Abstract
Background and Aims:
Lung cancer is the leading cause of cancer death worldwide. Cigarette smoking is the well-known risk factor for lung cancer. Epidemiological studies suggest that air pollution, especially particulate matter (PM) exposure, is associated with increased lung cancer risk and mortality independent of cigarette smoking.
Methods:
English-language publications focusing on PM, epigenetic changes, and lung cancer were reviewed. The epigenome serves as an interface between the environment and the genome. PM is one of the environmental factors that can cause epigenetic changes. The epigenome serves as an interface between the environment and the genome. Some of the epigenetic changes lead to increased disease susceptibility and progression. In cardiovascular disease and asthma, the association between PM exposure and the disease specific epigenetic changes has been identified. In lung cancer, the epigenetic changes in DNA methylation, histone modification and microRNA expression are commonly found, but the specific link between PM exposure and lung cancer remains incompletely understood.
Results:
The results of epidemiological studies indicate the important effects of PM exposure on lung cancer. PM2.5 is consistently associated with the increased lung cancer risk and mortality. Based on the epidemiological associations between PM exposure and lung cancer, PM-induced epigenetic changes may play important roles in the pathogenesis of lung cancer.
Conclusion:
In this review, we focus on the current knowledge of epigenetic changes associated with PM exposure and lung cancer. Better understanding of the link between PM exposure and lung cancer at the epigenomic level by comprehensive comparison approach may identify lung cancer early detection biomarkers and novel therapeutic targets.
Keywords: particulate matter, epigenetic changes, lung cancer
Introduction
In 2015, approximately 158,000 people will die from lung cancer in the United States (1). Cigarette smoking is the leading risk factor for lung cancer and accounts for more than 80% of all lung cancers. However, cigarette smoking rates have declined worldwide since the mid-1960s, whereas the lung cancer incidence and mortality rate continue to increase until 1990s, especially in women (1). Until recently, from 2005 to 2009, lung cancer incidence started to decrease among men in the United States and among women in certain regions in the United States (2). For decades, the concern of air pollution, especially the particulate matter (PM) exposure, as a risk factor of lung cancer has been raised, based on the timeline of industrialization, worsening air pollution and increasing lung cancer incidence despite the significant decrease of cigarette smoking rates. Several large cohort epidemiological studies were completed in Europe and the United States (3–6). The earlier studies focused on air pollution and the recent studies have more detailed information on the different components of air pollution, such as PM exposure levels. The association between air pollution and lung cancer risk is confirmed. However, the specific link between PM exposure and lung cancer remains incompletely understood.
In recent years, more attentions were focused on developing countries, such as China and India, for their larger populations and worse air quality. Air pollution with high levels of PM in some cities in China and India are sometimes 10 times worse than in the United States and European countries (7, 8). From the 2010 Global Burden of Disease (GBD 2010) Study, outdoor air pollution is among the top 10 risks of mortality worldwide and among the top 5 or 6 risks in the developing countries in Asia (9). Outdoor air pollution ranked 4th in mortality in East Asia and 6th in South Asia in 2010 (9). Air pollution and cigarette smoking are two separate risk factors but have very similar effects on human health. In contrast to the data in the United States, lung cancer incidence continues to increase in China. Lung cancer has surpassed liver cancer to become the leading cause of cancer death since 2008 (10). This represents a different trend of lung cancer incidence compared with the decreased incidence since 2005–2009 in the United States (2). The possible impact of air pollution, specifically the high PM levels, on lung cancer incidence has been in the headlines of public news media and scientific media. The fact that the outdoor air pollution is among the top risks of mortality highlights the importance and urgency of effective air pollution control (9).
In the United States, many efforts have been made to improve air quality. The Clean Air Act requires Environmental Protection Agency (EPA) established national ambient air quality standards since 1970. All the states are required to adopt enforceable plans to achieve the standards (11). In 1990, 20 years after the Clean Air Act in place, there were significant health benefits and economic benefits observed (12). The benefits continue to grow in the next 20 years from 1990 to 2010 (12). In Asia, Clean Air Asia was established in 2001 as a non-profit organization and it has been working on the implementation of strategies to improve air quality in Asian countries (13). It will take years to decades to see the health benefits from a better air quality.
In this review, we focus on the current knowledge of epigenetic changes associated with PM exposure and lung cancer. Better understanding of the link between PM exposure and lung cancer at the epigenomic level may serve as biomarkers of early detection and reveal novel therapeutic targets.
Association between PM exposure and lung cancer
Ambient air pollution has a complex category of components. The mixed components pose significant difficulties in identifying the causative agent. For example, air pollutants that are associated to adverse health effects in human include but not limit to PM, polycyclic aromatic hydrocarbons (PAHs), diesel exhaust particles (DEP) and cigarette smoke. Many of the pollutants can be part of the compositions of PM. In different regions, PM has different compositions. In order to have a standardized nomenclature, EPA has defined PM into three subsets based on the particle size: coarse particles, or PM10, refer to particles with a diameter smaller than 10 μm but larger than 2.5 μm; fine particles, or PM2.5, refer to particles smaller than 2.5 μm in diameter; ultrafine particles, or PM0.1, refer to those with a diameter less than 0.1 μm (14). PM10 is mostly deposited along the large airway due to gravity and is relatively easy to be cleaned by mucociliary clearance. Smaller particles (PM2.5 and PM0.1) were found to be more potent. They are small enough to be aspirated into alveoli, to be absorbed into lung vascular endothelial cells, and to be transported into the systemic circulation (15) (Fig. 1). The circulating PM2.5 may mediate systemic inflammation, therefore, cause further secretion of inflammatory cytokines and cellular damages.
Figure 1.
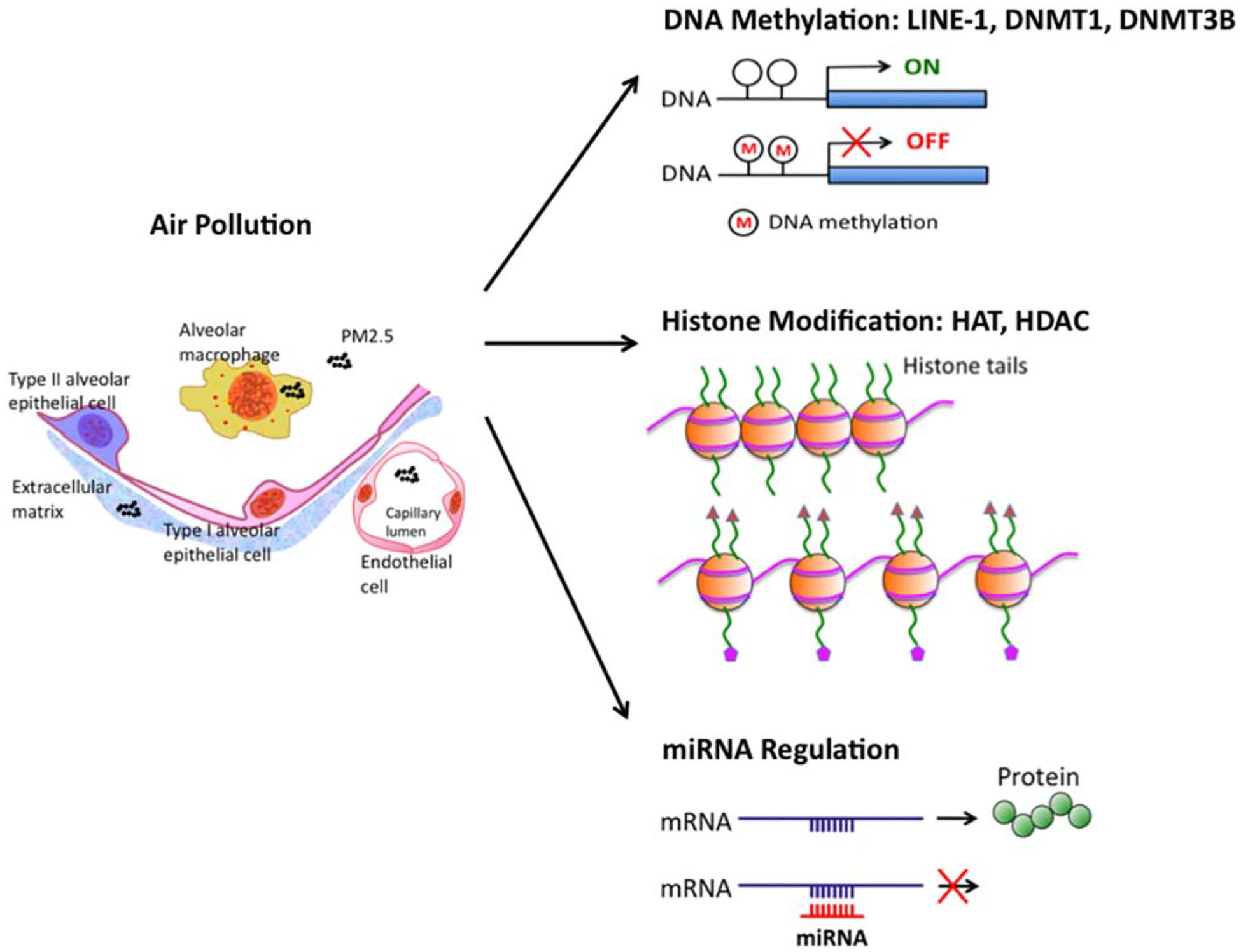
Epigenetic changes associated with PM exposure. PM2.5 is small enough to be aspirated into alveoli, to be absorbed either by alveolar macrophage or direct translocation into extracellular matrix and vascular endothelial cells, and to be transported into the systemic circulation. The circulating PM2.5 may induce epigenetic changes and systemic inflammation. PM2.5 exposure decreases DNA methylation of LINE-1, decreases DNMT1 and increased DNMT3B expression. PM10 exposure increases HAT activity and cigarette smoke decreases HDAC2 activity. PM2.5 exposure is associated with miRNA changes related to multiple inflammatory pathways.
PM2.5 contains a mixture of solid particles and liquid droplets, including black carbon, metals, nitrates and sulfates (16). PAHs and DEP can be part of the PM2.5, but they are usually discussed separately. There are outdoor and indoor sources of PM2.5. Industrial emissions and vehicle emissions are the common outdoor sources. Cigarette smoking, use of wood or nonsmokeless fuels for cooking or heating are the common indoor sources (14). Black carbon is formed by the incomplete combustion of fuels and biomass. Metals are mostly from industrial emissions. Common metals are iron, nickel and vanadium (17). PM2.5 can also be formed from chemical reactions of gases such as sulfur dioxide (SO2) and nitrogen oxides (NOx) include nitric oxide (NO) and nitrogen dioxide (NO2). In addition, PM2.5 from one region may circulate to another region by long-range transport. Thus, the outdoor and indoor sources, formation from chemical reactions and long-range transport from outside all contribute to regional PM2.5 levels.
Ambient air pollution has been proposed and studied to explain the increase in lung cancer risk and mortality in urban area (18, 19). One of the earlier cohort studies in 1995 identified the association between PM exposure and cardiopulmonary disease as well as lung cancer mortality, independent of cigarette smoking (20). The association between PM exposure and lung cancer mortality was re-demonstrated by the follow up study published by the same group in 2002 (4). In long-term exposure, each 10 μg/m3 increase in PM2.5 concentration was associated with 8% increased risk of lung cancer mortality (4). PM2.5 and SO2 were associated with all-cause, lung cancer and cardiopulmonary mortality (4, 20). More studies from Europe confirmed that PM exposure was associated with lung cancer risk due to NOx (5). A cohort study in life-long never-smokers showed that each 10 μg/m3 increase in PM2.5 concentration was associated with a 15–27% increase in lung cancer mortality in both men and women (21). Most recently, combined data from almost 313,000 people, the European study of cohorts for air pollution effects (ESCAPE) showed that long-term exposure to PM10 and PM2.5 were associated with an increased risk for lung cancer, particularly adenocarcinoma (6). A significant association between PM and lung cancer was found at a hazard ratio of 1.22 for each 10 μg/m3 increase of PM10 and a hazard ratio of 1.18 for each 5 μg/m3 increase of PM2.5 (6). Of note, PM exposure can increase the risk for lung cancer even below the levels recommended by European Union (6). In Japan, a large cohort study showed a lung cancer mortality hazard ratio of 1.24 for each 10 μg/m3 increase of PM2.5, after adjustment for confounding factors (22). They also found an association between SO2, NO2 and lung cancer mortality (22). Several studies revealed that traffic related NO2 are associated with increased lung cancer risk (5, 23). However, in the ESCAPE study, no association was found between lung cancer and NO2 or NOx (6).
The results of epidemiological studies indicate the important effects of PM exposure on human health and also raise many questions. Due to the different components in PM from different regions/countries, and the different information collected and studied, the results have some controversial aspects. More large-scale epidemiological studies, especially the comparison studies among different regions/countries, are needed to address these controversies and may eventually resolve the discrepancy observed. Due to the significantly delayed effects of PM exposure on human health, long-term epidemiological studies are essential. Nonetheless, so far PM2.5 is consistently associated with the increased lung cancer risk and mortality.
Epigenome and epigenetic changes
The constantly changing environment affects human health both at genomic and epigenomic levels. The epigenome serves as an interface between the environment and the genome. The epigenome is frequently changed in response to the environment, diet, exercise, disease and aging (24, 25). Some of the changes are transient and some of them are inheritable. For example, maternal exposure to air pollution during pregnancy has been shown to increase the risk of asthma in the offspring (26). It has been proven that some of the epigenetic changes lead to increased disease susceptibility and progression (27). As a result, epigenetic changes can be found in early stage of diseases or even before the disease can be detected by any other diagnostic studies. Therefore, understanding the epigenetic changes induced by PM exposure may provide an important tool for dissecting the association between PM exposure and lung cancer.
There are three main classes of epigenetic changes: DNA methylation, histone modification, and microRNA (miRNA) (28). All three of them can regulate gene expression simultaneously. DNA methylation is completed by three independent DNA methyltransferases (DNMTs): DNMT1, DNMT3A and DNMT3B (29). DNA methylation of cytosine residues in the CpG islands is one of the well-characterized epigenetic regulations in cancer. Genome-wide hypomethylation and gene specific hypermethylation of promoter of tumor suppressors are associated with cancer formation (30, 31). Histone modification, including acetylation, methylation, phosphorylation and ubiquitylation can regulate gene transcription levels. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) work together to balance histone acetylation (32). Genomic DNA is packaged in chromatin by associating with octamers of histone proteins (H2A, H2B, H3 and H4, two of each). Acetylation of histones reduces the affinity between histone proteins and DNA result in unpacking of DNA. Unpacked DNA with high accessibility for RNA polymerase II and transcription factor facilitates the transcriptional activation. Histone methyltransferases (HMTs) and histone demethylases (KDMs) work together to balance histone methylation. HMTs are enzymes that transfer methyl groups to the lysine or arginine on histone H3 and H4. KDMs (KDM1 to KDM6) remove methyl groups from the lysine residues (33). Increased transcription is found with high levels of acetylation and trimethylation of histone 3 lysine 4 (H3K4me3), lysine 36 (H3K36me3) and lysine 79 (H3K79me3) (34). DNA methylation and histone modification frequently regulate gene expression together at transcription levels.
On the other hand, miRNAs regulate gene expression at post-transcription levels. miRNAs are 20–22-nucleotide long RNAs that bind to mRNA, resulting in mRNA degradation or inhibition of protein translation (35). It has been speculated that some of the disease associated variants located in non-coding genomic DNA regions with unknown function may regulate certain miRNA levels. The miRNAs in turn regulate the translation of disease related proteins (36). In a number of cancers, miRNAs are identified to have multiple targets and contribute to malignant transformation by increasing oncogene levels or decreasing tumor suppressor gene levels (37).
PM-induced epigenetic changes
Recent studies have established that PM exposure can alter the epigenome, leading to dysregulation of gene expression (38, 39) (Fig. 1). PM2.5, PM10 and many compositions in PM were studied, either individually or in combination. It appears that PM exposure can cause epigenetic changes in all the three classes: DNA methylation, histone modification and miRNA.
After acute exposure to ambient PM2.5, DNA methylation of long interspersed nucleotide element (LINE)-1, a transposable element in the human genome, was decreased in blood samples (39). On the other hand, after chronic exposure to black carbon and sulfates, but not PM2.5, DNA methylation was decreased (40). Cigarette smoke, a component of PM, decreases DNMT1 and increased DNMT3B expression (41). This reflects the complex effects of PM exposure, based on their temporal and spatial variability.
PM10 exposure promotes inflammatory cytokine release in lung epithelial A549 cells by increasing HAT activity and acetylation of histone H4 (42). Co-treatment with HDAC inhibitors further enhanced the cytokine production, such as IL-8 (42). Cigarette smoke induced oxidative stress may cause reduction of HDAC2 (43). Exposure to DEP has been shown to decrease the HDAC1 activity to increase the acetylation of histone H4 (44). In alveolar macrophages, acute smoke exposure inhibits HDAC3 production (45). Taken together, the imbalance between HATs and HDACs leads to increased gene transcription of inflammatory cytokines in human bronchial epithelial cell line and chronic obstructive pulmonary disease (COPD) patients (44, 45).
Altered miRNA expression levels were found after the PM or DEP exposure in primary human airway epithelial cells (46, 47). Many of the miRNAs are associated with inflammatory or tumorigenesis pathways (46, 47). Altered miRNA expression levels have also been found after PM exposure in lung cancer A549 cells and in human blood (48–50). The changes in plasma miRNAs are thought to be involved in PM-induced cardiovascular disease risks (49). The relationship between PM-induced miRNA alterations and lung cancer risks is unclear.
Epigenetic changes associated with lung cancer
Genome-wide screen for DNA methylation and histone modification in lung cancer cell lines has provided some fundamental understanding of the epigenetic changes in lung cancer (51). In the presence of DNA methylation inhibitor and histone deacetylation inhibitor, coupled with gene expression profiling, several genes are found to be hypermethylated and downregulated in non-small cell lung cancer (NSCLC) cell lines (52). These genes function as tumor suppressors and the hypermethylation of these genes can be quantified and used as cancer biomarkers (52). In animal models, a 50% reduction in carcinogen-induced lung cancer development was observed after treatment with DNA methylation inhibitor and histone deacetylation inhibitor (53).
In human NSCLC tissues, mapping of DNA hypermethylation or hypomethylation revealed target genes that were silenced or activated, respectively (54). Of these, 11 CpG islands were methylated in 80–100% of the lung squamous cell carcinomas (54). In addition, aberrant DNA methylation can be detected noninvasively from sputum or plasma samples (55, 56). For example, the hypermethylation of the tumor suppressor, p16, may have clinical importance and may serve as a biomarker for early detection of NSCLC (55, 57). There are ongoing efforts to develop DNA methylation markers as a panel for early detection of lung cancer (58).
The pattern of histone modifications have been analyzed in human NSCLC (59). In stage I, large-cell or squamous cell carcinoma patients, higher levels of dimethylation of H3K4 predict better survival. In stage I, adenocarcinoma patients, lower levels of acetylation of H3K9 predict better survival (59). In stage II, patients with more than 5% of tumor cells with acetylation of H2AK5 have a better survival (59). One of the KDMs, histone H3 lysine 36 (H3K36) demethylase KDM2A, is frequently overexpressed in NSCLC tumors and cell lines and plays a role in the tumorigenic and metastatic processes (60). Several HDAC inhibitors are under investigation for their anti-tumor effects (61). However, how these specific histone modifications affect the expression of particular genes remains unknown.
miRNA expression profiles have been studied in lung cancer. Reduced expression of the let-7 miRNA has been found in human lung cancers (62). A fivemiRNA panel in sputum sample was studied recently for early detection of NSCLC (63). It was also found that miRNA expression profiles correlate with patient survival (64). These results indicate that miRNA expression profiles have diagnostic and prognostic values of lung cancer.
PM-induced epigenetic changes in lung cancer and other diseases
As discussed above, PM exposure can cause a wide range of epigenetic changes. Meanwhile, many epigenetic changes play important roles in lung cancer tumorigenesis. It becomes clear that the PM-induced epigenetic changes may contribute, at least partially, to the increased incidence of lung cancer in the United States after the decreased cigarette smoking but increased air pollution since mid-1960s. Similarly, lung cancer incidence continues to increase in the past decades with worsening PM exposure in China (10).
Based on our current knowledge, PM-induced epigenetic changes are likely to affect multiple genes in multiple pathways. To further complicate this, some PM components such as metals and PAHs, are known carcinogens themselves. They mediate the oxidative stress and DNA damage to promote tumorigenesis. Currently, the particular lung cancer specific epigenetic changes associated with PM exposure remain unclear.
In several other diseases, the association between PM exposure and the specific epigenetic changes are better understood. The PM levels are associated with an increase in emergency room visits and hospital admissions for cardiovascular diseases (65–67), with a strong association with the mortality rates (20, 66–68). It was found that the alterations of DNA methylation in LINE-1 are associated with PM exposure and the global DNA methylation changes contribute to PM-mediated cardiovascular mortality (39). The association between exposure to PAHs and childhood asthma and allergy are linked to hypermethylation of forkhead box P3 (FOXP3) in regulatory T cells (Treg) in asthmatic children (69). FOXP3 hypermethylation was associated with decreased Treg function and increased asthma severity and morbidity (69).
Future directions
The National Institutes of Health (NIH) roadmap epigenomics mapping consortium (70) is a major initiative that is intended to better understand of the role of epigenetic regulations in health and disease. Due to the complex constitution of PM2.5 and the complexity of human diseases, the bioinformatics tools, such as methylome sequencing (71), ChIP-seq (72) and miRNA-seq (73) are especially useful for providing unbiased information.
Epigenetic changes in DNA methylation, including genome-wide and promoter specific methylation, are currently the most investigated epigenetic changes in PM exposure research. Relatively fewer studies have been conducted on histone modification and miRNA expression. A comprehensive comparison study of DNA methylation, histone modification or miRNA profiling among the patients with high levels PM exposure lung cancer will elucidate the association between PM exposure and lung cancer. One of the important considerations is that different regions have different compositions of PM, which pose significant difficulties to reach conclusive results. By conducting comparison studies among multiple cities and multiple countries, we should be able to compare the PM compositions and analyze the epigenetic changes associated with them, with the goal of revealing disease specific epigenetic changes as biomarkers for lung cancer early detection and novel therapeutic targets.
Footnotes
Ethics
This is a review article. The manuscript has been read and approved by all co-authors. No human studies have been included.
Conflict of interest
No conflict of interest for all authors.
References
- 1.Cancer Fact and Figures 2015. American Cancer Society, 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. (Accessed 5 October 2015).
- 2.Lung cancer incidence trends among men and women—United States, 2005–2009. 2014. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6301a1.htm. (Accessed 5 October 2015). [PMC free article] [PubMed]
- 3.Dockery DW, Pope CA III, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24): 1753–9. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9): 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nafstad P, Haheim LL, Oftedal B, et al. Lung cancer and air pollution: a 27 year follow up of 16 209 Norwegian men. Thorax. 2003;58(12): 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14(9): 813–22. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet. 2010;375(9720): 1110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Li S, Tian Z, Pan X, Zhang J, Williams G. The burden of air pollution on years of life lost in Beijing, China, 2004–08: retrospective regression analysis of daily deaths. BMJ. 2013;347: f7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859): 2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest. 2013;143(4): 1117–26. [DOI] [PubMed] [Google Scholar]
- 11.Clean Air Act. Available at: http://www2.epa.gov/clean-airact-overview. (Accessed 5 October 2015).
- 12.Li J, Ewart G, Kraft M, Finn PW. The public health benefits of air pollution control. J Allergy Clin Immunol. 2012; 130(1): 22–3. [DOI] [PubMed] [Google Scholar]
- 13.Clean Air Asia. Available at: http://cleanairasia.org. (Accessed 5 October 2015).
- 14.United States Environmental Protection Agency. Particulate Matter (PM), 2014. Available at: http://www3.epa.gov/airquality/particlepollution/actions.html. (Accessed 5 October 2015).
- 15.Choi HS, Ashitate Y, Lee JH, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28(12): 1300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7): 989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179(12): 1115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh GK, Siahpush M, Williams SD. Changing urbanization patterns in US lung cancer mortality, 1950–2007. J Community Health. 2012;37(2): 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen AJ. Outdoor air pollution and lung cancer. Environ Health Perspect. 2000;108(Suppl. 4): 743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope CA III, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995; 151(3 Pt 1): 669–74. [DOI] [PubMed] [Google Scholar]
- 21.Turner MC, Krewski D, Pope CA III, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184(12): 1374–81. [DOI] [PubMed] [Google Scholar]
- 22.Katanoda K, Sobue T, Satoh H, et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol. 2011;21(2): 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyberg F, Gustavsson P, Jarup L, et al. Urban air pollution and lung cancer in Stockholm. Epidemiology. 2000;11(5): 487–95. [DOI] [PubMed] [Google Scholar]
- 24.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA. 2005;102(30): 10604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjornsson HT, Sigurdsson MI, Fallin MD, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299(24): 2877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126(3): 453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4): 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990): 457–63. [DOI] [PubMed] [Google Scholar]
- 29.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20(24): 3139–55. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143): 433–40. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2): 143–53. [DOI] [PubMed] [Google Scholar]
- 32.Robert T, Vanoli F, Chiolo I, et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Nature. 2011;471(7336): 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plass C, Pfister SM, Lindroth AM, Bogatyrova O, Claus R, Lichter P. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat Rev Genet. 2013;14(11): 765–80. [DOI] [PubMed] [Google Scholar]
- 34.Chi P, Allis CD, Wang GG. Covalent histone modifications– miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10(7): 457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RC, Feinbaum RL, Ambros V. The C. elegans hetero-chronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5): 843–54. [DOI] [PubMed] [Google Scholar]
- 36.Chorev M, Carmel L. Computational identification of functional introns: high positional conservation of introns that harbor RNA genes. Nucleic Acids Res. 2013; 41(11): 5604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10): 704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21(2): 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7): 572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madrigano J, Baccarelli A, Mittleman MA, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011;119(7): 977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu F, Killian JK, Yang M, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29(25): 3650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003;284(3): L533–40. [DOI] [PubMed] [Google Scholar]
- 43.Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007; 19(6): 694–700. [DOI] [PubMed] [Google Scholar]
- 44.Cao D, Bromberg PA, Samet JM. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37(2): 232–9. [DOI] [PubMed] [Google Scholar]
- 45.Winkler AR, Nocka KN, Williams CM. Smoke exposure of human macrophages reduces HDAC3 activity, resulting in enhanced inflammatory cytokine production. Pulm Pharmacol Ther. 2012;25(4): 286–92. [DOI] [PubMed] [Google Scholar]
- 46.Jardim MJ, Fry RC, Jaspers I, Dailey L, Diaz-Sanchez D. Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis-associated pathways. Environ Health Perspect. 2009; 117(11): 1745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleck B, Grunig G, Chiu A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190(7): 3757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollati V, Marinelli B, Apostoli P, et al. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect. 2010;118(6): 763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bollati V, Angelici L, Rizzo G, et al. Microvesicle-associated microRNA expression is altered upon particulate matter exposure in healthy workers and in A549 cells. J Appl Toxicol. 2015;35(1): 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fossati S, Baccarelli A, Zanobetti A, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25(1): 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shames DS, Girard L, Gao B, et al. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Med. 2006;3(12): e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong S, Fields CR, Su N, Pan YX, Robertson KD. Pharmacologic inhibition of epigenetic modifications, coupled with gene expression profiling, reveals novel targets of aberrant DNA methylation and histone deacetylation in lung cancer. Oncogene. 2007;26(18): 2621–34. [DOI] [PubMed] [Google Scholar]
- 53.Belinsky SA, Klinge DM, Stidley CA, et al. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63(21): 7089–93. [PubMed] [Google Scholar]
- 54.Rauch TA, Zhong X, Wu X, et al. High-resolution mapping of DNA hypermethylation and hypomethylation in lung cancer. Proc Natl Acad Sci USA. 2008;105(1): 252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62(8): 2370–7. [PubMed] [Google Scholar]
- 56.Hsu HS, Chen TP, Hung CH, et al. Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer. 2007;110(9): 2019–26. [DOI] [PubMed] [Google Scholar]
- 57.Belinsky SA, Nikula KJ, Palmisano WA, et al. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA. 1998;95(20): 11891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kagan J, Srivastava S, Barker PE, Belinsky SA, Cairns P. Towards clinical application of methylated DNA sequences as cancer biomarkers: A joint NCI’s EDRN and NIST workshop on standards, methods, assays, reagents and tools. Cancer Res. 2007;67(10): 4545–9. [DOI] [PubMed] [Google Scholar]
- 59.Barlesi F, Giaccone G, Gallegos-Ruiz MI, et al. Global histone modifications predict prognosis of resected non small-cell lung cancer. J Clin Oncol. 2007;25(28): 4358–64. [DOI] [PubMed] [Google Scholar]
- 60.Wagner KW, Alam H, Dhar SS, et al. KDM2A promotes lung tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin Invest. 2013;123(12): 5231–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gridelli C, Rossi A, Maione P. The potential role of histone deacetylase inhibitors in the treatment of non-small-cell lung cancer. Crit Rev Oncol Hematol. 2008;68(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 62.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11): 3753–6. [DOI] [PubMed] [Google Scholar]
- 63.Roa WH, Kim JO, Razzak R, et al. Sputum microRNA profiling: a novel approach for the early detection of non-small cell lung cancer. Clin Invest Med. 2012;35(5): E271. [DOI] [PubMed] [Google Scholar]
- 64.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3): 189–98. [DOI] [PubMed] [Google Scholar]
- 65.Pope CA III, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004; 109(1): 71–7. [DOI] [PubMed] [Google Scholar]
- 66.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA. 2008;299(18): 2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10): 1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7): 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nadeau K, McDonald-Hyman C, Noth EM, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4): 845–52. e10. [DOI] [PubMed] [Google Scholar]
- 70.Roadmap Epigenomics Project. 2014. Available at: http://www.roadmapepigenomics.org. (Accessed 5 October 2015).
- 71.Laird PW. Principles and challenges of genomewide DNA methylation analysis. Nat Rev Genet. 2010;11(3): 191–203. [DOI] [PubMed] [Google Scholar]
- 72.Schones DE, Zhao K. Genome-wide approaches to studying chromatin modifications. Nat Rev Genet. 2008;9(3): 179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1): 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


