In recent years, there has been substantial interest in the role of gut microbiota in the development of cardiovascular disease. Using metabolomic techniques, Trimethylamine-N-oxide (TMAO), was identified as one of the few microbiome-derived metabolites whose levels showed a strong correlation with increased risk of cardiovascular disease.1, 2 Choline-rich nutrients can be found in an omnivorous diets, particularly those rich in red meat. TMAO is formed from bacterial metabolism of choline via an intermediate, trimethylamine (TMA). TMA reaches the liver through portal circulation and undergoes hepatic oxidation through flavin monooxygenase 3 (FMO3).3 Mammals do not have the necessary enzyme, but gut microbes have TMA lyases, which can release the TMA from C-N bond of these nutrients and eventually generate TMAO.1 Interestingly, in mice, suppression of the intestinal microbiota blunted the formation of TMA from choline-rich diets, and prevented atherosclerosis.4
The effect of TMAO has been associated with vascular phenotypes of aging including endothelial dysfunction and vascular stiffness. TMAO, infused at a physiological level, promotes vascular inflammation through the activation of nuclear factor-kB (NF-kB) signaling in human aortic endothelial and smooth muscle cells.5 These changes beget the development of age-associated endothelial dysfunction.
Age-related changes in the gut microbiota occur progressively with time. The alpha-diversity of older individuals differs from those of young ones. Studies in community-dwelling older subjects show an increased occurrence of the microbial species known as Prevotella enterotype;6 this particular genus has been associated with high plasma TMAO levels in humans.4
Brunt et al.7 previously found that antibiotic treatment reversed endothelial dysfunction and enhanced nitric oxide availability in old mice through suppression of the gut microbiome. In this issue of the journal, Brunt et. al.8 adopted a different approach. Instead of using antibiotics to suppress gut microbiota which can produce off-target effects by altering the relative proportions of multiple genera; the authors supplemented TMAO in the diet of young mice and studied its impact on vascular oxidative stress and endothelial function. Interestingly, after TMAO supplementation, mice developed endothelial dysfunction as measured by decreased carotid artery endothelium-dependent vasodilation in response to acetylcholine.
The most important contribution of the present manuscript was the successful translation of their findings to humans. First, they reported high TMAO levels in the older study individuals. Second, they showed that brachial artery-flow mediated dilation was inversely correlated with TMAO levels supporting a relationship in humans. Finally, in harvested endothelial cells from old subjects with high TMAO, the authors reported elevated nitrotyrosine levels, a biomarker of vascular oxidative stress, figure. The suppression of oxidative stress via Tempol in mice or ascorbic acid in humans, restored endothelial function providing evidence that reactive oxygen species and subsequent vascular oxidative stress were the culprits; interestingly, the endothelial damage induced by these factors was reversible.
Figure. Schematic representation of the different phases involved in the production of Trimethylamine-N-oxide (TMAO) and its effect on age-related endothelial dysfunction.
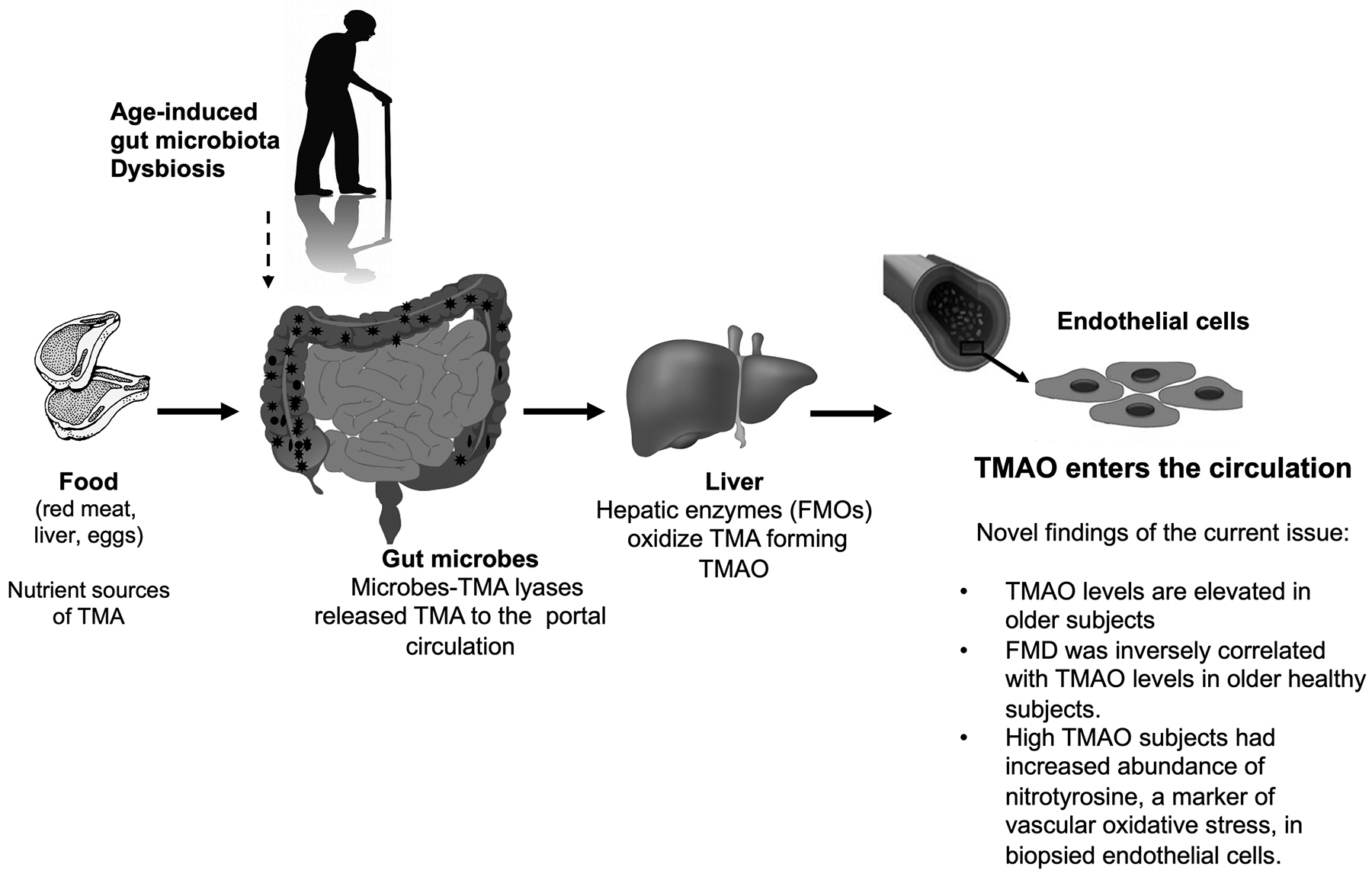
Red meat, liver, and eggs are rich in choline and carnitine. After ingestion, the gut microbiota uses these nutrients as carbo fuels, releasing TMA into the portal circulation. TMA is then converted into TMAO by flavin-containing monooxygenases (FOMs) in the liver. Aging associates with gut microbiota dysbiosis that alter the distribution of TMA producing species.
In this issue of the journal, Brunt et al. provided evidence that circulating TMAO is elevated in older adult and is associated with vascular oxidative stress and endothelial dysfunction.
Although the findings presented in this issue of the journal,8 supported an important role of TMAO in the development of age-associated endothelial dysfunction, further studies are needed to determine the mechanisms by which TMAO induces oxidative stress in the vasculature. TMAO does not appear to alter the most important pathways that contributes to vascular oxidation and eNOS uncoupling. In mice, TMAO supplementation did not affect activation of NFkB signaling, which was previously described,5 and does not appear to activate NADPH oxidases, which are major source of superoxide in the majority of experimental models of vascular endothelial dysfunction.9, 10 It remains unknown if the increased TMAO observed in the healthy old population was associated with specific changes in the proportion of species in the gut microbiota known to contribute to TMAO formation.
Interestingly, individuals on a vegan or vegetarian diet are known to have altered gut microbiota composition, which explains in part the decreased ability to produce TMAO even on a L-carnitine challenge (red meat).4 A pilot study, however, reported that fecal microbiota transplantation from a single lean vegan donor to obese subjects who effectively changed their gut microbiota, failed to elicit changes in TMAO production, and did not affect any parameters related to vascular inflammation.11
In conclusion, the paper by Brunt et al. advance the evidence of the role of TMAO in age-induced endothelial dysfunction, TMAO supplementation accelerates the aging of the vascular endothelium by inducing vascular oxidative stress. Future studies should evaluate if dietary restrictions that decrease the availability of choline-related nutrients such as meat, egg yolk, or liver improve endothelial function by reducing TMAO levels in older individuals.
Footnotes
Disclosures: None
Reference
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ and Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang WH and Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain MA, Fornasini G and Evans AM. Trimethylamine: metabolic, pharmacokinetic and safety aspects. Curr Drug Metab. 2005;6:227–240. [DOI] [PubMed] [Google Scholar]
- 4.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ and Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ and Shih DM. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP and O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. [DOI] [PubMed] [Google Scholar]
- 7.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vazquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R and Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. 2019;597:2361–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunt VE, Gioschia-ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR Hypertension. 2020;76:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R and Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. [DOI] [PubMed] [Google Scholar]
- 10.Channon KM and Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol. 2002;53:515–524. [PubMed] [Google Scholar]
- 11.Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL and Nieuwdorp M. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]


