Abstract
Accumulating preclinical and clinical evidence suggests that calcification is one of the body’s primary responses to injury and a key pathological feature of cardiovascular disease. Calcification activity can now be imaged using 18F-sodium fluoride (18F-NaF) positron emission tomography (PET) in combination with either computed tomography or magnetic resonance. These techniques allow visualization of calcification activity and, therefore, provide different information to the established macroscopic calcium imaged with computed tomography. Indeed, 18F-NaF PET has been used to investigate a wide range of valvular conditions, including aortic stenosis, mitral annular calcification, and bioprosthetic valve disease, as well as vascular conditions, including abdominal aortic aneurysm disease, coronary, and carotid atherosclerosis, peripheral vascular disease, and erectile dysfunction. In this brief review, we will focus on how 18F-NaF PET has improved our pathophysiological understanding of cardiovascular calcification and how it can be used as a marker of vascular calcification, providing a useful tool that can be utilized in clinical trials investigating the prediction of both disease progression and clinical events. Finally, we will discuss how 18F-NaF might be employed clinically to improve patient assessment and to guide decision-making.
Keywords: 18F-NaF, erectile dysfunction, positron emission tomography, tomography, vascular calcification
Highlights.
18F-sodium fluoride positron emission tomography can be used to study calcification activity across the vasculature.
It has been used to study (1) coronary artery disease, (2) carotid atherosclerosis, (3) abdominal aortic aneurysm disease, (4) aortic stenosis, (5) bioprosthetic valve degeneration and (6) erectile dysfunction.
Ongoing clinical trials are evaluating its ability to predict both disease progression and clinical events and to act as a biomarker evaluating drug efficacy.
Calcification is a key feature of atherosclerosis, heart valve disease, and peripheral vascular disease, that is governed in a highly regulated manner with similarities to skeletal bone formation. Computed tomography (CT), which detects large macroscopic deposits of calcium, has until recently been the only available noninvasive imaging modality allowing visualization of this process.1 However, imaging of this late stage means that the processes leading to calcium formation have often resolved. There is, therefore, interest in imaging the earlier stages of microcalcification where the disease process is active and therapeutic intervention may be more effective.
Please see www.ahajournals.org/atvb/atvb-focus for all articles published in this series.
Over the last 10 years, 18F-sodium fluoride (18F-NaF) positron emission tomography (PET) has emerged as a noninvasive quantitative imaging modality capable of imaging calcification activity in the vasculature. This has provided significant insight into the underlying pathophysiology of multiple different cardiovascular disease states and unique information regarding disease activity that may prove clinically useful in the future. In this brief review, we will talk about how 18F-NaF PET works and how this novel approach has been used to investigate a range of valvular and vascular diseases including aortic stenosis, mitral annular calcification (MAC), bioprosthetic valve degeneration, abdominal aortic aneurysm disease, erectile dysfunction, carotid atherosclerosis, and coronary artery disease (Figure 1).
Figure 1.
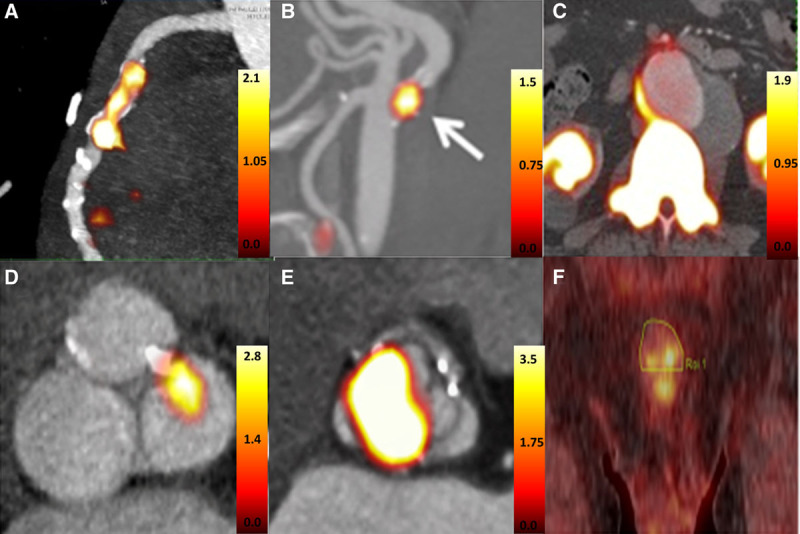
18F-fluoride uptake in different disease states. A, Patient with recent myocardial infarction (reprinted from Joshi et al.34 Copyright ©2014, the Authors), (B) patient with recent transient ischemic attack and uptake in the culprit carotid artery (reprinted from Vesey et al.39 Copyright ©2014, the Authors), (C) patient with abdominal aortic aneurysm (reprinted from Forsythe et al.42 Copyright ©2018, the Authors), (D) patient with mild aortic valve stenosis and corresponding 18F-NaF uptake in areas of leaflet calcification (image derived from Dweck et al17), (E) patient with aortic valve degeneration and corresponding 18F-NaF uptake (reprinted from Cartlidge et al.29 Copyright ©2019, the Authors), and (F) patient with erectile dysfunction and high penile uptake (reprinted from Nakahara et al.44 Copyright ©2019, Elsevier).
Understanding How 18F-NaF PET Works
CT is currently only able to identify macroscopic deposits of calcium with a diameter between 200 and 500 μm (CT calcium scoring).2,3 However, the 18F-NaF signal is higher in areas of microcalcification (<50 μm) than macrocalcific deposits for a similar mass of calcium. Newly developing calcium, adjacent or remote from macrocalcific deposits will pass through this microcalcification stage so that 18F-NaF effectively provides a marker of calcification activity, detecting new calcium formation beyond the resolution of CT.1,4 Initially used as bone tracer with increased uptake observed in conditions associated with high bone turnover and new bone formation,5–7 18F-NaF demonstrates favorable pharmacokinetic properties and has established an excellent safety profile. Following intravenous injection, roughly 70% of 18F-NaF is plasma-based, with the remaining 30 % found in erythrocytes. Because of its small size and negligible protein binding, one hour after administration only ≈10% of the injected 18F-NaF dose remains in the blood (SUVmean [SD]=1.13 [0.05] in right atrium).8–10 The mechanism of 18F-NaF uptake in bone is well understood. First, it diffuses via the capillary network into the bone extracellular fluid, and then it exchanges with hydroxyl groups on exposed regions of hydroxyapatite crystals on the bone surface to form fluorapatite.1 The intensity of the tracer uptake and as a result of the PET signal depends mainly on 2 factors: the bone blood flow and the surface area of exposed hydroxyapatite.1
Hydroxyapatite is also a key feature of vascular calcification, with a growing literature demonstrating that similar mechanisms govern 18F-NaF PET binding in the cardiovascular system. However, here blood flow is likely to be fairly constant, so the surface area of hydroxyapatite appears to be the major factor affecting 18F-NaF uptake. Indeed 18F-NaF binding is highest in areas of microcalcification compared with large macroscopic deposits due to the very high surface area of hydroxyapatite in these regions of powdery microcalcification.1,5,6,11 Unlike other PET tracers, 18F-NaF also demonstrates very low uptake in the myocardium, well below that observed even in the blood pool. This important characteristic facilitates clear visualization of regions of increased 18F-NaF uptake in areas of active calcification in the heart but also mediates precise coregistration via alignment of the ventricular blood pools on PET and contrast CT.10,12–14
Validation of the cardiovascular 18F-NaF signal has now been provided in multiple independent studies. Using excised atherosclerotic plaque, Cocker et al15 demonstrated the association between cardiovascular 18F-NaF PET uptake and the histological staining of hydroxyapatite with Goldner trichome. Aikawa et al16 confirmed that 18F-NaF binds predominantly to hydroxyapatite, whereas in the aortic valve, 18F-NaF has demonstrated a close association with areas of tissue with positive histochemical staining for alkaline phosphatase (r=0.65; P=0.04) and areas with positive immunohistochemical reactivity for osteocalcin (r=0.68; P=0.03).1,17 Irkle et al18 demonstrated that 18F-NaF binding is increased in regions of microcalcification, rather than large macroscopic deposits. This is predominantly due to surface area effects and because much of the hydroxyapatite crystal is internalized in the center of macroscopic deposit and, therefore, not available for 18F-NaF binding. Creager et al,1 confirmed that 18F-NaF binds to areas of microcalcification beyond the resolution of x-ray CT and that this binding was proportional to the surface area of hydroxyapatite in a controlled ex vivo model. Recently, Youn et al19 confirmed 18F-NaF binding to microcalcifications in the coronary arteries as well as the carotids.
In effect, 18F-NaF-PET detects the surface area of hydroxyapatite crystal and, therefore, provides different information to x-ray CT, which detects tissue density. Put another way, 18F-NaF detects microcalcifications and areas of calcification activity, whereas CT detects establish macroscopic deposits of calcium. The difference information provided by 18F-NaF PET compared with CT has now been demonstrated in multiple human studies in vivo across a range of different conditions, as discussed below. However, this observation was perhaps first described by Derlin et al20 in a study of 75 patients undergoing whole-body 18F-NaF PET/CT. They demonstrated that in the aorta, carotids, and femoral arteries, only 12% of all calcified plaques on CT demonstrated increased 18F-NaF uptake, whereas 75% of patients demonstrated increased 18F-NaF uptake remote from calcified CT plaques.
Valvular Applications
Aortic Stenosis and Bioprosthetic Valve Degeneration
Calcific aortic stenosis is the most common form of valve disease in the Western world and is set to become an increasing health burden with no available effective medical therapy.21 The only available treatment for patients that progress to severe symptomatic aortic stenosis remains surgical or transcatheter aortic valve replacement. There is, therefore, a pressing need to improve our pathophysiological understanding of the disease and to develop effective medical therapies capable of slowing disease progression.22,23
Dweck et al24 recruited 121 patients with a range of calcific aortic valve disease who underwent both 18F-FDG (fluorodeoxyglucose; a marker of inflammation) and 18F-NaF PET/CT imaging. 18F-NaF uptake activity was higher in aortic stenosis patients than controls and increased progressively with more advanced stages of aortic stenosis. Interestingly increased 18F-FDG activity was also detected, but uptake values were lower than 18F-NaF and had only a mild association with disease severity. Particularly, in the more advanced stages of the disease, calcification, rather than inflammation, had a more predominant role in the disease progressionr25 On this basis, we think it should be the target of future therapeutic interventions, although acknowledge that other processes such as fibrosis also determine valve stiffening and progression. Once again, in aortic stenosis, 18F-NaF activity was observed in a different distribution to the presence of calcium on CT. Moreover, 18F-NaF PET predicted where new areas of calcium on CT would develop on repeat scans performed 2 years later (Figure 2), consistent with it acting as marker of calcification activity and, therefore, providing powerful prediction of disease progression and future aortic valve replacement or cardiovascular mortality.26,27
Figure 2.
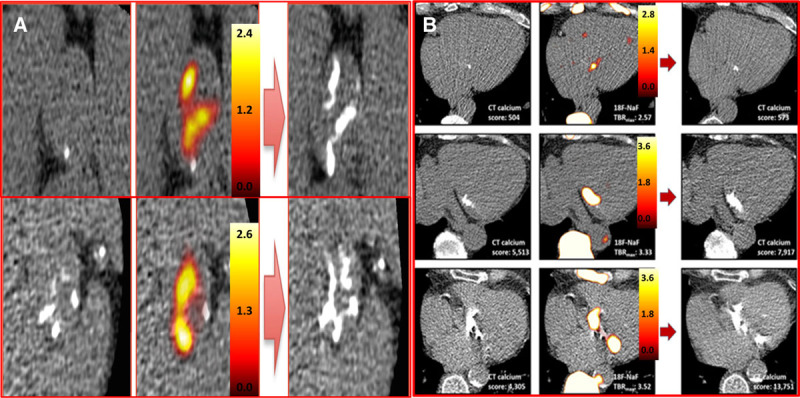
18F-sodium fluoride (18F-NaF) uptake predicts progression of aortic valve calcification and mitral annular calcification (MAC). A, Baseline calcium score (left) of 2 patients with aortic sclerosis (top) and moderate aortic stenosis (bottom). Fused 18F-NaF positron emission tomography (PET)–computed tomography (CT) scans (middle) show fluoride uptake in red and yellow. Follow-up CT at 2 y (right) indicated new areas of macroscopic calcium on the repeat CT in a similar distribution to the PET activity (reprinted from Jenkins et al47 with permission. Copyright ©2015, the Journal of the American College of Cardiology). B, Baseline calcium score (left) of patients with mild, moderate, and severe MAC. Fused 18F-NAF PET-CT scans (middle) show fluoride uptake in red and yellow. Similar to the aortic valve the baseline PET predicts where new macroscopic calcium in the mitral valve will develop on follow-up CT at 2 y (right) (reprinted from Massera et al.44 Copyright ©2019, the Authors).
Calcification also appears to have an important role in bioprosthetic valve degeneration, acting as a major pathological contributor to both progressive bioprosthetic valve narrowing and leaflet tears.28 Cartlidge et al29 examined 15 failed explanted bioprosthetic aortic valves using 18F-NaF PET/CT. All valves demonstrated 18F-NaF leaflet uptake that correlated with microcalcific and macrocalcific deposits within the valve leaflets and colocalized with regions of tissue degradation, pannus, and thrombus on histology. Moreover, they examined 71 patients without known bioprosthetic valve dysfunction. Similar to the ex vivo findings, increased 18F-NaF uptake colocalized with areas of spotty calcification, noncalcific leaflet thickening (suggestive of thrombus), and pannus observed on the CT. Twenty-four patients with increased 18F-NaF uptake at baseline demonstrated clear evidence of deteriorating bioprosthesis function after 2 years, whereas patients without uptake displayed no change in valve function. Ten patients developed new bioprosthetic valve failure; all 10 patients had increased 18F-NaF uptake at baseline.
Mitral Annular Calcification
MAC is a chronic process, the incidence of which is associated with advanced age, atherosclerosis, and altered mineral metabolism. It can lead to mitral valve dysfunction and is associated with an increased risk of adverse cardiovascular events; however, its pathophysiology remains incompletely understood.30,31 Massera et al32 investigated MAC using 18F-NaF and 18F-FDG positron emission tomography. The results were similar to those observed in aortic stenosis. Uptake of both tracers was higher in patients with MAC versus those with normal mitral valves and increased with disease severity. A strong correlation was observed between the baseline CT-MAC score and mitral annular 18F-NaF activity (r=0.79, P<0.001), whereas a moderate correlation was observed with 18F-FDG uptake (r=0.32, P=0.001). Similar to the aortic valve, areas of increased 18F-NaF activity on baseline imaging predicted where new regions of macroscopic calcium on CT would develop after 2 years. Indeed, there was a close association observed between baseline 18F-NaF and the change in CT calcium score (r=0.75, P<0.001), with 18F-NaF again emerging as a marker of disease activity and predictor of disease progression in this condition.
Vascular Applications
Coronary and Carotid Atherosclerosis
Dweck et al33 first described 18F-NaF uptake in the coronary arteries as a novel marker of microcalcification activity in subjects with and without aortic valve disease. Again 18F-NaF provided different information to CT. More than 40 % of patients with high coronary artery calcium scores (>1000 Agatston Units) did not demonstrate increased 18F-NaF uptake, signifying that 18F-NaF might be able to differentiate between active disease and advanced yet burnt out atheroma. Increased 18F-NaF uptake could be localized to individual coronary plaques and notably identified patients with both increased cardiac risk scores and a history of prior cardiovascular events. Joshi et al34 examined 40 patients with stable angina and 40 patients presenting with type 1 myocardial infarction, who all underwent 18F-NaF PET/CT. In the stable cohort, high tracer activity localized to individual coronary plaques in ≈40 % of patients. Using intravascular ultrasound and CT, these plaques had multiple adverse, unstable characteristics, including microcalcification, positive remodeling, and a large necrotic core. In a separate cohort of patients with carotid artery disease undergoing endarterectomy, increased 18F-NaF activity similarly colocalized to carotid plaques with histological adverse plaque features, including increased macrophage accumulation, cell death, and calcification. Finally, in the myocardial infarction cohort, 37 out of 40 patients demonstrated increased 18F-NaF uptake at the exact site of the culprit coronary plaque responsible for the event. By comparison, 18F-FDG was not able to provide similar discrimination of culprit coronary plaques.
Other studies have confirmed the localization of increased 18F-NaF to plaques with adverse characteristics including positive remodeling, low attenuation plaque (<30 HU), spotty calcification, obstructive coronary stenosis, and plaque volumes >100 mm3 determined by a range of different approaches, including CT, intravascular ultrasound, optical coherent tomography, and histology.19,35–37 More recently 18F-NaF PET activity has been associated with increased pericoronary adipose tissue density: an emerging marking of coronary plaque inflammation.38
Outside the heart, Vesey et al39 examined 18F-NaF and 18F-FDG uptake in the carotid arteries after transient ischemic attack or stroke. They recruited 26 patients following a recent cerebrovascular event. All individuals underwent PET/CT scanning using both tracers. 18F-NaF uptake was focal, readily identifiable, and localizable to plaque. The authors detected increased 18F-NaF within culprit lesions responsible for stroke compared with both the contralateral nonculprit carotid artery as well as arteries from patients with nonsymptomatic carotid disease. Similar discrimination was again not provided by 18F-FDG. In a smaller study of 9 patients, Quirce et al40 explored 18F-NaF and 18F-FDG uptake in patients with symptomatic carotid stenosis and showed again that 18F-NaF uptake appeared to be higher in the symptomatic carotid, whereas 18-FDG uptake was nondiscriminatory. Cocker et al41 examined 11 patients scheduled to have endarterectomy. They performed 18F-NaF PET/CT imaging of the carotid arteries <2 weeks before surgery and established greater 18F-NaF uptake in symptomatic versus nonsymptomatic plaque.
The retrospective identification of culprit plaque is only of limited clinical utility. More interesting is whether 18F-NaF can prospectively identify patients at increased risk of myocardial infarction so that preventative therapies might be institute to avoid such an event. The prognostic role of 18F-NaF is currently being evaluated in an ongoing prospective multicenter trial: PREFFIR (Prediction of Recurrent Events With 18F-Fluoride; https://www.clinicaltrials.gov; Unique identifier: NCT02278211).
Abdominal Aortic Aneurysms, Peripheral Artery Disease, and Erectile Dysfunction
18F-NaF PET has also been also explored in patients with abdominal aortic aneurysm disease, peripheral vascular disease, and erectile dysfunction. In the SOFIA3 study (18F-Sodium Fluoride Uptake in Abdominal Aortic Aneurysms), Forsythe et al42 first performed micro–PET-CT and histological analysis of excised abdominal aortic aneurysm disease tissue, establishing once more that 18F-NaF acts as a marker of microcalcification activity. Then they investigated 18F-NaF PET-CT imaging in patients with abdominal aortic aneurysm disease and control subjects. Increased 18F-NaF uptake was observed in the aneurysms compared with areas of nonaneurysmal aorta, providing important predictive information. Aneurysms in the highest 18F-NaF uptake tertile expanded 2.5× more rapidly than those in the lowest tertile. They were also nearly 3× as likely to experience abdominal aortic aneurysm disease repair or rupture. On multivariable analysis, 18F-NaF provided incremental prognostic information over conventional predictors of risk, including aneurysm size. Interestingly, CT calcium scoring was not predictive of either aneurysm expansion nor clinical events.
In peripheral vascular disease, Chowdhury et al43 performed a prospective observational study of 50 patients with symptomatic peripheral arterial disease undergoing 18F-FDG and 18F-NaF PET/CT of the superficial femoral artery before and 6 weeks after angioplasty. The investigators found that both tracers performed well in identifying patients who would develop restenosis within 12 months; indeed, there was clear separation of 18F-NaF activity in patients that did and did not go on to develop restenosis.
Finally, Nakahara et al44 investigated 18F-NaF uptake in the penile arteries of prostate cancer patients undergoing clinical PET scans. The investigators demonstrated that 18F-NaF uptake in the cavernous and dorsal penile arteries was associated with the both the presence of existing erectile dysfunction and the probability of its future development. Indeed, penile 18F-NaF activity was 30% higher in patients with compared with patients without erectile dysfunction.
Limitations
PET-CT has grown significantly over the last decade but still faces important challenges. Most notably is the expense of PET imaging, radiation exposure, and the relatively limited availability of this imaging modality. These may limit its widespread clinical adoption, although we note the increasing use of PET in oncology. In addition, there are technical challenges. In particular, PET imaging of the heart is complicated by cardiac, respiratory, and gross patient motion and how this effects image quality, particularly in small structures, such as the coronary arteries. Several motion correction methods have been proposed, leading to novel image reconstruction techniques, and the development of new software that can precisely read fused images. The first approach was to ECG-gate the images and only use PET data acquired in diastole when the heart is still (between 50% and 75% of the R-R interval).14 Although this improves motion artifact, this approach effectively ignores three-fourth of the total PET signal leading to increased noise. Doris et al45 used an alternative technique that modeled and then corrected for cardiac motion in the PET data using anatomy guided registration algorithm to preserve all of the PET data. Finally, Lassen et al,46 used an approach that corrected for cardiac, respiratory, and gross patient motion that in combination with background blood pool corrections markedly improved test-retest reproducibility of coronary 18F-NaF PET.
Clinical Trials
Although 18F-NaF PET has provided important pathophysiological insights and emerged consistently as a marker of vascular injury and predictor of disease progression, further work is required to demonstrate the incremental clinical utility of this imaging technique and to justify its relatively high costs. This question is being addressed in multiple prospective studies. As discussed, the PRE18FFIR Study is a multicenter observational study that will follow ≈700 high-risk patients with coronary artery disease to determine whether baseline 18F-NaF PET imaging can identify patients at increased risk of future myocardial infarction and disease progression. SALTIRE 2 (Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression; https://www.clinicaltrials.gov; Unique identifier: NCT02132036) will utilize 18F-NaF PET to determine if denosumab or bisphosphonate can reduce calcification activity and slow aortic stenosis progression in patients with mild and moderate aortic stenosis.
Conclusions
18F-NaF PET provides a marker of calcification activity across a range of cardiovascular conditions, providing potentially useful prediction of disease progression and clinical events. Although the technique has already provided some key pathophysiological insights and is being used as a surrogate efficacy end point in ongoing randomized clinical trials, large prospective studies are required to examine whether it can provide incremental information to justify its more widespread clinical use.
Sources of Funding
E. Tzolos was supported by a grant from Dr Miriam and Sheldon G. Adelson Medical Research Foundation. E. Tzolos was also supported by BHF Clinical Research Training Fellowship no. FS/17/51/33096. M.R. Dweck (FS/14/78/31020) is supported by the British Heart Foundation and is the recipient of Sir Jules Thorn Award for Biomedical Research (15/JTA).
Disclosures
None.
Footnotes
Nonstandard Abbreviations and Acronyms
- CT
- computed tomography
- PET
- positron emission tomography
- PREFFIR
- Prediction of Recurrent Events With 18F-Fluoride
For Sources of Funding and Disclosures, see page 1625.
References
- 1.Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC, Park MA, Lee LH, Singh SA, Alcaide-Corral CJ, et al. 18F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in positron emission tomography/computed tomography images. Circ Cardiovasc Imaging. 2019;12:e007835. doi: 10.1161/CIRCIMAGING.118.007835. doi: 10.1161/CIRCIMAGING.118.007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritman EL. Small-animal CT - its difference from, and impact on, clinical CT. Nucl Instrum Methods Phys Res A. 2007;580:968–970. doi: 10.1016/j.nima.2007.06.040. doi: 10.1016/j.nima.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baheza RA, Welch EB, Gochberg DF, Sanders M, Harvey S, Gore JC, Yankeelov TE. Detection of microcalcifications by characteristic magnetic susceptibility effects using MR phase image cross-correlation analysis. Med Phys. 2015;42:1436–1452. doi: 10.1118/1.4908009. doi: 10.1118/1.4908009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockisch A, Beyer T, Antoch G, Freudenberg LS, Kühl H, Debatin JF, Müller SP. Positron emission tomography/computed tomography–imaging protocols, artifacts, and pitfalls. Mol Imaging Biol. 2004;6:188–199. doi: 10.1016/j.mibio.2004.04.006. doi: 10.1016/j.mibio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31–37. doi: 10.1016/s0001-2998(72)80005-9. doi: 10.1016/s0001-2998(72)80005-9. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, Satyamurthy N, Barrio JR, Phelps ME. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 7.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoh CK, Hawkins RA, Dahlbom M, Glaspy JA, Seeger LL, Choi Y, Schiepers CW, Huang SC, Satyamurthy N, Barrio JR. Whole body skeletal imaging with [18F]fluoride ion and PET. J Comput Assist Tomogr. 1993;17:34–41. doi: 10.1097/00004728-199301000-00005. doi: 10.1097/00004728-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med. 2001;31:28–49. doi: 10.1053/snuc.2001.18742. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Doris MK, Andrews JPM, Bing R, Daghem M, van Beek EJR, Forsyth L, Shah ASV, Williams MC, Sellers S, et al. Molecular coronary plaque imaging using 18F-fluoride. Circ Cardiovasc Imaging. 2019;12:e008574. doi: 10.1161/CIRCIMAGING.118.008574. doi: 10.1161/CIRCIMAGING.118.008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 12.Andrews JPM, MacNaught G, Moss AJ, Doris MK, Pawade T, Adamson PD, van Beek EJR, Lucatelli C, Lassen ML, Robson PM, et al. Cardiovascular (18)F-fluoride positron emission tomography-magnetic resonance imaging: a comparison study [published online December 2, 2019]. J Nucl Cardiol. doi: 10.1007/s12350-019-01962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiecinski J, Berman DS, Lee SE, Dey D, Cadet S, Lassen ML, Germano G, Jansen MA, Dweck MR, Newby DE, et al. Three-hour delayed imaging improves assessment of coronary 18F-sodium fluoride PET. J Nucl Med. 2019;60:530–535. doi: 10.2967/jnumed.118.217885. doi: 10.2967/jnumed.118.217885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawade TA, Cartlidge TR, Jenkins WS, Adamson PD, Robson P, Lucatelli C, Van Beek EJ, Prendergast B, Denison AR, Forsyth L, et al. Optimization and reproducibility of aortic valve 18F-fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc Imaging. 2016;9:e005131. doi: 10.1161/CIRCIMAGING.116.005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocker MS, Spence JD, Hammond R, deKemp RA, Lum C, Wells G, Bernick J, Hill A, Nagpal S, Stotts G, et al. Canadian Atherosclerosis Imaging Network (CAIN) - Project II. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation: comparison to immunohistology and relationship to acuity of events. Int J Cardiol. 2018;271:378–386. doi: 10.1016/j.ijcard.2018.05.057. doi: 10.1016/j.ijcard.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 17.Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, Cowie WJ, Tsampasian V, Richardson H, Fletcher A, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 18.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn T, Al’Aref SJ, Narula N, Salvatore S, Pisapia D, Dweck MR, Narula J, Lin FY, Lu Y, Kumar A, et al. 18F-sodium fluoride positron emission tomography/computed tomography in ex vivo human coronary arteries with histological correlation. Arterioscler Thromb Vasc Biol. 2020;40:404–411. doi: 10.1161/ATVBAHA.119.312737. doi: 10.1161/ATVBAHA.119.312737. [DOI] [PubMed] [Google Scholar]
- 20.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, Klutmann S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 21.Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56:565–571. doi: 10.1016/j.pcad.2014.02.006. doi: 10.1016/j.pcad.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Brennan JM, Thomas L, Cohen DJ, Shahian D, Wang A, Mack MJ, Holmes DR, Edwards FH, Frankel NZ, Baron SJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439–450. doi: 10.1016/j.jacc.2017.05.060. doi: 10.1016/j.jacc.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 25.Dweck MR, Khaw HJ, Sng GK, Luo EL, Baird A, Williams MC, Makiello P, Mirsadraee S, Joshi NV, van Beek EJ, et al. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? Eur Heart J. 2013;34:1567–1574. doi: 10.1093/eurheartj/eht034. doi: 10.1093/eurheartj/eht034. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 27.Clavel MA, Burwash IG, Pibarot P. Cardiac imaging for assessing low-gradient severe aortic stenosis. JACC Cardiovasc Imaging. 2017;10:185–202. doi: 10.1016/j.jcmg.2017.01.002. doi: 10.1016/j.jcmg.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Cartlidge TR, Pawade TA, Dweck MR. Aortic stenosis and CT calcium scoring: is it for everyone? Heart. 2017;103:8–9. doi: 10.1136/heartjnl-2016-310297. doi: 10.1136/heartjnl-2016-310297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartlidge TRG, Doris MK, Sellers SL, Pawade TA, White AC, Pessotto R, Kwiecinski J, Fletcher A, Alcaide C, Lucatelli C, et al. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol. 2019;73:1107–1119. doi: 10.1016/j.jacc.2018.12.056. doi: 10.1016/j.jacc.2018.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 31.Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, Benjamin E Framingham Heart Study. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 32.Massera D, Trivieri MG, Andrews JPM, Sartori S, Abgral R, Chapman AR, Jenkins WSA, Vesey AT, Doris MK, Pawade TA, et al. Disease activity in mitral annular calcification. Circ Cardiovasc Imaging. 2019;12:e008513. doi: 10.1161/CIRCIMAGING.118.008513. doi: 10.1161/CIRCIMAGING.118.008513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJ, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 35.Kwiecinski J, Dey D, Cadet S, Lee SE, Tamarappoo B, Otaki Y, Huynh PT, Friedman JD, Dweck MR, Newby DE, et al. Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2020;21:58–66. doi: 10.1093/ehjci/jez152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitagawa T, Yamamoto H, Nakamoto Y, Sasaki K, Toshimitsu S, Tatsugami F, Awai K, Hirokawa Y, Kihara Y. Predictive value of 18F-sodium fluoride positron emission tomography in detecting high-risk coronary artery disease in combination with computed tomography. J Am Heart Assoc. 2018;7:e010224. doi: 10.1161/JAHA.118.010224. doi: 10.1161/JAHA.118.010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JM, Bang JI, Koo BK, Hwang D, Park J, Zhang J, Yaliang T, Suh M, Paeng JC, Shiono Y, et al. Clinical relevance of (18)F-sodium fluoride positron-emission tomography in noninvasive identification of high-risk plaque in patients with coronary artery disease. Circ Cardiovasc Imaging. 2017;10:e006704. doi: 10.1161/CIRCIMAGING.117.006704. [DOI] [PubMed] [Google Scholar]
- 38.Kwiecinski J, Dey D, Cadet S, Lee SE, Otaki Y, Huynh PT, Doris MK, Eisenberg E, Yun M, Jansen MA, et al. Peri-coronary adipose tissue density is associated with (18)F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging. 2019;11:30076–30072. doi: 10.1016/j.jcmg.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vesey AT, Jenkins WS, Irkle A, Moss A, Sng G, Forsythe RO, Clark T, Roberts G, Fletcher A, Lucatelli C, et al. Newby DE. (18)F-fluoride and (18)F-fluorodeoxyglucose positron emission tomography after transient ischemic attack or minor ischemic stroke: case-control study. Circ Cardiovasc Imaging. 2017;10:e004976. doi: 10.1161/CIRCIMAGING.116.004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quirce R, Martínez-Rodríguez I, Banzo I, Jiménez-Bonilla J, Martínez-Amador N, Ibáñez-Bravo S, López-Defilló J, Jiménez-Alonso M, Revilla MA, Carril JM. New insight of functional molecular imaging into the atheroma biology: 18F-NaF and 18F-FDG in symptomatic and asymptomatic carotid plaques after recent CVA. Preliminary results. Clin Physiol Funct Imaging. 2016;36:499–503. doi: 10.1111/cpf.12254. doi: 10.1111/cpf.12254. [DOI] [PubMed] [Google Scholar]
- 41.Cocker MS, Spence JD, Hammond R, Wells G, deKemp RA, Lum C, Adeeko A, Yaffe MJ, Leung E, Hill A, et al. Canadian Atherosclerosis Imaging Network (CAIN) [18F]-NaF PET/CT identifies active calcification in carotid plaque. JACC Cardiovasc Imaging. 2017;10:486–488. doi: 10.1016/j.jcmg.2016.03.005. doi: 10.1016/j.jcmg.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Forsythe RO, Dweck MR, McBride OMB, Vesey AT, Semple SI, Shah ASV, Adamson PD, Wallace WA, Kaczynski J, Ho W, et al. 18F-sodium fluoride uptake in abdominal aortic aneurysms: the SoFIA3 study. J Am Coll Cardiol. 2018;71:513–523. doi: 10.1016/j.jacc.2017.11.053. doi: 10.1016/j.jacc.2017.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury MM, Tarkin JM, Albaghdadi MS, Evans NR, Le EPV, Berrett TB, Sadat U, Joshi FR, Warburton EA, Buscombe JR, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease: a prospective clinical study. JACC Cardiovasc Imaging. 2020;13:1008–1017. doi: 10.1016/j.jcmg.2019.03.031. doi: 10.1016/j.jcmg.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakahara T, Narula J, Tijssen JGP, Agarwal S, Chowdhury MM, Coughlin PA, Dweck MR, Rudd JHF, Jinzaki M, Mulhall J, et al. 18F-fluoride positron emission tomographic imaging of penile arteries and erectile dysfunction. J Am Coll Cardiol. 2019;73:1386–1394. doi: 10.1016/j.jacc.2018.10.076. doi: 10.1016/j.jacc.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 45.Doris MK, Otaki Y, Krishnan SK, Kwiecinski J, Rubeaux M, Alessio A, Pan T, Cadet S, Dey D, Dweck MR, et al. Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol. 2020;27:494–504. doi: 10.1007/s12350-018-1317-5. doi: 10.1007/s12350-018-1317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lassen ML, Kwiecinski J, Dey D, Cadet S, Germano G, Berman DS, Adamson PD, Moss AJ, Dweck MR, Newby DE, et al. Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary 18F-NaF PET. Eur J Nucl Med Mol Imaging. 2019;46:2610–2620. doi: 10.1007/s00259-019-04437-x. doi: 10.1007/s00259-019-04437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins WS, Vesey AT, Shah AS, Pawade TA, Chin CW, White AC, Fletcher A, Cartlidge TR, Mitchell AJ, Pringle MA, et al. Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol. 2015;66:1200–1201. doi: 10.1016/j.jacc.2015.06.1325. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]


