Abstract
The dorsal hippocampus (DH) and medial prefrontal cortex (mPFC) are brain regions essential for processing and storing episodic memory. In rodents, the DH has a well-established role in supporting the consolidation of episodic-like memory in tasks such as object recognition and object placement. However, the role of the mPFC in the consolidation of episodic-like memory tasks remains controversial. Therefore, the present study examined involvement of the DH and mPFC, alone and in combination, in object and spatial recognition memory consolidation in ovariectomized female mice. To this end, we utilized two types of inhibitory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to inactivate the DH alone, the mPFC alone, or both brain regions concurrently immediately after object training to assess the role of each region in the consolidation of object recognition and spatial memories. Our results using single and multiplexed DREADDS suggest that excitatory activity in the DH and mPFC, alone or in combination, is required for the successful consolidation of object recognition and spatial memories. Together, these studies provide critical insight into how the DH and mPFC work in concert to facilitate memory consolidation in female mice.
Keywords: DREADD, CNO, SALB, Hippocampus, Memory, Object placement
1. Introduction
In humans, episodic memory is impaired during normal aging (Shing et al., 2010; Tulving, 1983), in neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases (Dubois et al., 2007; Williams-Gray, Foltynie, Lewis, & Barker, 2006), and in psychiatric disorders such as depression and PTSD (Dere, Pause, & Pietrowsky, 2010; Kleim & Ehlers, 2008; McNally, 2006; Moore & Zoellner, 2007). Given the substantial public health impacts of these disorders and limited therapeutic options currently available, it is of great interest and relevance to define the neurobiological basis of episodic memory formation. Mechanistic approaches for studying episodic memory are not feasible in humans, therefore, rodents provide a useful model for studying systems-level contributions of the neuronal populations that support the consolidation of episodic-like memories.
The formation of a memory for a particular event or episode involves the integration of information regarding what was encountered, when it happened, and where the encounter occurred. The successful consolidation of, and subsequent ability to retrieve, this information requires coordinated effort between the hippocampus and prefrontal cortex (Eichenbaum, 2017; Jin & Maren, 2015; Kitamura et al., 2017; Preston & Eichenbaum, 2013). Numerous species, including rodents, can encode and store episodic-like memories. Increasingly, object recognition and object placement tasks have been used to model the “what” (i.e., an object) and “where” (i.e., context or location within the testing arena) components of memory consolidation in rodents (Barker et al., 2017; Dere, Huston, & De Souza Silva, 2005; Eichenbaum, 2017; Ennaceur & Delacour, 1988; Ennaceur, 2010).
Interactions between the hippocampus and medial prefrontal cortex (mPFC) have been implicated in episodic-like memory (Warburton & Brown, 2015) and delayed spatial working memory (Churchwell & Kesner, 2011) in male rats, but the specific role of the mPFC alone, and its interactions with the dorsal portion of the hippocampus during object recognition and object placement memory formation remains controversial. For example, some data suggest that mPFC activation is required for spatial object tasks, such as object placement, but not for object recognition or temporal order object tasks (DeVito & Eichenbaum, 2010). Yet others have reported that mPFC inactivation after object training does impair object recognition memory consolidation (Akirav & Maroun, 2006). Behavioral studies aimed at addressing prefrontal-hippocampal interactions during episodic memory formation often involve a “functional disconnection” approach, which uses lesions of the mPFC and hippocampus to disrupt either ipsi- or contralateral projections between the two structures (Barker & Warburton, 2011; Barker et al., 2017; Floresco, Seamans, & Phillips, 1997; Wang & Cai, 2006). One study using this functional disconnection approach in male rats reported impaired performance in certain episodic-like memory tasks, such as the object-in-place recognition memory task and the temporal order memory task, but not in object location and object recognition tasks (Barker, Bird, Alexander, & Warburton, 2007). These findings suggest that a single lesion targeting the unilateral projections between the hippocampus and mPFC may not be sufficient to disrupt memory in all episodic-like tasks, as the brain may be able to compensate by utilizing indirect projections routed through the nucleus reuniens or entorhinal cortex to maintain hippocampal-prefrontal communication (Burwell & Amaral, 1998; Hoover & Vertes, 2007; Vertes, Hoover, Szigeti-Buck, & Leranth, 2007). Further, temporary inactivation of these structures (i.e., pharmacological or chemogenetic inhibition) may yield different behavioral results than permanent disruption (i.e., lesions).
The present study utilized a multiplexed chemogenetic DREADD (Designer Receptors Exclusively Activated by Designer Drugs) approach to determine the extent to which temporary inhibition of the dorsal hippocampus (DH) alone, mPFC alone, or both structures disrupts episodic-like memory consolidation in ovariectomized female mice. Adeno-associated viral vectors were used to deliver a mutated human Gi-coupled muscarinic receptor (hM4-DREADD; hM4Di) or kappa opioid receptor (KOR-DREADD; KORD) into excitatory neurons, which suppresses neuronal firing once the receptors are bound by their respective ligands (clozapine-n-oxide, CNO; salvinorin-B, SALB; (Armbruster, Li, Pausch, Herlitze, & Roth, 2007)). Because each DREADD is activated by a unique synthetic ligand, this approach allowed for discrete inactivation of the DH alone, mPFC alone, or coincident inactivation of these regions during memory formation in the same set of mice. We report that hM4Di-mediated inhibition of the DH 30 min before or immediately after object training impairs spatial, but not object recognition, memory consolidation. In a subsequent experiment, we utilized a multiplexed approach to deliver hM4Di to the mPFC and KORD to the DH, and found that hM4Di-mediated inhibition of the mPFC and KORD-mediated inhibition of the DH were each sufficient to impair spatial and object recognition memory consolidation. Finally, concurrent subthreshold suppression of neural activity in both the mPFC and DH disrupted consolidation in the object recognition and object placement tasks, suggesting that concurrent activity in these brain regions is required for both object recognition and spatial memory consolidation. These findings provide new insight into the neural circuitry that supports episodic memory formation, a type of memory whose function is compromised during aging and in numerous neuropsychiatric and neurodegenerative diseases.
2. Materials and methods
2.1. Subjects
The initial impetus for this work was a previous finding that bilateral DH infusion of a memory-enhancing dose of 17β-estradiol increased dendritic spine density in both the DH and mPFC of ovariectomized female mice (Tuscher, Luine, Frankfurt, & Frick, 2016), suggesting potentially important interactions between the DH and mPFC in mediating memory consolidation. To maintain consistency with this previous work, all experiments used young (912 week-old) female C57BL/6 mice (Taconic, Cambridge City, IN) who were ovariectomized as described previously (Kim, Szinte, Boulware, & Frick, 2016; Tuscher, Luine et al., 2016). Mice were housed in groups of up to 5 until surgery, after which they were singly housed. Mice were maintained on a 12 h light/dark cycle with ad libitum access to food and water. All experimental protocols and procedures were approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Surgery
2.2.1. General
Surgeries were conducted at least 3 weeks prior to behavioral testing. Mice were anesthetized with isoflurane (5% for induction, 2% for maintenance) in 100% oxygen and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Mice were ovariectomized as described previously (Kim et al., 2016; Tuscher, Luine et al., 2016) and injected with virus during the same surgical session.
2.2.2. DH DREADD surgeries
Immediately following ovariectomy, an incision was made in the scalp to expose the skull, and small perforations were made in the skull with a 26 ½ GA needle to create an opening for bilateral infusion of saline (n = 9) or virus (n = 13 eGFP, n = 13 DREADD) into the DH using a 10-μl Hamilton syringe and metal needle (Hamilton, Reno, NV). For our first experiment (Figs. 1–3), hM4Di virus (AAV-CaMKIIα-HA-hM4Di-IRES-mCitrine, 2.1 × 1012 particles/ml, serotype 8, UNC Vector Core, Chapel Hill, NC), eGFP control virus (AAV-CaMKIIα-eGFP, 2.1 × 1012 particles/ml, serotype 8, UNC Vector Core, Chapel Hill, NC), or saline was infused into the DH (−1.7 mm AP, ± 1.5 mm ML, −2.3 mm DV; 1.2 μl/hemisphere). Infusion volume and flow rate were controlled by a syringe pump (KD Scientific, Holliston, MA). The Hamilton syringe was first lowered to −2.3 mm ventral to the surface of the skull and held in place for two minutes to create a pocket for the first viral infusion. Three 0.4 μl infusions were delivered per hemisphere, one at −2.2 mm, one at −2.1 mm, and one at −1.9 mm DV. The Hamilton syringe was left in place for 2 min after each infusion to allow for diffusion of the virus, and was then slowly retracted before the process was repeated in the contralateral hemisphere. Mice received carprofen MediGel one day prior to surgery, as well as a s.c. injection of 5 mg/kg Rimadyl at the completion of surgery. Mice were allowed a minimum of three weeks for the virus to express and for surgical recovery prior to behavioral testing.
Fig. 1.
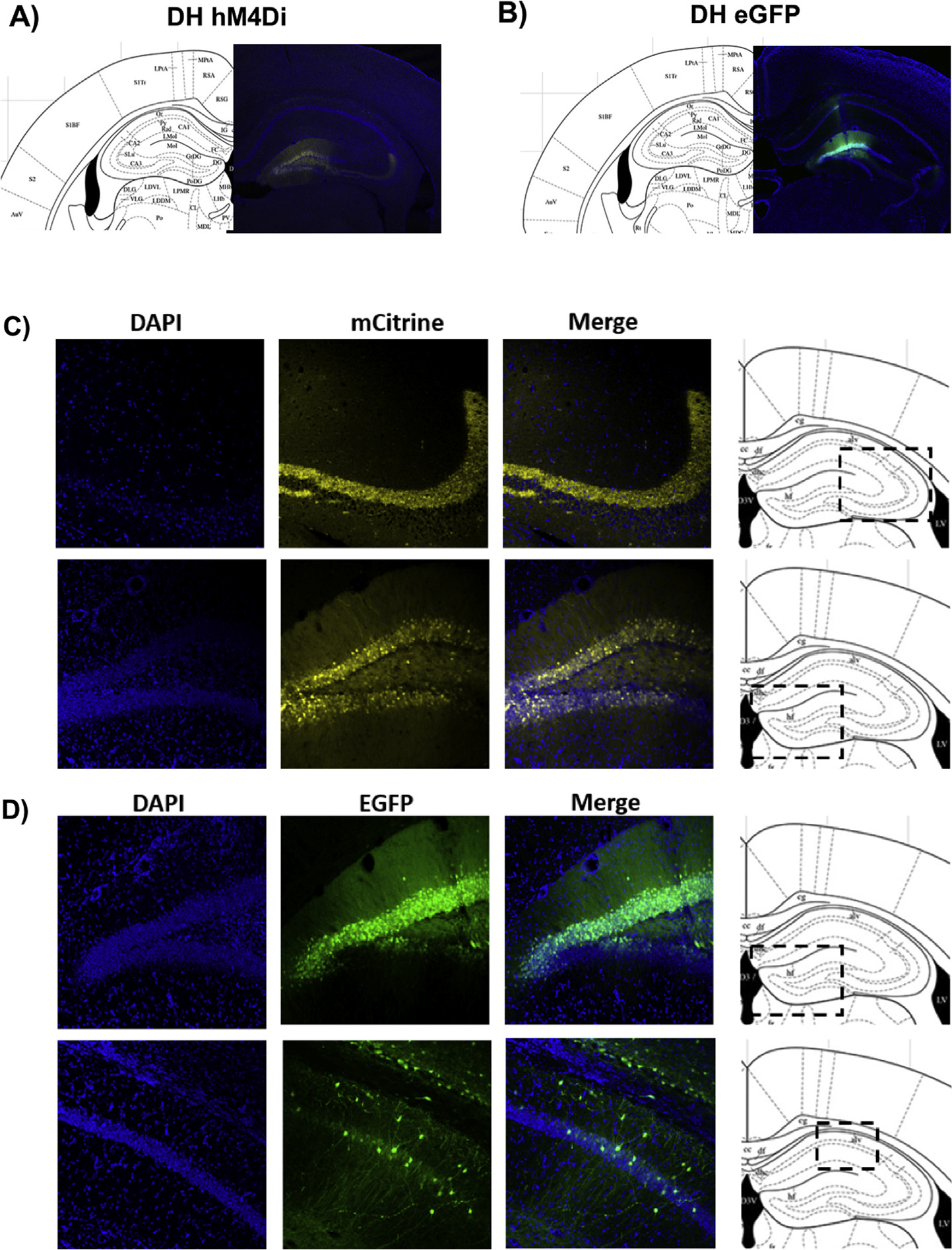
(A&C) Representative coronal sections (40 μm) of CaMKIIα-hM4Di-mCitrine DREADD or (B&D) CaMKIIα-EGFP control virus in female mouse brain 3 weeks post-injection demonstrate high levels of expression in the dentate gyrus, as well as weaker expression in CA1 and CA3. Blue puncta: DAPI; yellow: mCitrine-tagged DREADD virus; green: eGFP-tagged control virus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
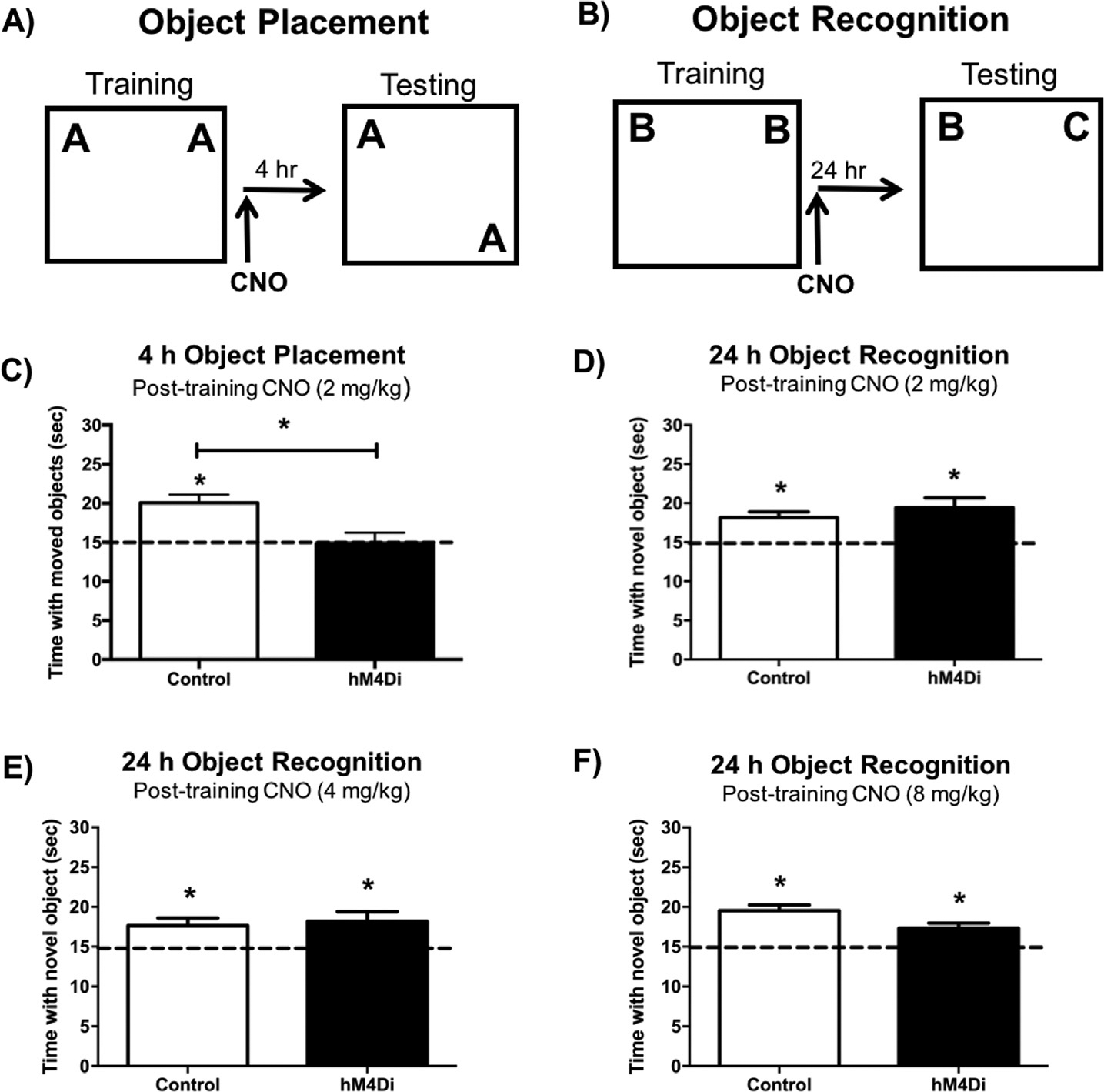
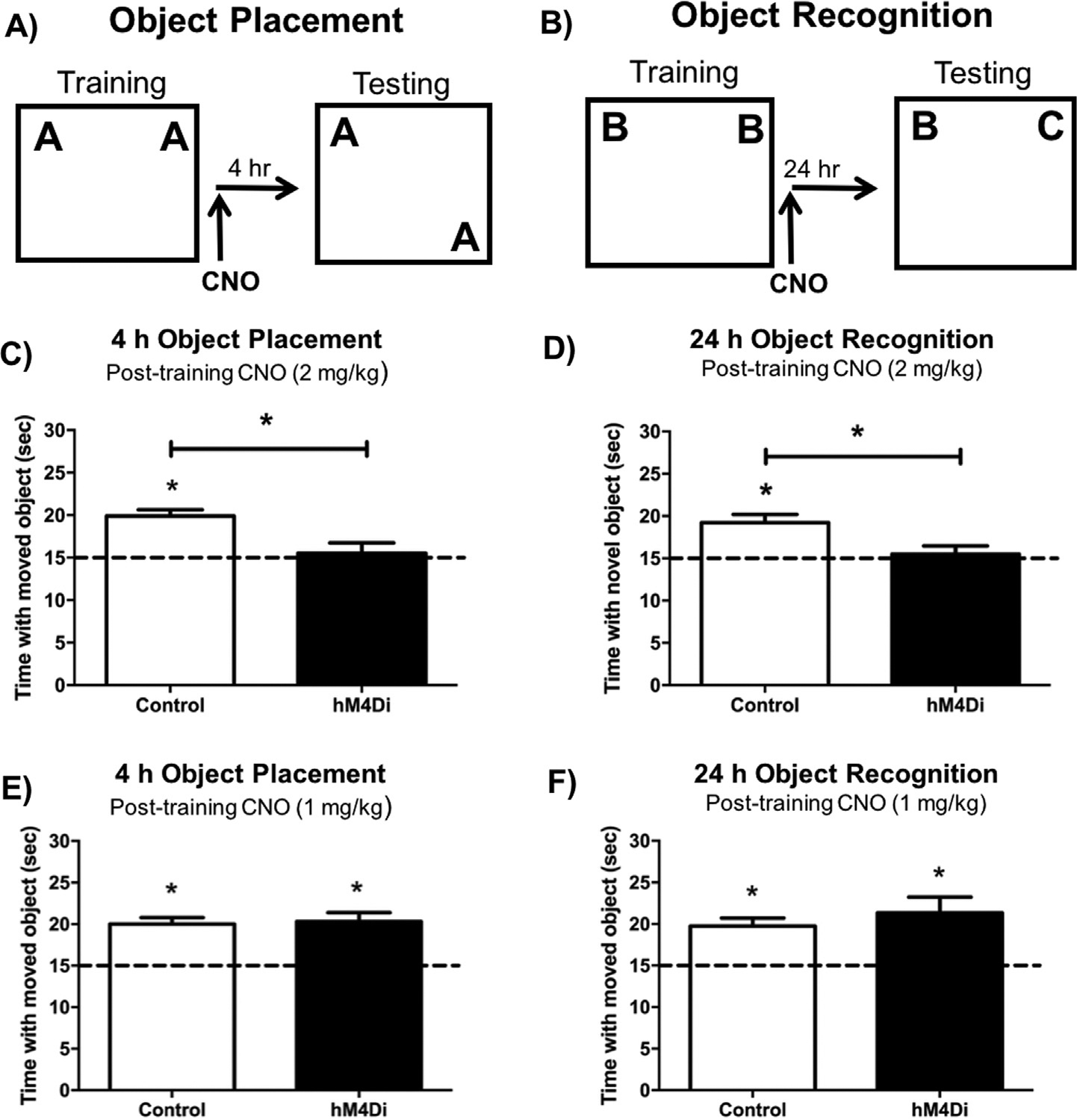
(A&C) In the object placement task, control mice administered 2 mg/kg CNO immediately post-training spent significantly more time than chance (15 s) with the moved object 4 h after training, whereas DH-hM4Di-expressing mice administered 2 mg/kg CNO did not. (B) In the object recognition task, control and DH-hM4Di mice administered 2 mg/kg (D), 4 mg/kg (E), or 8 mg/kg (F) CNO immediately post-training all spent significantly more time than chance (15 s) with the novel object during testing, suggesting intact object recognition memory 24 h after training. This finding suggests that post-training hM4Di-mediated inactivation of the DH impairs spatial memory consolidation, but does not affect object recognition memory consolidation, even at escalating doses of CNO. Bars represent the mean ± SEM, *p < 0.05 relative to chance or the Control group.
2.2.3. Double DREADD surgeries
For double DREADD surgeries (Figs. 4–8), two types of inhibitory DREADDs were used (i.e., hM4Di, KORD), each activated by a unique ligand, to examine the requirement of the mPFC, the DH, and concurrent activation of these brain regions during memory consolidation. All mice used for Figs. 4–8 received either eGFP control virus (n = 13), DREADD virus (n = 13), or saline infusions (n = 9) into both the mPFC and DH. For virus infusions into the mPFC, the same hM4Di DREADD described above, eGFP control virus, or saline (Sham condition) was infused into the mPFC (1.8 mm AP, ± 0.3 mm ML, −2.7 mm DV). mPFC virus infusions were conducted at the same rate as described for the DH (0.4 μl/2 min), however only 0.8 μl total was delivered per hemisphere (two 0.4 μl injections, one at −2.7 mm DV, one at −2.4 mm DV). These infusions targeted both the prelimbic and infralimbic regions of the mPFC. In the mPFC, infusions were separated by 8 min to allow for diffusion of the virus. During the same surgical session, mice were also bilaterally infused with an inhibitory KORD virus (AAV-CamKIIα-HA-KORD-IRES-mCitrine, 2.1 × 1012 particles/ml, serotype 8, UNC Vector Core), eGFP control virus (as described above), or saline (Sham condition) into the DH (−1.7 mm AP, ± 1.5 mm ML, −2.3 mm DV; 1.2 μl/hemisphere). This viral construct also targets the CaMKIIα promoter, and similar to the hM4Di DREADD, can be used to suppress excitatory neurotransmission (Vardy et al., 2015). Unlike the hM4-DREADD, the KORD-DREADD is activated by the synthetic ligand Salvinorin B (SALB), and can therefore be used for multiplexed modulation of behavior with CNO-activated DREADDs (Vardy et al., 2015). Thus, the use of both DREADDs permits determination of whether activation of mPFC alone, DH alone, or both mPFC and DH in concert is critical for memory formation in the same set of mice. Mice received carprofen MediGel 1 day prior to surgery, as well as a s.c. injection of 5 mg/kg Rimadyl at the completion of surgery, and were allowed a minimum of 3 weeks for the virus to express and for surgical recovery prior to behavioral testing.
Fig. 4.
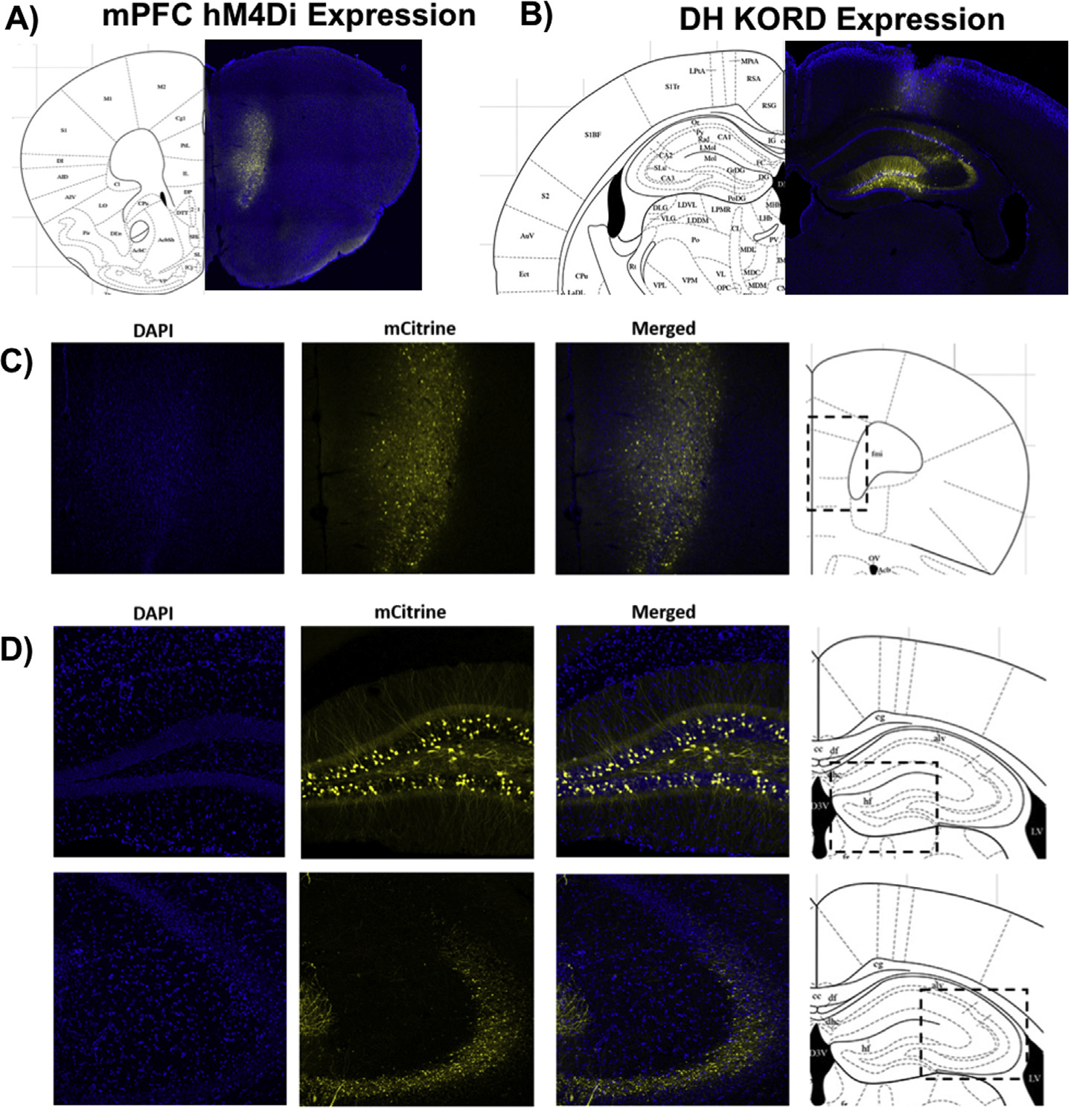
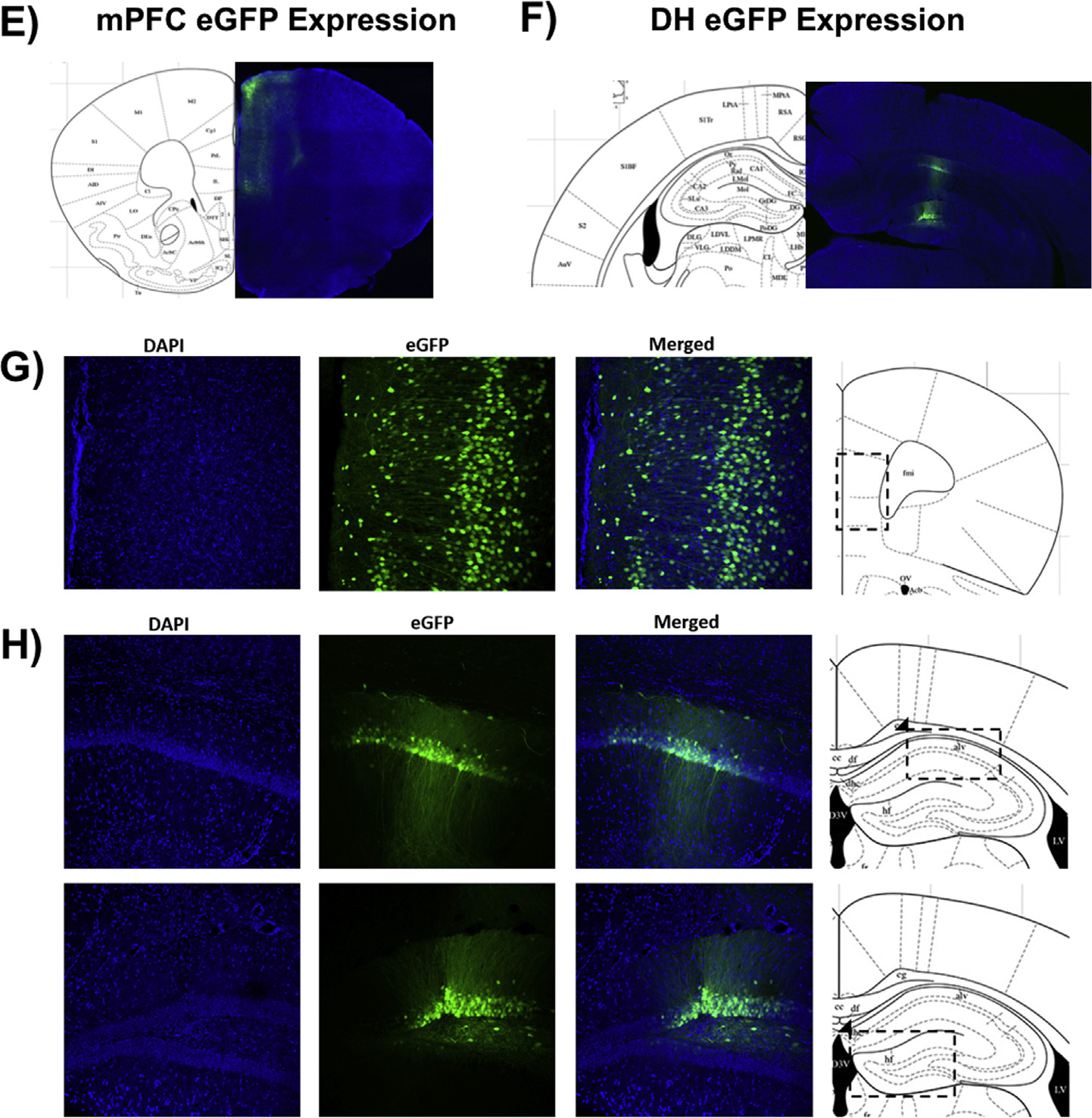
Representative coronal sections (40 μm) of CaMKIIα-hM4Di-mCitrine DREADD in the mPFC (A&C), CaMKIIα-KORD-mCitrine DREADD in the DH (B&D), and CaMKIIα-eGFP control virus in the mPFC (E&G) or DH (F&H) in female mouse brain 3 weeks post-injection. Blue puncta: DAPI; yellow: mCitrine-tagged DREADD virus; green: eGFP-tagged control virus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
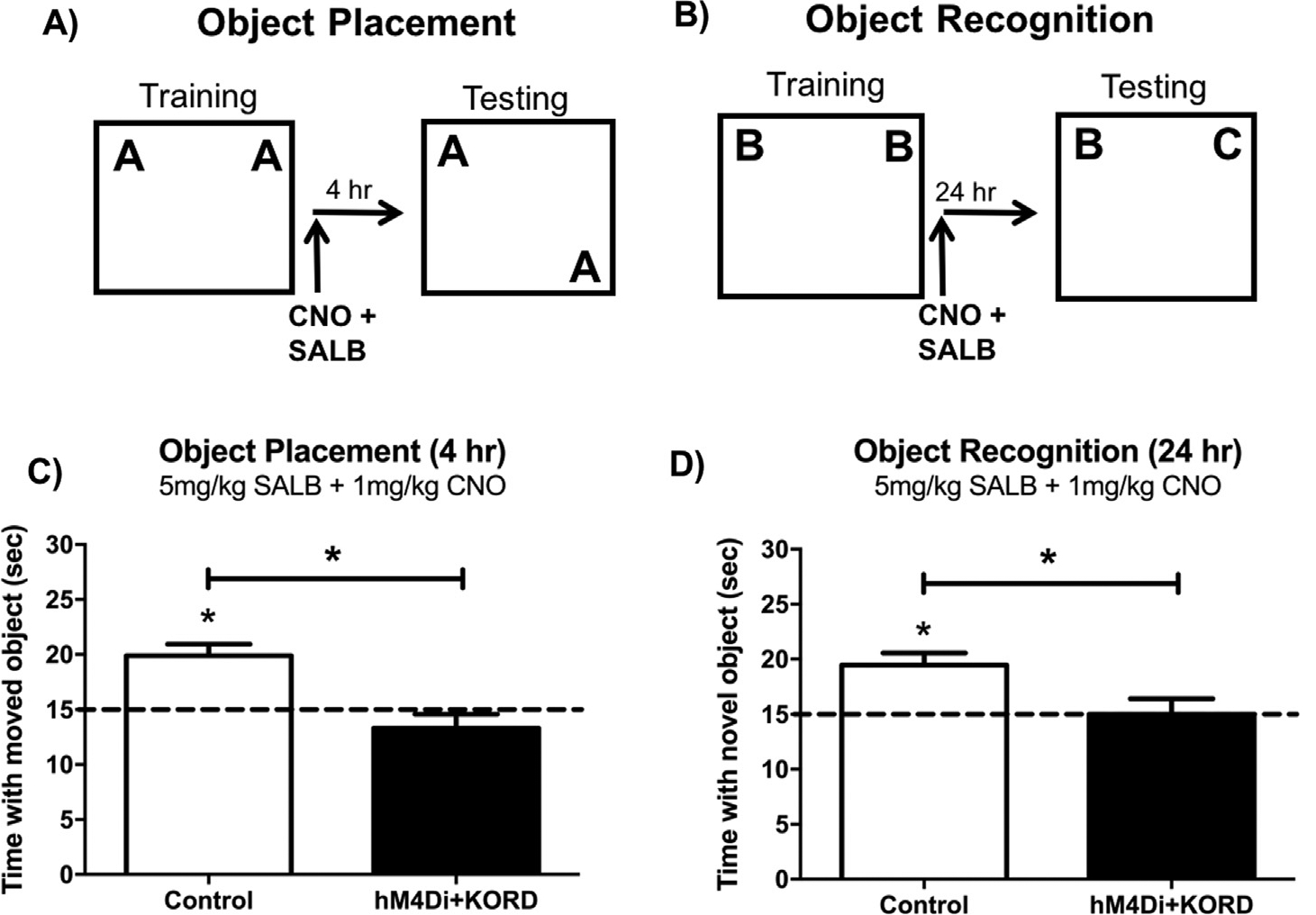
Experimental design for the object placement (A) and object recognition (B) subthreshold inactivation experiments. Doses of CNO and SALB that do not impair memory on their own impair OP (C) and OR (D) memory when co-administered to mice expressing KORD in the DH and hM4Di in the mPFC. Subthreshold doses of SALB (5 mg/kg) and CNO (1 mg/kg) do not impair memory in control mice in either task. This finding suggests that concurrent neural activity in both the DH and the mPFC is necessary for the consolidation of spatial and object recognition memories. Bars represent the mean ± SEM, *p < 0.05 relative to chance or the Control group.
2.3. Drugs, infusions, and injections
Stock solutions of CNO and SALB (Cayman Chemical, Ann Arbor, MI) were dissolved in 100% dimethyl sulfoxide (DMSO; Fisher Scientific, Pittsburgh, PA) at a concentration of 100 mg/ml, and stored in 10 μl aliquots at −20 °C. On the day injections were administered, CNO stock was thawed and diluted to a concentration of 1 or 2 mg/ml in a solution of sterile 0.9% saline containing 2% DMSO. SALB stock was thawed and diluted in 100% DMSO to a concentration of 5 or 10 mg/ml.
2.4. Behavioral testing
Object recognition (OR) and object placement (OP) were used to measure object recognition and spatial memory as described previously (Boulware, Heisler, & Frick, 2013; Fortress, Fan, Orr, Zhao, & Frick, 2013; Kim et al., 2016). Previous work (Cohen et al., 2013; Fernandez et al., 2008; Gresack & Frick, 2006; Li et al., 2004; Luine, Jacome, & Maclusky, 2003; Stackman, Cohen, Lora, & Rios, 2016; Walf, Koonce, & Frye, 2008) has established that each of these tasks involves the DH (see (Cohen & Stackman, 2015; Tuscher, Fortress, Kim, & Frick, 2015) for reviews). Three weeks after surgery, mice were handled for 1 min/day for 3 days prior to habituation. After the first day of handling, a Lego Duplo brick was placed in each home cage to habituate the mice to objects during the remaining handling days and habituation period. After 3 days of handling, mice were habituated to the behavioral apparatus for 2 consecutive days by allowing them to explore the empty white arena (60 cm × 60 cm × 47 cm) for 5 min/day. For the OR task, mice first accumulated 30 s exploring 2 identical objects placed 5 cm from the upper left and right corners of the arena during the training phase. Either 30 min prior to or immediately after training, mice were injected i.p. with CNO, SALB, or both ligands delivered in two separate syringes. Pre-training injections were used first to examine the effects of DREADD-mediated inhibition on memory acquisition and consolidation. Post-training injections were next used to pinpoint the effects of DREADD-mediated inactivation specifically to the memory consolidation period, while minimizing potential confounding effects on performance factors (e.g., motivation, anxiety) during training or retention testing (Frick & Gresack, 2003; McGaugh, 1989). OR memory was tested 24 h later by measuring the amount of time spent with the novel and familiar object. Intact OR memory consolidation is demonstrated if the mice spend more time than chance (15 s) with the novel object during testing. At the 24-hour time point, vehicle-infused ovariectomized females show intact object recognition (Boulware et al., 2013; Fortress et al., 2013), thereby permitting observation of the potential memory-impairing effects of DREADD-mediated inactivation. Training and testing for OP was identical to OR, except that testing was conducted 4 h after training, and involved moving one of the identical training objects to a new location in the arena (lower right or lower left corner) during testing. Intact spatial memory was demonstrated if mice spent more time than chance with the moved object. At the 4-hour delay, vehicle-infused ovariectomized females show intact OP memory (Boulware et al., 2013; Kim et al., 2016), which allowed any DREADD-mediated spatial memory impairments to be observed. All mice were trained and tested in both behavioral tasks. To counterbalance the order in which behavior was completed, half of the mice completed OR first, followed by OP, and the other half completed OP first, followed by OR. OR and OP training were separated by one week, and mice were trained with a unique set of objects for each task.
2.5. Histological verification of DREADD expression
Histology was performed to confirm comparable expression of hM4Di and KORD in both hemispheres of the mPFC and DH, respectively. Three weeks after surgery, a subset of mice (n = 3/group) were anesthetized with isoflurane and perfused with 4% paraformaldehyde (PFA) in 1× PBS to confirm viral expression in each cohort by the onset of behavioral training. Virus expression was verified in remaining mice (n = 10/group) after training and testing for the object tasks were complete. Whole mouse brains were then removed and post-fixed in 1× PBS/4% PFA overnight, followed by dehydration in a 1× PBS/30% sucrose solution until brains sank. Tissue was then sectioned on a cryostat (40 μm) and free-floated in 1× PBS until mounted onto microscope slides (VWR, Arlington Heights, IL) using aqueous mounting medium containing the nuclear stain DAPI. Fluorescent images were captured using an Olympus Fluoview FV1200 confocal microscope and accompanying software.
2.6. Data analysis
All statistical analyses were conducted using GraphPad Prism 6 (La Jolla, CA). To determine whether each group demonstrated intact memory for each behavioral task, OR and OP data were first analyzed using within-group one sample t-tests to determine if the time spent with the novel or moved object differed significantly from chance (15 s; (Boulware et al., 2013; Fortress et al., 2013; Kim et al., 2016)). This analysis was used because time spent with the objects is not independent; time spent with 1 object reduces time spent with the other object (Frick & Gresack, 2003). Student’s t tests were then used to determine significant between-group differences in performance between control and DREADD mice. Statistical significance for all analyses was determined as p ≤ 0.05.
3. Results
3.1. hM4Di-mediated inhibition of the DH impairs OP but not OR memory
Three weeks after surgery, brain tissue was collected from a subset of mice (n = 3) to verify eGFP and hM4Di expression in the DH at the initiation of behavioral testing (Fig. 1A–D). High levels of eGFP control virus and mCitrine-tagged DREADD virus were observed in the dentate gyrus, as well as weaker expression in CA1 and CA3. Viral expression was verified in the remaining mice after behavioral testing, and comparable expression was observed in both hemispheres. To test whether hM4Di-mediated inactivation of the DH impairs OP and OR memory formation, mice infused with saline (Sham), eGFP, or hM4Di into the DH received 2 mg/kg CNO i.p. 30 min before OP or OR training (Fig. 2A &B; n = 69/group). OP memory was tested four hours after training. Because Sham and eGFP controls did not differ (t(11) = 0.14, p = 0.89), they were combined into a single Control group and compared to the hM4Di group. Control mice administered 2 mg/kg CNO 30 min prior to training spent significantly more time than chance exploring the displaced object during OP testing (Control: t(12) = 5.80, p < 0.0001; Fig. 2C), demonstrating intact spatial memory and suggesting that 2 mg/kg CNO does not impair OP memory on its own in Control mice. However, CNO-treated hM4Di mice did not spend significantly more time than chance with the displaced object (hM4Di: t(8) = 0.09, p = 0.93; Fig. 2C), suggesting that spatial memory was impaired by hM4Di-mediated inhibition of the DH. Mice expressing hM4Di in the DH also spent significantly less time with the moved object than Control mice (t(20) = 3.24, p = 0.004; Fig. 2C), providing further evidence that spatial memory was impaired by DREADD-mediated suppression of the DH.
Fig. 2.
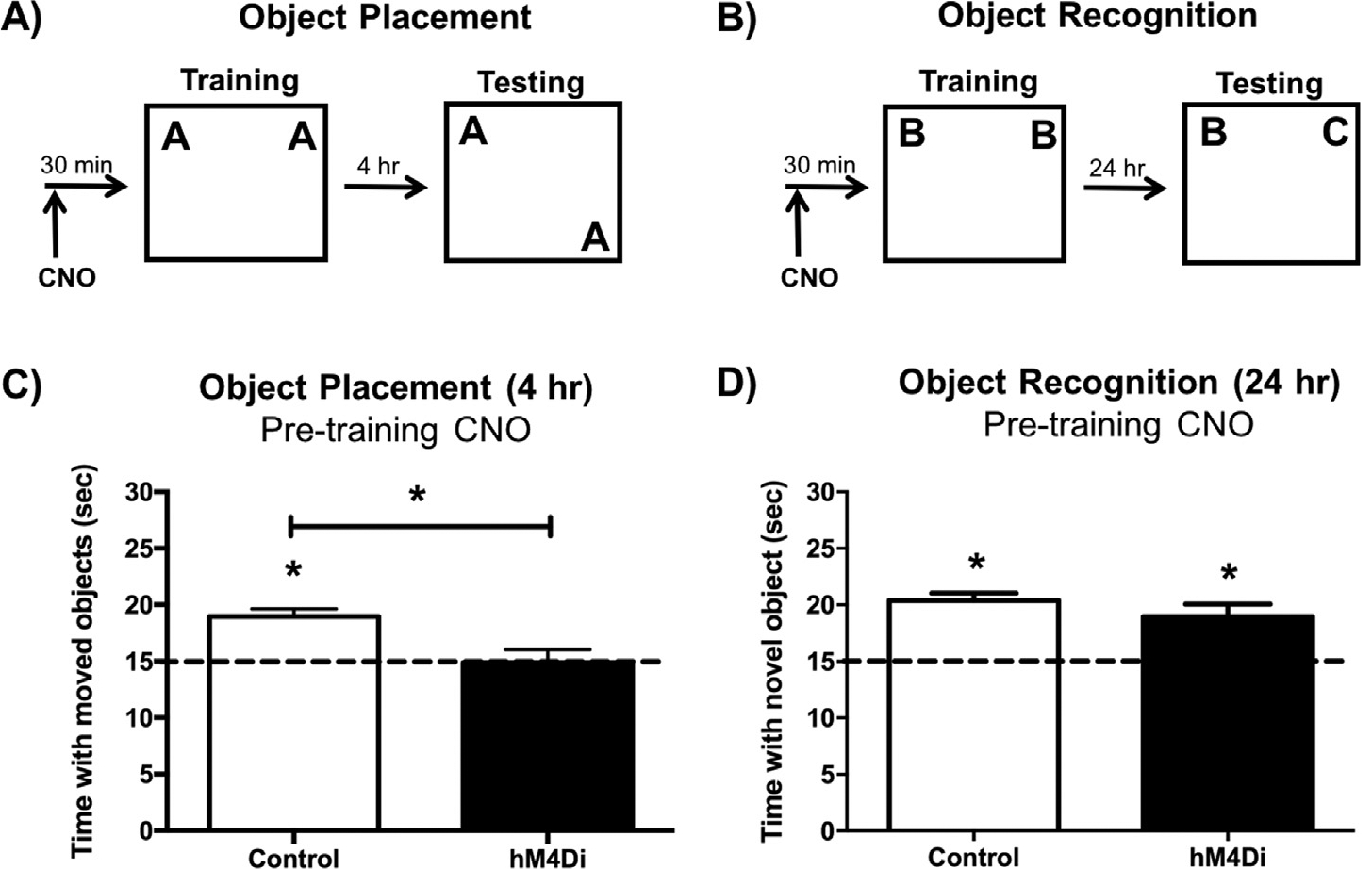
Experimental design for pre-training CNO injections using the object placement (A) and object recognition tasks (B). (C) In the object placement task, DH sham and eGFP control mice administered 2 mg/kg CNO 30 min before training spent significantly more time than chance (15 s) with the moved object 4 h after training, whereas DH-hM4Di-expressing mice administered 2 mg/kg CNO did not. (D) In the object recognition task, DH Sham, eGFP, and hM4Di mice administered 2 mg/kg CNO 30 min prior to training all spent significantly more time than chance (15 s) with the novel object during testing, suggesting intact object recognition memory 24 h after training. These findings suggest that pre-training hM4Di-mediated inactivation of the DH impairs spatial, but not object recognition, memory formation. Bars represent the mean ± SEM, *p < 0.05 relative to chance or the Control group.
OR memory was evaluated 24 h after training. In contrast to the OP task, CNO-treated Control and hM4Di mice all spent significantly more time than chance with the novel object during testing (Control: t(12) = 8.43, p < 0.0001; hM4Di: t(7) = 3.63, p = 0.008 Fig. 2D), and time spent with the novel object also did not differ between Control and hM4Di groups (t(19) = 1.21, p = 0.24), suggesting that all groups exhibited intact object recognition memory. Together, these data suggest that hM4Di-mediated inhibition of the DH, as driven by 2 m/kg CNO, impairs spatial memory but not object recognition memory.
Because CNO injections were administered prior to training, it was not clear if DREADD-mediated inhibition of the DH impaired acquisition or consolidation of OP memory formation. To target the consolidation period of memory formation, the same mice were trained in the OP task one week later with a new set of objects, and were injected with 2 mg/kg CNO immediately after training (Fig. 3A). Again, sham and eGFP control mice were combined into one Control group, as they did not statistically differ in time spent with the moved object (t(12) = 1.27, p = 0.23). Control mice spent significantly more time than chance with the moved object, demonstrating intact OP memory (Control: t(13) = 4.84, p = 0.0003; Fig. 3C), whereas hM4Di expressing mice administered 2 mg/kg CNO did not, suggesting that hM4Di-mediated inactivation of the DH impaired OP memory consolidation (hM4Di: t(8) = 0.08, p = 0.94; Fig. 3C). Control mice also spent significantly more time with the moved object during testing than the hM4Di group (t(21) = 3.07, p = 0.006; Fig. 3C), further supporting the notion that DREADD-mediated inhibition of the DH disrupts spatial memory consolidation.
To examine whether post-training hM4Di-mediated inactivation of the DH also impairs OR memory consolidation, we trained the same mice in the OR task with novel objects, and administered 2 mg/kg CNO immediately after training (Fig. 3B). Unlike OP, 2 mg/kg CNO did not impair OR memory consolidation in either group (Control: t(10) = 4.39, p = 0.001; hM4Di: t(6) = 3.41, p = 0.01; Fig. 3D) and Control and hM4Di groups did not differ from each other (t(16) = 0.91, p = 0.38). To test if higher doses of CNO could impair OR memory consolidation in mice expressing hM4Di DREADDs in the DH, we also administered 4 or 8 mg/kg CNO immediately after OR training. Neither the 4 mg/kg (Control: t(9) = 2.71, p = 0.02; hM4Di: t(8) = 2.60, p = 0.03; Fig. 3E), nor 8 mg/kg (Control: t(11) = 6.58, p < 0.0001; hM4Di: t(8) = 3.78, p = 0.01; Fig. 3F) dose of CNO impaired OR memory consolidation in the Control or hM4Di groups. Collectively, these data suggest hM4Di-mediated suppression of neural activity in the DH is sufficient to impair spatial, but not object recognition, memory consolidation.
3.2. hM4Di-mediated inhibition of the mPFC impairs OP and OR memory consolidation
To investigate the role of the mPFC alone, and its interactions with the DH, during object memory consolidation, a new set of mice was injected with the hM4Di inhibitory DREADD into the mPFC and another Gi-coupled inhibitory DREADD (kappa opioid receptor-DREADD; KORD) into the DH. Unlike the hM4-DREADD, the KOR-DREADD is activated by a distinct synthetic ligand (salvinorin-B; SALB), and can therefore be used for multiplexed modulation of behavior with CNO-activated DREADDs, such as hM4Di (Vardy et al., 2015). We used these two DREADD constructs to determine within the same mice whether activation of the mPFC alone, DH alone, or coincident activity in both regions is critical for memory consolidation. The injection of two different DREADD constructs activated by two distinct ligands enabled selective targeting of activity in two brain regions within the same mouse. This approach yielded three experimental groups: (1) mPFC-hM4Di + DH-KORD, (2) mPFC-eGFP + DH-eGFP, and (3) mPFC-Sham + DH-Sham (n = 610/group). Expression of hM4Di in the mPFC (Fig. 4A&C), KORD in the DH (Fig. 4B&D), and eGFP in both brain regions (Fig. 4FH), was verified by fluorescence microscopy 3 weeks after surgery (n = 3). Expression of mPFC-hM4Di and DH-KORD DREADDs were also detected at 6 weeks (Fig. 5A&B) and 18 weeks (Fig. 5C&D) post-infusion. Viral expression was verified in the mice tested in the studies below after behavioral testing, and comparable expression was observed in both hemispheres.
Fig. 5.
Representative coronal sections (40 μm) of CaMKIIα-hM4Di-mCitrine DREADD in the mPFC (A&C) or CaMKIIα-KORD-mCitrine in the DH (B&D) in female mouse brain 6 weeks (A&B) and 18 weeks (C&D) post-injection. Blue puncta: DAPI; yellow: mCitrine-tagged DREADD virus; green: eGFP-tagged control virus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To examine if mPFC activation alone is necessary for spatial memory consolidation, mice were trained in OP and then received an i.p. injection of CNO immediately after training (Fig. 6A). Sham and eGFP controls did not statistically differ from each other in time spent with the moved object after i.p. injection of either 1 mg/kg (t(13) = 0.07, p = 0.95) or 2 mg/kg CNO (t(11) = 1.11, p = 0.29), and thus were combined into a Control group. Mice expressing hM4Di in the mPFC spent no more time than chance with the displaced object during testing 4 h later when injected with 2 mg/kg CNO (hM4Di: t(5) = 0.40, p = 0.71; Fig. 6C), but not 1 mg/kg, CNO (hM4Di: t(8) = 5.04, p = 0.001; Fig. 6E). The Control group demonstrated intact spatial memory after i.p. injection of either 1 mg/kg CNO (Control: t(14) = 6.51, p < 0.0001; Fig. 6E) or 2 mg/kg CNO (Control: t(12) = 6.34, p < 0.0001; Fig. 6C), suggesting that both doses of CNO did not impair memory in Control mice. These findings suggest that hM4Di-mediated inhibition of the mPFC impairs OP memory after administration of 2 mg/kg CNO. Mice expressing hM4Di in the mPFC also spent significantly less time with the displaced object during testing than Control mice when injected with 2 mg/kg CNO (t(17) = 3.10, p = 0.006; Fig. 6C), further demonstrating DREADD-induced suppression of the mPFC disrupts spatial memory consolidation.
Fig. 6.
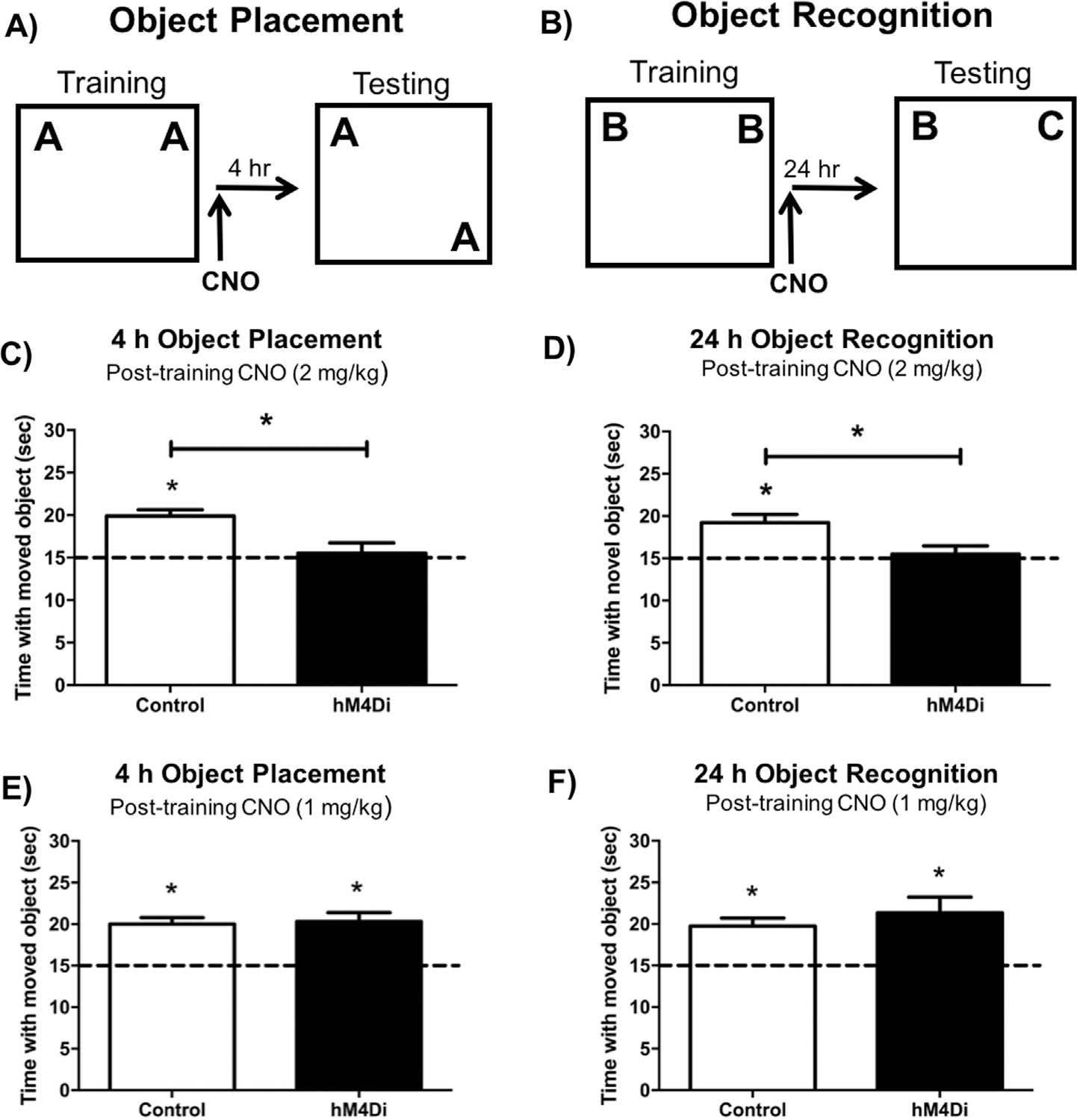
Experimental design for the object placement (A) and object recognition task (B). DREADD-mediated inhibition of the mPFC impaired both object placement (C) and object recognition (D) memory in mice expressing hM4Di in the mPFC that were administered 2 mg/kg CNO immediately after training, but not in control mice. A 1 mg/kg dose of CNO did not impair memory in the object placement (E) or the object recognition (F) task in control or hM4Di groups. These findings suggest that hM4Di-mediated inactivation of the mPFC in combination with 2 mg/kg CNO impairs spatial and object recognition memory consolidation. Bars represent the mean ± SEM, *p < 0.05 relative to chance or the Control group.
We next examined OR memory consolidation, and found that 2 mg/kg of CNO administered immediately after training impaired object recognition memory consolidation in mice expressing hM4Di in the mPFC, as these mice did not spend more time than chance with the novel object during testing (hM4Di: t(9) = 0.48, p = 0.64; Fig. 6D). In contrast, the Control group was not impaired by 2 mg/kg CNO when tested 4 h later (Control: t(19) = 4.25, p = 0.0004; Fig. 6D), and Controls did not statistically differ from each other in time spent with the novel object after i.p. injection of 2 mg/kg CNO (t(18) = 0.17, p = 0.87). mPFC-hM4Di mice injected with 2 mg/kg CNO immediately post-training also spent significantly less time with the novel object during testing than controls (t(28) = 2.37, p = 0.02; Fig. 6D), suggesting suppression of the mPFC impaired OR memory. Post-training injection of 1 mg/kg CNO did not impair OR memory consolidation in any treatment condition (hM4Di: t(5) = 3.32, p = 0.02; Control: t(12) = 4.80, p = 0.0004; Fig. 6F), and hM4Di and Control groups did not differ from each other (t(17) = 0.83, p = 0.42), demonstrating that the 1 mg/kg dose of CNO is behaviorally subeffective in both Control and DREADD-expressing mice. Collectively, these data suggest that suppression of mPFC neurotransmission by 2 mg/kg CNO disrupts both spatial and object recognition memory consolidation.
3.3. KORD-mediated inhibition of the DH impairs OP and OR memory consolidation
Our first series of experiments examining hM4Di-mediated inactivation of the DH indicated that DH activity is necessary for OP, but not OR, memory consolidation. However, numerous pharmacological studies suggest that DH activity is necessary for consolidation in these tasks (Baker & Kim, 2002; Broadbent, Squire, & Clark, 2004; Cohen et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Hammond, Tull, & Stackman, 2004; Zhao, Fan, Fortress, Boulware, & Frick, 2012). Therefore, we examined the effects of KORD-mediated DH inhibition on OR and OP memory to determine if the effects observed with the hM4Di DREADD would generalize to another DREADD construct. The same mice described above (Fig. 6) were trained in OP and OR with new sets of objects (Fig. 7A&B). Control mice injected immediately post-training with 10 mg/kg SALB (Control: t(16) = 4.10, p = 0.001; Fig. 7C) or 5 mg/kg SALB (Control: t(14) = 4.77, p = 0.0003; Fig. 7E) spent significantly more time than chance with the moved object during testing, and Sham and eGFP controls did not differ from each other when injected with either dose (5 mg/kg SALB: t(13) = 0.14, p = 0.89; 10 mg/kg SALB: t(17) = 1.06, p = 0.30), demonstrating that SALB does not impair OP memory consolidation in Control mice. In mice expressing KORDs in the DH, 10 mg/kg SALB impaired spatial memory consolidation, as these mice did not spend more time than chance with the displaced object during testing (KORD: t(8) = 1.35, p = 0.21; Fig. 7C). However, OP memory consolidation was not impaired in DH KORD-expressing mice by 5 mg/kg SALB (KORD: t(8) = 3.45, p = 0.01; Fig. 7E), suggesting this is a behaviorally subthreshold dose that is not sufficient to impair OP memory in control or DH-KORD expressing mice. Mice expressing KORD in the DH also trended toward spending significantly less time with the displaced object during testing than Control mice when injected with 10 mg/kg SALB (t(30) = 1.73, p = 0.09; Fig. 7C), further suggesting that DREADD-induced suppression of the DH disrupts spatial memory consolidation.
Fig. 7.
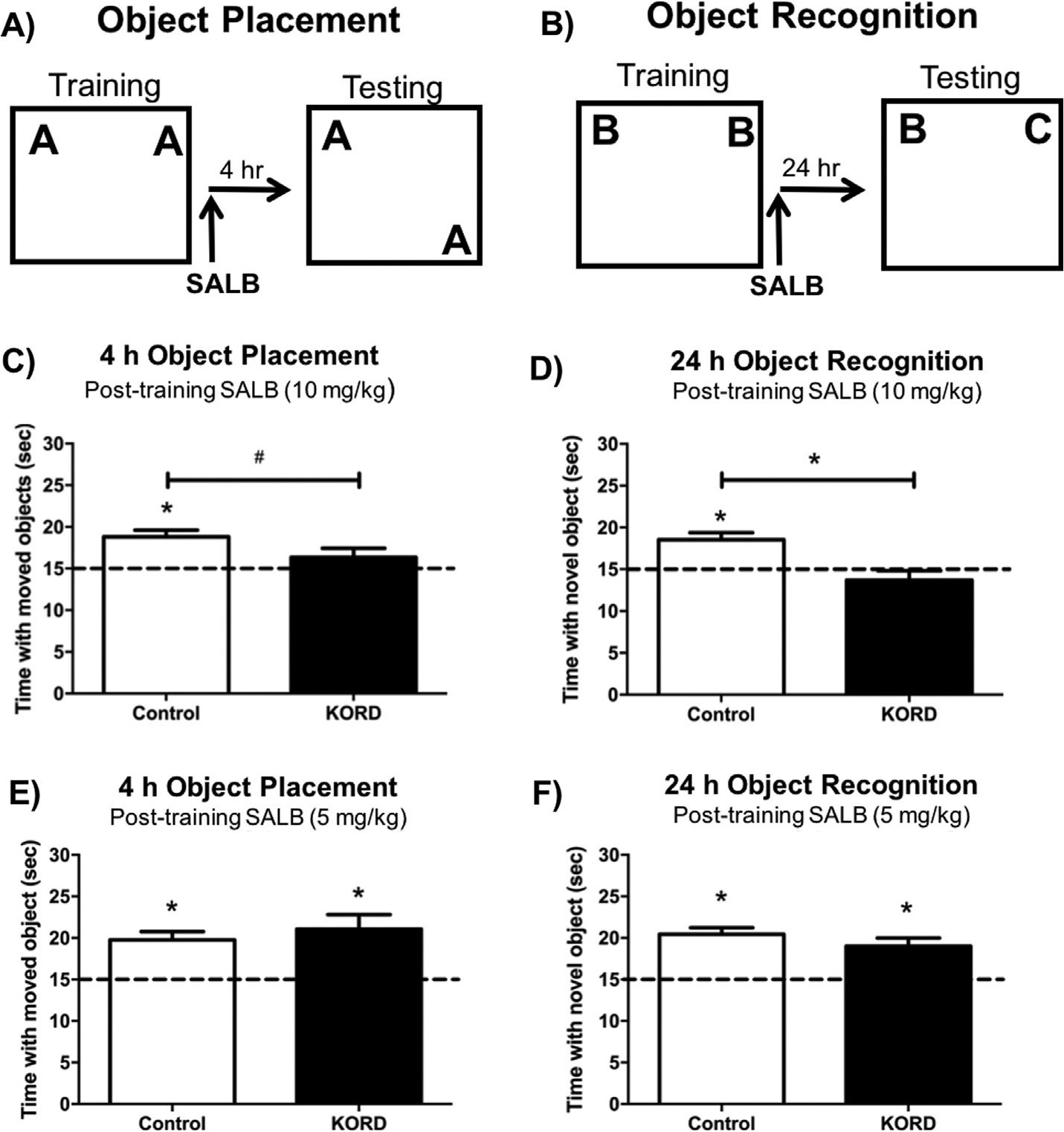
Experimental design for the object placement (A) and object recognition task (B). DREADD-mediated inhibition of the DH impaired both object placement (C) and object recognition (D) memory in mice expressing KORD in the DH that were administered 10 mg/kg SALB immediately after training. A 5 mg/kg dose of SALB did not impair memory in the object placement (E) or object recognition (F) task for control or KORD-expressing groups. This finding suggests that KORD-mediated inactivation of the DH in combination with 10 mg/kg SALB impairs spatial and object recognition memory consolidation. Bars represent the mean ± SEM, *p < 0.05 relative to chance or the Control group; #p = 0.08 relative to the Control group.
As in the OP task, Control mice injected with 10 mg/kg SALB (Control: t(12) = 2.22, p = 0.04; Fig. 7D) or 5 mg/kg SALB (Control: t(13) = 7.00, p < 0.0001; Fig. 7F), demonstrated intact OR memory, and Sham and eGFP controls did not differ from each other when injected with either dose of SALB (5 mg/kg SALB: t(13) = 0. 46, p = 0.65; 10 mg/kg SALB: t(18) = 0.75, p = 0.46). Also similar to OP, immediate post-training injection of 10 mg/kg SALB prevented DH-KORD mice from spending more time than chance with the novel object during testing 24 h later (KORD: t(8) = 1.14, p = 0.29; Fig. 7D), suggesting impaired object recognition memory consolidation. Again, OR was not impaired by 5 mg/kg SALB in DH-KORD mice (KORD: t(9) = 4.15, p = 0.002; Fig. 7F). Collectively, these findings show KORD-mediated suppression of the DH impairs both OP and OR memory consolidation. Mice expressing KORD in the DH also spent significantly less time with the novel object during testing than Control mice when injected with 10 mg/kg SALB (t(20) = 2.30, p = 0.03; Fig. 7C), further demonstrating DREADD-induced suppression of the DH disrupts object recognition memory consolidation. The fact that OR memory consolidation was impaired in the DH by KORD-mediated inactivation, but not hM4Di-mediated inactivation, suggests potentially interesting differences in the effects of these constructs and/or their relative expression in these two studies.
3.4. Concurrent subthreshold inhibition of the mPFC and DH impairs OP and OR memory
Finally, to examine the potential interaction between the DH and mPFC during object recognition and spatial memory consolidation, we used behaviorally subthreshold doses of CNO and SALB to concurrently suppress neurotransmission in the DH and mPFC. Importantly, neither dose of CNO (1 mg/kg; Fig. 6E&F) or SALB (5 mg/kg; Fig. 7E&F) used for this experiment was sufficient to impair memory in DREADD-expressing mice in either task when administered on its own. Thus, any memory impairments observed should be a result of combined disruption of the DH and mPFC. To this end, immediately after training with a new set of objects, mice were injected i.p. with 1 mg/kg CNO and 5 mg/kg SALB delivered in separate syringes. eGFP and Sham controls did not differ in time spent with the moved object (t(13) = 1. 50, p = 0.16) or novel object during testing (t(15) = 1. 04, p = 0.31), and were collapsed into one Control group. Control mice administered the combined subthreshold injections spent more time than chance with the moved object in OP (Control: t(13) = 4.65, p = 0.0005; Fig. 8C) and the novel object in OR (Control: t(15) = 4.07, p = 0.001; Fig. 8C), suggesting that spatial and object recognition memory were not impaired in Controls administered subthreshold doses of CNO and SALB. However, mice expressing hM4Di in the mPFC and KORD in the DH spent no more time than chance with the moved object during OP testing (mPFC-hM4Di + DH-KORD: t(8) = 1.33, p = 0.22; Fig. 8C) and the novel object during OR testing (mPFC-hM4Di + DH-KORD: t(8) = 0.01, p = 0.99; Fig. 8D) when injected with 1 mg/kg CNO and 5 mg/kg SALB immediately after training. Further, mPFC-hM4Di + DH-KORD mice administered 1 mg/kg CNO and 5 mg/kg SALB post-training also spent significantly less time with the moved object than Controls during OP testing (t(21) = 3.96, p = 0.0007; Fig. 8C), and with the novel object than Controls in the OR task (t(23) = 2.47, p = 0.02; Fig. 8D). These findings suggest that concurrent subthreshold disruption of neurotransmission in the mPFC and DH impairs spatial and object recognition memory consolidation.
4. Discussion
The goal of the present study was to determine the roles of the mPFC and DH, independently and in combination, in mediating object recognition and spatial memory consolidation. Using two different DREADD constructs, we found that inactivation of either the mPFC or the DH impaired the consolidation of both types of memory, although DH inactivation impaired object recognition only when using the KORD construct. These data suggest the primary importance of both the mPFC and DH in regulating object recognition and spatial memory consolidation. Notably, these brain regions appear to work in concert to mediate memory formation in the OR and OP tasks, as concurrent inactivation of both regions using subthreshold doses of DREADD ligands impaired consolidation in both tasks.
Our present findings that DH inactivation can disrupt OP memory using the hM4Di DREADD and both OP and OR using the KOR-DREADD are consistent with previously published evidence demonstrating that inhibiting DH function in rodents impairs performance in object tasks. For example, OP memory is impaired by NMDA and GABAA receptor blockade, as well as aromatase inhibition in the DH (Assini, Duzzioni, & Takahashi, 2009; Haettig et al., 2011; Larkin et al., 2008; Tuscher, Szinte et al., 2016). Similarly, OR memory consolidation is disrupted when the hippocampus is lesioned or pharmacologically inhibited by GABAA agonists, NMDA antagonists, or inhibitors of ERK/MAPK cell signaling, histone acetylation, and protein synthesis (Baker & Kim, 2002; Broadbent et al., 2004; Cohen et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Hammond et al., 2004; Zhao et al., 2012). Given previous studies demonstrating that DH inactivation impairs OR memory consolidation, it is perhaps not surprising that suppression of excitatory neural activity in the DH impaired memory consolidation in both OR and OP. However, despite numerous studies showing that DH activation is necessary for OR memory formation, some have reported that DH inactivation does not impair OR (Broadbent, Gaskin, Squire, & Clark, 2010; Forwood, Winters, & Bussey, 2005; Mumby, 2001; Squire, Wixted, & Clark, 2007; Winters, Forwood, Cowell, Saksida, & Bussey, 2004). Further, others have reported DH injection of hM4Di DREADDs impaired performance in the OP task, but did not impair OR memory in male mice (Lopez et al., 2016). Similarly, we also found that hM4Di-mediated inactivation of the DH was only sufficient to impair OP, but not OR, memory consolidation. Although it is not entirely clear why one inhibitory DREADD impaired OR whereas another form of inhibitory DREADD driven by the same CaMKII promoter did not, there are a couple of reasons why this might be the case. One possibility is that the proportion of neurons transduced by the DREADD may have differed by cohort. If a larger proportion of dentate gyrus neurons took up the KORD (relative to the hM4-DREADD), then a larger population of neurons may have been inhibited during KORD inactivation, and this could have resulted in greater disruption of excitatory neurotransmission. Alternatively, the distinct effects on behavior could be related to the different pharmacokinetic properties of each DREADD ligand. SALB administration reportedly results in rapid (within seconds) and acute neuronal silencing (Vardy et al., 2015; Hooker et al., 2009), whereas CNO-mediated inhibition takes 510 min to occur and does not peak until approximately 45 min later (Alexander et al., 2009; Urban and Roth, 2015). This more rapid onset of neuronal inhibition induced by SALB may have been more efficient at disrupting DH neural activity after training compared to CNO-based DREADDs. Another potential factor that could have contributed to our behavior observations is that spatial memory may rely more heavily on the DH than does object recognition memory (Broadbent et al., 2004; Squire et al., 2007; Wilson et al., 2013). Thus, spatial memory may be more susceptible to disruption when neuronal activity is suppressed in the DH, whereas perirhinal or parahippocampal regions may be able to compensate for DH disruption in recognition-based tasks (Aggleton, Albasser, Aggleton, Poirier, & Pearce, 2010; Albasser, Poirier, & Aggleton, 2010).
Our data also suggest that hM4Di-mediated inactivation of the mPFC immediately after training impaired both OR and OP memory consolidation. Although at least one study has implicated the mPFC as a critical locus for OR and OP memory consolidation (Akirav & Maroun, 2006), other mPFC inactivation studies have suggested that this region is involved in spatial object tasks but not OR (DeVito & Eichenbaum, 2010; Warburton & Brown, 2015). One potential factor that may contribute to this discrepancy is the length of the delay between training and testing. In studies concluding that mPFC activation was not necessary for OR, only a 50 min or 2-h delay was imposed between training and testing (Barker et al., 2007; DeVito & Eichenbaum, 2010). However, a study using a 24-h delay between training and testing reported that mPFC inactivation impaired OR memory (Akirav & Maroun, 2006). Therefore, the mPFC may be critical for the consolidation of long-term memories, but not short-term memories. This notion is consistent with our present findings, which indicate that recall after longer delays (i.e., 4 or 24 h) is impaired when neurotransmission is disrupted in the mPFC immediately after training.
Our finding that concurrent inhibition of the DH and mPFC disrupts OR and OP memory consolidation is distinct from previous work in male rats using a functional disconnection approach to examine hippocampal-prefrontal interactions during episodic-like memory tasks (Barker & Warburton, 2011; Barker et al., 2017; Floresco et al., 1997; Wang & Cai, 2006). Although sex differences in the circuitry that supports memory consolidation could potentially play a role, the discrepancy likely reflects differences in experimental approach. This report is the first to use multiplexed inhibitory DREADDs to partially inactivate both the DH and mPFC during memory formation to address whether concomitant activity in these regions is required for episodic-like memory consolidation. Given the numerous potential routes of communication between the DH and mPFC (Burwell & Amaral, 1998; Cenquizca & Swanson, 2007; Hoover & Vertes, 2007; Ye, Kapeller-Libermann, Travaglia, Inda, & Alberini, 2017), this approach prevented potential compensatory effects through alternate indirect routes (i.e., nucleus reuniens, entorhinal and perirhinal cortices) which could be utilized in functional disconnection studies that only disrupt either ipsi- or contralateral communication between these structures (Warburton & Brown, 2015). Importantly, we used doses of CNO (1 mg/kg; Fig. 6E&F) and SALB (5 mg/kg; Fig. 7E&F) that were not sufficient to impair object memory consolidation in either task when used alone to suppress neurotransmission in the mPFC or DH, respectively. Although our findings cannot definitively attribute memory impairment to blockade of a direct, monosynaptic connection between DH and mPFC, these data do provide support that concomitant neuronal activity is required in both brain regions for the successful consolidation of OR and OP memories. Future studies utilizing chemogenetic or optogenetic approaches to selectively target DH projection terminals in the mPFC (rather than silencing the entire mPFC) could be used to address whether direct DH efferent input into the mPFC is necessary for episodic-like memory formation.
The fact that concurrent disruption of neurotransmission in the mPFC and DH impaired memory consolidation in the present experiments is consistent with other research reporting that temporally-coordinated neuronal activity in these regions during periods of sleep and wakefulness in rodents is necessary for systems memory consolidation. For example, hippocampal input to the mPFC during sleep or slow-wave oscillations during rest periods after behavioral training are required for consolidation (Schwindel & McNaughton, 2011). During periods of wakefulness, neuronal firing in the DH and mPFC is phase-locked to hippocampal theta oscillations, and firing coherence is increased during spatial working memory tasks (Hyman, Zilli, Paley, & Hasselmo, 2005, 2010; Jones & Wilson, 2005). Further, reduced theta rhythm coherence between CA1 and mPFC in mice is correlated with poor performance in a spatial working memory task (Sigurdsson, Stark, Karayiorgou, Gogos, & Gordon, 2010). Given that hippocampal-prefrontal neural synchrony appears to be important for memory consolidation in the aforementioned studies, it follows that coinciding chemogenetic suppression of the DH and mPFC in the present study may have disrupted functional connectivity between the DH and mPFC, which ultimately impaired OR and OP memory consolidation.
Our present findings also align with recent work demonstrating that direct input from the dentate gyrus into the mPFC during contextual fear conditioning is necessary for establishing immature engram cells within the mPFC (Kitamura et al., 2017). Disruption of these DH-mPFC interactions during fear conditioning also prevents spine density increases later observed on eYFP-labeled engram cells in the mPFC at a remote memory test (Kitamura et al., 2017). This work and our current findings support the idea that communication between the DH and mPFC must be established during the consolidation period to support long-term memory formation. Other recent research investigating the necessity of DH-mPFC interactions during memory formation has shown that hM4Di-mediated inhibition of DH projection terminals in the prelimbic region of the mPFC prior to reactivation sessions prevents reactivation-induced increases in fear memory expression and memory-associated proteins in the mPFC (e.g., Arc, pCREB, and pCofilin protein; (Ye et al., 2017). Taken together, these studies and our present findings lend additional support to the idea that the DH and mPFC individually contribute to, and work together during, the successful consolidation of episodic-like memories.
These data complement our previous work in ovariectomized females showing that dorsal hippocampal infusions of estradiol increase dendritic spine density, not only in CA1 but also in the mPFC (Tuscher, Luine et al., 2016). However, the use of ovariectomized females here could limit the generalizability of these findings to females with low circulating levels of estradiol and males if there are significant sex differences in the circuitry underlying episodic memory formation that are largely driven by ovarian hormones. In humans, some neuroimaging evidence exists linking sex differences in episodic memory to differences in neural activity, however, these differences are relatively small and correlational in nature (Gron, Wunderlich, Spitzer, Tomczak, & Riepe, 2000; Nyberg, Habib, & Herlitz, 2000). Although subtle differences in the neuroanatomical correlates of episodic memory have been reported, behavioral performance in such tasks largely overlaps between the sexes (Cabeza et al., 1997; Jaeger et al., 1998; Nyberg et al., 2000). In rodents, few, if any, published studies have directly compared episodic memory in males and females, so whether sex differences in the underlying neural circuitry influence episodic memory formation remains an open question. Our observation here that inhibiting the DH or mPFC alone impairs episodic-like memory tasks in ovariectomized females is largely consistent with previously published work using male rodents (Akirav & Maroun, 2006; Baker & Kim, 2002; Cohen et al., 2013; Lopez et al., 2016). Studies examining putative interactions between the mPFC and DH in male rats have, thus far, not reported impairment in OR or OP tasks, however, the discordance between this research and our present findings likely reflects the different experimental approaches used, rather than direct support for the existence of sex differences in the circuitry that supports episodic memory. Previous studies examining episodic-like memory in male rats employed a functional disconnection approach (Barker & Warburton, 2011; Barker et al., 2007), which may have only partially disrupted potential ipsi- or contra-lateral projections between the mPFC and DH. In contrast, our multiplexed chemogenetic approach allowed for concurrent disruption of neural activity in both hemispheres of the DH and mPFC, thus preventing potential compensatory communication between these regions via indirect routes including the entorhinal cortex or nucleus reuniens of the thalamus (Burwell & Amaral, 1998; Hoover & Vertes, 2007; Vertes et al., 2007). Applying the same chemogenetic approach to male rodents could yield similar results, although this remains to be tested. Regardless of whether behavioral end points between males and females are similar, this outcome does not preclude the possibility that the synaptic, cellular, and molecular-level processes within the circuitry that supports episodic-like memory are distinct between the sexes. In fact, sex differences have been observed in the hippocampus and mPFC in morphology and function (see Juraska, Sisk, & DonCarlos, 2013; Koss & Frick, 2017 for reviews). However, sex differences on the cellular level do not necessarily translate into functional sex differences, as, for example, 17β-estradiol enhances hippocampal glutamatergic neurotransmission and memory consolidation in OR and OP among males and females, but via different receptor and cell-signaling mechanisms (Koss, Haertel, Philippi, & Frick, 2018; Oberlander & Woolley, 2016). Thus, sex differences in the circuitry underlying episodic memory may not produce sex differences in episodic memory if the circuity in each sex is optimally designed to mediate episodic memory formation in that sex.
In addition to improving our fundamental understanding of which brain regions support episodic-like memory consolidation, this work may also prove relevant for understanding how systems-level dysfunction contributes to mental health. For example, disruption of normal hippocampal-prefrontal communication has been implicated in a number of psychiatric and neurodegenerative disorders (Godsil, Kiss, Spedding, & Jay, 2013; Sampath, Sathyanesan, & Newton, 2017), many of which women are disproportionately at risk for developing, including depression, PTSD, Alzheimer’s, and Parkinson’s disease (Albert, Pruessner, & Newhouse, 2015; Dubois et al., 2007; Dye, Miller, Singer, & Levine, 2012; Solomon & Herman, 2009; Tolin & Foa, 2006; Williams-Gray et al., 2006; Zandi et al., 2002). As such, gaining a better understanding of how the hippocampus interacts with other brain regions to support healthy cognitive function will be essential for elucidating the systems-level basis of mental disorders, and for developing potential circuit-based therapeutic interventions.
In summary, the present study indicates that both the DH and the mPFC are required for the consolidation of object recognition and spatial memories, as suppressing neurotransmission in either brain region impairs performance in each of these tasks. In addition to the individual contribution of each brain region, our data also support the notion that these brain regions must act in concert to consolidate object recognition and spatial memories in ovariectomized female mice. Collectively, this work provides additional insight into the neurobiological basis of episodic-like memory formation, and may provide an important foundation for studying how circuit-level communication is compromised in certain neuropsychiatric disorders.
Acknowledgements
This work was supported by the University of Wisconsin-Milwaukee College of Letters & Science, a Research Growth Initiative Award (101X334) from the UWM Research Foundation to K.M.F., and a UWM Advanced Opportunity Placement Fellowship to J.J.T. We thank Jacqueline Haertel for assistance with data collection.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, & Pearce JM (2010). Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behavioral Neuroscience, 124(1), 55–68. 10.1037/a0018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, & Maroun M (2006). Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cerebral Cortex, 16(12), 1759–1765. 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, & Aggleton JP (2010). Qualitatively different modes of perirhinal-hippocampal engagement when rats explore novel vs. familiar objects as revealed by c-Fos imaging. European Journal of Neuroscience, 31(1), 134–147. 10.1111/j.1460-9568.2009.07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Pruessner J, & Newhouse P (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology, 59, 14–24. 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, & Roth BL (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron, 63, 27–39. 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, & Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of National Academy of Sciences USA, 104(12), 5163–5168. 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assini FL, Duzzioni M, & Takahashi RN (2009). Object location memory in mice: Pharmacological validation and further evidence of hippocampal CA1 participation. Behavioural Brain Research, 204(1), 206–211. 10.1016/j.bbr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Baker KB, & Kim JJ (2002). Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory, 9, 58–65. 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Banks PJ, Scott H, Ralph GS, Mitrophanous KA, Wong LF, … Warburton EC (2017). Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nature Neuroscience, 20(2), 242–250. 10.1038/nn.4472. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, & Warburton EC (2007). Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. Journal of Neuroscience, 27(11), 2948–2957. 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2011). When is the hippocampus involved in recognition memory? Journal of Neuroscience, 31(29), 10721–10731. 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, & Frick KM (2013). The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. Journal of Neuroscience, 33(38), 15184–15194. 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, & Clark RE (2010). Object recognition memory and the rodent hippocampus. Learning and Memory, 17(1), 5–11. 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, & Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of National Academy of Sciences USA, 101(40), 14515–14520. 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, & Amaral DG (1998). Perirhinal and postrhinal cortices of the rat: Interconnectivity and connections with the entorhinal cortex. Journal of Comparative Neurology, 391(3), 293–321. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, … Craik FI (1997). Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience, 17(1), 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, & Swanson LW (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews, 56(1), 1–26. 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, & Kesner RP (2011). Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behavioural Brain Research, 225(2), 389–395. 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, & Stackman RW Jr. (2013). The rodent hippocampus is essential for nonspatial object memory. Current Biology, 23(17), 1685–1690. 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, & Stackman RW Jr. (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behavioural Brain Research, 285, 105–117. 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, & De Souza Silva MA (2005). Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiology of Learning and Memory, 84(3), 214–221. 10.1016/j.nlm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dere E, Pause BM, & Pietrowsky R (2010). Emotion and episodic memory in neuropsychiatric disorders. Behavioural Brain Research, 215(2), 162–171. 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- DeVito LM, & Eichenbaum H (2010). Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behavioural Brain Research, 215(2), 318–325. 10.1016/j.bbr.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, … Scheltens P (2007). Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology, 6(8), 734–746. 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Dye RV, Miller KJ, Singer EJ, & Levine AJ (2012). Hormone replacement therapy and risk for neurodegenerative diseases. International Journal of Alzheimer’s Disease, 258454. 10.1155/2012/258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nature Reviews Neuroscience, 18(9), 547–558. 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2010). One-trial object recognition in rats and mice: Methodological and theoretical issues. Behavioural Brain Research, 215(2), 244–254. 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, & Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, … Frick KM (2008). Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. Journal of Neuroscience, 28, 8660–8667. 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, & Phillips AG (1997). Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. Journal of Neuroscience, 17(5), 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, & Frick KM (2013). Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in dorsal hippocampus. Learning and Memory, 20(3), 147–155. 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, & Bussey TJ (2005). Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus, 15(3), 347–355. 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Frick KM, & Gresack JE (2003). Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral Neuroscience, 117, 1283–1291. 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, & Jay TM (2013). The hippocampal-prefrontal pathway: The weak link in psychiatric disorders? European Neuropsychopharmacology, 23(10), 1165–1181. 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Gresack JE, & Frick KM (2006). Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacology Biochemistry and Behavior, 84, 112–119. 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gron G, Wunderlich AP, Spitzer M, Tomczak R, & Riepe MW (2000). Brain activation during human navigation: Gender-different neural networks as substrate of performance. Nature Neuroscience, 3(4), 404–408. 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, & Wood MA (2011). HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learning and Memory, 18(2), 71–79. 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, & Stackman RW (2004). On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory, 82(1), 26–34. 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function, 212(2), 149–179. 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Munro TA, Beguin C, Alexoff D, Shea C, Xu Y, & Cohen BM (2009). Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence. Neuropharmacology, 57, 386–391. 10.1016/j.neuropharm.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, & Hasselmo ME (2005). Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus, 15(6), 739–749. 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, & Hasselmo ME (2010). Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience, 4, 2. 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, VanValin RD Jr., Kemmerer DL, Murphy BW, & Wack DS (1998). Sex differences in brain regions activated by grammatical and reading tasks. Neuroreport, 9(12), 2803–2807. [DOI] [PubMed] [Google Scholar]
- Jin J, & Maren S (2015). Prefrontal-hippocampal interactions in memory and emotion. Frontiers in Systems Neuroscience, 9, 170. 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, & Wilson MA (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology, 3(12), e402. 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, & DonCarlos LL (2013). Sexual differentiation of the adolescent rodent brain: Hormonal influences and developmental mechanisms. Hormones and Behavior, 64(2), 203–210. 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, & Frick KM (2016). 17beta-estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. Journal of Neuroscience, 36(11), 3309–3321. 10.1523/JNEUROSCI.0257-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, … Tonegawa S (2017). Engrams and circuits crucial for systems consolidation of a memory. Science, 356(6333), 73–78. 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim B, & Ehlers A (2008). Reduced autobiographical memory specificity predicts depression and posttraumatic stress disorder after recent trauma. Journal of Consulting and Clinical Psychology, 76(2), 231–242. 10.1037/0022-006X.76.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Haertel JM, Philippi SM, & Frick KM (2018). Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17b-estradiol. eNeuro, 5(5), 1–14. 10.1523/ENEURO.0267-18.2018. September/October 2018,e0267-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, & Frick KM (2017). Sex differences in hippocampal function. Journal of Neuroscience Research, 95(12), 539–562. 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- Larkin AE, Fahey B, Gobbo O, Callaghan CK, Cahill E, O’Mara SM, & Kelly AM (2008). Blockade of NMDA receptors pre-training, but not post-training, impairs object displacement learning in the rat. Brain Research, 1199, 126–132. 10.1016/j.brainres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, … McEwen BS (2004). Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences, USA, 101, 2185–2190. 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AJ, Kramar E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, … Wood MA (2016). Promoter-specific effects of DREADD modulation on hippocampal synaptic plasticity and memory formation. Journal of Neuroscience, 36(12), 3588–3599. 10.1523/JNEUROSCI.3682-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, & Maclusky NJ (2003). Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology, 144(7), 2836–2844. 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (1989). Dissociating learning and performance: Drug and hormone enhancement of memory storage. Brain Research Bulletin, 23, 339–345. [DOI] [PubMed] [Google Scholar]
- McNally RJ (2006). Cognitive abnormalities in post-traumatic stress disorder. Trends in Cognitive Sciences, 10(6), 271–277. 10.1016/j.tics.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Moore SA, & Zoellner LA (2007). Overgeneral autobiographical memory and traumatic events: An evaluative review. Psychological Bulletin, 133(3), 419–437. 10.1037/0033-2909.133.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG (2001). Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research, 127, 159–181. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, & Herlitz A (2000). Brain activation during episodic memory retrieval: Sex differences. Acta Psychologica (Amst), 105(23), 181–194. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, & Woolley CS (2016). 17beta-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. Journal of Neuroscience, 36(9), 2677–2690. 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum H (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23(17), R764–773. 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Sathyanesan M, & Newton SS (2017). Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatric Disease and Treatment, 13, 1509–1519. 10.2147/NDT.S136122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindel CD, & McNaughton BL (2011). Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Progress in Brain Research, 193, 163–177. 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Muller V, Li SC, & Lindenberger U (2010). Episodic memory across the lifespan: The contributions of associative and strategic components. Neuroscience and Biobehavioral Reviews, 34(7), 1080–1091. 10.1016/j.neubiorev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, & Gordon JA (2010). Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature, 464(7289), 763–767. 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, & Herman JP (2009). Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiology and Behavior, 97, 250–258. 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, & Clark RE (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience, 8(11), 872–883. 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW Jr., Cohen SJ, Lora JC, & Rios LM (2016). Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiology of Learning and Memory, 133, 118–128. 10.1016/j.nlm.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Tulving E (1983). Elements of episodic memory. Oxford: Oxford University Press. [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, & Frick KM (2015). Regulation of object recognition and object placement by ovarian sex steroid hormones. Behavioural Brain Research, 285, 140–157. 10.1016/j.bbr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, & Frick KM (2016). Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. Journal of Neuroscience, 36(5), 1483–1489. 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, & Frick KM (2016). Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Hormones and Behavior, 83, 60–67. 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, & Roth BL (2015). DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic tools with therapeutic utility. Annual Review of Pharmacology and Toxicology, 55, 399–417. 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, DiBerto JF, Giguere PM, … Roth BL (2015). A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron, 86(4), 936–946. 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, & Leranth C (2007). Nucleus reuniens of the midline thalamus: Link between the medial prefrontal cortex and the hippocampus. Brain Research Bulletin, 71(6), 601–609. 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, & Frye CA (2008). Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiology of Learning and Memory, 89, 513–521. 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, & Cai JX (2006). Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research, 175(2), 329–336. 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Warburton EC, & Brown MW (2015). Neural circuitry for rat recognition memory. Behavioural Brain Research, 285, 131–139. 10.1016/j.bbr.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Lewis SJ, & Barker RA (2006). Cognitive deficits and psychosis in Parkinson’s disease: A review of pathophysiology and therapeutic options. CNS Drugs, 20(6), 477–505. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, & Ainge JA (2013). Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus, 23(5), 352–366. 10.1002/hipo.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, & Bussey TJ (2004). Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. Journal of Neuroscience, 24(26), 5901–5908. 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, & Alberini CM (2017). Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nature Neuroscience, 20(1), 52–61. 10.1038/nn.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, & Breitner JCS (2002). Hormone replacement therapy and incidence of Alzheimer disease in older women. Journal of the American Medical Association, 288, 2123–2129. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, & Frick KM (2012). Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. Journal of Neuroscience, 32(7), 2344–2351. 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


