Abstract
Purpose of review
Pediatric acute kidney injury (AKI) in critically ill patients is associated with increased morbidity and mortality. Emerging data support that the incidence of pediatric AKI in the ICU is rising. For children with severe AKI, renal replacement therapy (RRT) can provide a lifesaving supportive therapy. The optimal timing to deliver and modality by which to deliver RRT remain a point of discussion within pediatric (and adult) literature. This review discusses the use of RRT for pediatric patients in the ICU. We discuss the most recent evidence-based methods for RRT with a focus on continuous RRT.
Recent findings
The feasibility of dialyzing the smallest infants and more medically complex children in the ICU is dependent on the advancements in dialysis access and circuit technology. At present, data indicate that upward of 27% of children in the ICU develop AKI and 6% require RRT. Newer dialysis modalities including prolonged intermittent hemodialysis and continuous flow peritoneal dialysis as well as newer dialysis technologies such as the smaller volume circuits (e.g., Cardio-Renal Pediatric Dialysis Emergency Machine, Newcastle Infant Dialysis and Ultrafiltration System) have made the provision of dialysis safer and more effective for pediatric patients of a variety of sizes.
Summary
Renal replacement in the ICU requires a multidisciplinary team approach that is facilitated by a pediatric nephrologist in conjunction with intensivists and skilled nursing staff. Although mortality rates for children on dialysis remain high, outcomes are improving with the support of the multidisciplinary team and dialysis technology advancements.
Keywords: acute kidney injury, pediatric, renal replacement therapy
INTRODUCTION
Acute kidney injury (AKI) is an increasingly frequent health complication, occurring in one in three hospitalized children worldwide [1,2]. In a recent prospective study of over 4000 children admitted to pediatric intensive care units (PICUs) across four continents, AKI developed in 26.7% of children of whom 5.8% required renal replacement therapy (RRT) [2], with modalities including peritoneal dialysis, hemodialysis, and continuous RRT (CRRT). Pediatric AKI is associated with prolonged critical care admissions, greater mechanical ventilation needs, and longer overall hospitalizations [3–5]. The incidence of chronic kidney disease after pediatric AKI is as high as 20–50% with 10–12% of children requiring chronic dialysis within 5 years after hospital discharge [6–8]. Furthermore, the presence of AKI is associated with significantly higher mortality in hospitalized children with mortality rates as high at 30–50% in children requiring RRT [9,10]. However, it is well accepted that effective and appropriately prescribed RRT is associated with significantly better survival [11,12].
In this review, we will provide current literature-supported indications for RRT and discuss the use of RRT in parallel with the unique technical challenges associated with providing RRT in the smallest of children.
INDICATIONS FOR DIALYSIS IN THE ICU
The optimal timing of initiation of RRT for patients remains unknown. RRT is not risk-free, and careful consideration to use must be given on an individual basis. Overall, RRT is generally safe – even in infants – although it is not without risk for complication such as allergic reactions to system components, hypotension, fatal arrhythmias, complications of anticoagulation, and complications due to vascular access [13]. There is a strong consensus that the benefits of RRT outweigh the risks for universal indications such as life-threatening hyperkalemia, acidosis, intoxications/ingestions, hyperammonemia/inborn errors of metabolism, severe symptomatic uremia, oliguria/anuria, and volume overload associated with AKI. It is also well established that there is no well defined serum creatinine, cystatin C, or blood urea nitrogen value in pediatric patients that indicates the need for dialysis; however, emerging urinary biomarkers are increasingly being used to predict the need for dialysis. For example, urine neutrophil gelatinase-associated lipocalin has been shown to accurately detect AKI earlier and predict the need for dialysis in children after cardiac surgery better than serum creatinine alone [14]. Finally, several studies have evaluated the approach to RRT for sepsis-induced AKI. Cumulatively, there has been no definitive advantage shown with initiation of RRT for sepsis-induced AKI alone [11].
Fluid overload
Fluid overload may co-occur with AKI; for example, due to need for aggressive fluid resuscitation in septic patients or those with left ventricular cardiac dysfunction. Fluid overload is strongly associated with heightened morbidity and mortality in pediatric critical care patients; however, the underlying physiology leading to poor outcome is yet to be fully delineated [15,16]. Those with overt fluid overload not responsive to diuretics require consideration of RRT. Evaluation and team-based discussion for RRT should occur in the setting of more than 10% cumulative fluid overload. Initiation of RRT is advised if cumulative fluid overload is more than 20% [17]. Cumulative percentage fluid overload can be calculated as follows:
When evaluating daily fluid goals and fluid overload, the multidisciplinary team must ensure that adequate nutritional volume can be provided. Inability to provide nutrition due to fluid overload should signal strong consideration to early RRT implementation to prevent further fluid and electrolyte disturbance.
TECHNICAL CHALLENGES AND CONSIDERATIONS TO PROVIDING RENAL REPLACEMENT THERAPY IN THE PEDIATRIC INTENSIVE CARE UNITS
The decision to offer RRT to the smallest pediatric patients is complicated by a multitude of challenges that require a multidisciplinary team approach: pediatric patient size and a limited availability of appropriately sized dialysis catheters; lack of machines designed for neonates and small pediatric patients; and certainly, the technical difficulties associated with access placement in the smallest of patients.
Patient size is a primary factor in choosing an RRT modality in the ICU. Size, particularly small size, may lead to significant technical challenges with placement of dialysis access. Currently available CRRT machines approved for use in the United States are approved by the Food and Drug Administration for patients weighing more than 25 kg; however, off-label use occurs routinely in pediatric nephrology care for children less than 25 kg given lack of otherwise feasible dialytic options. In neonates, peritoneal dialysis is often considered a first-line dialytic modality to support critically ill neonates with AKI, oliguria, and fluid overload. Peritoneal dialysis has distinct advantages to blood-based methods of RRT in that there is no vascular access required, no extracorporeal circuit, and no requirement for anticoagulation to provide RRT.
With use of filtration-based modalities, including CRRT and intermittent hemodialysis (IHD), it is possible that the circuit extracorporeal circuit volume in neonates and small children could be greater than 10–15% of a child’s blood volume. This necessitates the use of packed red blood cells to prime the circuit [18]. Blood priming a dialysis circuit increases the risk of reactions such as hypotension, hyperkalemia, hypocalcemia, and/or coagulopathy at circuit initiation [19]. These reactions are potentiated by both bradykinin release when packed red blood cells contact the hemodialysis filter membrane and due to the citrate containing preservatives in the blood products. Preparatory maneuvers can be done to diminish the bradykinin effect associated with blood priming a circuit such as administration of bicarbonate during circuit initiation, buffering the red cells used for blood prime, and/or administering the blood cells for blood prime postfilter [20]. Blood priming a circuit also sensitizes the immune system to blood antigens, an important concern for patients who may be more likely to require kidney transplantation in their future.
Dialysis access may be technically challenging to obtain for neonates and small children. The minimum recommended catheter size for hemodialysis is a 7-French double lumen [21]. With consideration given to Poiseuille’s law (resistance to flow through a tube is inversely proportional to the fourth power of its radius), the wider and shorter the lumen the higher the likelihood of achieving optimal blood flows and successful dialysis [22]. Catheters are generally dual lumen and composed of either silicone or polyurethane material. The preferred location for catheter placement is the right internal jugular vein. Catheter insertion in the right internal jugular vein is associated with a lower risk for complications compared with other potential catheter insertion sites [23–25]. Placement of the catheter in the subclavian vein increases risk of subclavian stenosis which, in turn, may prevent future attempts at creation of an arteriovenous (AV) fistula in the upper extremity [26]. The catheter tip should be located at the junction of the superior vena cava and right atrium (RA) or in the RA to provide adequate blood flow for RRT.
An additional consideration with vascular catheter placement is the use of a tunneled versus non tunneled access. Tunneled vascular catheters have the primary advantage of long-term use and feasibility for use at hospital discharge in outpatient dialysis units compared with temporary catheters. Temporary catheters carry a higher risk of infections and thrombosis and often require replacement due to dysfunction whereas tunneled vascular catheters are associated with longer patency and lower infection rates compared with nontunneled (temporary) vascular catheters [26,27]. Whereas a temporary catheter can be placed at the bedside by the PICU team, tunneled catheters do require more extensive surgery to insert and remove, such that nontunneled catheters may be a more appropriate acute option if short-term access (<1 week) is anticipated [26]. The ICU and nephrology teams should consider the risk for long-term loss of central vascular access with placement of large bore catheters and risk for subsequent stenosis of central vessels. The concern may be heightened in a patient the primary team believes may someday require long-term RRT in transition to organ transplant.
For successful initiation of peritoneal dialysis, the majority of infants and children with AKI undergo placement of a double cuffed peritoneal dialysis catheter with a downward or lateral positioned exit site that is placed at a distance from any ostomy site and the general diaper area to decrease the risk of peritonitis [28]. Successful placement of the peritoneal dialysis catheter depends upon an exit site supported by subcutaneous tissue to prevent leakage. Whereas infants more than 2–3 kg are candidates for a double cuffed peritoneal dialysis catheter, smaller infants less than 2–3 kg often use a single cuffed or acute catheter because of their lack of substantial subcutaneous tissue [29]. The thin abdominal wall of low birth weight infants can lead to a high incidence of leakage and the need for catheter replacement [30,31]. Placement of peritoneal dialysis catheters in infants less than 1 kg may require novel positioning and be accompanied by considerable surgical (technical) difficulties associated with access placement in extremely low birth weight infants. In infants weighing less than 1000 g, case series data report use of single cuff vascular catheters, intravenous cannulas, and/or umbilical venous catheters placed in the abdomen as modified peritoneal dialysis catheters [32,33]; however, one case report does document use of standard peritoneal dialysis equipment in novel positioning for acute peritoneal dialysis in an infant weighing less than 1000 g [34] (Fig. 1).
FIGURE 1.
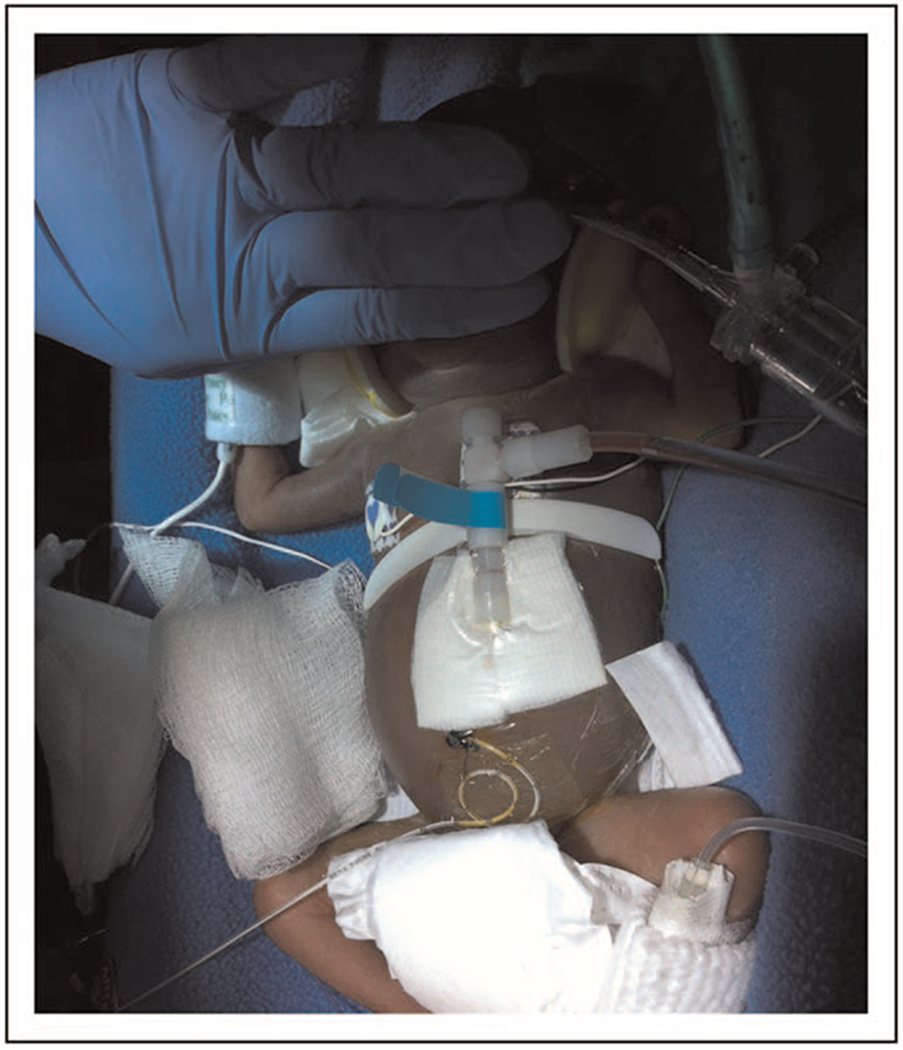
Size is a critical limitation for use of dialysis equipment in neonates. An 800-g infant is pictured here with use of standard peritoneal dialysis equipment [34]. Note that standard equipment was placed in the left upper quadrant to allow for adequate positioning of the catheter within the peritoneum.
MODALITIES OF RENAL REPLACEMENT THERAPY COMMONLY USED IN THE ICU
The modality chosen to deliver RRT in the pediatric ICU patient is often guided by a variety of factors including institutional resources, local expertise, patient characteristics, treatment goals, and physician preference.
Continuous renal replacement therapy
CRRT is often the preferred modality for management of the critically ill, hemodynamically unstable patient needing dialysis in the ICU [35]. CRRT shares many principles of solute clearance and ultrafiltration with IHD but at much lower flow rates [21]. It reduces the risk of hypotension and increased intracranial pressure described with IHD via gradual solute and fluid removal [21]. Generally, CRRT provides the same solute clearance over 24 h that is provided by one IHD session. CRRT provides more efficient clearance than peritoneal dialysis with easier to control fluid removal which allows for greater liberalization of fluid intake and nutrition [36]. Disadvantages of CRRT primarily relate to the technical difficulties with providing therapy to neonates, infants, and small children as most commonly available CRRT circuits are not designed for use in persons weighing less than 20 kg [37]. At present, the most widely used CRRT circuits have extracorporeal volume circuits of 80–90 ml or well over 10% of an infant’s blood volume making patients at significantly greater risk for hypotension and requiring blood priming of the dialysis tubing and filter as described above. However, the Cardio-Renal Pediatric Dialysis Emergency Machine, the Newcastle Infant Dialysis and Ultrafiltration System, and the Aquadex show promising results as a smaller volume CRRT circuits (20–40 ml) specifically for use in neonates and small children and are becoming increasingly available [38▪▪,39,40▪].
CRRT requires careful anticoagulation management to maintain the circuit continuously at the slower blood flow rates characteristic of CRRT compared with IHD. Well defined citrate and heparin-based anticoagulation protocols have been defined for CRRT [41]. An inherent benefit of citrate is that anticoagulation is limited to the circuit rather than systemically delivered to the patient. Recent data have also shown longer circuit survival and reduced bleeding risks in children receiving citrate anticoagulation compared with heparin anticoagulation for CRRT [42–44]. Though of note, citrate anticoagulation protocols may need to be adjusted in the setting of liver dysfunction given that citrate is metabolized within the liver and can accumulate in liver failure.
In contrast to IHD, CRRT clearance of small solutes is determined by the replacement/dialysis fluid rate. A standard dose for CRRT is a dialysis fluid rate of 2000 ml/1.73m2/h though in the setting of hyperammonemia, high-dose rates of 4000–8000 ml/1.73 m2/h have been found to lead to more rapid clearance of ammonia with subsequent reduction in dialysate rate (500ml/h or 2000–4000 ml/h/1.73 m2) after consistent fall in ammonia levels to less than 400 μmol/l over 12–24 h of therapy [45,46].
Hemodialysis
The hemodialysis prescription must be calculated and individualized for each patient to provide adequate solute clearance and fluid removal. Assessment and adjustment of the prescription is needed prior to each therapy in the PICU setting [47]. Components of the hemodialysis prescription include the dialyzer, tubing (i.e., the hemodialysis circuit), blood flow rate, dialysate composition, dialysate flow rate, treatment duration and frequency, fluid removal goal, and anticoagulation. Similar to CRRT, dialyzer and tubing contain a fixed extracorporeal blood volume and should be chosen to maximize solute clearance, yet not exceed 10% of the patient’s total blood volume [48].
A hollow-fiber design within the dialyzer is the most common currently in use and contains thousands of hollow fibers in parallel structure, similar to the human capillary network. Small solute clearance is dependent on the clearance characteristics of the dialyzer, as determined by the surface area of the dialyzer [48]. Successful dialysis minimizes blood volume retained in the dialyzer but yet provides adequate solute clearance and fluid removal across the dialyzer. The tubing chosen provides a circuit from the patient to the dialyzer and with a return flow to the patient. Consideration should be given to size of tubing needed based on patient age (e.g., infant versus adolescent) [47].
In contrast to CRRT, the blood flow rate for hemodialysis determines the solute clearance: higher blood flows increase solute clearance by optimizing diffusion and convection. The PICU and nephrology teams must balance the benefits of a higher blood flow to increase solute clearance with the potential for cardiovascular compromise at higher blood flow rates.
Dialysate solution typically includes sodium, potassium, calcium, chloride, magnesium, bicarbonate, and glucose. Dialysate flow rate, like blood flow rate, is a determinant of solute clearance. To maximize the bidirectional transport of small solutes between the blood and dialysate, the dialysate flow rate should be at a rate of at least 1.5–2 times the blood flow rate [48].
Treatment duration and frequency may vary for the individual patient in the PICU; however, daily RRT may be needed in the acute setting. The length of each treatment is determined by the amount of solute clearance and fluid removal needed. It should be noted that accurate assessment of dry weight in children undergoing acute hemodialysis can be difficult but this assessment is essential to reverse the complications of fluid overload including hypertension and cardiovascular morbidity [49]. The use of noninvasive monitoring (NIVM) during hemodialysis treatments can reduce intradialytic symptoms (e.g., hypotension, headache, nausea, vomiting, cramping) while achieving dry weight [50]. NIVM uses hematocrit-guided ultrafiltration algorithms for safe and accurate fluid removal. These algorithms allow the bedside dialysis nursing staff and physician team to adjust fluid removal goals in real-time based on blood volume changes during the hemodialysis treatment. This is particularly helpful with younger children who are not able to verbalize symptoms of rapid or excessive fluid removal.
Using standard hemodialysis machines, hemodialysis delivered over 6–12h daily with low ultrafiltration rates, or prolonged intermittent RRT (PIRRT) has been described in the management of pediatric AKI. Survival rates for children with AKI receiving PIRRT have been reported as similar to those of children with AKI receiving CRRT (~50%) [51]. PIRRT is described as a mechanism to combine the slow sustained modality of continuous venovenous hemodialysis, ensuring hemodynamic stability and better biochemical clearance along with the cost effectiveness of conventional IHD [52]. Thus, PIRRT maybe particularly beneficial in smaller children where there is specific need to reduce the hemodynamic instability associated with traditional IHD or the increased clotting and blood loss associated with CRRT [53]; however, this modality may be limited in implementation by availability of nursing staff in many pediatric centers.
Heparin is the standard anticoagulant used for hemodialysis treatments with unfractionated heparin typically given as an initial bolus (25–50 U/kg) followed by a continuous infusion during dialysis [48]. In cases of coagulopathy, consideration can be given to use of isotonic saline flushes paired without systemic anticoagulation.
Peritoneal dialysis
Peritoneal dialysis is a safe, effective, and inexpensive form of RRT for pediatric AKI. It is quite feasible for ages ranging from neonates to adolescents. However, its use has declined with the availability of the advancements of extracorporeal therapies described above to support smaller infants and children [54]. Advantages of peritoneal dialysis, which are especially pertinent for infants and small children, include avoidance of vascular access as a critical point for children who are inherently at high-risk for end stage kidney disease and potentially in need of vessel preservation for chronic dialysis in the future [55]. Hemodynamic instability is infrequent as a result of a more physiologic and less proinflammatory process as compared with hemodialysis and in particular is more suitable for children who have peritoneal membrane surface areas greater than adults creating a more efficient gradient for solute and fluid removal. Peritoneal dialysis is also associated with better preservation of residual kidney function compared with hemodialysis. The dialysate contains dextrose and can provide a source of supplemental calories for the patient undergoing peritoneal dialysis, especially for whom hypoglycemia with fluid restriction may be a problem. The typical prescriptions in the setting of AKI involves high volume peritoneal dialysis in which each session lasts 24 h and is repeated daily, with a dwell time of 30–50 min, dwell volume of 800–1200 ml/m2, and 18–22 exchanges/24h [54]. In addition to standard prescriptions, more novel peritoneal dialysis prescriptions can be considered in the setting of inadequate clearance or ultrafiltration. Limited data suggest that continuous flow peritoneal dialysis (CFPD), using a fixed intraperitoneal volume and continuous flow of dialysate into and out of the peritoneal cavity using two peritoneal catheters or one double lumen catheter, could be advantageous in hypermetabolic states. To accomplish continuous flow, a dialysate flow rate is maintained with fresh peritoneal dialysis solution using an adapted CRRT set-up. In a study on the use of CFPD in children with AKI admitted to the ICU, CFPD was shown to be nine-fold more effective than conventional peritoneal dialysis for ultrafiltration and at least threefold to five-fold more effective for urea and creatinine clearance [54,56]. Technical challenges are higher than with standard peritoneal dialysis, including variable and unpredictable ultrafiltration rates, risk for infection with two catheters, complex mechanical setup requiring modified CRRT equipment, as well as unknown long-term effects of high dialysate flow rate on the peritoneum [57].
Limitations to peritoneal dialysis in the setting of AKI include the potential for mechanical obstruction or dysfunction, pericatheter leakage, infection, and hernias secondary to increased intraperitoneal pressure [58]. Delayed initiation of peritoneal dialysis for a period of 48–72 h or longer after peritoneal dialysis catheter insertion, and use of low fill volumes (10 ml/kg) at peritoneal dialysis initiation are most ideal to reduce the risks of these complications [3].
MULTIDISCIPLINARY DELIVERY OF RENAL REPLACEMENT THERAPY IN THE ICU
Pediatric RRT care is a unique component of critical care and should be provided in a pediatric center that is equipped with pediatric dialysis support, including a multidisciplinary nursing and physician team from both intensive care and pediatric nephrology specialties to deliver appropriate therapy. As noted previously, the equipment for RRT and a patient’s individual prescription require modifications across all age and weight populations. The multidisciplinary team should review fluid goals, including goals for fluid removal, on a daily basis and ensure there is continuous attention to adequacy of dialysis access. Providing hemodialysis and CRRT to the smallest patients is an intense and time-consuming process that requires attention to detail to ensure patient safety is a top priority. Nursing staff providing continuous bedside therapies, such as peritoneal dialysis or CRRT, should receive ongoing education to reinforce competency in the modality. AKI in the PICU may also require optimization of nutritional status in conjunction with registered dieticians to ensure optimal nutrition and metabolic status both during AKI and in renal recovery. Each of these unique aspects within the pediatric critical care population must be addressed to provide safe and effective RRT.
CONCLUSION
Provision of RRT in the pediatric critical care setting is challenging – our patients may range from less than 1kg to nearly 100 kg. There is a dearth of pediatric-specific equipment for RRT provision. Delivery of care in a center specializing in pediatric nephrology, with trained pediatric dialysis and critical care nurses, benefits the child and family through provision of multifaceted resources necessary to facilitate safe and effective provision of RRT.
KEY POINTS.
AKI may lead to need for RRT in the setting of metabolic sequelae (e.g., hyperkalemia and acidosis) and morbidity and mortality associated with fluid overload.
Filtration-based methods of RRT, such as continuous RRT or hemodialysis, may be technically challenging in infants due to lack of pediatric-specific machines and difficulty with obtaining adequate vascular access.
Provision of RRT in the ICU requires interdisciplinary planning between the ICU and pediatric nephrology teams as well as trained pediatric nursing staff to provide safe and effective RRT.
Acknowledgements
Financial support and sponsorship
K.S. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2TR002490 and grant 2015213 from the Doris Duke Charitable Foundation. L.A.H. is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK110443).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Kellum JA, Lameire N, Aspelin P, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2:1–138. [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017; 376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Galasso L, Picca S, Guzzo I. Dialysis modalities for the management of pediatric acute kidney injury. Pediatr Nephrol 2020; 35:753–765. [DOI] [PubMed] [Google Scholar]
- 4.Vaewpanich J, Akcan-Arikan A, Coss-Bu JA, et al. Fluid overload and kidney injury score as a predictor for ventilator-associated events. Front Pediatr 2019; 7:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessey E, Morissette G, Lacroix J, et al. Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol 2018; 13:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg AX, Suri RS, Barrowman N, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 2003; 290:1360–1370. [DOI] [PubMed] [Google Scholar]
- 7.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 2012; 59:523–530. [DOI] [PubMed] [Google Scholar]
- 8.Sigurjonsdottir VK, Chaturvedi S, Mammen C, Sutherland SM. Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm? Pediatr Nephrol 2018; 33:2047–2055. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CW, Symons JM. Acute kidney injury: can we improve prognosis? Pediatr Nephrol 2010; 25:2401–2412. [DOI] [PubMed] [Google Scholar]
- 10.Uber AM, Sutherland SM. Acute kidney injury in hospitalized children: consequences and outcomes. Pediatr Nephrol 2020; 35:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romagnoli S, Ricci Z, Ronco C. CRRT for sepsis-induced acute kidney injury. Curr Opin Crit Care 2018; 24:483–492. [DOI] [PubMed] [Google Scholar]
- 12.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000; 356:26–30. [DOI] [PubMed] [Google Scholar]
- 13.Shingarev R, Wille K, Tolwani A. Management of complications in renal replacement therapy. Semin Dial 2011; 24:164–168. [DOI] [PubMed] [Google Scholar]
- 14.Bojan M, Vicca S, Lopez-Lopez V, et al. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol 2014; 9:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001; 107:1309–1312. [DOI] [PubMed] [Google Scholar]
- 16.Askenazi DJ, Koralkar R, Hundley HE, et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol 2013; 28:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010; 55:316–325. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein SL. Overview of pediatric renal replacement therapy in acute renal failure. Artif Organs 2003; 27:781–785. [DOI] [PubMed] [Google Scholar]
- 19.Pasko DA, Mottes TA, Mueller BA. Pre dialysis of blood prime in continuous hemodialysis normalizes pH and electrolytes. Pediatr Nephrol 2003; 18:1177–1183. [DOI] [PubMed] [Google Scholar]
- 20.Brophy PD, Mottes TA, Kudelka TL, et al. AN-69 membrane reactions are pH-dependent and preventable. Am J Kidney Dis 2001; 38:173–178. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland SM, Alexander SR. Continuous renal replacement therapy in children. Pediatr Nephrol 2012; 27:2007–2016. [DOI] [PubMed] [Google Scholar]
- 22.Bunchman T, Gardner J, Kershaw D, Maxvold N. Vascular access for hemodialysis or CVVH(D) in infants and children. Nephrol Dial Transplant 1994; 23:314–317. [Google Scholar]
- 23.Cimochowski GE, Worley E, Rutherford WE, et al. Superiority of the internal jugular over the subclavian access for temporary dialysis. Nephron 1990; 54:154–161. [DOI] [PubMed] [Google Scholar]
- 24.Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant 1991; 6:722–724. [DOI] [PubMed] [Google Scholar]
- 25.Vanholder R, Ringoir S. Vascular access for hemodialysis. Artif Organs 1994; 18:263–265. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein SL, Macierowski CT, Jabs K. Hemodialysis catheter survival and complications in children and adolescents. Pediatr Nephrol 1997; 11:74–77. [DOI] [PubMed] [Google Scholar]
- 27.Coryell L, Lott JP, Stavropoulos SW, et al. The case for primary placement of tunneled hemodialysis catheters in acute kidney injury. J Vasc Interv Radiol 2009; 20:1578–1581quiz 1582. [DOI] [PubMed] [Google Scholar]
- 28.Zaritsky J, Warady BA. Peritoneal dialysis in infants and young children. Semin Nephrol 2011; 31:213–224. [DOI] [PubMed] [Google Scholar]
- 29.Zurowska AM, Fischbach M, Watson AR, et al. Clinical practice recommendations for the care of infants with stage 5 chronic kidney disease (CKD5). Pediatr Nephrol 2013; 28:1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulthard MG, Vernon B. Managing acute renal failure in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1995; 73:F187–F192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal E, Edefonti A, Murer L, et al. Peritoneal dialysis in infants: the experience of the Italian Registry of Paediatric Chronic Dialysis. Nephrol Dial Transplant 2012; 27:388–395. [DOI] [PubMed] [Google Scholar]
- 32.Macchini F, De Carli A, Testa S, et al. Feasibility of peritoneal dialysis in extremely low birth weight infants. J Neonatal Surg 2012; 1:52. [PMC free article] [PubMed] [Google Scholar]
- 33.Stojanovic VD, Bukarica SS, Antic JB, Doronjski AD. Peritoneal dialysis in very low birth weight neonates. Perit Dial Int 2017; 37:389–396. [DOI] [PubMed] [Google Scholar]
- 34.Harshman LA, Muff-Luett M, Neuberger ML, et al. Peritoneal dialysis in an extremely low-birth-weight infant with acute kidney injury. Clin Kidney J 2014; 7:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltramo F, DiCarlo J, Gruber JB, et al. Renal replacement therapy modalities in critically ill children. Pediatr Crit Care Med 2019; 20:e1–e9. [DOI] [PubMed] [Google Scholar]
- 36.Ricci Z, Goldstein SL. Pediatric continuous renal replacement therapy. Contrib Nephrol 2016; 187:121–130. [DOI] [PubMed] [Google Scholar]
- 37.Nada A, Bonachea EM, Askenazi DJ. Acute kidney injury in the fetus and neonate. Semin Fetal Neonatal Med 2017; 22:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.▪▪.Vidal E, Cocchi E, Paglialonga F, et al. Continuous veno-venous hemodialysis using the Cardio-Renal Pediatric Dialysis Emergency Machine™: first clinical experiences. Blood Purif 2019; 47:149–155. [DOI] [PubMed] [Google Scholar]; The article describes successful use of a pediatric-specific machine for designed to provide continuous venovenous hemodialysis for neonates and infants.
- 39.Coulthard MG, Crosier J, Griffiths C, et al. Haemodialysing babies weighing <8 kg with the Newcastle Infant Dialysis and Ultrafiltration System (NIDUS): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 2014; 29:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.▪.Menon S, Broderick J, Munshi R, et al. Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol 2019; 14:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]; Menon et al. report the use of an ultrafiltration device to provide a range of dialytic therapies in neonates and small children in a critical care setting.
- 41.Brophy PD, Somers MJ, Baum MA, et al. Multicentre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 2005; 20:1416–1421. [DOI] [PubMed] [Google Scholar]
- 42.Sik G, Demirbuga A, Annayev A, Citak A. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill children. Int J Artif Organs 2019; 43:234–241. [DOI] [PubMed] [Google Scholar]
- 43.Rico MP, Fernandez Sarmiento J, Rojas Velasquez AM, et al. Regional citrate anticoagulation for continuous renal replacement therapy in children. Pediatr Nephrol 2017; 32:703–711. [DOI] [PubMed] [Google Scholar]
- 44.Raymakers-Janssen PAMA, Lilien M, van Kessel IA, et al. Citrate versus heparin anticoagulation in continuous renal replacement therapy in small children. Pediatr Nephrol 2017; 32:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spinale JM, Laskin BL, Sondheimer N, et al. High-dose continuous renal replacement therapy for neonatal hyperammonemia. Pediatr Nephrol 2013; 28:983–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanudel M, Avasare S, Tsai E, et al. A biphasic dialytic strategy for the treatment of neonatal hyperammonemia. Pediatr Nephrol 2014; 29:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischbach M, Edefonti A, Schroder C, Watson A. Hemodialysis in children: general practical guidelines. Pediatr Nephrol 2005; 20:1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller D, Goldstein SL. Hemodialysis in children with end-stage renal disease. Nat Rev Nephrol 2011; 7:650–658. [DOI] [PubMed] [Google Scholar]
- 49.Patel HP, Goldstein SL, Mahan JD, et al. A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol 2007; 2:252–257. [DOI] [PubMed] [Google Scholar]
- 50.Jain SR, Smith L, Brewer ED, Goldstein SL. Noninvasive intravascular monitoring in the pediatric hemodialysis population. Pediatr Nephrol 2001; 16:15–18. [DOI] [PubMed] [Google Scholar]
- 51.Sethi SK, Bansal SB, Khare A, et al. Heparin free dialysis in critically sick children using sustained low efficiency dialysis (SLEDD-f): a new hybrid therapy for dialysis in developing world. PLoS One 2018; 13:e0195536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinha R, Sethi SK, Bunchman T, et al. Prolonged intermittent renal replacement therapy in children. Pediatr Nephrol 2018; 33:1283–1296. [DOI] [PubMed] [Google Scholar]
- 53.Ali US, Arya MK. Efficacy and safety of prolonged daily hemodialysis in critically ill children weighing less than 10 kg. Hemodial Int 2020; 24:108–113. [DOI] [PubMed] [Google Scholar]
- 54.Vasudevan A, Phadke K, Yap HK. Peritoneal dialysis for the management of pediatric patients with acute kidney injury. Pediatr Nephrol 2017;32:1145–1156. [DOI] [PubMed] [Google Scholar]
- 55.Sanderson KR, Warady BA. End-stage kidney disease in infancy: an educational review. Pediatr Nephrol 2020; 35:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raaijmakers R, Schroder CH, Gajjar P, et al. Continuous flow peritoneal dialysis: first experience in children with acute renal failure. Clin J Am Soc Nephrol 2011; 6:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verrina E, Perri K. Pediatric dialysis In: Technical aspects and prescription of peritoneal dialysis in children. 2nd ed Boston, MA: Springer; 2012. [Google Scholar]
- 58.Sanderson K, Zaritsky J, Warady B. Peritoneal dialysis in children In: Magee C, Tucker JK, Singh AK, editors. Core concepts in dialysis and continuous therapies. Switzerland: Springer; 2016. pp. 135–154. [Google Scholar]


