Abstract
Background:
Exposure to household air pollution generated as a result of cooking and heating is a leading contributor to global disease. The effects of cookstove-generated air pollution on adult lung function, however, remain uncertain.
Objectives:
We investigated acute responses in lung function following controlled exposures to cookstove-generated air pollution.
Methods:
We recruited 48 healthy adult volunteers to undergo six 2-hour treatments: a filtered-air control and emissions from five different stoves with fine particulate matter (PM2.5) targets from 10 to 500 µg/m3. Spirometry was conducted prior to exposure and immediately, 3 hours, and 24 hours post-exposure. Mixed-effect models were used to estimate differences in post-exposure lung function for stove treatments versus control.
Results:
Immediately post-exposure, lung function was lower compared to the control for the three highest PM2.5-level stoves. The largest differences were for the fan rocket stove (target 250 µg/m3; forced vital capacity [FVC]: −60 mL, 95% CI −135, 15; forced expiratory volume [FEV1]: −51 mL, 95% CI −117, 16; mid-expiratory flow [FEF25–75]: −116 mL/s, 95% CI −239, 8). At 3 hours post-exposure, lung function was lower compared to the control for all stove treatments; effects were of similar magnitude for all stoves. At 24 hours post-exposure, results were consistent with a null association for FVC and FEV1; FEF25–75 was lower relative to the control for the gasifier, fan rocket, and three stone fire.
Conclusions:
Patterns suggesting short-term decreases in lung function follow from exposure to cookstove air pollution even for stove exposures with low PM2.5 levels.
Keywords: air pollution, cookstoves, spirometry, FEV1, FVC, controlled exposure
Introduction
Nearly 40% of the world’s population cooks over open fires or with rudimentary stoves that burn solid fuels, which generates high levels of household air pollution (Bonjour et al. 2013). Exposures to this pollution were estimated to contribute to approximately 59 million disability-adjusted life-years in 2017, including over 1.6 million premature deaths - primarily in the form of respiratory and cardiovascular diseases (Stanaway et al. 2018). Approaches to reduce disease burden from household air pollution have included switching to cookstoves designed to emit lower levels of fine particulate matter mass (PM2.5) and/or carbon monoxide (CO). Cookstove emissions can vary substantially across different stoves and fuels however (Bilsback et al. 2018), and it is unclear how these differences in exposures across stoves contribute to different health responses. Although some research has demonstrated the potential for improved stoves to reduce human exposures (Thomas et al. 2015) and associated pulmonary effects (Fullerton et al. 2011; da Silva et al. 2012), other studies have not demonstrated the expected health benefits following dissemination of improved stoves (Romieu et al. 2009; Smith-Sivertsen et al. 2009; Smith et al. 2011; Mortimer et al. 2017).
Controlled human exposure studies conducted in laboratory settings may have increased internal validity compared to observational based field studies, primarily due to reduction of confounding and other biases. This allows for generation of data that can support mechanistic understanding of the relationships between exposure and health effects. Controlled exposure studies can also provide information about between-stove differences in health endpoints (e.g., exposure-response data) that is not feasible to collect in the field. Using crossover designs in a controlled exposure setting allows for comparison of multiple treatments within the same participant, which provides statistical efficiency and eliminates time-invariant (i.e., population-level) confounders. However, it is not feasible to evaluate long-term exposures or clinically-significant endpoints or mortality in a controlled exposure study. Instead, exposures and measured health outcomes must be acute, transient, and subclinical. Measurement of translatable risk markers can provide connection between laboratory studies and field studies.
Lung function metrics measured through spirometry can provide insight on pulmonary inflammation following air pollution exposures. The deposition of air pollutants, particularly PM2.5, throughout the respiratory tract can cause acute pulmonary irritation and tissue damage, provoking a cascade of cellular injury and inflammation that can lead to development and exacerbation of obstructive lung diseases like COPD and asthma (U.S. EPA 2009; Brook et al. 2010; Perez-Padilla et al. 2010; Gordon et al. 2014). Pulmonary inflammation can trigger activation of the autonomic nervous system and contribute to development of systemic inflammation, which can lead to a number of non-respiratory impacts such as cardiovascular disease (U.S. EPA 2009). Small, transient changes in lung function following air pollution exposures in controlled settings may have minimal clinical relevance on an individual level or for healthy populations. They are, however, relevant indicators that can inform our understanding of biological pathways which contribute to the exposure-response when extrapolated to real-world settings where diverse populations are exposed over their lifetime. Further, implications for public health could be considerable if the effects scale with chronic exposures, given the large number of individuals exposed globally.
The objective of this work was to investigate markers of acute change in lung function following 2-hour controlled exposures to cookstove-generated air pollution from five different cookstoves (with PM2.5 levels from 10 to 500 µg/m3) compared to a filtered-air control. We chose cookstoves that represent a range of technologies available in the real world, to allow for comparisons of effects across stoves that represent real choices for cookstove users world-wide. Stoves included a woodburning three-stone fire (a “lack of technology”), and three different types of improved efficiency woodburning stove designs, and a liquified petroleum gas stove (an improved fuel option). We used a 2-hour exposure window to mimic exposures of a typical cooking event while balancing logistical and ethical considerations of a controlled exposure study. Spirometry, a measurement that can be conducted in both laboratory and field settings and has implications towards long-term pulmonary health, was used as a marker of acute health response.
Materials and Methods
Detailed study methods are reported in a previous publication (Fedak et al. 2019) and in the online supplemental material; brief methods are provided herein. All protocols were approved by the Colorado State University Institutional Review Board.
Participants
We recruited 48 young, healthy, never-smoker adults from the Fort Collins, Colorado, USA area to participate between October 2016 and January 2018. Eligibility criteria included age (18 to 35 years), weight/body mass index (BMI) within an average healthy range, normal spirometry (defined as spirometry values greater than 70% of the predicted value for the age/gender at a pre-study screening exam), no medication/drug use, no history of inflammatory or chronic diseases, no regular pollution exposures (including no use of wood stoves for home heating/cooking or occupation within the food industry), and ability to complete the study protocols (see online supplemental material). All participants provided written informed consent.
Study Design
Participants were exposed to six 2-hour treatments (one per study session). Treatments were a filtered air control (PM2.5 target: 0 µg/m3) and five cookstoves: liquefied petroleum gas (LPG; 10 µg/m3), gasifier (35 µg/m3), forced-draft fan rocket elbow (fan rocket; 100 µg/m3), natural-draft rocket elbow (250 µg/m3), and three stone fire (500 µg/m3). Participants were scheduled for treatments in groups of four per study session. Sessions were scheduled with a minimum 2-week period between treatments; missed sessions were made up at the end of the sequence. Treatment order was determined using a Williams square design - a Latin square crossover that is balanced for treatment orders and carry-over effects and therefore robust to confounding by personal-level and external factors (e.g., sex, ambient conditions) (Williams 1949; Jones B and Kenward 2014; Kenward 2015). Participants were asked to abstain from alcohol, caffeine, smoke exposures, strenuous exercise, and medications 24 to 72 hours before and during study sessions.
Each study session, participants arrived at the facility in the morning for baseline health measurements. Participants then underwent a 2-hour whole-body exposure within a controlled-environment chamber (participants were not informed of treatment type). Additional health measurements were conducted immediately post-exposure and at 3 and 24 hours post-exposure.
During each exposure period, PM2.5 mass concentration, CO and oxygen levels, temperature, and relative humidity in the chamber were monitored continuously. Separate tests were conducted to characterize additional pollutants present in the emissions for each treatment (PM2.5 mass, particle number size distributions [10 to 500 nm], elemental and organic carbon concentrations [EC, OC], nitrogen oxides [NOx], carbonyls, and select volatile organic compounds [benzene, toluene, ethylbenzene, and xylenes]) (see online supplemental material).
Health and Additional Measurements
Participants performed spirometry at each health measurement time using an ultrasonic device (Easy on-PC, ndd Medizintechnik AG, Zurich, Switzerland) according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (Miller et al. 2005). The lung function metrics used for analysis were FVC, FEV1, FEV1/FVC, and FEF25–75. For all tests that met the ATS/ERS quality criteria (Miller et al. 2005), we used the largest FVC and FEF25–75 values from the acceptable trials within that test and the FEV1 and FEV1/FVC value from the trial with the largest FVC. For tests that did not meet the ATS/ERS quality criteria, a board-certified pulmonologist (JB) reviewed the spirographs to determine which values, if any, could be used in analyses.
We administered surveys to assess potential confounders such as alcohol and caffeine consumption, sleep quantity, and mode of commute to the facility. Ambient meteorological and pollutant data were obtained via local monitors.
Data Analysis
Mixed-effect regression models were used to estimate the effect of the stove exposures on each lung function metric at each post-exposure time point, presented as difference relative to the control (Bates et al. 2015). Models included a baseline lung function term to account for differences in pre-exposure lung function across the six sessions (Vickers and Altman 2001), a random person intercept to account for repeated measures among participants, and a random day intercept to account for non-independence of data for participants who experienced treatments together (e.g., the four participants scheduled on the same study day). Inclusion of individual-level or external confounders is theoretically not necessary due to the study design, which eliminates the potential for confounding by time-invariant individual-level factors, and analysis model, which incorporates terms (e.g., the baseline and random effects) that encompass and control for differences due to time-varying factors (e.g., day-to-day differences in participant diet/activities, weather, ambient exposures) (Jones B and Kenward 2014; Kenward 2015). However, we examined descriptive statistics and conducted sensitivity analyses to confirm there were no associations between potential confounders and treatment type occurring by chance and model results were not sensitive to the potential confounders. We also evaluated alternative models that contained additional design structure variables and/or were run using limited datasets (e.g., excluding data from make-up sessions, only including tests that met ATS/ERS quality criteria) as additional sensitivity analyses (see online supplemental material).
Data processing and statistical analyses were performed in R (version 3.3.1, The R Foundation for Statistical Computing).
Results
Participants, Exposures, and Health Measurement Times
A total of 269 treatments were administered across 47 participants (39 participants completed all six treatments; missing data rate 8%; see online supplemental material). Participants were young (average age 27 years, range 21 to 36) with normal BMI (average 23.4 kg/m2, range 19.4 to 28.7) and normal baseline lung function (average FVC: 4.9 L [range 3.1 to 8.1], FEV1: 3.9 L [2.9 to 6.1], FEF25–75: 3.9 L/s [2.2 to 6.3]) (see Table 1). As anticipated based on the population from which we recruited, participants predominately self-identified as non-Hispanic white (42/47 participants; 89%).
Table 1.
Description of Study Participants.*
| Variable (units) | Statistic | All (n=47) |
Female (n=22) | Male (n=25) |
|---|---|---|---|---|
| BMI (kg/m2) | Mean [SD] min, max |
23.4 [2.3] 19.4, 28.7 |
23.5 [2.6] 19.7, 28.7 |
23.3 [2.0] 19.4, 26.0 |
| Age (years) | Mean [SD] min, max |
27.4 [3.6] 20.5, 36.1 |
27.5 [3.4] 22.8, 34.0 |
27.4 [3.9] 20.5, 36.1 |
| Number of sessions conducted* | Total sessions | 269 | 129 | 140 |
| Participants with data for all six treatments* | Percent | 81 | 86 | 76 |
| Baseline FVC (liters) | Mean† [SD] min, max |
4.9 [1.1] 3.1, 8.1 |
4.2 [0.5] 3.1, 5.4 |
5.5 [1.0] 4.0, 8.1 |
| Baseline FEV1 (liters) | Mean† [SD] min, max |
3.9 [0.8] 2.9, 6.1 |
3.4 [0.4] 2.9, 4.4 |
4.3 [0.7] 3.3, 6.1 |
| Baseline FEV1/FVC (ratio) | Mean† [SD] min, max |
0.8 [0.1] 0.6, 1.0 |
0.8 [0.1] 0.7, 1.0 |
0.8 [0.1] 0.6, 0.9 |
| Baseline FEF25–75 (liters/s) | Mean† [SD] min, max |
3.9 [1.1] 2.2, 6.3 |
3.5 [0.9] 2.2, 5.4 |
4.2 [1.1] 2.2, 6.3 |
A participant session was counted if they had data for baseline measurement and at least one post-exposure measurement.
Mean of each individuals’ average baseline health measurement across their completed study sessions. Participants were required to have normal spirometry at a pre-study screening exam (results not included here), defined as spirometry values greater than 70% of the predicted value for the age/gender.
Definition of abbreviations: BMI = body mass index; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEF25–75 = mid-expiratory flow rate (average rate at 25th-75th percent of the pulmonary volume); SD = standard deviation
The mean PM2.5 concentrations for the 2-hour treatments were close to the targets for each treatment (see Table 2; average difference from target: filtered air control, +1 µg/m3; LPG: −2 µg/m3; gasifier: +11 µg/m3; fan rocket: −5 µg/m3; rocket elbow: +4 µg/m3; three stone fire: −36 µg/m3). Carbon monoxide levels, which did not have a target, generally increased with PM2.5 from a mean of 2 ppm for the control to 9 ppm for the three stone fire. Results from additional pollutant characterization tests indicated that other pollutants generally increased with increasing PM2.5 concentration; however there were some exceptions. The rocket elbow stove produced high elemental carbon concentrations (and higher ratio of elemental carbon to total PM2.5) as well as high nitrogen oxide compared to other stoves; nitrogen oxide levels were also high for the fan rocket stove. Ultrafine particle (<100nm) number fraction was considerably higher for the LPG stove than all others, driven primarily by a high count of particles 10 to 30 nm in size. Carbonyl concentrations were also higher for the LPG stove than the gasifier and fan rocket, at a similar concentration as the rocket elbow stove. More detail is reported previously (Fedak et al. 2019) and in the online supplemental material.
Table 2.
Distributions of the Individual Mean Two-Hour Pollutant Exposures Measured during Treatments among 47 participants.
| Treatment* | Participants Completing Treatment (n) | PM2.5 level (µg/m3) | CO level (ppm) | ||
|---|---|---|---|---|---|
| Mean [SD]† | Min, Max Individual Exposure‡ | Mean [SD]† | Min, Max Individual Exposure‡ | ||
| Control | 46 | 1 [2] | −1§, 9 | 2 [2] | 1, 10 |
| LPG | 45 | 8 [3] | 3, 13 | 3 [1] | 1, 6 |
| Gasifier | 43 | 46 [9] | 30, 76 | 5 [3] | 1, 14 |
| Fan rocket | 44 | 95 [9] | 77, 111 | 8 [2] | 5, 12 |
| Rocket elbow | 45 | 254 [9] | 236, 276 | 6 [2] | 3, 11 |
| Three stone fire | 46 | 464 [39] | 367, 531 | 9 [4] | 4, 20 |
Target PM2.5 levels for each treatment were: HEPA-filtered air (0 µg/m3), LPG (10 µg/m3), gasifier (35 µg/m3), fan rocket (100 µg/m3), rocket elbow (250 µg/m3), and three stone fire (500 µg/m3). CO did not have a target level.
Measured pollutant mean is of the participants’ two-hour average values, calculated by determining the two-hour average of the one-second exposure data for each participant and then averaging across all participants for each treatment.
Min and max individual values are the lowest and highest two-hour average value measured for a single participant.
Negative values are a result of a DustTrak calibration artifact.
Definition of abbreviations: SD = standard deviation; LPG = liquefied petroleum gas; PM2.5 = fine particulate matter mass less than 2.5 µm in diameter, CO = carbon monoxide; ppm = parts per million
Due to our study protocols that involved several other health measurements, post-exposure spirometry occurred on average 30 minutes after the nominally reported measurement series start time of immediately, 3 hours, and 24 hours post-exposure (see online supplemental material).
Model Results
The effect estimates and 95% confidence intervals (CIs) for the mean difference in post-exposure lung function for each stove type compared to the filtered-air control are presented in Table 3 and Figure 1. No confounding variables were included in the analysis as there was no evidence of any meaningful associations between observed potential confounders and the treatments (see online supplemental material). Sensitivity analyses were consistent with the primary model (see online supplemental material).
Table 3.
Mean Differences in Lung Function for Each Stove Treatment Compared to Control at Each Measurement Time. Effect estimate is the difference in lung function value for the stove treatment compared to the control, accounting for baseline (pre-exposure).
| Treatment | Baseline value mean (SD)† | Effect Estimate (95% confidence interval) as compared to control* | ||
|---|---|---|---|---|
| Immediate post-exposure | 3 hours post-exposure | 24 hours post-exposure | ||
| FVC (ml) | Difference in FVC (ml) | |||
| LPG | 4854 (1024) | 5 (−69, 80) | −39 (−114, 35) | 9 (−74, 91) |
| gasifier | 4867 (1043) | 19 (−56, 93) | −21 (−95, 54) | 12 (−72, 96) |
| fan rocket | 4879 (1148) | −60 (−135, 15) | −30 (−105, 45) | 12 (−72, 95) |
| rocket elbow | 4898 (1064) | −40 (−114, 35) | −8 (−82, 67) | 1 (−83, 84) |
| three stone fire | 4860 (1070) | −42 (−116, 32) | −21 (−96, 53) | 26 (−56, 108) |
| FEV1 (ml) | Difference in FEV1 (ml) | |||
| LPG | 3860 (750) | 3 (−64, 69) | −68 (−128, −7) | 7 (−62, 76) |
| gasifier | 3873 (803) | 7 (−59, 74) | −53 (−114, 8) | −4 (−74, 66) |
| fan rocket | 3873 (852) | −51 (−117, 16) | −68 (−129, −7) | −15 (−84, 55) |
| rocket elbow | 3895 (816) | −24 (−91, 42) | −39 (−99, 22) | 0 (−69, 69) |
| three stone fire | 3887 (793) | −27 (−93, 39) | −39 (−99, 21) | −14 (−82, 54) |
| FEF25–75 (ml/s) | Difference in FEF25–75 (ml/s) | |||
| LPG | 3836 (987) | −44 (−167, 79) | −122 (−255, 11) | 39 (−78, 156) |
| gasifier | 3844 (1151) | −13 (−137, 110) | −74 (−208, 59) | −63 (−182, 56) |
| fan rocket | 3823 (1079) | −116 (−239, 8) | −114 (−249, 21) | −81 (−199, 37) |
| rocket elbow | 3862 (1148) | −68 (−191, 55) | −56 (−190, 77) | 35 (−83, 153) |
| three stone fire | 3915 (1126) | −103 (−225, 19) | −31 (−164, 102) | −88 (−204, 27) |
| FEV1/FVC (%) | Difference in FEV1/FVC (%) | |||
| LPG | 79.8 (6.4) | 0.0 (−0.9, 0.8) | −0.8 (−1.7, 0.2) | 0.0 (−0.9, 0.9) |
| gasifier | 79.4 (5.8) | −0.3 (−1.2, 0.6) | −0.9 (−1.9, 0.0) | 0.0 (−1.0, 0.9) |
| fan rocket | 79.4 (6.7) | −0.1 (−1.0, 0.7) | −0.5 (−1.4, 0.5) | −0.4 (−1.3, 0.5) |
| rocket elbow | 79.4 (6.4) | −0.2 (−1.0, 0.7) | −0.6 (−1.6, 0.3) | 0.2 (−0.8, 1.1) |
| three stone fire | 79.7 (6.8) | 0.5 (−0.4, 1.4) | 0.2 (−0.8, 1.1) | −0.2 (−1.1, 0.7) |
All estimates are adjusted for baseline (pre-exposure) values.
Control value at baseline [mean(SD)]: FVC: 4875 (1081) mL; FEV1: 3864 (787) mL; FEV1/FVC ratio: 79.4 (6.6)%; FEF25–75: 3832 (1101) mL/s.
Figure 1.
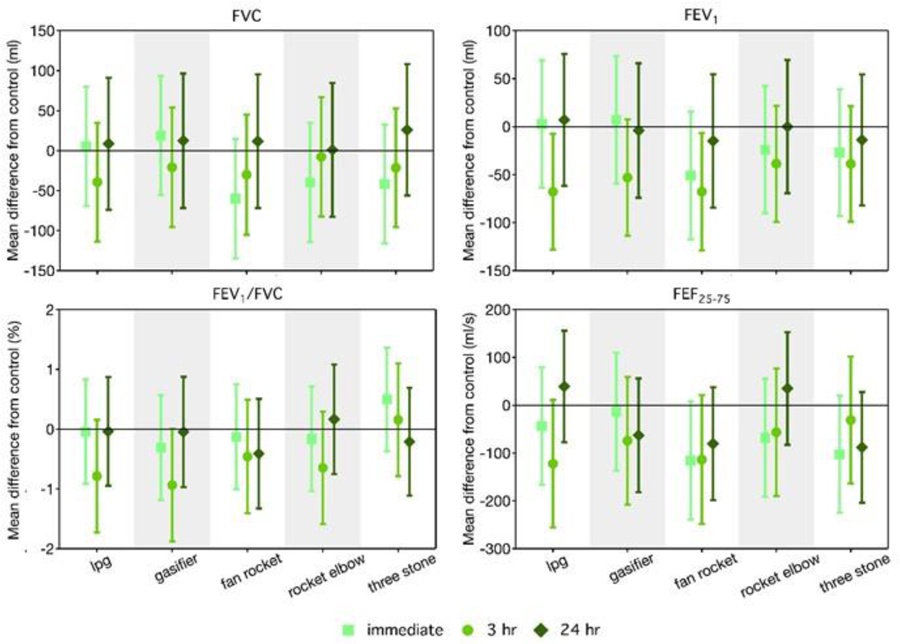
Effect Estimates and 95% Confidence Intervals for Spirometry Metrics by Stove Type and Post-Exposure Time Point. Effect estimate is the difference in lung function value for the stove treatment compared to the filtered air control at the given post-exposure measurement time, accounting for the baseline (pre-exposure) lung function. Units: FVC = mL, FEV1 = mL, FEV1/FVC = %, FEF25–75 = mL/s.
No statistically significant differences in FVC were observed for any stove or measurement type point. Immediately post-exposure, FVC values were lower than the control (by 40 to 60 mL) for the three higher PM2.5-level treatments (fan rocket, rocket elbow, three stone fire) but not the two lower PM2.5-level treatments (LPG, gasifier); results were nonsignificant for all stoves though suggestive for the fan rocket. Three hours after exposure, FVC was non-significantly lower for all stoves relative to the control, though effect estimates were small in magnitude (8 to 39 mL) with no discernible exposure-response pattern across stove types. At 24 hours post exposure, differences in FVC compared to the control were consistent with a null hypothesis for all stoves; effect estimates suggested higher FVC for the treatments than the control (by 1 to 26 mL).
FEV1 values immediately post-exposure were lower (not significant) than the control for the three higher PM2.5-level treatments (by 24 to 51 mL; suggestive for the fan rocket stove) but not the two lower PM2.5-level treatments. At 3 hours post exposure, the differences were more pronounced: all stove treatments had lower FEV1 values than the control, by 39 to 68 mL. The difference in FEV1 at 3 hours was largest for the three lower PM2.5-level treatments and statistically significant for the LPG and fan rocket stove and suggestive for the gasifier. At 24 hours post exposure, differences in FEV1 compared to the control were consistent with a null hypothesis for all stoves.
No statistically significant differences in FEF25–75 were observed for any stove or measurement type point, though patterns were consistent with those for FEV1. Immediately post-exposure, FEF25–75 was lower for stove treatments compared to the control (by 68 to 116 mL/s) for the three higher PM2.5-level treatments. At 3 hours post exposure, FEF25–75 was lower for all stove treatments compared to the control (by 31 to 122 mL/s), with the largest effects for the three lower PM2.5-level treatments. At 24 hours post-exposure, for the gasifier, fan rocket, and three stone fire treatments, but not the LPG or rocket elbow, a lower FEF25–75 was observed compared to the control.
No clear patterns of differences were seen in the FEV1/FVC ratio across stoves at the immediate or 24-hour post exposure measurements. At 3 hours post-exposure, effect estimates indicate small, nonsignificant differences in ratio (<1% lower) compared to the control for all treatments other than the three stone fire.
Discussion
We observed weak evidence of lower FVC, FEV1, FEF25–75, and FEV1/FVC following controlled exposure to cookstove air pollution immediately and 3 hours post-exposure (but not at 24 hours), compared to a filtered-air control. Lower lung function immediately post-exposure may be indicative of a pulmonary irritant mode of action; more delayed (e.g., 3-hour) responses may suggest a secondary inflammatory mode of action. While we observed some small differences in response among lower PM2.5-emitting stoves versus higher PM2.5-level emitting stoves, our results do not suggest an acute exposure-response relationship exists between cookstove exposure defined by PM2.5 mass and lung function. However, our generally null results are difficult to interpret in light of previous studies.
Several controlled exposure studies evaluating different pulmonary-health relevant markers (e.g., airway inflammatory markers, exhaled NO, clinical irritation symptoms) have indicated potential for wood smoke air pollution to elicit acute respiratory tract inflammation and irritation (Barregard et al. 2008; Riddervold et al. 2012; Stockfelt et al. 2012), demonstrating plausibility for a lung function response. Yet, in the three other studies that have evaluated lung function impacts following controlled wood smoke exposures, authors report no differences between smoke exposures and clean-air control (Sehlstedt et al. 2010; Ghio et al. 2012; Riddervold et al. 2012). In light of these studies, our results add to consistency in null results for acute lung function responses post-exposure. However, compared to these studies, our work evaluated different post-exposure follow-up times (capturing different points of the mechanistic pathway, which may have different expected effect magnitudes), had a larger sample size (which gives more power to detect small changes), and tested multiple exposure treatment levels, including ones at higher PM2.5 levels (which allows for consideration of a trend across exposure levels and contributes to more statistical power, presuming a larger effect at higher exposure). This lack of consistency makes interpretation across studies challenging.
Observational field studies generally support an association between exposure to cookstove-generated air pollution and reduced lung function. Cross-sectional studies in Malawi, Brazil, India, Nigeria, and Mexico have found decreased FVC and FEV1 among biomass/wood users compared to other fuel types (e.g., kerosene, charcoal, LPG) (Regalado et al. 2006; Desalu et al. 2010; Fullerton et al. 2011; da Silva et al. 2012; Revathi et al. 2012; Ibhafidon et al. 2014). Conversely, similar studies in Ecuador, Honduras, and Guatemala have found no associations or inverse associations between lung function (FVC and FEV1) and cookstove exposures (Rinne et al. 2006; Diaz et al. 2007; Clark et al. 2009). Studies that have considered acute changes following short duration biomass cooking exposures in Nigeria and Bangladesh have not found any changes in FEV1 or FVC (Oluwole et al. 2013; Medgyesi et al. 2017). Longer-term intervention studies in China, Mexico, and Guatemala have been more consistent in demonstrating improved lung function (or lessened decline in lung function) following use of lower PM2.5-emitting stoves (Romieu et al. 2009; Smith-Sivertsen et al. 2009; Zhou et al. 2014). In the RESPIRE trial in Guatemala, no associations were found between assignment of a plancha stove intervention and FEV1, FVC, or FEV1/FVC at follow-up through 18 months (Smith-Sivertsen et al. 2009). However, when exposure was defined as exhaled CO in breath measured concurrently with spirometry, investigators observed a 35 mL decrease in FEV1 (95% CI −61, −9 mL) and a 26 mL decrease in FVC (95% CI −57, 6 mL) – yet no change in FEV1/FVC – for each one unit increase in natural log transformed CO (Pope et al. 2015).
Differences in smoke composition may contribute to different pulmonary responses observed across various field and controlled exposure studies, as characteristics beyond PM2.5 concentration (for example, CO, NOx, or ultrafine particle fraction) can elicit health responses (Abolhassani et al. 2009; Traboulsi et al. 2017). Our characterization tests (conducted independent from the controlled exposures) showed that some co-emitted pollutants did not scale with increasing PM2.5 concentrations across the different treatments (Fedak et al. 2019). The multipollutant nature of our treatments may be a contributing factor to our lack of observed exposure-response across stove types, which might otherwise be expected due to the increasing PM2.5 levels. We chose cookstoves that represent a range of technology available in the field as feasible alternatives to three-stone fires, and set target PM2.5 levels based on what is reasonably expected for the stove under real operating conditions (Fedak et al. 2019). While the choice to use different stove types as the exposure sources and administering a multipollutant exposure precludes the ability to make exposure-response conclusions from our data, it allows us to address a realistic research question of how different stoves, with different multipollutant exposures, impact health. Future work - both observational field studies and controlled exposure studies - would benefit from including characterization of multiple health-relevant particle and gas phase pollutants in the studied cookstove exposures, to aid in comparison across studies and stove/fuel types and establishment of an exposure-response relationship across stove designs.
There are several limitations to our study design that may suggest alternative explanations for our generally null results beyond a true lack of association between cookstove-generated air pollution and respiratory effects. First, the post-exposure times for health measurements (approximately 30 minutes, 3.5 hours, and 24 hours post-exposure) were chosen based on the other health endpoints measured within our study that are not reported here (e.g., cardiovascular endpoints) (Langrish et al. 2012; Fedak et al. 2019); for logistical purposes, we maintained the same times for measurement of lung function. It is possible that these times do not correspond to peak responses within the mechanistic pathway for lung function changes and our study was not adequately powered to detect sub-peak responses (CIs suggest that our study was not powered to detect differences smaller than 3–4% of the total average FVC or FEV1). Our results suggest that lung function decreases within a few hours following exposure and then returns to baseline within 24 hours post-exposure; the largest response may occur at a time we did not capture, such as between 3 hours and 24 hours post-exposure. While previous null-result studies considered times of 4, 6, or 20 hours post-exposure (Sehlstedt et al. 2010; Ghio et al. 2012; Riddervold et al. 2012), our study may have been better powered to detect responses at these times due to the aforementioned differences in our study design. Further, the method of measuring lung function using spirometry lacks precision and is an insensitive endpoint in healthy individuals. This results in measurement error that reduces our statistical power, (increases standard errors and widens confidence intervals). Spirometry can also be influenced by the technician performing the test, as effective ‘coaching’ might result in a better effort from the participant. While technicians were not fully blinded to the treatment type, we do not believe that our technicians impacted their administration of the tests based on treatment. All technicians were trained to follow a standard procedure for testing, in compliance with the ATS guidelines, and we used a spirometry device that contained automatic features to ensure quality was met; further, which technician administered the test was independent and random with respect to the participants and treatments and often multiple technicians were present to provide oversight. Finally, we measured effects from acute exposures in a population of young, healthy, predominantly white individuals from a single community where air pollution exposures in their everyday life are low (Good et al. 2016). While this population was feasible to study from an ethical and logistical standpoint and allowed minimization of biases to contribute to strong internal validity, it does lack some generalizability towards other populations. The lack of observation of acute responses in this population does not preclude the occurrence of responses among different populations (e.g., children, elderly, or non-white) or with chronic exposures. Responses to air pollution exposures may be different among cookstove users of different racial groups (Jones MR et al. 2015; Sack et al. 2017); generalizability to populations of cookstove users of different racial backgrounds may be limited.
We included more treatments and a larger sample size than previous studies of wood smoke, ambient air pollution, and diesel exhaust (e.g., Sallsten et al. 2006; Barregard et al. 2008; Sehlstedt et al. 2010; Riddervold et al. 2011; Forchhammer et al. 2012; Ghio et al. 2012; Riddervold et al. 2012; Stockfelt et al. 2012; Bonlokke et al. 2014), which provided us with the ability to compare effects across a wider exposure range within a single study. While our research focus was on household air pollution, we included lower PM2.5 levels than many previous studies, comparable to ambient air pollution levels in cities throughout the U.S. and Europe. The results of this study have strong internal validity, as the balanced Williams square crossover design and protocols that restricted participant behavior contributed to a lack of confounders and allowed for more efficient analyses.
This study was designed to measure pulmonary responses to cookstove-generated air pollution exposures in a way that allows comparison of responses across several different cookstove designs with limited interaction, confounding, or other bias. We aimed to complement the data that can be ascertained from traditional epidemiologic designs (e.g., case-control or cohort studies in populations of cookstove users) by contributing to an understanding of the initial, acute pulmonary responses that occur with different real-life relevant exposures. Our research explored adverse health responses to stove emissions in healthy individuals whose normal exposure is low (Good et al. 2016). From a public health perspective, a more relevant research question is whether reducing or eliminating exposures among individuals who have been exposed to higher levels throughout their lifetime – such as through changes in cooking practices in communities using traditional cookstoves – can result in reduced or eliminated health burden. For the results of this study to translate towards this research question, we assume that the health impacts of cookstove-generated air pollution are reversible (e.g., removing exposure will result in an inverse response to that seen from being exposed) and that responses in those chronically exposed follow a similar mechanistic pathway as responses in those without chronic exposure. However, this may not be the case. Regardless, our study is not irrelevant to the target population of cookstove users. Individuals’ first exposures to cookstove emissions occur when young and generally healthy; further, exposures can be intermittent and transient, as they peak with cooking events. Information related to the initial, acute responses that occur with single exposures is relevant towards understanding the physiological pathways and potential for chronic effects with more lasting exposures.
Supplementary Material
Acknowledgements:
We are grateful to the volunteer participants of this study, the nurses and doctors from Heart Center of the Rockies who supported the project, and student technicians who operated our controlled exposure facility.
This work was supported by the NIH under Grant R01ES023688.
Footnotes
Disclosure of interest: The authors report no conflict of interest.
References
- Abolhassani M, Guais A, Chaumet-Riffaud P, Sasco AJ, Schwartz L. 2009. Carbon dioxide inhalation causes pulmonary inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 296(4):L657–L665. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, Olin AC. 2008. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occupational and Environmental Medicine. 65(5):319–324. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4 [sparse matrix methods; linear mixed models; penalized least squares; Cholesky decomposition]. J Stat Softw 67(1):48. [Google Scholar]
- Bilsback KR, Eilenberg SR, Good N, Heck L, Johnson M, Kodros JK, Lipsky EM, L’Orange C, Pierce JR, Robinson AL et al. 2018. The Firepower Sweep Test: A novel approach to cookstove laboratory testing. Indoor air 28(6):936–949. [DOI] [PubMed] [Google Scholar]
- Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Pruss-Ustun A, Lahiff M, Rehfuess EA, Mishra V, Smith KR. 2013. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect 121(7):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonlokke JH, Riddervold IS, Gronborg TK, Skogstrand K, Hougaard DM, Barregard L, Sigsgaard T. 2014. Systemic effects of wood smoke in a short-term experimental exposure study of atopic volunteers. J Occup Environ Med 56(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- Clark ML, Peel JL, Burch JB, Nelson TL, Robinson MM, Conway S, Bachand AM, Reynolds SJ. 2009. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. International Journal of Environmental Health Research. 19(5):357–368. [DOI] [PubMed] [Google Scholar]
- da Silva LF, Saldiva SR, Saldiva PH, Dolhnikoff M. 2012. Impaired lung function in individuals chronically exposed to biomass combustion. Environ Res 112:111–117. [DOI] [PubMed] [Google Scholar]
- Desalu OO, Adekoya AO, Ampitan BA. 2010. Increased risk of respiratory symptoms and chronic bronchitis in women using biomass fuels in Nigeria. Jornal Brasileiro de Pneumologia 36(4):441–446. [DOI] [PubMed] [Google Scholar]
- Diaz E, Bruce N, Pope D, Lie RT, Diaz A, Arana B, Smith KR, Smith-Sivertsen T. 2007. Lung function and symptoms among indigenous Mayan women exposed to high levels of indoor air pollution. Int J Tuberc Lung Dis 11(12):1372–1379. [PubMed] [Google Scholar]
- Fedak KM, Good N, Walker ES, Balmes J, Brook RD, Clark ML, Cole-Hunter T, Devlin R, L’Orange C, Luckasen G. 2019. Acute Effects on Blood Pressure Following Controlled Exposure to Cookstove Air Pollution in the STOVES Study. Journal of the American Heart Association. 8(14):e012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer L, Moller P, Riddervold IS, Bonlokke J, Massling A, Sigsgaard T, Loft S. 2012. Controlled human wood smoke exposure: Oxidative stress, inflammation and microvascular function. Particle and Fibre Toxicology. 9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton DG, Suseno A, Semple S, Kalambo F, Malamba R, White S, Jack S, Calverley PM, Gordon SB. 2011. Wood smoke exposure, poverty and impaired lung function in Malawian adults. Int J Tuberc Lung Dis 15(3):391–398. [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, Case M, Dailey LA, Richards J, Berntsen J, Devlin RB, Stone S, Rappold A. 2012. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occupational and Environmental Medicine. 69(3):170–175. [DOI] [PubMed] [Google Scholar]
- Good N, Molter A, Ackerson C, Bachand A, Carpenter T, Clark ML, Fedak KM, Kayne A, Koehler K, Moore B et al. 2016. The Fort Collins Commuter Study: Impact of route type and transport mode on personal exposure to multiple air pollutants. J Expo Sci Environ Epidemiol 26(4):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam K-bH, Mortimer K, Asante KP, Balakrishnan K, Balmes J et al. 2014. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2(10):823–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhafidon LI, Obaseki DO, Erhabor GE, Akor AA, Irabor I, Obioh I. 2014. Respiratory symptoms, lung function and particulate matter pollution in residential indoor environment in Ile-Ife, Nigeria. Niger Med J 55(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Kenward M. 2014. Design and analysis of cross-over trials. Third Edition. Boca Raton, Florida: CRC Press. [Google Scholar]
- Jones MR, Diez-Roux AV, O’Neill MS, Guallar E, Sharrett AR, Post W, Kaufman JD, Navas-Acien A. 2015. Ambient air pollution and racial/ethnic differences in carotid intima-media thickness in the Multi-Ethnic Study of Atherosclerosis (MESA). J Epidemiol Community Health. 69(12):1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward MG. 2015. Crossover designs In: Wright JD, editor. International Encyclopedia of the Social & Behavioral Sciences (Second Edition). Oxford, England: Elsevier; p. 349–353. [Google Scholar]
- Langrish JP, Bosson J, Unosson J, Muala A, Newby DE, Mills NL, Blomberg A, Sandstrom T. 2012. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med 272(3):224–239. [DOI] [PubMed] [Google Scholar]
- Medgyesi DN, Holmes HA, Angermann JE. 2017. Investigation of acute pulmonary deficits associated with biomass fuel cookstove emissions in rural Bangladesh. International Journal of Environmental Research and Public Health. 14(6):641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P et al. 2005. Standardisation of spirometry. The European respiratory journal. 26(2):319–338. [DOI] [PubMed] [Google Scholar]
- Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, Havens D, Pope D, Bruce NG, Nyirenda M et al. 2017. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. The Lancet 389(10065):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole O, Arinola GO, Ana GR, Wiskel T, Huo D, Olopade OI, Olopade CO. 2013. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Global journal of health science. 5(4):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Padilla R, Schilmann A, Riojas-Rodriguez H. 2010. Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis 14(9):1079–1086. [PubMed] [Google Scholar]
- Pope D, Diaz E, Smith-Sivertsen T, Lie RT, Bakke P, Balmes JR, Smith KR, Bruce NG. 2015. Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environmental Health Perspectives. 123(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regalado J, Perez-Padilla R, Sansores R, Paramo Ramirez JI, Brauer M, Pare P, Vedal S. 2006. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med 174(8):901–905. [DOI] [PubMed] [Google Scholar]
- Revathi M, Kutty TK, Annamalai N. 2012. Pulmonary function in rural women exposed to biomass fuel. Journal of Pulmonary and Respiratory Medicine. 2(7):1–4. [Google Scholar]
- Riddervold IS, Bonlokke JH, Molhave L, Massling A, Jensen B, Gronborg TK, Bossi R, Forchhammer L, Kjaergaard SK, Sigsgaard T. 2011. Wood smoke in a controlled exposure experiment with human volunteers. Inhal Toxicol 23(5):277–288. [DOI] [PubMed] [Google Scholar]
- Riddervold IS, Bonlokke JH, Olin AC, Gronborg TK, Schlunssen V, Skogstrand K, Hougaard D, Massling A, Sigsgaard T. 2012. Effects of wood smoke particles from wood-burning stoves on the respiratory health of atopic humans. Particle and Fibre Toxicology. 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne ST, Rodas EJ, Bender BS, Rinne ML, Simpson JM, Galer-Unti R, Glickman LT. 2006. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural Ecuador. Respiratory Medicine. 100(7):1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Riojas-Rodriguez H, Marron-Mares AT, Schilmann A, Perez-Padilla R, Masera O. 2009. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med 180(7):649–656. [DOI] [PubMed] [Google Scholar]
- Sack C, Vedal S, Sheppard L, Raghu G, Barr RG, Podolanczuk A, Doney B, Hoffman EA, Gassett A, Hinckley-Stukovsky K et al. 2017. Air pollution and subclinical interstitial lung disease: The Multi-Ethnic Study of Atherosclerosis (MESA) air-lung study. European Respiratory Journal. 50(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallsten G, Gustafson P, Johansson L, Johannesson S, Molnar P, Strandberg B, Tullin C, Barregard L. 2006. Experimental wood smoke exposure in humans. Inhal Toxicol 18(11):855–864. [DOI] [PubMed] [Google Scholar]
- Sehlstedt M, Dove R, Boman C, Pagels J, Swietlicki E, Löndahl J, Westerholm R, Bosson J, Barath S, Behndig AF et al. 2010. Antioxidant airway responses following experimental exposure to wood smoke in man [journal article]. Particle and Fibre Toxicology. 7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Sivertsen T, Diaz E, Pope D, Lie RT, Diaz A, McCracken J, Bakke P, Arana B, Smith KR, Bruce N. 2009. Effect of reducing indoor air pollution on women’s respiratory symptoms and lung function: The RESPIRE Randomized Trial, Guatemala. Am J Epidemiol 170(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, McCracken JP, Weber MW, Hubbard A, Jenny A, Thompson LM, Balmes J, Diaz A, Arana B, Bruce N. 2011. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): A randomised controlled trial. The Lancet 378(9804):1717–1726. [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F et al. 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockfelt L, Sallsten G, Olin AC, Almerud P, Samuelsson L, Johannesson S, Molnar P, Strandberg B, Almstrand AC, Bergemalm-Rynell K et al. 2012. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal Toxicol 24(1):47–59. [DOI] [PubMed] [Google Scholar]
- Thomas E, Wickramasinghe K, Mendis S, Roberts N, Foster C. 2015. Improved stove interventions to reduce household air pollution in low and middle income countries: A descriptive systematic review. BMC Public Health. 15:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traboulsi H, Guerrina N, Iu M, Maysinger D, Ariya P, Baglole CJ. 2017. Inhaled pollutants: The molecular scene behind respiratory and systemic diseases associated with ultrafine particulate matter. Int J Mol Sci 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- mU.S. EPA. 2009. Integrated Science Assessment (ISA) for particulate matter (Second External Review Draft, Jul 2009). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-08/139B, 2009. [Google Scholar]
- Vickers AJ, Altman DG. 2001. Analysing controlled trials with baseline and follow up measurements. B Med J 323(7321):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ. 1949. Experimental Designs Balanced for the Estimation of Residual Effects of Treatments. Aust J Sci Res Ser A 2(2):149–168. [Google Scholar]
- Zhou Y, Zou Y, Li X, Chen S, Zhao Z, He F, Zou W, Luo Q, Li W, Pan Y et al. 2014. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med 11(3):e1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


