Abstract
The sorting receptor SorLA is highly expressed in neurons and is also found in other polarized cells. The receptor has been reported to participate in the trafficking of several ligands, some of which are linked to human diseases, including the amyloid precursor protein, TrkB and lipoprotein lipase (LpL). Despite this, only the trafficking in non-polarized cells has been described so far. Due to the many differences between polarized and non-polarized cells, we examined the localization and trafficking of SorLA in epithelial Madin-Darby canine kidney (MDCK) cells and rat hippocampal neurons. We show that SorLA is mainly found in sorting endosomes and on the basolateral surface of MDCK cells and in the somatodendritic domain of neurons. This polarized distribution of SorLA respectivly depends on an acidic cluster and an extended version of this cluster and involves the cellular adaptor complex AP-1. Furthermore, we show that SorLA can mediate transcytosis across a tight cell layer.
Keywords: intracellular trafficking, adaptor protein, cell polarity, epithelial cell, sorting, AP1, Neurons, SorLA, LR11
INTRODUCTION
The type-1 transmembrane sorting receptor SorLA is a member of the Vps10p-domain family [1, 2]. SorLA is highly expressed in the nervous system during development and adulthood, but is also found in tissues such as the kidney, mammary gland, and intestine. A common feature of these tissues is the presence of and functional dependence upon polarized cells [3]. Polarized cells differ from other cells in their division of the plasma membrane and cytoplasm into separate regions with distinct compositions of proteins, such as apical versus basolateral in epithelial and endothelial cells or axonal versus somatodendritic in neurons. Polarization of cells is critical for a number of processes, including directed cell migration, neuronal signal transmission, and separation of the external milieu from the interior of the organism. Defects are consequently associated with a range of disorders, such as cystic fibrosis [4], Hermansky-Pudlak syndrome [5], familial hypercholesterolaemia [6] and cancer [7].
The trafficking of SorLA in non-polarized cells is mediated by sorting motifs in the cytoplasmic tail. SorLA is mainly intracellular, and at any given time, less than 10% is present at the cell surface [8]. Following essential propeptide-cleavage in the trans-Golgi network (TGN), mature SorLA can follow a direct route to the cell membrane or it can be forwarded to endosomes. Like the mannose-6-phosphate receptor, SorLA transports ligands directly to the endo-lysosomal pathway [9]. Interactions with the retromer complex ensures recycling of SorLA back to the TGN. Additionally, SorLA can mediate fast internalization from the plasma membrane [10]. Till now, SorLA trafficking in polarized cells has not been studied. Polarized trafficking of membrane proteins differs from non-polarized trafficking in a number of ways. Basolateral and somatodendritic trafficking is mainly promoted by cytoplasmic tyrosine-based (YXXØ) and dileucine-based sorting signals [11–16], while apical and axonal trafficking signals are generally believed to be a more heterogeneous group containing both protein motifs and glycosylations as well as attached GPI-anchors [17]. The sorting of many polarized membrane proteins is initiated at the TGN (the direct biosynthetic delivery route), but sorting can also take place by selective endocytic retrieval through recycling endosomes (the indirect route) [18, 19]. In addition, some receptors can traffic between apical and basolateral membranes in a process known as transcytosis.
SorLA mediates trafficking of a diverse range of ligands, such as LpL, amyloid precursor protein and TrkB [9, 20, 21]. Given that several of these proteins are polarized sorted and localized in neurons and epithelial cells [21–23] and transcytosed across epithelial and endothelial cells [24, 25], a description of polarized SorLA trafficking in these cells is highly relevant. In this paper we show that newly synthesized SorLA is localized at the basolateral membrane and in sorting endosomes of epithelial MDCK cells and in the somatodendritic domain of hippocampal neurons. The polarity of SorLA is dependent upon an acidic cluster in MDCK cells and an extended version of this motif in neurons, as well as interactions with the adaptor protein complex AP-1.
RESULTS
SorLA localization in epithelial cells and neurons
To test if SorLA displays polarized localization and trafficking, we used the epithelial MDCK cell line and primary rat hippocampal neurons as models. MDCK cells were grown on Transwell filters in order to induce the formation of tight junctions and obtain a polarized cell structure. The tightness of the tight junctions was followed daily by measuring fluorescein diffusion over the membrane as described in Materials and Methods. It has previously been shown that SorLA is a highly active endocytic receptor, though only a small amount is found on the cell surface. In order to stain the fraction of surface localized SorLA in MDCK cells, we incubated both sides of live SorLA transfectants with goat anti-SorLA at 0°C. Using the well-described polarization marker, E-cadherin (E-cad), we found that SorLA was mainly found on the basolateral membrane where it co-localized with E-cad (Fig. 1A, upper panel). In fixed and permeabilized cells, intracellular SorLA is primarily associated with vesicles in the subapical cytoplasmic area (Fig. 1A, lower panel).
FIGURE 1. Polarization of SorLA in epithelial cells and rat hippocampal neurons.
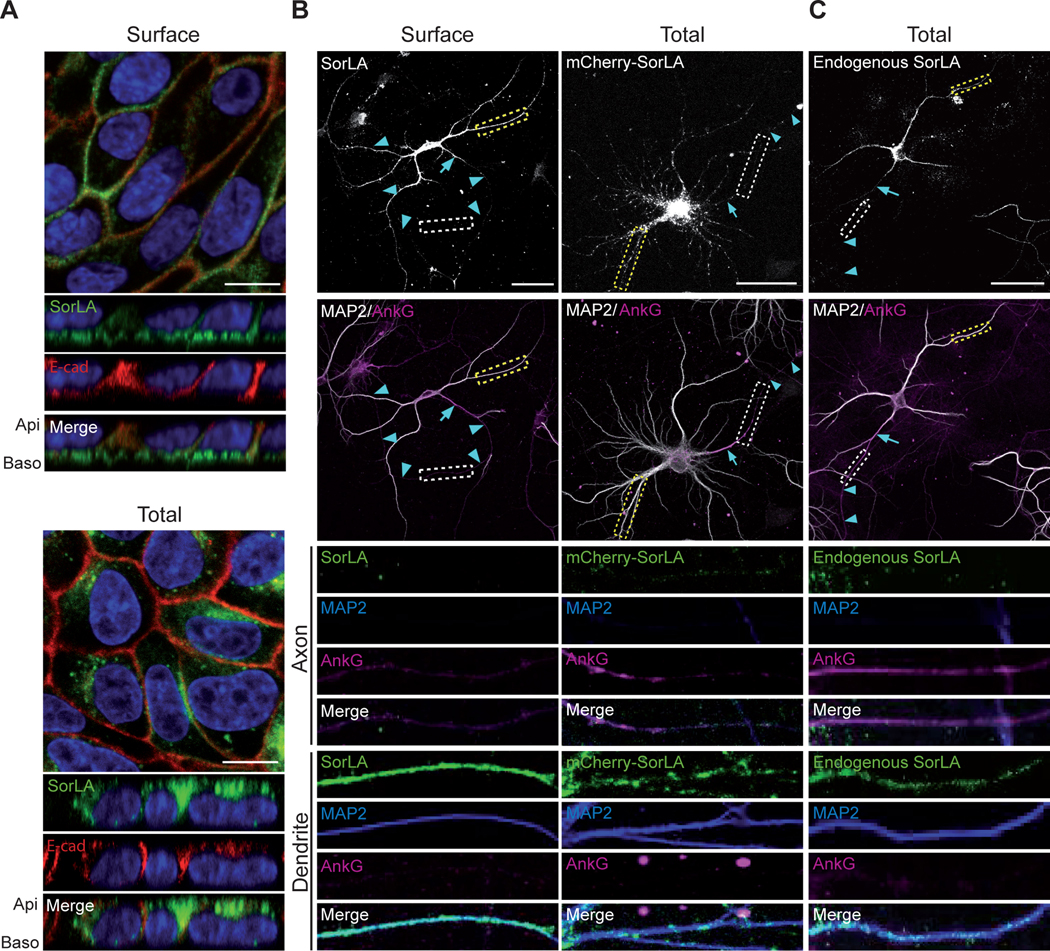
A) The localization of full-length SorLA (green) visualized by immunohistochemical staining in polarized and stably transfected MDCK CCL34 cells. The upper panel shows the surface-associated fraction, while the lower panel shows a total staining. E-cad (red) marks the basolateral membrane. The images are from the middle of a z-stack, and images of the xz-dimension are shown below. B) The localization of full-length or mCherry-tagged SorLA tail in hippocampal rat neurons at DIV 10. The left panel shows the surface-staining of full-length SorLA, while the right panel shows a total staining of mCherry-SorLA. C) Total staining of endogenous SorLA, using a monoclonal antibody (anti-LR11). White and yellow boxes frame segments of the axon and a dendrite, respectively, and enlargements are shown below. MAP2 is used to identify dendrites and AnkG to identify the axon. Arrow: axon initial segment. Arrowheads: axon. Scalebars: 10 μm for MDCK cells and 50 μm for neurons. The close-ups of axons in B and C are rotated to align the part of the axon branching from the soma at the left side.
Like epithelial cells, neurons are polarized, with both the plasma membrane and the cytoplasm divided into an axonal and a somatodendritic part. The surface distribution of neuronal SorLA was visualized in cells transfected with the full-length construct (Fig. 1B, left panel), and for a total staining of SorLA in neurons, we used a chimeric mCherry-SorLA construct, containing an N-terminally placed ER signal peptide fused to the mCherry sequence followed by the complete transmembrane and C-terminal domain of SorLA (Fig. 1B right panel). We observed that the majority of SorLA is restricted from entering the axon identified by the scaffolding protein ankyrin G (AnkG), and is mainly found in the somatodendritic part of the cell, identified by MAP2 (Fig. 1B). In addition, we stained for endogenous SorLA to confirm that the chimera and full length receptors localize in comparable ways (Fig. 1C).
Internalization and subcellular localization of SorLA
To follow the internalization and subsequent intracellular trafficking in MDCK cells, cell surface-associated SorLA receptors were saturated with goat anti-SorLA. We have previously compared these antibodies with single Fab fragments and saw no change in trafficking of SorLA caused by binding of anti-SorLA [10]. After a wash with cold PBS, the cells were either fixed or further incubated with pre-warmed medium at 37°C in order to restart the endocytic machinery. At different time points (5, 15 and 45 min) samples were taken and fixed. As shown in Figure 2A, the basolaterally localized SorLA displays fast internalization whereafter SorLA gathers in perinuclear vesicles. In similar experiments, SorLA’s trafficking after internalization was followed using the subcellular marker EEA1 for early endosomes (Fig. 2B), p230 and MPR300 for TGN, GS15 for late endosomes, lamp1 for lysosomes (not shown), and transferrin receptor (TfR) for common recycling endosomes (CRE) (Fig. 2C). Based on observations from similar experiments in HEK 293 cells [10], we expected to observe a high degree of SorLA colocalizing with EEA1 after clathrin-mediated endocytosis, followed by transport to late endosomes and TGN. After 5 minutes, very little SorLA appeared to co-localize with EEA1. SorLA was found in distinct vesicles; however, the majority of these were localized in the lower part of the cells and overlapped only slightly with the EEA1-positive endosomes. After 30–45 minutes, SorLA had traversed the cells and a minor increase in colocalization with EEA1 was seen (Fig. 2B). The average Manders coefficient increased from 0.38 at 0 minutes to 0.58 after 30 minutes (N>400, P<0.0001). Previously analysis of SorLA trafficking in 293 HEK cells, showed a high degree of co-localization few minutes after internalization [10]. The observed delay in EEA1/SorLA co-localization in MDCK cells could therefore reflect that SorLA is transcytosed to the apical membrane followed by internalization to EEA1 positive endosomes. TfR was used as a marker for CRE. A total staining showed some colocalization of SorLA and mCherry-tagged TfR (Manders coefficient 0.66 at 45 min, N>1700) (Fig. 2C). SorLA has in other cell types been shown to shuttle between endosomes and the TGN [9], but in MDCK cells, only sporadic staining of SorLA was observed in TGN and in lysosomes. In neurons, SorLA is likewise subject to fast endocytosis, and shows colocalization with EEA1 at all time points between 5 and 60 minutes, with the highest degree of overlap after 15 minutes (Manders coeffecient 0.57) (Fig. 2D).
FIGURE 2. Internalization of SorLA.

A) The surface of polarized MDCK cells was labelled with SorLA antibody, and internalization was followed for various periods of time. B) Cells, treated as in A, were also stained for the cellular marker EEA1 (for sorting endosomes). The enlargements and arrows indicates areas of co-localization. C) Cells transfected with SorLA and mCherry-TfR were immunostained against SorLA and mCherry, as a marker of CRE. Arrows indicate areas of colocalization. D) Endocytosis of 20C11 anti-SorLA in neurons after 5–60 minutes. Arrows indicate areas of colocalization with EEA1 in the enlargements shown. Scalebars: 10 μm for MDCK cells and 50 μm for neurons.
An acidic cluster mediates polarization
Previous studies have demonstrated that there are at least four functional sorting motifs in the cytoplasmic tail of SorLA. In 293 HEK cells and SHSY5Y cells, the FANSHY motif has been shown to mediate retrograde transport of SorLA [26], the acidic cluster is involved in internalization as well as TGN/endosome trafficking, whereas the C-terminal four amino acids (MVIA) and the dileucine-like MI motif slightly affects TGN/endosome trafficking [10, 27]. To identify motifs in SorLA’s cytoplasmic domain responsible for polarization in neurons and epithelial cells, we generated a set of truncated and point-mutated SorLA constructs (Fig. 3A). In stably transfected MDCK cells, we found that disruption of the acidic cluster (AC) was enough to hamper the basolateral polarization of surface localized SorLA (Fig. 3B, first column and 3D). Expectedly, this construct also has a significantly slower internalization rate. 41.6% of the AC is internalized after 15 min compared to 97.8% for WT. Complete truncation of the cytoplasmic tail (STOP) blocks the endocytosis of SorLA most efficiently. Only 5.5% is internalized after 15 minutes, and this amount could very well reflect background or nonspecific uptake of the receptor. In primary neurons, the acidic cluster itself is only partially responsible for keeping SorLA somatodendritic, and the mutant consequently has a polarity index of 3.15, which is approximately 20% lower than WT SorLA (4.05), and not significantly different (Fig. 3C and E). Likewise, mutation of a short version of the combined acidic cluster/dileucine-like motif (sACLL) only leads to a very modest reduction of polarity to 2.90. For complete loss of polarity in neurons, the entire acidic cluster in combination with the dileucine-like motif (ACLL) must be changed to alanines, resulting in a polarity index of 1.36, similar to that of the STOP mutant (1.18). This resembles the basolateral trafficking of furin in MDCK cells, which is dependent on a combination of an acidic cluster and a short phenylalanine/isoleucine motif [28]. These results identify an acidic cluster as a common determinant of polarization, while also highlighting differences in the sequence requirements for polarized sorting in neurons and epithelial cells.
FIGURE 3. Motifs involved in SorLA polarization and trafficking.

A) The cytoplasmic domains of wildtype SorLA and the mutants used in the following experiments. The red lines highlight known sorting motifs in SorLA. B) The apical and basolateral surfaces of MDCK CCL34 cells stably expressing the various SorLA mutants was labelled with antibody, whereafter internalization was followed for 0–30 minutes. Green: SorLA, red: E-cadherin, blue: hoechst nuclear stain. Scalebars: 10 μm C) A total staining of various mCherry-tagged SorLA mutants in hippocampal neurons. White box: axon. Yellow box: dendrite (enlargements shown below). MAP2 and AnkG identify dendrites and axon, respectively. Arrow: axon initial segment. Arrowheads: axon. Scalebars: 50 μm. D and E) The polarity index of MDCK cells or hippocampal neurons expressing wildtype or mutant SorLA was calculated. Error bars show the standard error of mean. *: P<0.0001 (compared to WT).
Surface targeting of SorLA is unaffected by the acidic cluster
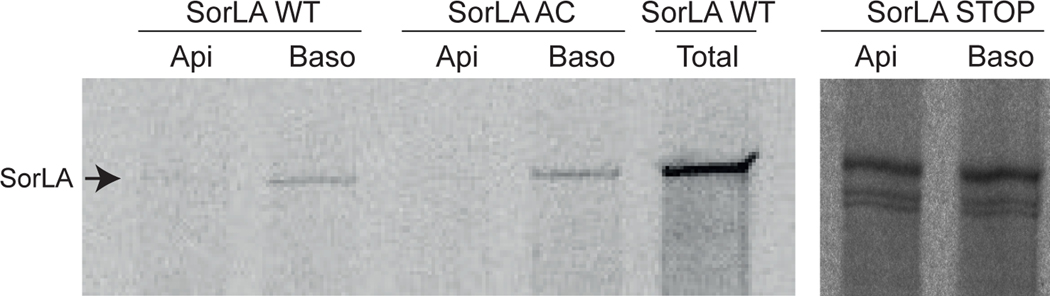
To determine what part of the trafficking itinerary was affected by the acidic cluster, MDCK cells expressing SorLA WT or AC were grown on filters, labelled with 35S and chased for 20 minutes, before SorLA was immunoprecipitated from either the basolateral or the apical side (Fig. 4). Clearly, SorLA WT as well as SorLA AC is found only on the basolateral side, demonstrating that the acidic cluster has no effect on the initial polarization of SorLA. This indicates that newly synthesized SorLA is sent to the basolateral surface, whereafter the acidic cluster affects endocytosis and thereby prevents following transport to the apical surface, retrieval from the apical membrane or both.
FIGURE 4. SorLA is secreted to the basolateral side.
A) Metabolically labeled SorLA (250 kDa, arrowhead) was immunoprecipitated from the apical or basolateral side of MDCK cells expressing SorLA WT, AC or STOP. The two minor bands that are present in the STOP construct, are most likely degradation products, as there are also observed in Western blotting. In addition, the total amount of SorLA was precipitated as a positive control.
AP-1 is essential for neuronal polarization of SorLA
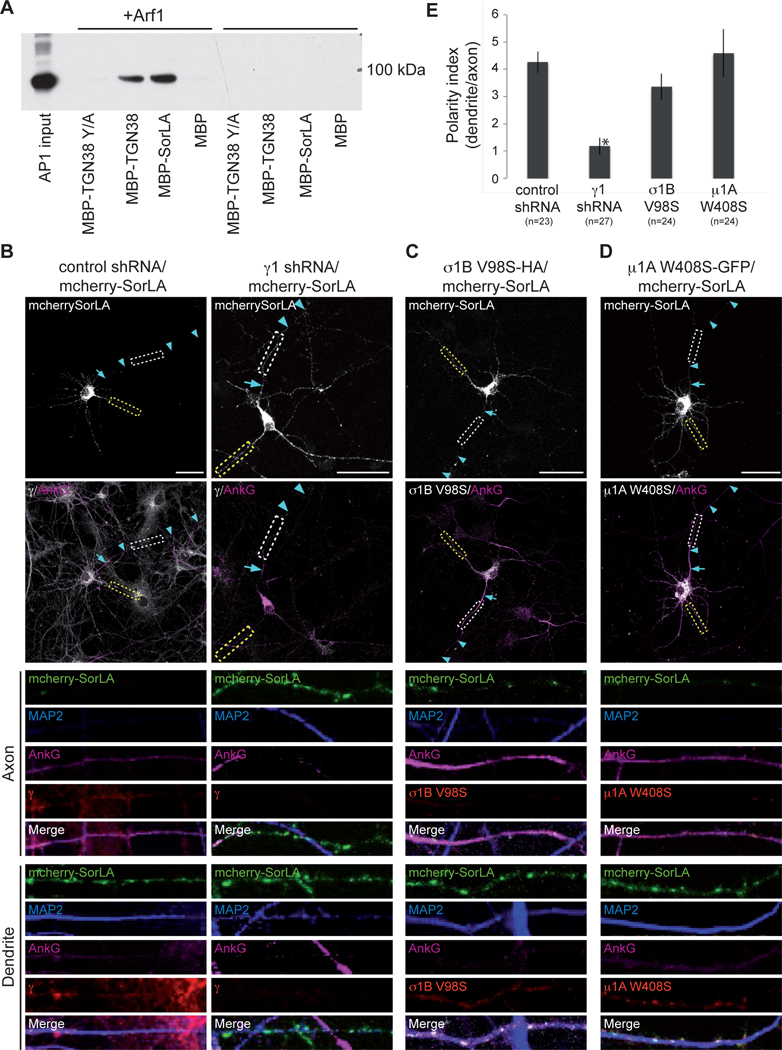
The adaptor protein complex AP-1 has previously been reported to be involved in polarized trafficking [12–14, 16]. Interactions between AP-1 and SorLA were verified using a recently described pull-down assay based on interactions between GST-6His-tagged AP-1 core and SorLA tail fused with maltose binding protein (MBP) in the presence of 6His-tagged Arf1, which greatly enhances the strength of the interactions by inducing a conformational activation of AP-1 [14, 19, 29]. Briefly, the AP-1 core consisted of four subunits, namely human σ1C, N-terminally 6-His-tagged human β1, mouse γ1-GST and mouse μ1A and was expressed in E.coli. Purified AP-1 bound to MBP-fused cargo was collected on amylose beads and identified by immunoblotting with anti-GST. TGN38, which interacts with AP-1 through a YXXØ-type motif, was used as a positive control, and the mutant Y/A as a negative control. We were able to show strong interactions between AP-1 and both SorLA tail and TGN38 in the presence of Arf1 (Fig. 5A). We would have liked to test the binding of SorLA mutants to AP-1, but were unfortunately unable to purify MBP-tagged mutants due to a very high degree of cleavage of the point-mutated SorLA tails. Due to the non-canonical appearance of the motif mediating polarization of SorLA, we decided to test several of the AP-1 subunits, which are known to interact with different types of sorting motifs. AP-1 generally recognizes tyrosine-based (YXXØ) and dileucine-based (D/EXXXLL/I) motifs. Tyrosine-based motifs bind to the μ-subunit, while dileucine-based motifs bind to a combination of γ and σ [16, 30]. γ1 shRNA was used to knock down the entire AP-1 complex, and as expected, this led to complete de-polarization of SorLA, showing the importance of AP-1 for SorLA polarized trafficking (Fig. 5B and E). The involvement of the single subunits σ and μ in SorLA binding was tested by overexpression of dominant negative mutants that are incorporated into AP-1 complexes, but are unable to bind ligands. μ1A W408S has previously been shown to be essential for binding of proteins with YXXØ-type motifs [13]. Likewise, the σ1B V98S is critical for interactions with D/EXXXLL/I-type motifs [14]. When SorLA was co-transfected with the dominant negative σ1B V98S subunit, we saw a modest decrease of polarity (approximately 20%), while co-expression with dominant negative μ1A W408S had no effect.
FIGURE 5. SorLA interacts with AP-1.
A) Purified wildtype MBP-fused SorLA tail was used to pull down purified AP-1A core complex in the absence and presence of Arf1. Binding of the core complex was analysed by SDS-PAGE and western blotting with antibodies to GST to detect the GST-tagged γ-subunit of the AP-1 complex. MBP-TGN38 and MBP-TGN38 Y/A are positive and negative controls, respectively. AP-1 input represents the amount of AP-1 complex used for the pull-down assay. B) Hippocampal neurons were transfected with mCherry-SorLA and control-or γ1-shRNA at DIV 4 and fixated at DIV 8. C) Neurons transfected with μCherry-SorLA and HA-tagged σ1B V98S. D) Neurons transfected with mCherry-SorLA and GFP-tagged μ1A W408S. White and yellow boxes frame axon and dendrite, and enlargements are shown below. Arrows indicate the axon initial segment, and arrowheads show the axon. Scalebars: 50 μm. E) Polarity index of the cells in B, C, and D. Error bars show the standard error of mean. *: P<0.0001 (compared to control or WT).
SorLA can mediate transcytosis
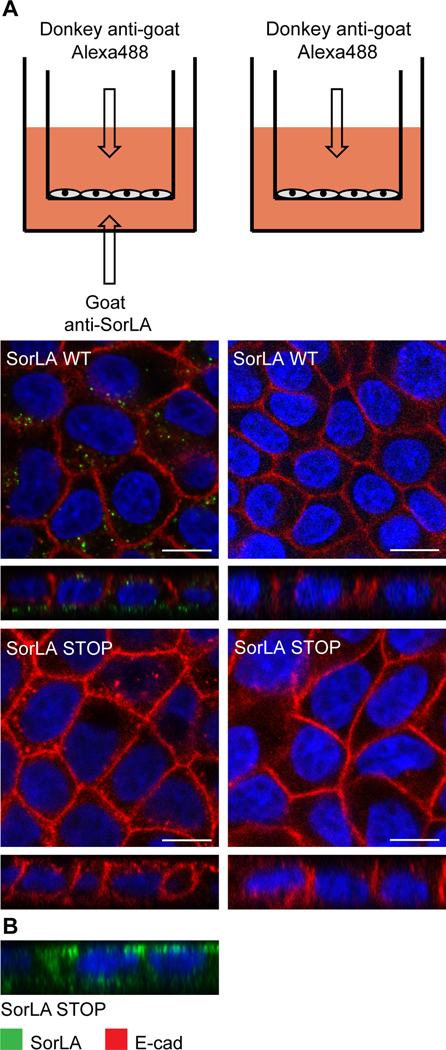
The experiments examining internalization and intracellular trafficking of SorLA in MDCK cells demonstrated that SorLA departs from the basolateral membrane of MDCK cells, after which a fraction ends up in early endosomes. Due to the close relationship between endocytosis and transcytosis, and because retrograde receptors are segregated from recycling receptors in the early endosome, we wanted to test if SorLA can go all the way from the basolateral to the apical side and thereby has the ability to function as a transcytotic receptor. Ideally, we would like to follow the natural SorLA ligand LpL in a tight polarized MDCK barrier. Due to the sensitive dimeric nature of LpL that is easily destroyed on labeling, we designed a setup, where we added goat anti-SorLA to the basolateral chamber of a tight cell layer and Alexa-488-labeled donkey anti-goat to the apical chamber. If primary anti-SorLA is subjected to transcytosis it would bind the labeled secondary antibody on the apical side, after which it would be internalized. After 90 minutes of incubation, vesicles were visible in the SorLA-transfected MDCK cells, indicating that SorLA can mediate transcytosis (Fig. 6A, left panel). The tightness of the cell layer was tested before the experiments using fluorescein, and to make sure that the observed vesicles were not a trace of this dye and that the secondary antibody was not internalized itself, the experiment was repeated without primary anti-SorLA (Fig. 6A, right panel). A labelled ligand would have allowed us to quantify to rate of transcytosis. Unable to do this, we instead counted the number of vesicles, and found on average more than 10 per cell in SorLA WT cells with primary and secondary antibody (N=1563) and less than 0.015 in SorLA WT cells without primary antibody (N=1513) (P<0.0001). As an additional control for the tightness of the monolayer, and to show that the added anti-SorLA was actively transported by SorLA, the experiment was also repeated with the SorLA STOP construct, which is unable to internalize (Fig. 6A, lower part). Figure 6B is a simultaneous total staining of the SorLA STOP transfected cells to verify that the cells actually express this SorLA construct. Staining are seen on the apical as well as the basolateral membrane, confirming the fact that SorLA STOP is observed in approximately equally amount on each side (Fig. 4).
FIGURE 6. Basolateral to apical transcytosis in MDCK cells.
A) MDCK-I cells stably transfected with WT SorLA and SorLA STOP were incubated at 37° C with goat anti-SorLA at the basolateral side and donkey anti-goat 488 at the apical side (left panel) or without goat anti-SorLA as control (right side). The corresponding experimental setup is shown at the top. The SorLA signal obtained in cells expressing wildtype SorLA shows that SorLA can mediate transcytosis across a tight cell layer. B) A membrane staining made simultaneously demonstrates that the SorLA-STOP cells were expressing SorLA. Scalebars: 10 μm.
LpL is known to be present in milk, although its function there remains to be elucidated. LpL is secreted from the epithelial cells of mammary gland tubulo-alveolar lobes as a part of casein micelles and possibly also of milk fat globules. It has been generally assumed that the main source of LpL in the mammary gland are the mammary epithelial cells [31], but the enzyme is also synthesized by underlying parenchymal cells, such as adipocytes and other cell types as reported for murine mammary glands [32].Thus both secretion of LpL produced in the mammary epithelial cells and active transcytosis through the epithelial cells of LpL from nearby sources may occur. To determine whether SorLA can be involved in transcytosis of LpL across a physiological barrier, we first tested for SorLA expression in the epithelial cells in the lactiferous ducts of mammary glands from mice. Paraffin sections from WT and SorLA knockout mice were stained using goat anti-SorLA, demonstrating a strong specific staining in the epithelium (Fig. 7A). The absence of SorLA in the mammary gland tissue from SorLA knockout mice was furthermore verified by western blotting (Fig. 7B). Next, lactating WT and SorLA knockout mice were anesthesized at day 3 post partum. Oxytocin was injected subcutaneously to start the milk ejection reflex, and 50–100 μL milk were collected from each mouse. The LpL activity was measured from each mouse in triplicates (Fig. 7C). As demonstrated in the histogram, a significantly reduced amount of LpL is secreted into milk in SorLA knockout mice. We have previously shown that SorLA binds newly synthesized LpL in the TGN and transports it directly to late endosomes and lysosomes, whereby it escapes the cell surface. This leads to a significant decrease in LpL activity [9]. If SorLA was presumed to encounter LpL in the TGN of mammary gland epithelial cells, we would therefore expect it to direct LpL to the endo-lysosomal system, and the activity would consequently decrease. Instead, we see an increased activity, and due to the fact that the transcytotic pathway does not cross the TGN, we believe that the increased LpL activity in milk from WT mice compared to SorLA knockout mice represents SorLA mediating transcytosis of LpL secreted by underlying parenchymal cells. We assume that the lower level of LpL activity in milk from SorLA knockout mice represents the amount of LpL produced in the epithelium.
FIGURE 7: SorLA transcytosis in mammary epithelial cells.
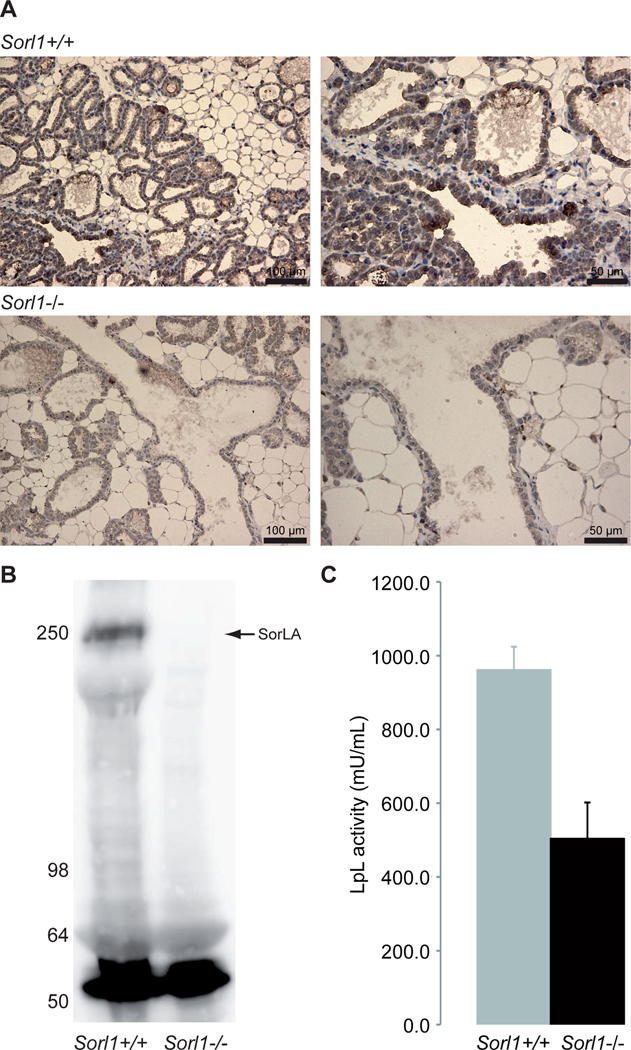
A) Lactating WT and SorLA knockout (−/−) C57BL/6 mice with three days old pups were PFA perfused, and the mammary glands were removed and embedded in paraffin. 2 μm thin sections were stained with goat anti-SorLA and donkey anti-goat-HRP. Nuclei were stained using Mayer’s hematoxyline. B) Western blot of mammary tissue isolated from lactating WT and SorLA knockout mice at day 3 post partum, blottet with antibody against SorLA (mouse anti-LR11) and developed using ECL. The arrow indicates the position of SorLA, and the band around 55 kDa is most likely IgG. C) WT (n=15) and SorLA knockout (n=8) lactating mice were subjected to anesthesiology at day 3 post partum. 50–100 μl milk were milked form each mice, and the LpL activity was measured from each mouse in triplicates. As demonstrated in the histogram, a significant reduction of LpL is secreted to the milk in SorLA knockout mice, indicating that SorLA is involved in the transcytosis of LpL. Error bars show standard error of mean. P<0.0005.
DISCUSSION
SorLA is expressed in a range of polarized cells, especially in the nervous system. Our results demonstrate that the receptor is excluded from the apical membrane and found mainly in sorting endosomes as well as on the basolateral membrane domain in cultured MDCK cells. Likewise, SorLA is found in the soma and dendrites of hippocampal neurons, and is largely excluded from the axon. While trafficking to the basolateral membrane of epithelial cells is relatively well described, polarized trafficking in neurons remains poorly understood. Discovering that basolateral and somatodendritic trafficking resemble each other and share some mechanisms has contributed to our understanding of neuronal polarized trafficking. The tyrosine-based YXXØ-type motifs and dileucine-based D/EXXXLL/I-type motifs mediate basolateral trafficking in epithelial cells and likewise target proteins to the somatodendritic domain of neurons [13, 14]. In addition, the existence of several non-canonical sequences, including combinations of acidic cluster and smaller motifs, indicates that other mechanisms may also be causing basolateral sorting [28, 33–35].
We show that the polarization of SorLA in epithelial cells is dependent upon a cluster of acidic residues. The initial polarization of newly synthesized SorLA occurs independent of the acidic cluster, as was demonstrated using metabolic labeling and immunoprecipitation. Hence, we assume the acidic cluster affects endocytosis and thereby the transport from the basolateral to the apical membrane and/or retrieval from the apical membrane (Fig. 8). In neurons, surprisingly, disruption of the acidic cluster was not enough to abolish the somatodendritic polarity of SorLA. Neither was disruption of the dileucine-like MI-motif, and we had to combine these in order to lose the polarity of SorLA completely. This shows first of all that a pure acidic cluster can mediate basolateral polarization, which has not been reported before. The closest resemblance may be the low-density lipoprotein receptor or furin, which are basolateral due to the combination of a tyrosine-based motif and a short acidic cluster or an acidic cluster and phenylalanine/isoleucine, respectively [28, 36]. The acidic cluster of SorLA has previously been shown to mediate endocytosis in CHO cells, while the MI-motif was assigned little or no function [10]. Furthermore, this is, to our knowledge, the first time a non-canonical motif has been shown to facilitate somatodendritic polarity in neurons. The discrepancy between the motifs indicates that despite the close relationship between basolateral and somatodendritic polarity, there are still differences.
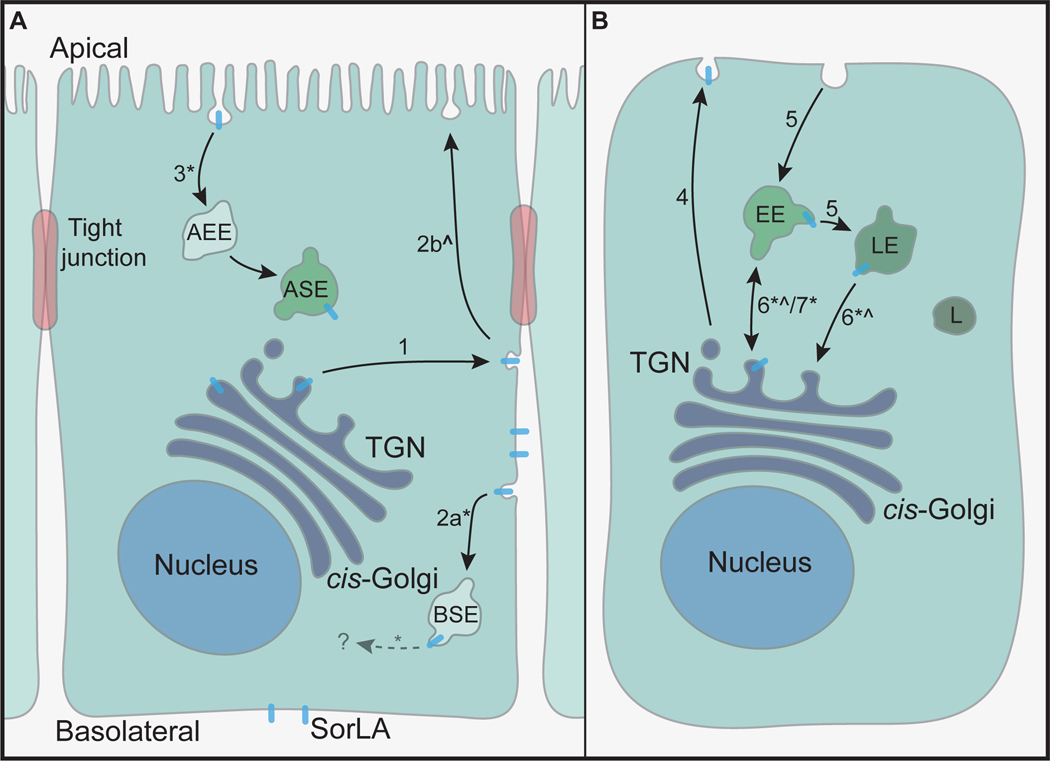
FIGURE 8. SorLA localization and trafficking in non-polarized and polarized cells.
SorLAs trafficking in polarized (A) compared to non-polarized (B) cells. In polarized cells, SorLA is secreted to the basolateral plasma membrane (1), a transport that may go through common recycling endosomes. The initial polarized transport is independent of the acidic cluster. Afterwards, SorLA is internalized, either to basolateral sorting endosomes (2a) and the common endosomal system, or sent via specialized transcytotic endosomes to the apical membrane (2b), as indicated by the relatively low polarity index. From the apical membrane, proteins are internalized via EEA1-positive apical early endosomes to apical sorting endosomes (3). In non-polarized cells, SorLA is either secreted (4) and afterwards internalized to the endosomal system (5) and returned to the TGN for recycling (6) or sent directly from the TGN to the endosomal system (7). Approximately 10% is localized at the plasma membrane at steady-state. * indicates a step where AP1 is believed to be involved, while ^ indicates a step possibly involving Vps35.
Abbreviations: AEE (apical early endosomes), EE (early endosomes), LE (late endosomes), TGN (trans Golgi network), BSE (basolateral sorting endosomes), ASE (apical sorting endosomes), L (lysosomes).
Tyrosine-based and dileucine-based motifs are generally recognized by members of the heterotetrameric adaptor protein family, and the clathrin-associated AP-1 has hence been shown to mediate both basolateral and somatodendritic sorting of transmembrane receptors, including the transferrin receptor and several neuron-specific glutamate receptors [13, 14]. Three out of the four subunits of AP-1 have more than one isoform, giving rise to many possible combinations. Much attention has been focused on the μ-subunits, which occur as two isoforms named μ1A and μ1B. These two isoforms define AP-1 complex variants known as AP-1A and AP-1B. While AP-1A is ubiquitous, AP-1B is only expressed in epithelia, and they appear to have distinct as well as overlapping functions [18, 19, 37]. Our results demonstrate the ability of AP-1A to bind SorLA and to mediate biosynthetic trafficking of SorLA in neurons. Given that expression of a dominant negative μ1A subunit in neurons had no effect on the polarity of SorLA, we do not expect AP-1B to have a distinct effect, if any, on SorLA trafficking in epithelial cells.
It was previously shown that the SorLA cytoplasmic tail can pull down the AP γ1 subunit from brain lysates[10]. We confirmed SorLA’s binding by its ability to pull down the entire AP-1 core complex. In addition, SorLA’s dependence upon AP-1 for polarization was demonstrated with knockdown using shRNA. It should be emphasized that due to the low transfection rate (which is typical for primary neurons) it is not possible to quantify the rate of the shRNA knockdown of the single neuron. The detected effect could be more pronounced than observed in our experiments. Due to the non-canonical nature of the sorting motif, it was not clear to us which AP-1 subunit was responsible for interactions with SorLA. In order to determine the subunits responsible for the interaction, we therefore co-transfected SorLA with the dominant-negative subunits μ1A W408S or σ1B V98S. Clearly, the mutation of the μ1A subunit, which generally binds YXXØ-type motifs, had no effect on the polarity of SorLA. Co-expression with dominant-negative σ1, on the other hand, lead to a small decrease in the polarity, but not to complete non-polarization. Binding of dileucine-based motifs to AP-1 has been reported to take place at the juncture of the γ and σ subunits and therefore involve both of these [16]. Based on our current results, we suggest that SorLA can bind to a combination of the AP-1 γ and σ subunits in a way that resembles the dileucine-based motifs, but a crystallographic study would be able to definitively determine this. The closely related receptor sortilin was recently shown to be able to bind to μ as well as σ and γ subunits [38]. It is therefore possible that SorLA can bind AP1 in a similar way. This would explain the relatively weak effect we see of co-transfecting SorLA with dominant negative σ. Furthermore, it could explain why mutation of the acidic cluster alone is insufficient to disrupt the polarity of SorLA. A new study, possibly combining yeast 2-and 3-hybrid experiments, would be needed to clarify this.
Acidic clusters were discovered as a sorting motif in non-polarized cells in 1995 and polarized cells in 1999 [28, 39]. Their interactions with adaptor proteins is still discussed, and while some suggest a direct interaction with AP-1 [40, 41], others claim that interactions with phosphofurin acidic cluster-sorting protein 1 (PACS) mediate the binding between AP-1 and cargo [42]. We have previously shown that while PACS is able to bind SorLA, it has no effect on internalization, and knockdown does not affect the beta-hexosaminidase sorting by a MPR300/SorLA chimera [10]. Later reports have demonstrated that PACS, nonetheless, seems to affect the retrograde transport of SorLA from endosomes to the TGN [43]. Hence, we cannot rule out that PACS may play a role in the interaction between AP-1 and SorLA.
According to our studies, SorLA is initially sent to the basolateral, but not the apical membrane, as demonstrated using metabolic labeling and immunostaining. Internalizations showed that after the following endocytosis from the basolateral membrane, a fraction of SorLA eventually goes to EEA1-positive endosomes, while other fractions colocalize with TfR-positive CRE. Surprisingly, almost no overlap was found with late endosomes and TGN, which are typical destinations for canonical retrograde transport receptors. We therefore tested SorLA’s ability to mediate trafficking from the basolateral to the apical membrane. Due to the fragile nature of LpL and other known SorLA ligands, we demonstrated transcytosis using a monoclonal SorLA antibody, but supplemented this with an experiment showing decreased transport of LpL across the epithelial membrane of the mammary gland of SorLA knockout mice compared to WT mice. It is known that SorLA interacts with the retromer Vps26-Vsp29-Vps35 core complex, and we found some degree of co-localization with Vps35 (not shown), which previously has been used as a marker of transcytosis of the polymeric immunoglobulin receptor [26, 44]. However we were not able to verify this observation, due to lack of functional antibodies recognizing canine Vps26 and 29. We therefore believe that SorLA has the potential to be involved in transcytosis of ligands in vivo, but retromer involvement in this remains to be demonstrated.
According to our experiments, SorLA is mainly found in the basolateral endosomes and membrane in epithelial cells, and the corresponding somatodendritic part of neurons. The calculated polarity index (Basolateral:Apical, app. 4:1) for WT SorLA is significantly higher than for cells expressing the non-polarized SorLA STOP mutant (Basolateral:Apical, app. 1:1). At the same time, the polarity indexes are markedly lower than those reported for other somatodendritic proteins, such as the transferrin receptor and the glutamate receptors NR2A, NR2B, GluR1 and GluR2, which all have polarity indexes ranging from 6.6 to 11.8 [13]. The polarity indexes reflect the distribution of SorLA at a given time, and is thus a snapshot. The relatively low polarity index could therefore both be caused by a higher degree of traffic to the axon or a very fast retrograde transport of SorLA in the axon. Either way, this indicates that SorLA primarily plays roles in the basolateral/somatodendritic parts, but as we see it, does not exclude the possibility of SorLA also carrying out a function in the apical/axonal parts of cells.
In summary, we show for the first time that SorLA is basolateral/somatodendritic when expressed in epithelial or neuronal cells. The polarity is caused by the presence of an acidic cluster and additional C-terminal amino acids in SorLA’s cytoplasmic domain and its interaction with AP-1 (Fig. 8). Our data thus describe a novel motif for mediating polarized traffic. In addition, it points out that there may be subtle differences between otherwise similar mechanisms of trafficking, despite the likelihood that the same adaptor is involved in the transport. The ability of SorLA to mediate transcytosis opens up the possibility that SorLA has new physiological roles yet to be described. In future studies it will be important to further investigate SorLA’s potential role in ligand transport, both intracellular and transcytotic. This includes transport across the blood-brain barrier, where SorLA is expressed. Furthermore, knowledge of the structural detail behind the binding of the acidic cluster to AP-1 will elucidate the nature of this interaction, and, most likely indicate what causes the difference between the motif needed for polarity in epithelial cells and neurons.
MATERIALS AND METHODS
Cell culture, transfection and antibodies
CCL-34 MDCK (NBL-2) cells (ATCC, Manassas, VA) were maintained in MEM (Gibco, Carlsbad, CA) with 50 U/mL penicillin (Invitrogen, Carlsbad, CA), 50 μg/mL streptomycin (Invitrogen), 10% fetal calf serum (Invitrogen) and 2 mM glutaMAX™ (Invitrogen). MDCK_frt (gift from R. Fenton, Aarhus University, Denmark) were maintained in DMEM with 50 U/mL penicillin (Invitrogen, Carlsbad, CA), 50 μg/mL streptomycin (Invitrogen), 100 μg/ml zeocin (Invitrogen) and 10% fetal calf serum (Invitrogen). CCL-34 cells were transfected with wildtype or mutant SorLA constructs using FuGene (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Stable clones were selected by incubation in growth medium containing 100 μg/mL zeocin (Invitrogen), and protein expression was evaluated by western blotting and immunofluorescence. MDCK_frt cells were transfected with Metafectene pro (Biontex, München, Germany) according to the manufacturer’s protocol. Stable clones were selected with 50 μg/ml Hygromycin B (InvivoGen, Toulouse, France).
Primary cultures of rat hippocampal neurons were prepared from Sprague-Dawley rats on embryonic day 18, as previously described [45]. Briefly, hippocampi were dissected, dissociated with trypsin and plated onto poly-L-lysine treated coverslips in DMEM with 10% horse serum at a density of 30,000 cells per coverslip. After 4 hours, the culture medium was changed to Neurobasal medium (Gibco) with B-27 (Gibco) and Glutamax (Gibco). Cells were transfected with various constructs at day in vitro (DIV) 4 using Lipofectamine 2000 (Invitrogen), and analyzed at DIV 10 unless otherwise stated.
The primary antibodies used were the following: a polyclonal rabbit antibody against soluble SorLA, polyclonal goat anti-sorLA, monoclonal mouse anti-sorLA were all produced by the authors and has previously been described in [9, 10]. Monoclonal mouse anti-SorLA (Anti-LR11, BD Biosciences), mouse anti E-cad (rr1, Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit anti-MAP2 (Santa Cruz Biotechnology, Dallas, TX), goat anti-AnkG (P-20, Santa Cruz Biotechnology), mouse anti-γ-adaptin (BD Biosciences, San Jose, CA), rabbit anti-EEA1 (ab2900, Abcam, Cambridge, England), rabbit anti-mCherry (GTX59788, GeneTex, Irvine, CA), rabbit anti-Lamp1 (ab24179, Abcam), mouse anti-P230 (BD 611281, BD Biosciences), mouse anti-gs15 (BD 610961, BD Biosciences), and goat anti-Vps35 (EB06268, Everest Biotech, Oxfordshire, UK). As secondary antibodies, Alexa Flour-tagged antibodies (Invitrogen) were used. In addition, Hoechst 33342 nucleic acid stain (Sigma-Aldrich, St. Louis, MO) was used.
Constructs and vectors
The coding sequence of SorLA was expressed in eukaryotic cell lines using the expression vectors pcDNA3.1/(−)zeo (Invitrogen) and pcDNA5/FRT (Invitrogen). Constructs were inserted into the full length pcDNA5/FRT construct using a unique NsiI site in the SorLA coding sequence. mCherry-TfR was expressed using the pPAmCherry vector (Addgene, Cambridge, MA) The mCherry-SorLA tail was expressed using the pSECTAG2b/hyg vector (Invitrogen) which encodes a signal peptide. Insertion of the mCherry coding sequence was followed by insertion of the transmembrane and cytoplasmic domains of SorLA (amino acids A2136-A2214 (accession number U60975)). SorLA mutants were generated by either site-directed mutagenesis (Quikchange, Agilent, Santa Clara, CA) or chemical synthesis (Eurofins, Ebersberg, Germany), and resulting fragments were verified by DNA sequencing. ShRNA encoding plasmids targeting γ1 were a kind gift from T. Ryan (Weill Cornell Medical College, New York, NY). μ1A-GFP was expressed using pEGFP-N1 and σ1B tagged at the C-terminus with three copies of HA (σ1B –HA) was expressed in the pCI-neo (Promega, Fitchburg, WI) vector as described previously [14]. The MBP-tagged proteins used for pull-down were expressed in a modified pHis2 vector encoding MBP [29], and plasmids encoding the recombinant AP-1 core and Arf1Δ1−16-Q71L have been described previously [19, 29].
Polarization and evaluation of MDCK cells
MDCK cells (CCL-34 and _frt transfectants) were grown on 12 mm Millicell-HA filters with a pore size of 0.45 μm (Millipore, Billerica, MA). 105 cells were seeded on each filter and subsequently cultured in 24-well trays for 2–4 days to obtain a confluent monolayer. Tightness of the cell layer was evaluated each day with a modified fluorescein leakage test [46] where 10 μg/L sodium-fluorescein (Sigma-Aldrich) was added to the apical side of cells for 15 min. at 37°C before measuring the degree of leakage into the basolateral media fraction using an ELISA reader. The cell layer was considered tight when the leakage was less than 5% compared to a control filter without cells. Cells grown on 0.4 μm PET 12 well hanging culture inserts (Millipore) were evaluated by trans-epithelial electrical resistance measurements. Briefly, filters were placed in an Endohm-12 culture cup (World Precision Instruments, Sarasota, FL), and resistance was measured in triplicates according to manufacturers instructions using an EVOM voltohmmeter (World Precision Instruments). Cells with background-substracted resistances above 100 Ω were considered tight.
Immunofluorescence microscopy
Polarized MDCK cells were fixed with 4% paraformaldehyde in PBS, quenched with 50 mM ammonium chloride in PBS, and permeabilized with 0.25% saponin in PBS, followed by incubation with relevant antibodies (see antibody section). The filters supporting the MDCK cells were cut out and mounted onto glass slides using DAKO fluorescence mounting media (DAKO, Glostrup, Denmark). Unless otherwise indicated, neurons were fixed on DIV 10, using PBS with 4% paraformaldehyde and 4% w/v sucrose, followed by permeabilization with 0.2% Triton X-100 in PBS containing 1 mM MgCl2, 0.1 mM CaCl2 (PBS+), and 0.2% gelatin, and blocking in PBS+ with 0.2% gelatin. Cells were incubated with primary and afterwards secondary antibodies in PBS+ with gelatin, and mounted using Fluoromount G (Electron Microscopy Sciences, Hatfield, PA). The cells were analyzed on Zeiss LSM 710 or 780 confocal microscopes (Carl Zeiss, Heidelberg, Germany) using a 40x (N.A. 1.4) or 63x objectiv (N.A. 1.2), and images were evaluated using the ImageJ software [47].
Internalization and transcytosis
For internalization, MDCK cells were placed on ice for 30 min, incubated with goat anti-sorLA added to both sides of the filter for 90 min at 0°C, washed with cold PBS, and returned to the incubator for various times before the cells were fixed and stained with relevant antibodies. For transcytosis, the primary antibody was added to the basolateral side of a tight cell layer while an appropriate secondary antibody was added to the apical side. The cells were incubated for 90 min at 37°C, washed twice in PBS, and fixed as described above. In neurons, the surface was stained with monoclonal 20C11 anti-SorLA added to fixed, but unpermeabilized cells. For endocytosis, 20C11 was added directly to the medium for 5–60 minutes at 37°C, followed by wash and fixation. Colocalization analysis and quantification was performed using the Imaris 8.0.1 software (Bitplane, Belfast, UK) or the ZEN 8.1 software (Carl Zeiss).
Metabolic labeling and immunoprecipitation
MDCK cells expressing SorLA WT or AC were grown on filters. Tightness was tested daily using trans-epithelial electrical resistance measurements. When cells were tight, they were labeled metabolically. Briefly, cells were washed and preincubated for 30 min. in cysteine-and methionine-free modified Eagle’s media (Sigma) with 2% dialyzed fetal bovine serum. Biolabeling was performed in the same medium using 11.1 MBq/mL [35S]L-cysteine and [35S]L-methionine (pro-mix, GE Healthcare, Buckinghamshire, UK) for 20 minutes at 37°C. After this chase period, the cells were then transferred to ice for 30 min. to arrest vesicular receptor trafficking before incubation with polyclonal rabbit anti-SorLA at either the basolateral or the apical side for 90 min. on ice. Next, cells were washed and 2 mM of the cross linker dithiobis(succinimidyl propionate) (DSP, Life Technologies, Carlsbad, CA) was added for 60 min. on ice before 100 mM Tris-HCl, pH 7.5 was added for 15 min. to quench the reaction. Cells were washed and lyzed for 10 min. in 1% Triton X-100, 150 mM NaCl and 50 mM Tris-HCl, pH 7.5. For immunoprecipitation, lysates were incubated for 2 hours at 4°C with 75 μL GammaBind-G-sepharose beads (GE Healthcare) that had been preincubated with pure medium or polyclonal anti-SorLA (total lysate, as positive control). The beads were washed five times in PBS with 0.05% Tween-20 and prepared for SDS-PAGE by boiling for 3 min. in 50 μsL reducing sample buffer (10 mM dithiothreitol and 2.5% SDS). Gels were developed using the LAS-3000 system (Fujifilm, Stamford, CT).
Protein purification and pull-down assay
MBP-fused cargos TGN38 (WT and Y/A mutant) and SorLA cytoplasmic domain, Arf1Δ1−16-Q71L, and AP-1 complex were purified as described earlier [14, 19, 29]. Briefly described, protein was first purified from bacterial lysate using Ni-NTA beads (Qiagen). Additional affinity purification was performed on glutathione-Sepharose 4B resin (GE Healthcare) in the case of AP-1 complex. Further purification of all proteins was performed using size exclusion chromatography on ÄKTA (GE Healthcare). Purified proteins were frozen in liquid nitrogen and stored at −80 °C till used. Interaction of cargo proteins with AP-1 complex in the absence or presence of Arf1Δ1−16-Q71L was monitored using a pull down assay [14, 19, 29]. In brief, 20 μg of MBP-tagged cargo protein was immobilized on amylose resin (50 μL) (New England Biolabs, Ipswich, MA) in immobilization buffer (20 mM Tris.HCl pH 7.4, 200 mM NaCl, 0.3 mM TCEP supplemented with Complete protease inhibitor cocktail (Roche)). The resin was washed with immobilization buffer, and incubated with 0.05 μM of AP-1 complex precleared on amylose resin, in immobilization buffer supplemented with 0.25 % v/v Triton X-100 and 0.15% w/v BSA, in the absence or presence of 10 μM Arf1Δ1−16-Q71L and 1 mM GTP overnight at 4 °C. The pull-down was washed four times with immobilization buffer supplemented with 0.1 % v/v Triton-X-100, and was subjected to SDS-PAGE followed by western blotting.
Mouse tissue staining
WT C57BL/6N and SorLA knock out mice (previously described in [48]) were anaesthetized with isoflurane and in vivo perfused with 4% paraformaldehyde through the left ventricle, whereafter relevant tissues were collected and post-fixed in 4% paraformaldehyde for one hour. The tissues were dehydrated in ethanol and xylene, infiltrated and embedded in paraffin, and cut in 2 μm sections. Tissue sections were collected on superfrost+ slides (Hounisen, Skanderborg, Denmark) and dried at 60°C.
Tissue was deparaffinated in xylene and ethanol, blocked in methanol with hydrogen peroxide, and rinsed in ethanol and water. Before staining, sections were boiled in TEG-buffer, pH 9, quenched with 50 mM ammonium chloride and blocked in 1% BSA, 0.2% gelatin and 0.05% saponin in PBS. The tissue was incubated with relevant primary and afterwards HRP-conjugated secondary antibodies (DAKO) in 0.1% BSA and 0.3% Triton X-100 in PBS. Antibodies were detected with di-amino benzedine (Kem-En-Tec, Taastrup, Denmark) and hydrogen peroxide, and nuclei were stained with Mayers hematoxylin (VWR, Søborg, Denmark). The sections were finally dehydrated in ethanol and xylene and mounted with Eukitt mounting medium (Sigma-Aldrich) The tissue sections were analyzed with a Leica phase contrast microscope using 20x (N.A. 0.25) or 40x (N.A. 0.4) objectives (Leica, Solms, Germany).
Collection of mouse milk samples
Mouse milk samples were collected on day 3 post partum. Prior to collection, the mice were removed from their pups and fasted for 90 min. The mice were anesthetized by isoflurane inhalation before 250 μL 3 U/mL oxytocin (abcam) was injected intraperitoneally. After 5 min. the mice were milked using a glass pipette connected to an electric pump (Medela, McHenry, IL). The milk was kept on ice during the milking procedure and afterwards immediately frozen at −80°C. Each mouse was milked only after their first birth to avoid repeaters.
LpL activity measurements
LpL activity was measured as previously described [49]. Briefly, 2 μL mouse milk was incubated with 200 μL of a solution containing a phospholipid-stabilized emulsion of soybean triacylglycerols, with the same composition as Intralipid (10%, Fresenius-KABI, Uppsala, Sweden), into which 3H-labeled triolein had been incorporated by the manufacturer. PMFS-treated, heat-inactivated rat serum was present as a source of apolipoprotein CII (5%), BSA as a fatty acid acceptor (6%), heparin to stabilize the lipase (3.33 U/mL), 0.1 M NaCl, and 0.15 M Tris-HCl, pH 8.5. Incubations were performed in a water-bath at 25°C and exact time was noted. Reactions were stopped by addition of a mixture containing isopropanol, heptane and sulfuric acid (40:48:3.1) and water. After vortexing, the heptane phase was mixed with alkaline-ethanol and heptane. At the end, the amount of labeled fatty acids in the lower phase was measured in a scintillation counter (Wallac Win Spectral, Perkin Elmer, Waltham, MA). All samples were measured in triplicates. One milliunit of lipase activity corresponds to the release of 1 nmol of fatty acids per minute.
Statistics
Polarity indexes were calculated by measuring the intensity of axon and three dendrites per neuron, or basolateral and apical membranes of MDCK cells. Using the ImageJ software [47], a segmented line was drawn, and the intensity per area was determined. All measurements were calculated as percent of the total per cell, and finally the dendritic value was divided by the axonal to give the polarity index. Values were compared and the statistical significance of the differences compared to the wildtype was evaluated with a two-tailed Student’s t-test for unpaired data. All bar diagrams show the mean value ± the standard error of mean. The total number of cells (n) is indicated below each bar.
Use of animals
Sprague-Dawley rats (Harlan Laboratories) were temporarily kept at the NIH animal facility. Animal work was conducted under protocol #13–011 following the United States regulations and guidelines set forth by the NIH, the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Government Principles for the Utilization and Care of Vertebrate Animals used in Testing, Research and Training.
Acknowledgements:
We would like to thank F. Kamakh, N Mahler, X. Zhu and N. Tsai for expert technical assistance and T. Ryan and R. Fenton for kind gifts of reagents or cells.
Funding
The work was supported by the Alfred Benzon Foundation (to SCK), The Lundbeck Foundation (project number R108-A10265 (to SCK) and project number R93-A8846), The Lundbeck Foundation Research Initiative on Blood Brain and Drug Delivery, The Danish Diabetes Academy, Novo Nordisk Foundation (MK) and The Intramural Program of NICHD, NIH. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication
Footnotes
Conflict of Interest:The authors declare that they have no conflicts of interest with the content of the article.
References
- 1.Jacobsen L, Madsen P, Moestrup SK, Lund AH, Tommerup N, Nykjaer A, Sottrup-Jensen L, Gliemann J. & Petersen CM (1996) Molecular characterization of a novel human hybrid-type receptor that binds the alpha2-macroglobulin receptor-associated protein, The Journal of biological chemistry. 271, 31379–83. [DOI] [PubMed] [Google Scholar]
- 2.Mazella J. (2001) Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking?, Cellular signalling. 13, 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Bryant DM & Mostov KE (2008) From cells to organs: building polarized tissue, Nature reviews Molecular cell biology. 9, 887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro CM (2011) Measurements of intracellular calcium signals in polarized primary cultures of normal and cystic fibrosis human airway epithelia, Methods Mol Biol. 742, 113–26. [DOI] [PubMed] [Google Scholar]
- 5.Di Pietro SM & Dell’Angelica EC (2005) The cell biology of Hermansky-Pudlak syndrome: recent advances, Traffic. 6, 525–33. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto UM, Hubbard AL & Mellman I. (2001) A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor, Cell. 105, 575–85. [DOI] [PubMed] [Google Scholar]
- 7.Godde NJ, Pearson HB, Smith LK & Humbert PO (2014) Dissecting the role of polarity regulators in cancer through the use of mouse models, Experimental cell research. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J. & Petersen CM (2001) Activation and functional characterization of the mosaic receptor SorLA/LR11, The Journal of biological chemistry. 276, 22788–96. [DOI] [PubMed] [Google Scholar]
- 9.Klinger SC, Glerup S, Raarup MK, Mari MC, Nyegaard M, Koster G, Prabakaran T, Nilsson SK, Kjaergaard MM, Bakke O, Nykjaer A, Olivecrona G, Petersen CM & Nielsen MS (2011) SorLA regulates the activity of lipoprotein lipase by intracellular trafficking, Journal of cell science. 124, 1095–105. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen MS, Gustafsen C, Madsen P, Nyengaard JR, Hermey G, Bakke O, Mari M, Schu P, Pohlmann R, Dennes A. & Petersen CM (2007) Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA, Molecular and cellular biology. 27, 6842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifacino JS (2014) Adaptor proteins involved in polarized sorting, The Journal of cell biology. 204, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS & Rodriguez-Boulan E. (2012) Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B, Proceedings of the National Academy of Sciences of the United States of America. 109, 3820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farias GG, Cuitino L, Guo X, Ren X, Jarnik M, Mattera R. & Bonifacino JS (2012) Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons, Neuron. 75, 810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Farias GG & Bonifacino JS (2015) Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1, Molecular biology of the cell. 26, 218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasiecka ZM & Winckler B. (2011) Mechanisms of polarized membrane trafficking in neurons --focusing in on endosomes, Molecular and cellular neurosciences. 48, 278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattera R, Boehm M, Chaudhuri R, Prabhu Y. & Bonifacino JS (2011) Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants, The Journal of biological chemistry. 286, 2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X, Surma MA & Simons K. (2012) Polarized sorting and trafficking in epithelial cells, Cell research. 22, 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS & Rodriguez-Boulan E. (2012) The clathrin adaptor AP-1A mediates basolateral polarity, Developmental cell. 22, 811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Mattera R, Ren X, Chen Y, Retamal C, Gonzalez A. & Bonifacino JS (2013) The adaptor protein-1 mu1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells, Developmental cell. 27, 353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsen C, Glerup S, Pallesen LT, Olsen D, Andersen OM, Nykjaer A, Madsen P. & Petersen CM (2013) Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein, The Journal of neuroscience : the official journal of the Society for Neuroscience. 33, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohe M, Hartl D, Fjorback AN, Klose J. & Willnow TE (2013) SORLA-mediated trafficking of TrkB enhances the response of neurons to BDNF, PloS one. 8, e72164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haass C, Koo EH, Capell A, Teplow DB & Selkoe DJ (1995) Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals, The Journal of cell biology. 128, 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simons M, Ikonen E, Tienari PJ, Cid-Arregui A, Monning U, Beyreuther K. & Dotti CG (1995) Intracellular routing of human amyloid protein precursor: axonal delivery followed by transport to the dendrites, J Neurosci Res. 41, 121–8. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, Owens NC, Rege SV, Si G, Ahuja A, Zhu D, Miller CA, Schneider JA, Maeda M, Maeda T, Sugawara T, Ichida JK & Zlokovic BV (2015) Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance, Nat Neurosci. 18, 978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanchette-Mackie EJ, Masuno H, Dwyer NK, Olivecrona T. & Scow RO (1989) Lipoprotein lipase in myocytes and capillary endothelium of heart: immunocytochemical study, The American journal of physiology. 256, E818–28. [DOI] [PubMed] [Google Scholar]
- 26.Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, Willnow TE, Christensen EI, Mobley WB, Nykjaer A. & Andersen OM (2012) Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing, The Journal of neuroscience : the official journal of the Society for Neuroscience. 32, 1467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobsen L, Madsen P, Nielsen MS, Geraerts WP, Gliemann J, Smit AB & Petersen CM (2002) The sorLA cytoplasmic domain interacts with GGA1 and −2 and defines minimum requirements for GGA binding, FEBS letters. 511, 155–8. [DOI] [PubMed] [Google Scholar]
- 28.Simmen T, Nobile M, Bonifacino JS & Hunziker W. (1999) Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster, Molecular and cellular biology. 19, 3136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren X, Farias GG, Canagarajah BJ, Bonifacino JS & Hurley JH (2013) Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1, Cell. 152, 755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T. & Bonifacino JS (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins, Science. 269, 1872–5. [DOI] [PubMed] [Google Scholar]
- 31.Camps L, Reina M, Llobera M, Vilaro S. & Olivecrona T. (1990) Lipoprotein lipase: cellular origin and functional distribution, The American journal of physiology. 258, C673–81. [DOI] [PubMed] [Google Scholar]
- 32.Jensen DR, Bessesen DH, Etienne J, Eckel RH & Neville MC (1991) Distribution and source of lipoprotein lipase in mouse mammary gland, Journal of lipid research. 32, 733–42. [PubMed] [Google Scholar]
- 33.Dempsey PJ, Meise KS & Coffey RJ (2003) Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants, Experimental cell research. 285, 159–74. [DOI] [PubMed] [Google Scholar]
- 34.Dillon C, Creer A, Kerr K, Kumin A. & Dickson C. (2002) Basolateral targeting of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal but independent of the C-terminal ERBIN-binding domain, Molecular and cellular biology. 22, 6553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He C, Hobert M, Friend L. & Carlin C. (2002) The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, including a dominant signal with a polyproline core, The Journal of biological chemistry. 277, 38284–93. [DOI] [PubMed] [Google Scholar]
- 36.Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E. & Gonzalez A. (2007) Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes, Molecular biology of the cell. 18, 4872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY & Guo X. (2014) Adaptor protein complexes and intracellular transport, Bioscience reports. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baltes J, Larsen JV, Radhakrishnan K, Geumann C, Kratzke M, Petersen CM & Schu P. (2014) sigma1B adaptin regulates adipogenesis by mediating the sorting of sortilin in adipose tissue, Journal of cell science. 127, 3477–87. [DOI] [PubMed] [Google Scholar]
- 39.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ & Bonifacino JS (1995) An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface, The EMBO journal. 14, 4961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirst J, Borner GH, Antrobus R, Peden AA, Hodson NA, Sahlender DA & Robinson MS (2012) Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system, Current biology : CB. 22, 1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia X, Singh R, Homann S, Yang H, Guatelli J. & Xiong Y. (2012) Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef, Nature structural & molecular biology. 19, 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA & Thomas G. (2001) PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic, The EMBO journal. 20, 2191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgert T, Schmidt V, Caglayan S, Lin F, Fuchtbauer A, Fuchtbauer EM, Nykjaer A, Carlo AS & Willnow TE (2013) SORLA-dependent and -independent functions for PACS1 in control of amyloidogenic processes, Molecular and cellular biology. 33, 4308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verges M, Luton F, Gruber C, Tiemann F, Reinders LG, Huang L, Burlingame AL, Haft CR & Mostov KE (2004) The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor, Nature cell biology. 6, 763–9. [DOI] [PubMed] [Google Scholar]
- 45.Kaech S. & Banker G. (2006) Culturing hippocampal neurons, Nature protocols. 1, 2406–15. [DOI] [PubMed] [Google Scholar]
- 46.Cottin M. & Zanvit A. (1997) Fluorescein leakage test: a useful tool in ocular safety assessment, Toxicology in vitro : an international journal published in association with BIBRA. 11, 399–405. [DOI] [PubMed] [Google Scholar]
- 47.Rasband WS http://imagej.nih.gov/ij/in, U.S. National Institutes of Health, Bethesda, Maryland. [Google Scholar]
- 48.Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A. & Willnow TE (2005) Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein, Proceedings of the National Academy of Sciences of the United States of America. 102, 13461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergo M, Olivecrona G. & Olivecrona T. (1996) Forms of lipoprotein lipase in rat tissues: in adipose tissue the proportion of inactive lipase increases on fasting, The Biochemical journal. 313 (Pt 3), 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]