Abstract
Background:
There is a lack of data exploring the use and optimal timing of immunotherapy and chemoradiation (CRT) in node positive cervical cancer. Further translational research into mechanisms of response and resistance to immunotherapy in advanced cervical cancer is warranted. Primary Objective(s) To determine if sequencing of atezolizumab and CRT result in differential immune activation, as determined by clonal expansion of T cell receptor beta (TCRB) repertoires in peripheral blood on day 21.
Study Hypothesis:
There is a difference for clonal expansion of T cell receptor beta repertoires in the peripheral blood at day 21 between the priming and concurrent atezolizumab and CRT in Arm A vs. the concurrent atezolizumab and CRT in Arm B. Trial Design Locally advanced cervical cancer patients with lymph node positive disease will be randomized on this open label, randomized trial with two experimental arms. Arm A will get one dose of atezolizumab prior to cisplatin CRT, and then two subsequent doses of atezolizumab during the CRT, and Arm B will get 3 doses during CRT. Patients will be followed for 2 years to assess outcomes.
Major Inclusion/Exclusion Criteria:
Patients must have histologically confirmed newly diagnosed advanced cervical cancer (squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma): FIGO 2009 clinical stages IB2/IIA with positive para-aortic nodes, or FIGO 2009 clinical stages IIB/IIIB/IVA with positive pelvic or para-aortic lymph nodes (PALN). Exclusion criteria include those who had a prior hysterectomy or lymph node dissection. Primary Endpoint(s) Clonal expansion of T cell receptor beta (TCRB) repertoires in peripheral blood on day 21. The sample size will be 40 patients. We estimate accrual to finish by the summer of 2020 with presentation of results to follow in 2021.
Trial Registration:
Introduction
Cervical cancer affects an estimated 12,900 women and accounts for approximately 4,100 deaths in the United States, and 266,000 deaths globally each year. Locally advanced cervical cancer is defined as stage FIGO 2018 IB3 (tumor confined to the cervix measuring ≥4cm), stage II (tumor invading beyond the uterus upper 2/3 of vagina or invasion of the parametria), or stage III (positive lymph nodes, tumor extending to pelvic sidewall, lower 1/3 of vagina, or causing hydronephrosis), and stage IVA (tumor invading mucosa of bladder or rectum or extending beyond the true pelvis). Patients diagnosed with locally-advanced cervical cancer have a higher risk of recurrence and worse survival than the early stage patients. Patients presenting with para-aortic lymph node (PALN) metastases at diagnosis represent a particularly poor prognostic group with 5-year survival of approximately 40% across the stages1. Therefore, there is an unmet need for therapeutic treatment options for patients with PALN, given the dismal survival with current therapeutic options available. Immunotherapy added to radiation is promising in the field of oncology for synergistic effects, and the opportunity to study this combination in cervical cancer is the basis for the clinical trial NRG Oncology GY017, Anti PD-L1 (atezolizumab) as an Immune Primer and Concurrently with Extended Field Chemoradiotherapy for Node Positive Locally Advanced Cervical Cancer2.
Cervical cancer has been identified to be direct consequence of infection by specific human papilloma virus (HPV) subtypes, predominantly subtypes 16 and 18 3–5. Despite the initial host immune response to HPV antigens, cervical cancers often develop multiple resistance mechanisms that allow for immune escape, which include local immune suppression through a number of mechanisms, including tumor infiltration with various immunosuppressive cell populations and expression of immunosuppressive molecules, as well as induction of T cell dysfunction, associated with expression of immune checkpoints 6.
Overcoming the multiple mechanisms of immunosuppression will thus require multi-modality treatments, including therapies directed at the immunosuppressive microenvironment and therapies targeting T cell dysfunction. Emerging data from multiple studies highlight pro-immunogenic effects of radiation on the tumor and tumor microenvironment. Early data suggest that the immune system, in particular CD8+ T cells, have a key role for tumor cell death within a radiation field 7,8. Radiation therapy causes migration of dendritic cells, cross-penetration of tumor antigens, which can result in T cell activation and proliferation 9. Furthermore, radiation therapy increases the density of tumor infiltrating lymphocytes (TIL) within a tumor likely by extravasation of TIL within the vasculature of tumors and chemokines activation 9,10. It is known that radiation therapy alters the T cell repertoire of peripheral T cell clones 11. There is thus a strong rationale for combination of radiation with immune checkpoint blockade. There are limited data surrounding the optimal dose and fractionation needed to provoke an ideal immune response when combining immunotherapy with radiation in cervical cancer. Sequencing of CTLA-4 blockade with immunotherapy in preclinical models demonstrate that when anti-CTLA-4 is delivered prior to RT, there is increased efficacy compared to delivery after RT12. Studies have also demonstrated that RT increases PD-L1 expression, which may act as a negative feedback mechanism preventing T-cell-mediated tumor rejection 13,14. One may thus hypothesize that similarly, administration of PD-1 or PD-L1 blockade prior to radiation may prime the immune system to resist the immunosuppressive effects of PD-L1. On the other hand, one may also argue that activation of tumor-specific T cells with PD-1/PD-L1 blockade prior to radiation may expose the cells to cytotoxic effects of radiation and dampen the immune response.
Immune checkpoint blockade has been studied in metastatic and persistent cervical cancer with promising outcomes. Pembrolizumab was granted US FDA approval in June 2018 based on the results of KEYNOTE-158 (Clinical trial NCT02054806) which evaluated patients with recurrent and metastatic CC15. Ninety-eight patients with an ECOG performance status 0–1 were treated with 200mg pembrolizumab q3w for up to 24 months or until confirmed disease progression, intolerable toxicity or death. PD-L1 positivity, defined by PD-L1 expression score ≥1, was seen in 83% of patients15. Treatment related toxicity was generally acceptable with 11% of patients having a grade 3–4 AEs15. At a median follow up of 10.3 months the ORR was 13% with CR in 3 patients and PR in 10 patients15. 17 patients had stable disease and the disease control rate was 31%. Of those who responded, nearly 70% (9/13) had a response lasting over 9 months15. The phase I/II study Checkmate 358 of nivolumab in CC (NCT02488759) patients with ecurrent or mestastatic CC with ≤2 prior systemic therapies were treated with nivolumab 240 mg every 2 weeks until progression or unacceptable toxicity16. In the 19 CC patients with a median survival of 21.9 months, the ORR was 26.3%, regardless of PD-L1 expression, disease control rate was 68%, grade 3–4 toxicity was 15.8%, suggestive of clinical activity with a manageable safety profile16.
There remains a paucity of clinical data on the ideal sequencing of immunotherapy and radiation in locally advanced cervical cancer. Furthermore, biologic insights into peripheral and intratumoral immune changes in response to radiation and immune checkpoint blockade in human tumors are generally lacking. To address these questions, in this clinical trial we are exploring the biologic and clinical consequences of differential sequencing of PD-L1 blockade and CRT in patients with newly diagnosed locally advanced cervical cancer. We hypothesize that differences in sequencing of atezolizumab and chemoradiation will result in differential immune activation, as determined by clonal expansion of T cell receptor beta (TCRB) repertoires in peripheral blood in response to therapy, which will be predictive of clinical outcomes.
Methods and Trial Design
This study has two treatment arms as outlined below.
Arm A: receives one dose of intravenous (IV) atezolizumab prior to cisplatin chemotherapy and radiation therapy, and then two subsequent doses of atezolizumab administered every 3 weeks during the chemotherapy and radiation therapy. Arm B: receives 3 doses of atezolizumab administered every 3 weeks during the cisplatin chemotherapy and radiation therapy.
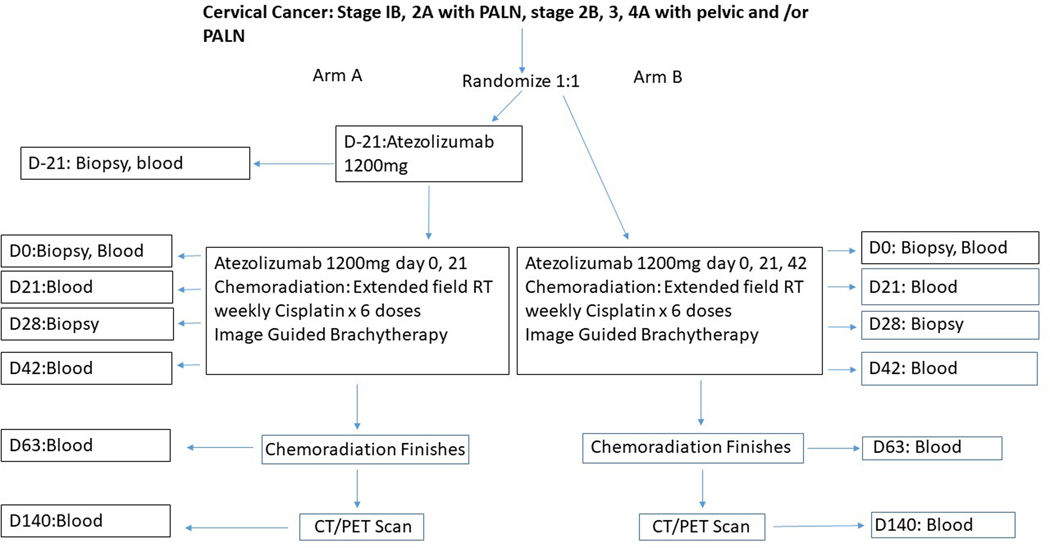
The schema of the clinical trial is shown in Figure 1.
Figure 1:
Schema of the clinical trial. CT/PET, computed tomography/positron emission tomography; D, day; PALN, paraaortic lymph nodes; RT, radiation therapy.
The NRG GY17 clinical trial is funded by the National Cancer Institute and the NRG Oncology cooperative groups. The trial is open at select phase I gynecology oncology NRG Oncology institutions.
Participants
Patients with histologically confirmed newly diagnosed advanced cervical cancer (squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma): FIGO 2018 stage IB3/IIA positive para-aortic nodes, or FIGO 2018 stages IIB/IIIB/IVA with positive pelvic or para-aortic lymph nodes (PALN). Pelvic or PALN nodal status is confirmed by PET/CT scan, fine needle biopsy, extra peritoneal biopsy, or laparoscopic biopsy. The PALN must be inferior to the T12/L1 interspace. Patients must have an ECOG performance status ≤2, and have normal organ and marrow function. Patients ineligible for the trial include those who have received prior radiation therapy to the pelvis or abdominal cavity, PALN radiation, or previous therapy of any kind for this malignancy.
Primary Objectives
To determine whether differences in sequencing of atezolizumab and chemoradiation result in differential immune activation, as determined by clonal expansion of T cell receptor beta (TCRB) repertoires in peripheral blood on day 21.
Secondary Objectives
To investigate the feasibility of administration of the anti PD-L1 antibody (atezolizumab) as an immune primer and concurrent with chemoradiation (CRT) therapy in patients with locally advanced cervical cancer. To determine the nature and degree of toxicity of the anti PD-L1 antibody (atezolizumab) administered as an immune primer and concurrent with chemoradiation (CRT) therapy in patients with locally advanced cervical cancer.To examine the changes in TCR clonality, diversity, and frequency in peripheral blood and tissue and correlate this with clinical outcomes, such as the exploratory response assessment on the post-treatment PET-CT scan and 2-year disease-free survival (DFS).To assess the predictive value of baseline and on-treatment PD-L1 expression in the tissue in each treatment arm for clinical outcomes using post-treatment PET-CT scan and 2-year DFS as the outcome measures.
Exploratory Objectives
To explore baseline and on-treatment blood and tissue biomarkers that could predict response to the combination therapy, as correlated to the exploratory clinical endpoint of the week 12 (day 140) PET –CT scan and 2-year DFS. To explore the response assessment on the exploratory and optional post-treatment week 12 (day 140) PET-CT scan and the clinical 2-year DFS.
Sample Size and Statistical Methods
The primary objective of this study is to determine whether there is a difference of anti-tumor immune response between the two treatment arms through as defined by T cell receptor beta (TCRB) clonal expansion in peripheral blood at day 21. It is hypothesized that a treatment with increased clinical benefit will 1) have evidence of oligoclonal TCR expansion in peripheral blood and/or 2) show expansion of tumor-associated TCR clones in peripheral blood in response to therapyAll eligible patients treated on Arm A (dose at day −21 and dose at day 0) and Arm B (dose at day 0) and have TCRB measurements on day 21 will be evaluable. For the primary objective, there will be a comparison of the overall TCR diversity and the numbers of expanded tumor-associated TCR clones between the arms at day 21 by 2-sided t-test at 10% significance level. A sample size of 40 patients (20 in each treatment arm) will give this study a 90% power to detect an effect size of 0.95 by a two-sided t-test at significance level of 0.1.
Dose-limiting toxicities (DLTs) will be defined by drug related adverse effects that meet specific criteria as evaluated by NCI CTCAE v.5 unless clearly unrelated to study therapy (e.g., disease progression). As this regimen is being developed as a front-line therapy, the goal is for patients to complete all treatments. Thus, all cycles will be considered in determining DLT. Specifically, the DLT period for Arm A will start with the priming dose of atezolizumab and last until 30 days after the completion of CRT; for Arm B the DLT period will start with CRT and last until 30 days after the completion of CRT.
Discussion
This phase I trial will have a potential to change the treatment landscape of locally advanced node positive cervical cancer, and will offer novel insights into optimal sequencing and use of immunotherapy with CRT. This trial specifically includes patients at highest risk for failure after standard chemoradiation, including patients with positive para-aortic LN, which represents the population of locally advanced cervical cancer patients with highest unmet need. In addition to the efficacy and translational assessments, this trial will provide safety data for immunotherapy administered both pre- and concurrent with CRT. The trial is being offered at selected high-volume cervical cancer centers through the NRG Oncology clinical trial cooperative group with support from the NRG Oncology and NCI/CTEP programs in partnership with Genentech. Assessment of the primary translational endpoints will be performed in partnership with Adaptive Biotechnologies. Unique to the trial design is the heavy focus on translational endpoints as the primary outcome measures. All patients will undergo required biopsies and blood collection before and during immunotherapy and CRT, which will allow to assess dynamic changes in the tumor microenvironment and TCR repertoires and correlate these to long term outcomes.
We expect that patients will be able to complete their standard CRT in a timely fashion. Nevertheless, we anticipate that the study will present several potential challenges and will require close monitoring by the study team. The study team will be actively monitoring for safety issues during the trial enrollment in order to identify early signs of immune-mediated toxicities and institute a treatment plan to maximize patient safety. For example, it is often difficult to distinguish between RT induced GI acute toxicity, and immune related colitis based on symptoms alone. We plan to use our NRG Oncology workflow of patient communication, study coordinators, effective and timely reporting to the study team, and the temporal space of symptoms to distinguish between the RT-related and immunotherapy-related side effects. We anticipate that the use of modern RT techniques such as intensity modulated radiation therapy and image guided brachytherapy will help to minimize the risk of GI toxicities and maximize safety of the combined modality therapy.
Despite the advances in treatment of locally advanced cervical cancer, presence of lymph node metastases continues to portend high risk of disease recurrence. We believe that findings from the NRG GY017 trial will generate early clinical and translational signals of activity of PD-L1 blockade administered in combination with chemoradiation in cervical cancer and will provide invaluable insights into appropriate sequencing of radiation and immunotherapy, which will serve as a foundation for future clinical trial designs and clinical practice.
References
- 1.Macdonald OK, Chen J, Dodson M, et al. : Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol 32:411–6, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Dyer BA, Zamarin D, Eskandar RN, et al. : Role of Immunotherapy in the Management of Locally Advanced and Recurrent/Metastatic Cervical Cancer. J Natl Compr Canc Netw 17:91–97, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Clifford GM, Smith JS, Plummer M, et al. : Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 88:63–73, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, et al. : Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–27, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cogliano V, Baan R, Straif K, et al. : Carcinogenicity of human papillomaviruses. Lancet Oncol 6:204, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–54, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone HB, Peters LJ, Milas L: Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst 63:1229–35, 1979 [PubMed] [Google Scholar]
- 8.Lee Y, Auh SL, Wang Y, et al. : Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114:589–95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharabi AB, Lim M, DeWeese TL, et al. : Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 16:e498–509, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Hallahan D, Kuchibhotla J, Wyble C: Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 56:5150–5, 1996 [PubMed] [Google Scholar]
- 11.Twyman-Saint Victor C, Rech AJ, Maity A, et al. : Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young KH, Baird JR, Savage T, et al. : Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 11:e0157164, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. : Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74:5458–68, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Deng L, Liang H, Burnette B, et al. : Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–95, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung HC, Schellens JHM, Delord J-P, et al. : Pembrolizumab treatment of advanced cervical cancer: Updated results from the phase 2 KEYNOTE-158 study. Journal of Clinical Oncology 36 (suppl; abstr 5522), 2018 [Google Scholar]
- 16.Hollebecque A MT, Moore K et al. : An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. Journal of Clinical Oncology 35, no. 15_suppl (May 20 2017) 5504–5504., 2017 [Google Scholar]