines, inflammation, lipidomics, metabolic syndrome, obesity, sex
Abstract
Objective:
Metabolic dysregulation and inflammation are important consequences of obesity and impact susceptibility to cardiovascular disease. Anti-inflammatory therapy in cardiovascular disease is being developed under the assumption that inflammatory pathways are identical in women and men, but it is not known if this is indeed the case. In this study, we assessed the sex-specific relation between inflammation and metabolic dysregulation in obesity.
Approach and Results:
Three hundred two individuals were included, half with a BMI 27 to 30 kg/m2 and half with a BMI>30 kg/m2, 45% were women. The presence of metabolic syndrome was assessed according to the National Cholesterol Education Program-ATPIII criteria, and inflammation was studied using circulating markers of inflammation, cell counts, and ex vivo cytokine production capacity of isolated immune cells. Additionally, lipidomic and metabolomic data were gathered, and subcutaneous fat biopsies were histologically assessed.
Metabolic syndrome is associated with an increased inflammatory profile that profoundly differs between women and men: women with metabolic syndrome show a lower concentration of the anti-inflammatory adiponectin, whereas men show increased levels of several pro-inflammatory markers such as IL (interleukin)-6 and leptin. Adipose tissue inflammation showed similar sex-specific associations with these markers. Peripheral blood mononuclear cells isolated from men, but not women, with metabolic syndrome display enhanced cytokine production capacity.
Conclusions:
We identified sex-specific pathways that influence inflammation in obesity. Excessive production of proinflammatory cytokines was observed in men with metabolic syndrome. In contrast, women typically showed reduced levels of the anti-inflammatory adipokine adiponectin. These different mechanisms of inflammatory dysregulation between women and men with obesity argue for sex-specific therapeutic strategies.
Highlights.
We identified sex-specific pathways that influence inflammation in obesity and metabolic syndrome.
Women show lower concentrations of the anti-inflammatory adiponectin in the presence of metabolic syndrome.
In men, the presence of metabolic syndrome was associated with increased monocyte-derived circulating cytokines (mainly IL [interleukin]-6) and hyperresponsive circulating immune cells.
The prevalence of overweight and obesity has increased dramatically over the last few decades. In 2016, globally 39% of adults were overweight (BMI>25 kg/m2) and 13% were obese (BMI>30 kg/m2),1 while in developed countries, the percentages are often even higher. Overweight and obesity are strongly associated with atherosclerotic cardiovascular disease (CVD), diabetes mellitus, and several types of cancer.2 Two important mechanism that drive CVD development in obesity are metabolic dysregulation and systemic inflammation, with low-grade chronic inflammation contributing to the development of metabolic syndrome and its complications.3 The metabolic syndrome serves as an indicator of obesity-related metabolic dysregulation and is strongly associated with atherosclerotic CVD.4
There are important sex differences in the pathophysiology of atherosclerotic CVD, with sex-specific risk factors and differences in the prevalence and treatment.5 Differences in sex hormones and sex-specific effects of adipokines are all implicated in these differences, but knowledge on sex-specific regulation of inflammation and metabolic dysregulation in obesity is sparse.
We have previously shown that sex, age, genes, and the environment are important determinants of systemic inflammatory processes in healthy individuals.6,7 While it is implied that the same mechanisms control inflammation in women and men with regard to CVD, this has never been systematically investigated. In this study, we investigated the impact of sex on the relation between inflammation and metabolic syndrome in overweight women and men. We further explored the role of circulating metabolites (metabolome) and circulating hormones on sex-specific effects. We specifically focused on 3 groups of inflammatory parameters: plasma adipokine and cytokine concentrations, immune cell subpopulations in the blood, and the ex vivo cytokine production capacity of circulating immune cells (such as monocytes and T cells). These distinct inflammatory phenotypes are all known to be important in the pathophysiology of CVD but are differentially regulated.8
Methods and Materials
Cohort and Measurements
Anonymized data and materials have been made publicly available at the Human Functional Genomics Project (HFGP) website and can be accessed at https://hfgp.bbmri.nl/. As part of the HFGP,9 we recruited a cohort of 302 overweight or obese individuals between 55 and 82 years of age, with a BMI >27 kg/m2 at screening, of mostly Western European ancestry, termed the 300-Obesity (300-OB) cohort. Most of these participants previously took part in the Nijmegen Biomedical Study—Non-Invasive Measurements of Atherosclerosis 1.10 Detailed information about the cohort is available in the Methods in the Data Supplement. All participants were included between the year 2014 and 2016.
All individuals filled out questionnaires, which included questions about lifestyle, environmental factors, and medication usage. If used, participants temporarily discontinued lipid-lowering therapy 4 weeks before the measurements. Blood samples were taken in the morning following an overnight fast. All women were postmenopausal and were not receiving hormonal replacement therapy. All participants received detailed printed and oral information and subsequently gave written informed consent. The study was approved by the Ethical Committee of the Radboud University (nr. 46846.091.13). Experiments were conducted according to the principles expressed in the Declaration of Helsinki.
The Metabolic Syndrome
In our study, the metabolic syndrome is defined by the National Cholesterol Education Program ATP III criteria as the presence of any 3 of the following 5 traits.11
Abdominal obesity, defined as a waist circumference in men ≥102 cm (40 in) and in women ≥88 cm (35 in).
Serum triglycerides (TG) ≥150 mg/dL (1.7 mmol/L) or drug treatment for elevated triglycerides.
Serum high-density lipoprotein cholesterol <40 mg/dL (1 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women or drug treatment for low high-density lipoprotein cholesterol.
Blood pressure ≥130/85 mm Hg or drug treatment for elevated blood pressure.
Fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or drug treatment for elevated blood glucose.
Blood glucose, triglycerides, total cholesterol, and high-density lipoprotein cholesterol were measured using standard laboratory procedures. Before measuring systolic and diastolic blood pressure, participants took 30 minutes of supine rest.
Detailed information on the measurements is available in the Methods in the Data Supplement.
Circulating Mediators
Circulating mediators (cytokines and adipokines) were measured in human EDTA plasma using ELISA.
Stimulation Experiments
PBMC Stimulation Experiments
Isolation of peripheral blood mononuclear cells (PBMCs) was performed as described in Oosting et al.12
Whole Blood Stimulation Experiments
A volume of 100 μL of heparin blood was added to a 48-well plate (Corning) containing 400 µL stimulus (final volume 500 µL/well) for 48 hours at 37°C and 5% CO2.
ELISA Analysis
Cytokine concentrations after stimulation were measured using commercially available ELISA kits.
Stimuli and Cytokines
Table I in the Data Supplement lists the concentrations of the stimuli used. IL (interleukin)-1β, IL-6, TNF-α, and IL-1Ra were measured after 24-hour stimulation with these stimuli, and IFNγ, IL-17, and IL-22 were measured after 7 days of stimulation only for Candida albicans and Staphylococcus aureus stimulation. The choice of proinflammatory mediators was based on extensive literature linking cytokines and adipokines to inflammation and CVD complications. Stimulation of PBMCs was performed with a comprehensive set of stimuli containing both purified innate immune stimuli that are associated with chronic inflammation (eg, LPS, oxidized LDL [low-density lipoprotein]) and microorganism that are the source of microbial ligands that translocate in the circulation at the intestinal level.
Cell Count Data
Immune cell counts were determined in fresh whole blood EDTA samples using the Sysmex XE-5000.
Metabolomics
Untargeted Metabolomics
Blood was collected in EDTA tubes and plasma was extracted. Flow injection electrospray time-of-flight mass spectrometry was performed by General Metabolomics (1 Broadway, Cambridge, MA 02142) to identify metabolic features based on m/z. Details of the procedure can be found in Fuhrer et al.13 A total of 1339 m/z signals could be assigned to one or more metabolites.
Lipidomics
A high-throughput nuclear magnetic resonance metabolomics platform (Nightingale’s Biomarker Analysis Platform) was used to quantify a total of 231 lipid and metabolite measures. Most of these measures were very highly correlated to other measures from the same platform. Groups of lipoprotein particle characteristics were therefore made based on a correlation between variables of at least r>0.75 and expert knowledge. This led to 17 groups (see Table II in the Data Supplement), for each group, a representative variable was selected to represent the whole group of measurements. This led to easier interpretation and less strict multiple testing correction.
Adipose Tissue Analysis
Subcutaneous adipose tissue biopsies were obtained under local anesthesia by needle biopsies performed 6 to 10 cm lateral to the umbilicus in the right lower quadrant, after an overnight fast. The morphometry of individual fat cells was assessed using digital image analyses as described previously.14 For each participant, the adipocyte cell diameters of all adipocytes in 4 microscopic fields of view were counted and measured. To detect macrophages, adipose tissue sections were incubated with a CD68-monoclonal antibody (Serotec, Oxford, United Kingdom). The percentage of macrophages was expressed as the total number of macrophages divided by the total number of adipocytes counted in 15 random microscopic fields of view. A crown-like structure was defined as an adipocyte surrounded by at least 3 macrophages.15 To robustly quantify adipose tissue inflammation among study participants using histology, several parameters were assessed and combined into an adipose tissue inflammation score, as the phenotype by which inflammation in the adipose tissue presents is quite heterogenous.16,17 This assessment led to a score including the following parameters: a mean adipocyte diameter above the average diameter of the cohort (>51.7 µm [mean diameters in the 300-OB] cohort) was defined as one point in the adipose tissue inflammation score (AT score), the percentage of macrophages above the average of the cohort (>12.6% [mean in the 300-OB cohort]) was defined as one point in the AT score and the presence of crown-like structures was defined as one point in the AT score. Hence, the adipose tissue inflammation score ranged from 0 (no inflammation) to 3 (severely inflamed).
Quantification and Statistical Analysis
Data and Software Availability
The R code (via R programming language18) used for the analyses will be made available upon request. Multiple testing correction was performed using the Benjamini-Hochberg false discovery rate (FDR) procedure.19
For the metabolic pathway analysis, we used an adaptation (Fast Gene Set Enrichment Analysis20) of Gene Set Enrichment Analysis.21 The pathways provided by the KEGG pathway database22 were used for enrichment analysis. Interesting pathways were visualized using Pathview.23
For details on the statistical analysis, see Methods in the Data Supplement.
Results
300-OB Cohort
Figure 1 provides an overview of the clinical characteristics of the cohort, separated by sex: 55% are men and 45% are women, with a similar BMI distribution in both sexes (Figure 1B and Table X in the Data Supplement). Metabolic syndrome is defined as having 3 out of 5 cardiovascular risk factors mentioned in Figure 1E through 1I.11 The prevalence of metabolic syndrome was 54.8% in women and 53.9% in men (Figure 1C), and a larger percentage of women had abdominal obesity and low high-density lipoprotein cholesterol (Figure 1G and 1H). Some individuals also had other obesity-related comorbidities (Figure 1D).
Figure 1.
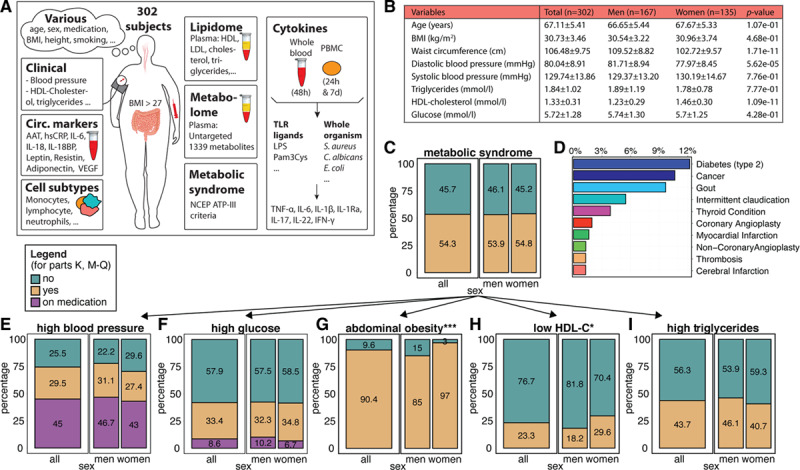
Characteristics of the 300-OB cohort. A, Overview of some of the parameters measured in the 300-OB cohort. Each outlined square indicates a different data type and schematically describes the different measurements. circ. markers, circulating markers of inflammation. B, Baseline characteristics, also split by sex. C, Percentage of individuals with the metabolic Syndrome as defined by the National Cholesterol Education Program (NCEP)-ATP III criteria. Left: overall percentage, right: subdivided into women and men. Yellow: people with the condition, green: people without the condition. D, Percentage of individuals with other conditions. E–I, Percentage of individuals with each of the 5 criteria used in defining metabolic syndrome, displayed in a similar style to (C): purple indicates people on medication for the condition (E) high blood pressure, (F) high glucose, (G) abdominal obesity, (H) low HDL cholesterol (HDL-c), (I) high triglycerides *P value ≤0.05 calculated using χ2 test. ***P value ≤0.005.
Association Between Markers of Inflammation in Overweight/Obese Individuals
The sex-specific values of circulating inflammatory markers and adipokines are depicted in Figure 2A. We previously reported the effects of age and sex on inflammation in a healthy cohort7 (n=489; age, 27.4±12.5 years; BMI, 22.7±2.7 kg/m2). Overall, in the present study, we observed similar effects of age (Figure 2B; Table III in the Data Supplement), that is, IL (interleukin)-6 and IL-18BP circulating concentrations increase with age. In addition, we observed an effect of sex on various parameters; higher levels of hsCRP (high-sensitive CRP), leptin, and adiponectin were seen in women (Figure 2B).
Figure 2.
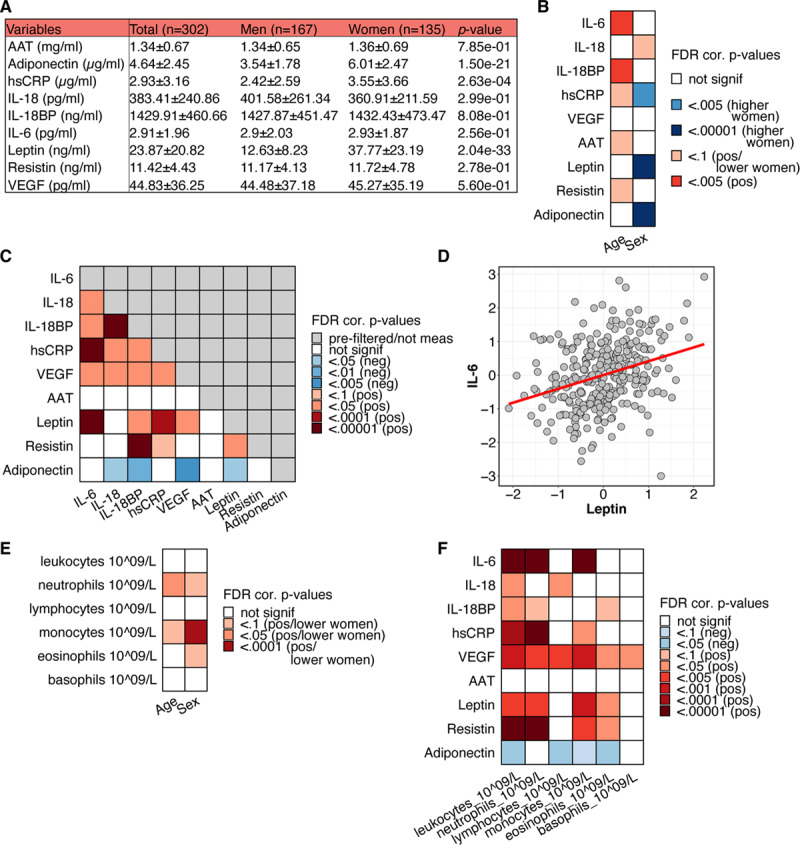
Associations of various immune and host parameters in the 300-OB cohort. A, Mean and SDs of several circulating inflammatory markers for women and men. B, P values of associations between age and sex with markers of inflammation* (BH-false discovery rate [FDR] multiple testing corrected). C, P values of associations between markers of inflammation, corrected for age, sex, and season (BH-FDR multiple testing corrected). D, Example association between IL (interleukin)-6 and leptin. E, P values of associations between age and sex with immune cell counts* (BH-FDR multiple testing corrected). F, P values of associations between immune cell numbers and circulating markers of inflammation, corrected for age, sex, and season (BH-FDR multiple testing corrected). Note: all P values were calculated using linear regression and testing the null hypothesis that β=0 for relationship between x and y, where x and y are the parameters of interest. The data in the example plots (D) were corrected for age, sex, and season and transformed using IRT (see section Methods). *Associations with age corrected for sex and season, associations with sex corrected for age and season.
To better understand the regulation of inflammation in obesity, we evaluated the association between different markers of inflammation using FDR corrected P values based on linear regression models. Age, sex, and season were added as co-factors, as they are known to have an influence on the immune system.7 Most markers of inflammation show positive associations with one another, specifically IL-6, IL-18, IL-18BP, hsCRP, VEGF, and leptin (Figure 2C; Table IV in the Data Supplement). IL-6 concentrations show particularly strong associations with leptin (Figure 2D, adjusted β=0.31) and hsCRP (adjusted β=0.53), which is in line with previous studies.24,25 We observed a negative association of adiponectin with several inflammatory markers (Figure 2C), in line with its known anti-inflammatory properties and its decrease with increasing BMI.26 Associations of these markers of inflammation are mostly the same between women and men (Table V in the Data Supplement).
In addition to circulating inflammatory markers, we also measured immune cell populations. Absolute numbers of neutrophils and monocytes increase with age, and men generally have higher numbers of neutrophils, eosinophils, and especially monocytes (Figure 2E; Table VII in the Data Supplement), in line with previous findings.26,27
We found strong associations between most inflammatory markers and the number of leukocytes, neutrophils, and monocytes, most of them positive (Figure 2F; Table VII in the Data Supplement).
The Impact of Sex on Metabolic Syndrome and Inflammation
The presence of metabolic syndrome was positively associated with several inflammatory markers IL-6, IL-18, IL-IL-18BP, hsCRP, leptin, and VEGF (Figure 3A; Table VIII in the Data Supplement). Importantly, IL-6 and leptin show profound sex-specific effects: only in men, these are higher in the presence of the metabolic syndrome, whereas in women, there is no association with the metabolic syndrome (Figure 3A, 3B, 3E, and 3F). Similar trends are observed for hsCRP and IL-18, although the interaction between sex and metabolic syndrome was not significant when testing based on a linear regression model with an interaction term (Figure 3A and 3B; Table VIII and IX in the Data Supplement). In contrast to the proinflammatory mediators, the anti-inflammatory adiponectin was lower in individuals with metabolic syndrome. Interestingly, this effect was significantly stronger in women compared with men, as opposed to IL-6 and leptin (Figure 3A, 3B, and 3G). To validate this finding, we measured adiponectin levels in a subset of the Nijmegen Biomedical Study—Non-Invasive Measurements of Atherosclerosis 1 cohort28 of 441 participants, filtering for a BMI>27 kg/m2. The individuals in this cohort were on average 4.4 years younger than the 300-OB cohort (2.8 years younger on average for men and 6.0 years for women). In this cohort, 33% of the subjects suffered from the metabolic syndrome (Additional details can be found in Table X in the Data Supplement). We were able to confirm the same pattern of a lower concentration of adiponectin in women with the metabolic syndrome but not in men (Figure 3H).
Figure 3.
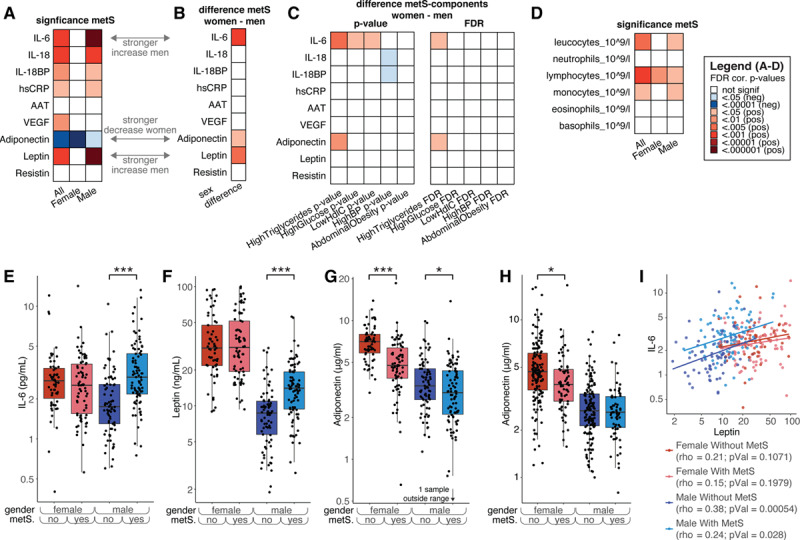
The effect of metabolic syndrome (MetS) on immune parameters. A, Significance of the difference in levels of several inflammatory markers between individuals with and without MetS. Red indicates that the marker is significantly higher in individuals with metabolic syndrome and blue means it is higher in those without. The first column is for all individuals, and the second and third are for women and men, respectively (false discovery rate [FDR] corrected per column). B, Significance of the interaction effect between sex and metabolic syndrome. Red indicates that the increase of women with metabolic syndrome vs those without was significantly lower than men with metabolic syndrome vs those without, that is, red means men show a stronger increase than women or women show a stronger decrease than men. C, Interaction effects for each individual medical condition used to score metabolic syndrome with sex. Each individual is scored as either having the condition or not, and interaction effects are calculated in a similar way as in (B). Similarly, red means men show a stronger increase than women, or women show a stronger decrease than men; blue means the opposite. The left part shows the P values not corrected for multiple testing, and the right part shows the FDR-corrected P values. D, Same as (A), but for cell counts. E–H, Example plots split into women and men with and without metabolic syndrome. E, IL-6, (F) leptin, (G) adiponectin. H, Similar to (G), but for the validation cohort. I, Plot showing Spearman correlations between leptin and IL-6 for the 4 categories: men with MetS, men without MetS, women with MetS, and women without MetS. Note: P values in (A and D) were calculated using linear regression by testing the null hypothesis that β=0 for relationship between metabolic syndrome status (independent variable) and a continuous parameter (dependent variable), see Methods for details. The interaction effects of (B and C) were calculated using linear regression, with the null hypothesis that β=0 for the interaction effect between sex and metabolic syndrome status (independent variable) and a continuous parameter (dependent variable).
In addition, as inflammation originating from the adipose tissue is one of the main driving forces for systemic low-grade inflammation present in obesity,29 we examined the possible existence of sex-specific differences in the relation between circulating leptin, adiponectin, and adipose tissue inflammation. We calculated AT scores for the 300-OB cohort based on fat tissue taken from the abdominal subcutaneous fat depot. After adjustment for age, pack years, and season,7 AT scores showed significant association with circulating levels of leptin and IL-6 in men but not women (standardized βmen=0.22, Pmen<0.001, Pinteraction_sex:leptin=0.02 for leptin; βmen=0.25, Pmen<0.001, Pinteraction_sex:IL6=0.07 for IL-6), while in women AT scores were more strongly negatively associated with circulating adiponectin (standardized βwomen=−0.28; Pwomen<0.0001). This provides further evidence of sex-specific regulation of systemic low-grade inflammation.
As IL-6 and leptin concentrations are correlated, and the concentrations of these markers are elevated specifically in men with metabolic syndrome, we assessed the strength of this correlation separately by sex and presence/absence of metabolic syndrome. We observed that leptin and IL-6 are only correlated in men but not in women, and most strongly in men without metabolic syndrome (Figure 3I).
The 5 factors (Figure 1E through 1I) that define metabolic syndrome might not have an equal contribution to the sex-specific effects described in the previous paragraphs. We therefore evaluated each of these factors separately, using the cut-off levels as defined by National Cholesterol Education Program ATP-III criteria (see Methods). Having high versus normal triglycerides levels seems to be the most important parameter in explaining the sex-specific effects of metabolic syndrome on both IL-6 in men and adiponectin in women (Figure 3D, compared with Figure 3B). The sex-specific changes in leptin with metabolic syndrome cannot be explained by any single parameter, and the full definition of metabolic syndrome is needed to explain that effect.
We also observed changes in the number of immune cell subtypes in individuals with metabolic syndrome: specifically, total leukocytes, lymphocytes, and monocytes numbers are higher in individuals with metabolic syndrome (Figure 3E). Importantly, these changes in both absolute amounts of cell subtypes with metabolic syndrome are not sex-dependent (Table XI in the Data Supplement).
Differential Regulation of Cytokine Production Capacity in Women and Men
Cytokine production capacity of PBMCs, as an indicator of the intrinsic inflammatory responsiveness, has previously been linked to the presence of atherosclerosis.30,31 Production of the proinflammatory cytokines IL-1β and IL-6 and the anti-inflammatory cytokine IL-1Ra show associations with circulating levels of IL-6 and hsCRP (Figure I in the Data Supplement). Interestingly, in line with higher inflammatory markers in men with metabolic syndrome, there is a strong trend toward higher monocyte-derived inflammatory cytokine production capacity specifically in men, but not women, with metabolic syndrome (Figure 4A, left, Figure 4B, Tables XII and XIII in the Data Supplement). This effect does not seem to be limited to a single PBMC stimulation, and is most apparent for the cytokines IL-6 and IL-1β, and less so for TNF-α. Again, high triglycerides is the main factor of metabolic syndrome showing sex-specific effects, with only men with high triglycerides showing increased levels of cytokine production capacity (Figure 4A and 4C; Figure II and Table XIV in the Data Supplement). There were no differences in the cytokine production capacity of IL-1Ra and the lymphocyte derived cytokines IL-22, IL-17, and IFNγ between women or men with or without metabolic syndrome (data not shown).
Figure 4.
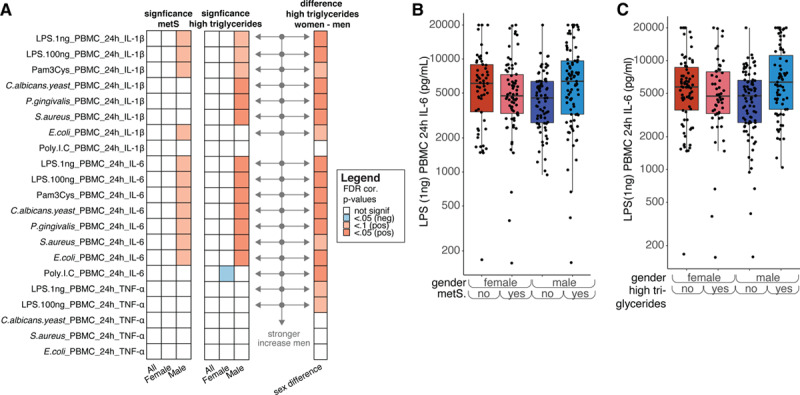
A, Left, same as (A) but for IL (interleukin)-6, IL-1β, and TNFα production in peripheral blood mononuclear cells (PBMCs) after different stimulations. Middle, similar to the heatmap on the left but for high triglycerides instead of metabolic syndrome (right) similar to (D) but solely plotted for high triglycerides (false discovery rate [FDR] corrected). The arrows mark those cytokines after stimulation that show a significantly stronger increase in men than in women. Note: if men show a nonsignificant difference between men with and without MetS and women also show a nonsignificant difference, the increase in men show with getting MetS can still be significantly different from the change that women show. B, Example plot showing the effect of metabolic syndrome and sex on IL-6 production in response to Candida albicans stimulation. C, Example plot showing the effect of having high triglycerides and sex on IL-6 production in response to C albicans stimulation. Note: P values were calculated in a similar fashion as for Figure 3.
Changes in the Metabolome of Individuals With Metabolic Syndrome
Metabolic dysregulation and systemic inflammation are important mechanisms that drive CVD development in obesity. To further explore whether the composition of circulating lipoproteins could contribute to the sex-specific association of the metabolic syndrome with IL-6, leptin, adiponectin, and cytokine production capacity, we measured lipoprotein subclasses, lipoprotein particles sizes, apolipoproteins and lipoprotein content using the Nightingale platform (Table II in the Data Supplement). A principle component analysis shows separation between both women and men and between individuals with and without metabolic syndrome (Figure 5A). The principle component analysis and a linear regression analysis show that women have larger concentrations of HDL particles, HDL triglycerides, ApoA1, and cholesterol (Figure 5B and Table XV in the Data Supplement), and many lipoproteins have altered composition and concentration in subjects with metabolic syndrome, for example, decreases in HDL particles and increases in LDL particles, with an increase in triglycerides (Figure 5C, left). Women and men show the same patterns of change when comparing individuals with and without metabolic syndrome (Figure 5C, right).
Figure 5.
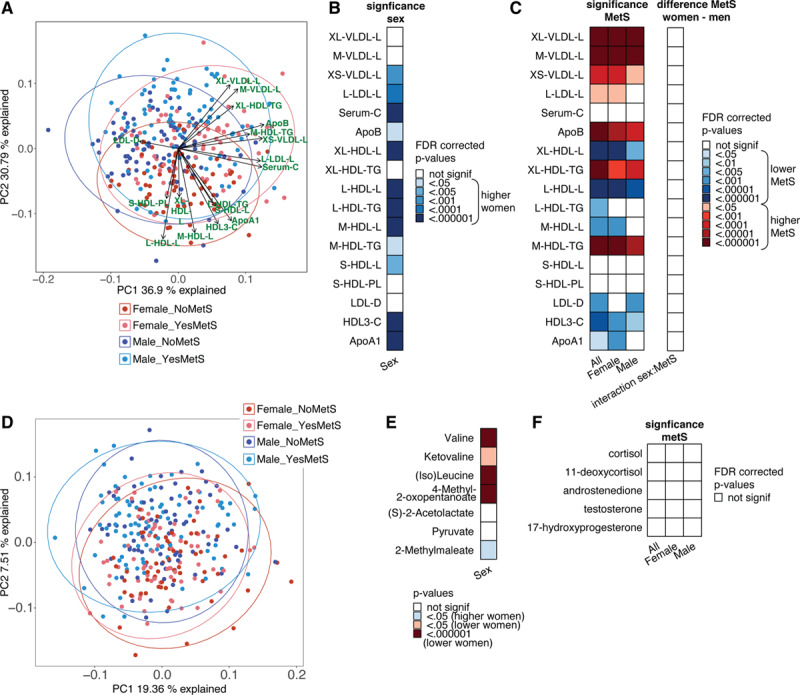
A, Principle component analysis (PCA) plot of PC1 vs PC2 for the lipidomics data. B, Lipid groups that are significantly different between women and men. Blue indicates higher levels in women. C, Left, significance (false discovery rate [FDR]-corrected P values) of the difference of lipid concentrations between individuals with or without metabolic syndrome. Calculated for the whole 300-OB cohort and calculated for women a women separately. Right, FDR-corrected P values for the interaction effect between metabolic syndrome and sex in predicting the lipids, that is, showing if the increase/decrease of these lipids seen in metabolic syndrome is the same in women and men. D, PCA plot of the metabolomics data after inverse rank transformation and standardization. The first 2 PCs are plotted, and the samples are split into 4 groups: women without metabolic syndrome (“Female_NoMetS”), women with metabolic syndrome (“Female_YesMetS”), men without metabolic syndrome (“Male_NoMetS”), men with metabolic syndrome (“Female_YesMetS”). PC2 shows some separation between women and men. E, Significance of sex differences for the 7 metabolites from the valine, leucine, and isoleucine biosynthesis that showed significant association with metabolic syndrome. Several of these metabolites also show clear differences between women and men. F, Significance (FDR-corrected P values) of the difference in hormone concentrations between individuals with or without metabolic syndrome. Note: P values in (B) and (E) were calculated using linear regression by testing the null hypothesis that β=0 for relationship between sex (independent variable) and a continuous parameter (dependent variable), see Methods for details. P values in (C [left]) and (F) were calculated using linear regression by testing the null hypothesis that β=0 for relationship between metabolic syndrome status (independent variable) and a continuous parameter (dependent variable), see Methods for details. The interaction effect of (C [right]) was calculated using linear regression, with the null hypothesis that β=0 for the interaction effect between sex and metabolic syndrome status (independent variable) and a continuous parameter (dependent variable). C indicates total cholesterol; CE, cholesterol esters; D, mean diameter; FC, free cholesterol; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; L, large; L, total lipids; LDL, low-density lipoprotein; M, medium; P, concentration of particles; PL, phospholipids; S, small; Serum-C, serum cholesterol; TG, triglyceride; VLDL, very-low-density lipoprotein; XL, very large; and XS, extremely small.
To further explore metabolic changes associated with the metabolic syndrome beyond lipidomics, we used untargeted metabolomics assay that detected 1339 metabolite signals. We evaluated the association of metabolic syndrome with these metabolites. First, we applied principle component analysis to these data to see if metabolic syndrome and/or sex impact on the variation of this data. PC2 shows a clear separation between women and men (Figure 5D). However, using these untargeted analyses, no large differences between individuals with and without metabolic syndrome can be observed. Nonetheless, using a regression model, we found many metabolites that were associated with metabolic syndrome. The associations for all metabolite hits with metabolic syndrome are listed in Table XVI in the Data Supplement. The top pathway found in a pathway analysis was valine, leucine, and isoleucine biosynthesis though it did not reach statistical significance after multiple testing correction (Table XVII in the Data Supplement). It is interesting to note that 5 out of the 7 metabolites of the “valine, leucine and isoleucine biosynthesis” pathway that are significantly associated with metabolic syndrome, also show significantly different levels between women and men (Figure 5E; Table XVIII in the Data Supplement). However, these metabolites did not show a sex-specific association with metabolic syndrome (Figure 5E).
Hormonal Changes Do Not Explain Sex Differences
The cause of the differences in inflammatory profiles between women and men with metabolic syndrome is not yet known, but one hypothesis is that it might involve differential homeostasis of sex hormones. To evaluate the potential role of sex hormones in the metabolic syndrome we measured baseline levels of several steroid hormones: testosterone, androstenedione, cortisol, 11-deoxycortisol, and 17-hydroxyprogesterone (Table XIX in the Data Supplement). We found no significant difference in hormone levels associated with the metabolic syndrome in both women and men (Figure 5F).
Plasma Metabolome Effects on Inflammatory Markers
To explore whether circulating metabolites influence inflammatory parameters and account for the sex-differences in inflammation in relation to metabolic syndrome, we evaluated the relationship between metabolites and inflammation. We see the strongest associations for adiponectin and IL-18BP, but hsCRP, resistin, and IL-6 also show many associations with metabolites (Figure 6A; Table XX in the Data Supplement).
Figure 6.
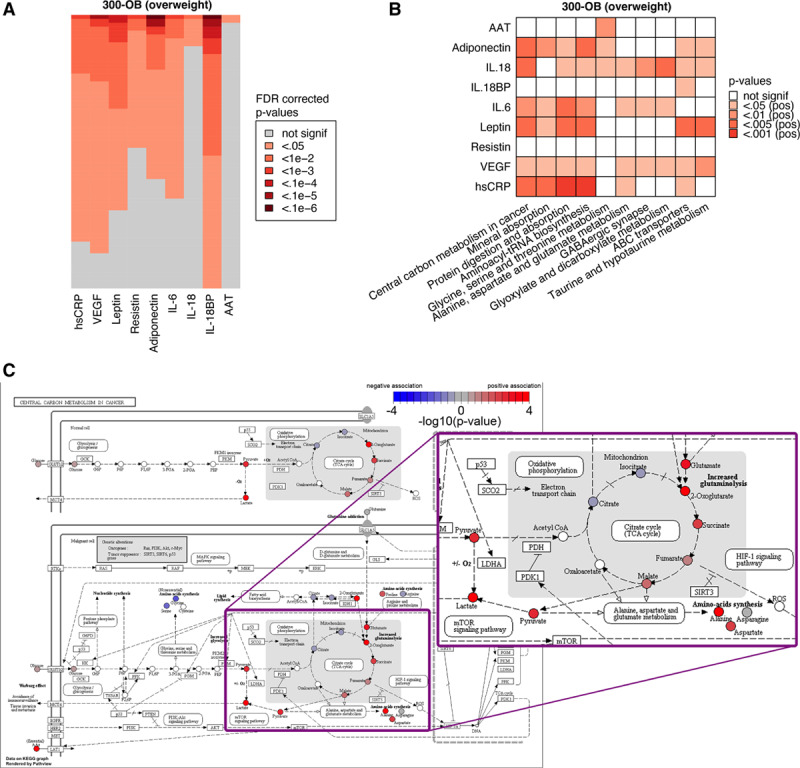
Metabolic comparison between normal weight and overweight individuals. A, Visualization of the top 5% of metabolites (lowest P values) associated with each of the circulating markers of inflammation to give an impression of the number of significant metabolites. For each marker, the false discovery rate (FDR)-corrected P values were sorted independently to show the relative number of significant metabolites for each marker. B, Top 10 metabolic pathways associated with circulating markers of inflammation in the 300-OB cohort. See Methods for details. C, Example pathway plot of Central Carbon Metabolism in Cancer for VEGF in the 300-OB cohort. Significance is indicated as −log10 (P values), any P value below P=1×10−4 is rounded up to 1×10−4 for this visualization. Red indicates a positive association, blue indicates a negative association, and white indicates that the metabolite was not present in our data. Visualization was performed using Pathview.23 The portion of the figure showing the TCA cycle is shown as a zoomed-up inset.
The results for the top 10 pathways that correlate with inflammatory markers are displayed in Figure 6B. Pathway analysis was performed using an adapted version of Gene Set Enrichment Analysis.21 The central carbon metabolism in cancer pathway is particularly interesting as an important part of it is the TCA cycle, which in the last years has been shown to have a strong impact on inflammation and the function of immune cells.32,33 However, although we identified important correlations between metabolites and inflammation, these associations were not sex-specific and could not explain the sex-differences in inflammatory markers in individuals with metabolic syndrome (Table XXI in the Data Supplement).
Discussion
In this study, we aimed to understand the various regulatory layers of the adverse immunometabolic effects of overweight and obesity, and in particular which parts of that regulation differ between women and men. Therefore, we characterized in detail a cohort of 302 individuals (approximately equally distributed between women and men, with or without metabolic syndrome) with overweight and obesity in terms of circulating inflammatory markers, immune cell counts, immune cell responsiveness, circulating metabolomics/lipidomics, and histological analysis of subcutaneous fat biopsies. The most important finding was that there is a sex-specific association of metabolic syndrome with various inflammatory parameters: metabolic syndrome is associated with lower circulating concentrations of the anti-inflammatory adiponectin, whereas only in men the presence of metabolic syndrome is associated with increased monocyte-derived circulating cytokines (mainly IL-6), and increased leptin. For adiponectin, these sex-specific associations were validated in an independent cohort. Adipose tissue inflammation is a central feature of metabolic syndrome, and we found that adipose tissue inflammation was positively associated with circulating leptin and IL-6 only in men, while in women adiponectin was negatively associated with the AT score. In addition, metabolic syndrome was associated with hyperresponsive circulating immune cells only in men. This suggests that the role of inflammation and the immune system in the adverse cardiometabolic consequences of obesity is different in women and men. Further analyses revealed sex-specific differences circulating metabolites, lipoprotein composition, and hormones, but these differences did not explain the differential association of inflammatory markers with metabolic syndrome between women and men. These findings call for a sex-specific approach with regard to inflammation as a pharmacological target to prevent CVD in obese individuals and for further research to unravel the mechanisms that drive the sex-specific differences.
The metabolic syndrome serves as an indicator of metabolic dysregulation, is strongly associated with the development of atherosclerotic CVD,5 and is often accompanied by a chronic low-grade inflammation.34 Differences in a selection of inflammatory parameters between women and men that have metabolic syndrome have been reported previously in small studies. Sarbijani et al35 showed in a group of 40 subjects that men with metabolic syndrome have higher IL-6 levels than those without, though it should be noted that for women and men the characteristics of the case and control groups were different in this study. Ahonen et al36 showed that absolute differences in adiponectin in individuals with and without the metabolic syndrome were larger in women than men, although the significance of this difference was not reported. However, a systematic overview of the differences between women and men with metabolic syndrome is still lacking. The strength of our current study is that it provides a more comprehensive description of the sex-specific associations of metabolic syndrome with circulating inflammatory markers and immune cell phenotype. Additionally, we strengthened these findings by studying adipose tissue inflammation and an independent cohort and explored potential underlying mechanisms. One of the most important observations of our study is the difference in the dysregulation of inflammation between women and men: in women, the presence of metabolic syndrome is characterized by a lack of the anti-inflammatory adipokine adiponectin, while in men it presents as an excess of proinflammatory mediators such as IL-6 and leptin. Interestingly, IL-6 and leptin are only correlated in men, further suggesting a differential regulation of these inflammatory markers in women. We were able to validate the sex specific associations of adiponectin levels in women and men with metabolic syndrome in an independent cohort. These different inflammatory phenotypes in women and men with metabolic syndrome can have important therapeutic consequences, suggesting sex-specific approaches.
In addition to systemic inflammation, there is accumulating evidence that the phenotype of circulating immune cells contributes to adverse cardiometabolic effects. We and others have recently reported that circulating monocytes have an enhanced cytokine production capacity in patients with risk factors for atherosclerosis, including familial hypercholesterolemia and elevated lipoprotein (a),37,38 and in patients with established coronary atherosclerosis.30,31 Interestingly, in the 300-OB cohort, a similar hyperresponsiveness of circulating PBMCs was present in men with metabolic syndrome compared with men without metabolic syndrome, whereas in women, these differences were not observed. In men, the presence of metabolic syndrome was associated with a higher production of IL-1β and IL-6 after stimulation with a wide range of inflammatory stimuli. This might well be related to the observation that circulating IL-6 is higher in men with metabolic syndrome as IL-6 is associated with ex vivo production of IL-1β and IL-6 in response to several stimuli (Figure IA in the Data Supplement).
To gain insight into the mechanism responsible for the sex-specific association of metabolic syndrome with adipokines, and inflammatory parameters, we first studied the association between adipose tissue inflammation and circulating adipokine and cytokine levels. Leptin and adiponectin are both produced in adipose tissue. Leptin is at the interface between metabolism and inflammation in fat tissue; leptin production by the adipose tissue facilitates the secretion of proinflammatory cytokines, and these, in turn, promote the release of leptin from adipocytes.39 Adipose tissue inflammation is a well-known feature of metabolic syndrome. Of great interest, we were able to demonstrate a positive association of adipose tissue inflammation with leptin and IL-6 specifically in men and a negative association of adipose tissue inflammation with adiponectin in women. However, the causality between adipose tissue inflammation and these circulating adipokines and cytokines needs to be further established. We hypothesize that the increased adipose tissue inflammation associated with metabolic syndrome in men results in peripheral leptin resistance, which in turn leads to increased leptin levels and also impacts other inflammatory parameters. Conversely, in women, the increased adipose tissue inflammation limits adiponectin production. It is important to realize that the adipose tissue biopsy was obtained from the abdominal subcutaneous fat depot in both sexes, while men and women are known to have a difference in (abdominal) fat distribution: with the same BMI, men have on average a higher ratio of visceral/subcutaneous adipose tissue compared with women.40 This difference might impact on systemic inflammation and needs to be included in future analyses.
Additionally, to gain more insight into what specific features of the metabolic syndrome drive the observed associations with circulating adipokines and cytokines, we individually evaluated the 5 parameters defining metabolic syndrome. We showed that circulating triglycerides concentration seem to be the most important condition driving the sex-specific association. In women with high TGs, adiponectin levels are lower, whereas in men with high TGs, plasma IL6 is higher. In addition, also the augmented cytokine production capacity of PBMCs in men with metabolic syndrome is mainly dependent on the presence of high TGs. Further lipidomic analyses showed that this differential effect is not because of sex-differences in the composition of circulating lipoproteins. Following high-fat loads, circulating triglycerides are associated with circulating cytokine levels such as IL-6 and with activation of circulating innate immune cells.41,42 However, it is currently unclear why triglycerides are associated with higher IL-6 and higher cytokine production capacity only in men.
As has been shown previously that for example testosterone can have immunomodulatory effect,43 we tested the hypothesis that circulating hormones contribute to the observed sex-specific differences in inflammatory parameters by measuring the serum concentrations of 5 circulating steroid hormones, of which 4 show strong differential concentrations in men and women, including 17-OH-progesteron and testosterone. For these hormones, we did not observe any differential association with triglycerides or the metabolic syndrome nor an association with circulating cytokines and cytokine-production capacity that could explain our findings. It is important to realize that we were only able to test a selection of hormones and that we cannot exclude effects of progesterone or estrogens. Also, though changes in sex hormones with the metabolic syndrome do not explain the observed sex differences, contrasting baseline levels of steroid hormones between women and men might still play a role these differences (Table XIX in the Data Supplement).
Following these observations of a differential association of adipokines, inflammatory markers, and immune cell responsiveness, we further explored the role of other circulating metabolites that might play a role in metabolic syndrome. Metabolites are the end point of many biological processes, and the metabolome thereby provides a snap-shot of the current physiological state. We observed strong differences between women and men in circulating metabolites. In our study, the valine, leucine, and isoleucine biosynthesis pathway was associated with the presence of metabolic syndrome and is likely to be the most biologically relevant in terms of association with inflammatory parameters. This pathway has previously linked to metabolic syndrome, poor metabolic health, insulin resistance, and type II diabetes mellitus.44,45 However, women and men showed the same associations between these metabolite concentrations and metabolic syndrome. Lipid profiles of women and men with metabolic syndrome showed similar patterns: while levels of most lipid markers were different between women and men, and these levels changed with metabolic syndrome, these changes were the same in women and men. This suggests that even though metabolites play an important role in metabolic syndrome, this role is similar in both sexes.
There are several limitations to the current study. First, this is an observational study, and although we validated some of the major findings in an independent cohort, our data do not provide information about causality and mechanisms. Second, all individuals in the 2 cohorts studied here were of Western European descent, and it is difficult to extrapolate these data to other populations. Third, we used metabolic syndrome as indicator of cardiometabolic dysregulation. It is important to realize that there are various definitions for metabolic syndrome and this syndrome is heterogeneous. To optimize external validation, we used the most frequently used definition of the National Cholesterol Education Program ATP III. In addition, we performed all analyses separately for the various components of the metabolic syndrome, indicating triglycerides as major factor that contributes to the sex-specific differences. Finally, we have only studied a selection of circulating hormones and cannot exclude modulating effects of unmeasured hormones as an explanation of our findings.
In conclusion, in this first study, we comprehensively analyzed the regulation of inflammation in overweight individuals, and we showed that there is as strong sex-dependent association of metabolic syndrome with circulating markers of inflammation. Importantly, we demonstrate that inflammatory dysregulation in women and men with obesity and metabolic syndrome is mediated by different mechanisms, which relate to adipose tissue inflammation. Women show defective anti-inflammatory mechanisms (adiponectin), whereas men have higher concentrations of proinflammatory mediators (leptin, IL-6) and their myeloid cells show a hyperresponsive phenotype. These findings strongly argue for more in vitro and in vivo studies aimed at unraveling mechanisms that underlie this sex-specific inflammatory regulation. Moreover, these findings suggest that women and men might benefit from a differential sex-specific anti-inflammatory pharmacological intervention to prevent the adverse cardiometabolic effects of obesity.
Acknowledgments
We thank all of the volunteers in the 300-OB cohort for their participation. We thank Rosanne van Deuren for helpful discussions about the project. We thank Rinke Stienstra for developing the adipose tissue inflammation score.
Sources of Funding
This study was supported by an IN-CONTROL CVON grant (CVON2012-03 and CVON2018-27) to M.G. Netea, L.A.B. Joosten, J.H.W. Rutten, N.P. Riksen). The Human Functional Genomics Project is supported by a European Research Council (ERC) Consolidator grant (ERC 310372) to M.G. Netea. M.G. Netea is further supported by a Netherlands Organization for Scientific Research Spinoza Grant [NWO SPI 94-212]. L.A.B. Joosten is supported by a Competitiveness Operational Programme grant of the Romanian Ministry of European Funds (HINT, ID P_37_762; MySMIS 103587). N.P. Riksen is recipient of a grant of the ERA-CVD Joint Transnational Call 2018, which is supported by the Dutch Heart Foundation (JTC2018, project MEMORY); 2018T093.
Disclosures
None.
Supplementary Material
Footnotes
Nonstandard Acronyms and Abbreviations
- AT score
- adipose tissue inflammation score
- CVD
- cardiovascular disease
- hsCRP
- high-sensitive CRP
- LDL
- low-density lipoprotein
- MetS
- metabolic syndrome
- PBMC
- peripheral blood mononuclear cell
These authors contributed equally to this article.
These authors share senior authorship.
For Sources of Funding and Disclosures, see page 1799.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.314508.
References
- 1.World Health Organization. Obesity and overweight. 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoletti R, Bolego C, Poli A, Cignarella A. Metabolic syndrome, inflammation and atherosclerosis. Vasc Health Risk Manag. 2006;2:145–152. doi: 10.2147/vhrm.2006.2.2.145. doi: 10.2147/vhrm.2006.2.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 5.Regitz-Zagrosek V, Kararigas G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol Rev. 2017;97:1–37. doi: 10.1152/physrev.00021.2015. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Oosting M, Smeekens SP, Jaeger M, Aguirre-Gamboa R, Le KTT, Deelen P, Ricaño-Ponce I, Schoffelen T, Jansen AFM, et al. A Functional Genomics Approach to Understand Variation in Cytokine Production in Humans. Cell. 2016;167:1099–1110.e14. doi: 10.1016/j.cell.2016.10.017. doi: 10.1016/j.cell.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AFM, Lemmers H, et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell. 2016;167:1111–1124.e13. doi: 10.1016/j.cell.2016.10.018. doi: 10.1016/j.cell.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, Joosten LA, Li Y, Kumar V, Oosting M, Smeekens S, Jaeger M, Ter Horst R, Schirmer M, Vlamakis H, et al. Understanding human immune function using the resources from the Human Functional Genomics Project. Nat Med. 2016;22:831–833. doi: 10.1038/nm.4140. doi: 10.1038/nm.4140. [DOI] [PubMed] [Google Scholar]
- 10.Galesloot TE, Vermeulen SH, Swinkels DW, de Vegt F, Franke B, den Heijer M, de Graaf J, Verbeek ALM, Kiemeney LALM. Cohort Profile: The Nijmegen Biomedical Study (NBS). Int J Epidemiol. 2017;46:1099–1100j. doi: 10.1093/ije/dyw268. doi: 10.1093/ije/dyw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Program National Cholesterol Education. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation. 2002;106:3143. doi: 10.1161/circ.106.25.3143. [PubMed] [Google Scholar]
- 12.Oosting M, Buffen K, Cheng SC, Verschueren IC, Koentgen F, van de Veerdonk FL, Netea MG, Joosten LAB. Borrelia-induced cytokine production is mediated by spleen tyrosine kinase (Syk) but is Dectin-1 and Dectin-2 independent. Cytokine. 2015;76:465–472. doi: 10.1016/j.cyto.2015.08.005. doi: 10.1016/j.cyto.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrer T, Heer D, Begemann B, Zamboni N. High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal Chem. 2011;83:7074–7080. doi: 10.1021/ac201267k. doi: 10.1021/ac201267k. [DOI] [PubMed] [Google Scholar]
- 14.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Müller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–287. doi: 10.1007/s11154-014-9301-0. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 17.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R-project.org/ [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R STAT SOC B. 1995;57:289–300. [Google Scholar]
- 20.Korotkevich G, Sukhov V, Sergushichev A. Fast gene set enrichment analysis [published online June 20, 2016]. bioRxiv. doi: 10.1101/060012. http://biorxiv.org/content/early/2016/06/20/060012. [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1831. doi: 10.1093/bioinformatics/btt285. doi: 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stelzer I, Zelzer S, Raggam RB, Prüller F, Truschnig-Wilders M, Meinitzer A, Schnedl WJ, Horejsi R, Möller R, Weghuber D, et al. Link between leptin and interleukin-6 levels in the initial phase of obesity related inflammation. Transl Res. 2012;159:118–124. doi: 10.1016/j.trsl.2011.10.001. doi: 10.1016/j.trsl.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Lee YJ, Park B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine (Baltimore) 2019;98:e15340. doi: 10.1097/MD.0000000000015340. doi: 10.1097/MD.0000000000015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holewijn S, den Heijer M, van Tits LJ, Swinkels DW, Stalenhoef AF, de Graaf J. Impact of waist circumference versus adiponectin level on subclinical atherosclerosis: a cross-sectional analysis in a sample from the general population. J Intern Med. 2010;267:588–598. doi: 10.1111/j.1365-2796.2009.02192.x. doi: 10.1111/j.1365-2796.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 29.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 30.Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–236. doi: 10.1016/j.atherosclerosis.2016.10.019. doi: 10.1016/j.atherosclerosis.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. doi: 10.1084/jem.20150900. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qatanani M, Tan Y, Dobrin R, Greenawalt DM, Hu G, Zhao W, Olefsky JM, Sears DD, Kaplan LM, Kemp DM. Inverse regulation of inflammation and mitochondrial function in adipose tissue defines extreme insulin sensitivity in morbidly obese patients. Diabetes. 2013;62:855–863. doi: 10.2337/db12-0399. doi: 10.2337/db12-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams NC, O’Neill LAJ. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front Immunol. 2018;9:141. doi: 10.3389/fimmu.2018.00141. doi: 10.3389/fimmu.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Sarbijani HM, Khoshnia M, Marjani A. The association between Metabolic Syndrome and serum levels of lipid peroxidation and interleukin-6 in Gorgan. Diabetes Metab Syndr. 2016;10(1 Suppl 1):S86–S89. doi: 10.1016/j.dsx.2015.09.024. doi: 10.1016/j.dsx.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Ahonen T, Saltevo J, Laakso M, Kautiainen H, Kumpusalo E, Vanhala M. Gender differences relating to metabolic syndrome and proinflammation in Finnish subjects with elevated blood pressure. Mediators Inflamm. 2009;2009:959281. doi: 10.1155/2009/959281. doi: 10.1155/2009/959281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekkering S, Stiekema LCA, Bernelot Moens S, Verweij SL, Novakovic B, Prange K, Versloot M, Roeters van Lennep JE, Stunnenberg H, de Winther M, et al. Treatment with Statins Does Not Revert Trained Immunity in Patients with Familial Hypercholesterolemia. Cell Metab. 2019;30:1–2. doi: 10.1016/j.cmet.2019.05.014. doi: 10.1016/j.cmet.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 38.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation. 2016;134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr Immunol Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–647. doi: 10.2337/dc11-1583. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Zhao SP, Wen T, Zhou HN, Hu M, Li JX. Postprandial hypertriglyceridemia associated with inflammatory response and procoagulant state after a high-fat meal in hypertensive patients. Coron Artery Dis. 2008;19:145–151. doi: 10.1097/MCA.0b013e3282f487f3. doi: 10.1097/MCA.0b013e3282f487f3. [DOI] [PubMed] [Google Scholar]
- 42.Hyson DA, Paglieroni TG, Wun T, Rutledge JC. Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clin Appl Thromb Hemost. 2002;8:147–155. doi: 10.1177/107602960200800211. doi: 10.1177/107602960200800211. [DOI] [PubMed] [Google Scholar]
- 43.Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation. 2008;5:32. doi: 10.1186/1742-2094-5-32. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017;25:43–56. doi: 10.1016/j.cmet.2016.09.018. doi: 10.1016/j.cmet.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. doi: 10.1161/CIRCULATIONAHA.114.013116. doi: 10.1161/CIRCULATIONAHA.114.013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code (via R programming language18) used for the analyses will be made available upon request. Multiple testing correction was performed using the Benjamini-Hochberg false discovery rate (FDR) procedure.19
For the metabolic pathway analysis, we used an adaptation (Fast Gene Set Enrichment Analysis20) of Gene Set Enrichment Analysis.21 The pathways provided by the KEGG pathway database22 were used for enrichment analysis. Interesting pathways were visualized using Pathview.23
For details on the statistical analysis, see Methods in the Data Supplement.


