Abstract
Adenosine is a ubiquitous neuromodulator that plays a role in sleep, vasodilation, and immune response and manipulating the adenosine system could be therapeutic for Parkinson’s disease or ischemic stroke. Spontaneous transient adenosine release provides rapid neuromodulation; however, little is known about the effect of sex as a biological variable on adenosine signaling and this is vital information for designing therapeutics. Here, we investigate sex differences in spontaneous, transient adenosine release using fast-scan cyclic voltammetry to measure adenosine in vivo in the hippocampus CA1, basolateral amygdala, and prefrontal cortex. The frequency and concentration of transient adenosine release were compared by sex and brain region, and in females, stage of estrous. Females had larger concentration transients in the hippocampus (0.161 ±0.003 μM) and the amygdala (0.182±0.006 μM) than males (hippocampus: 0.134 ±0.003, amygdala: 0.115±0.002 μM), but the males had a higher frequency of events. In the prefrontal cortex, the trends were reversed. Males had higher concentrations (0.189±0.003 μM) than females (0.170±0.002 μM), but females had higher frequencies. Examining stages of the estrous cycle, in the hippocampus, adenosine transients are higher concentration during proestrus and diestrus. In the cortex, adenosine transients were higher concentration during proestrus, but were lower during all other stages. Thus, sex and estrous cycle differences in spontaneous adenosine are complex, and not completely consistent from region to region. Understanding these complex differences in spontaneous adenosine between the sexes and during different stages of estrous is important for designing effective treatments manipulating adenosine as a neuromodulator.
Keywords: Adenosine, Sex Differences, in vivo, Fast scan cyclic voltammetry, FSCV, Estrous cycle
Graphical Abstract

Introduction
Adenosine is an endogenous purine nucleoside that plays roles in sleep, vasodilation, and immune response (Latini and Pedata 2001; Fredholm et al. 2005). In the brain, adenosine builds up in the extracellular space after the breakdown of ATP, but is also directly released through synaptic vesicles and membrane transporters (Wall and Dale 2008; Etherington et al. 2009; Corti et al. 2013). Adenosine builds up on the timescale of minutes during stressful events in the brain (Park and Gidday 1990); for example, acting in a neuroprotective role during ischemia (Villarreal et al. 2003). Adenosine also signals on a rapid timescale, lasting a few seconds (Klyuch et al. 2012; Nguyen et al. 2014; Nguyen and Venton 2015; Ross et al. 2014). The faster mode of adenosine transiently modulates phasic dopamine (Ross and Venton 2015) and blood flow, which is correlated to oxygen release (Wang and Venton 2017; Wang and Venton 2019b). Adenosine based therapeutics have been proposed as treatments for several neurological diseases (Cunha 2016), and many of these diseases where adenosine could act as neuroprotective agent have sex differences. For example, ischemia occurs less frequently in women until the age of 80 (Siegel et al. 2010), schizophrenics have sex differences in severity of symptoms and response to therapeutics (Abel et al. 2010), and Parkinson’s disease is twice as prevalent in men than women, as estrogen has a protective effect on striatal dopamine loss in females (Gillies et al. 2014). Rapid adenosine might be important in providing neuromodulation in these diseases, and for schizophrenia and Parkinson disease, could provide a non-dopaminergic drug target, which reduces the development of tolerance to the treatment (Schwarzschild et al. 2006). However, almost all adenosine research in the brain has been performed in males, so there is little to no information about how adenosine signaling varies with regard to sex as a biological variable or during the female estrous cycle.
A few studies have examined sex differences regulated by adenosine, typically focusing on differences in adenosine receptors. In mice, there are sex differences in the regulation of heart rate, body temperature, and locomotor activity caused by differences in adenosine A1 receptor expression (Yang et al. 2007). Additionally, A1 and A2A receptors regulate the level of severity of learning deficits that accompany attention-deficit hyperactivity disorder, and those deficits vary between the sexes (Pires et al. 2009). Adenosine has also been implicated in the differences in cocaine addiction between males and females, with an A2A antagonist having greater effects on motivation in females (Doyle et al. 2012). There are sex differences in the metabolic enzyme adenosine deaminase after stroke, with males having reduced adenosine deaminase activity compared to females, which may provide males with more neuroprotection because less adenosine is broken down (Tavilani et al. 2008). These studies suggest there are sex differences in the adenosine system but no studies have monitored adenosine levels as a function of biological sex.
Sex differences are found in both regional brain structure and neurochemistry that result in behavioral changes (Gorski et al. 1978; Barker et al. 2009; Schwarz et al. 2012). In the prefrontal cortex, sex differences are found in microglial colonization (Schwarz et al. 2012), corticolimbic dopamine and serotonin systems (Duchesne et al. 2009), stress induced dysfunction (Shansky et al. 2004), and cognitive function (Overman 2004). In the hippocampus, sex differences have been observed in the effects of stress on dendritic spine density (Shors et al. 2001) and the expression of serotonin receptors (Zhang et al. 1999). In the basolateral amygdala, there are sex differences in extracellular concentrations of dopamine and serotonin (Mitsushima et al. 2006). Given that many sex differences have been observed for neurotransmitters and neuromodulators, we examined sex differences in adenosine in these three regions.
In this work, we explored sex differences in spontaneous, transient adenosine release in vivo for the first time. The concentration and frequency of transient adenosine release events was compared in the CA1 region of the hippocampus, the medial prefrontal cortex, and the basolateral amygdala. There were significant differences in both transient concentration and frequency between the sexes in all regions, with females having larger, less frequent transients in the hippocampus and amygdala, and males having larger, less frequent transients in the prefrontal cortex. We then explored the effect of estrous cycle in the hippocampus and prefrontal cortex, finding concentration and frequency of spontaneous adenosine transients vary with changes in stage of estrous. In the hippocampus, adenosine concentrations are highest during proestrus, when estrogen levels peak. These results reveal complex sex and estrous cycle differences in the frequency and concentration of spontaneous adenosine release which should be considered in developing treatments targeting the adenosine system.
Materials & Methods
Materials
All reagents were purchased from Fisher Scientific (Fair Lawn, NJ, USA) unless otherwise noted. Phosphate buffered saline was adjusted to pH 7.4 and was made with sodium chloride (NaCl, S271–3, 131.25 mM), sodium phosphate from Sigma Aldrich (St. Louis, MO, USA) (NaH2PO4, monohydrate, S9683–250G, 10.0 mM), potassium chloride (KCl, P217–500, 3.0 mM), sodium sulfate (Na2SO4, anhydrous, S421–1, 2.0 mM), calcium chloride, (CaCl2, dihydrate, Sigma Aldrich, 223506–500G, 1.2 mM), magnesium chloride (MgCl2, hexahydrate, M33–500, 1.2 mM).
Electrodes and fast-scan cyclic voltammetry
Carbon-fiber microelectrodes were prepared as previously described (Huffman and Venton 2008). In brief, a carbon fiber (T-650, Cytec Engineering Materials, West Patterson, NJ, USA) of 7 μm in diameter was aspirated into a glass capillary (1.2 × 0.68 mm) and pulled using a vertical pipette puller (model PE-21; Narishige, Tokyo, Japan) into two electrodes. The protruding carbon fiber was cut to 100–125 μm with a scalpel. Electrical connection was made by backfilling the capillary with 1 M KCl. The silver-silver chloride reference electrodes were made in house by electrodepositing chloride onto a silver wire (7440–22-4, Acros Organics, New Jersey, USA). Fast-scan cyclic voltammetry (FSCV) was used to detect and quantitate adenosine on a subsecond time scale (Swamy and Venton 2007; Venton and Cao 2020). The FSCV waveform was applied and data were collected through computer controlled HDCV software (University of North Carolina, Chapel Hill, NC, USA). A Dagan ChemClamp potentiostat (Dagan Corporation, Minneapolis, MN, USA) was used to apply the potential in conjunction with a Pine Research WaveNeuro headstage (AB01HS2-P, Pine Research Instrumentation, Durham, NC, USA). The applied waveform was from –0.40 V to 1.45 V and back at 400 V/s, for every 100 milliseconds against Ag/AgCl reference. Applying the waveform produces a large background current, thus data were background subtracted (10 cyclic voltammograms averaged) to remove non-Faradaic currents. Electrodes were post-calibrated with 1.0 μM adenosine in phosphate buffered saline immediately following animal experiments and the currents were used to estimate the concentrations of adenosine in vivo.
Animal experiments
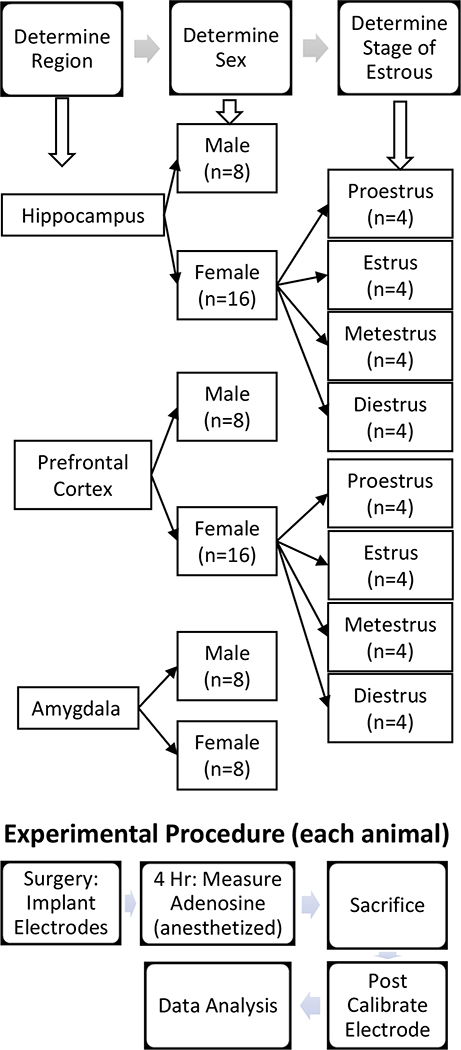
All animal experiments were conducted in accordance with protocol number 3517–10-17 approved by the Institutional Animal Care and Use Committee of the University of Virginia. A flowchart of the general animal experiment workflow can be seen in Figure 1. Animal welfare was monitored daily by animal care staff. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) between 250–425 grams and female Sprague-Dawley rats (RRID: RGD_734476 Charles River Laboratories, Wilmington, MA, USA) between 225–375 grams were housed in 12/12-hour light/dark cycles, fed ad libitum, and provided environmental enrichment. A power analysis was conducted to calculate acceptable sample size. In this determination, it was assumed that there was a confidence level of 95% and that minimum observable difference would be 20%. When calculated using a comparison of means with the cortex data, it determined that for a mean concentration of 0.244 μM with a standard deviation of 0.310, a minimum of 1055 transients would have to be observed, which equated to about 4 rats. The data presented are from 8 males per brain region and 8 females in the amygdala and 16 females from both the cortex and the hippocampus. Surgeries were performed in the morning, during the beginning of the light cycle. Estrous cycle determination was made by vaginal swab with sterile calcium alginate tipped applicators moistened with saline. Swabs were taken in the morning during the beginning of the light cycle, smeared on glass slides, and examined under light microscope in accordance with the Organization for Economic Cooperation and Development Histopath guidance, Part 5 (OECD 2008). No randomization was performed to allocate subjects in the study. Animals were assigned to different experimental groups by the experimenter without any blinding procedure. Study was not pre-registered.
Figure 1. Flow chart of experimental procedure and groups for animal experiments.
The experimenter decided the region that would be studied and the sex of the animal was recorded. If the animal was female, stage of estrous was determined by vaginal swab after the animal had been anesthetized. Surgery was performed under urethane anesthesia and the electrodes implanted. Spontaneous adenosine was measured for 4 hr. under anesthesia in the selected brain region. The animal was sacrificed by decapitation and a post experiment calibration of the electrode performed. The data were analyzed by software to find the adenosine transients and then statistical analysis performed.
Experiments were performed while animals were anesthetized to minimize impact to the animals. Urethane is a commonly used anesthesia for non-survival voltammetry surgery and fits well with our experimental timeline. Prior to the anesthetic injection with urethane (1.5 g/kg, i. p.), rats were initially anesthetized with isoflurane (1 mL/100 g rat weight). Surgical areas were exposed by shaving around the surgical sites. The rat was then placed in a stereotaxic frame and 250 μL of bupivacaine (Sensorcaine, MPF, APP Pharmaceuticals, LLC; Schaumburg, IL, USA) was injected subcutaneously at the top of the skull prior to incision to ensure no pain was inflicted. Holes were drilled in the skull for the placement of both working and reference electrodes (Paxinos and Watson 2007). The working carbon-fiber microelectrode was placed in the CA1 region of the hippocampus (in mm from bregma): AP: −2.5, ML: +2.0, DV: –3.0, the prefrontal cortex (in mm from bregma): AP: +2.7, ML: +0.8, DV: –3.0, or the basolateral amygdala (in mm from bregma): AP: −2.8, ML: +4.5, DV: –8.2. The Ag/AgCl reference electrode was placed on the contralateral side. The rat’s body temperature was regulated with a temperature-controlled heating pad and thermistor probe (FHC; Bowdoin, ME, USA). Rats were sacrificed via decapitation under anesthesia. One male and four female rats died during the course of the experiment.
Data collection and analysis
Electrodes were implanted and equilibrated for at least 30 min with the applied waveform prior to data collection. Data were excluded if fewer than 10 transients were observed within the initial 30 min. If robust transients were not found, a new electrode was inserted, up to five new electrodes for each animal. Eighty-two animals were excluded based on these criteria. After adenosine transients were identified, the electrode placement was optimized and data were collected for 4 h. An automated algorithm was previously developed to identify transient adenosines without bias using MATLAB, which resulted in a more rapid analysis than was possible manually (Borman et al. 2017). Here, to further separate the signal from the noise, data was also analyzed with a second custom-written MATLAB program that verified the presence of the secondary oxidation peak at an acceptable prominence. The original program analyzed the data for the presence of a secondary oxidation peak, but did not evaluate the quality and position of those peaks, and the secondary program cut down on false positives (i.e. identifying changes as adenosine that were not).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). Welch’s t-test was used to compare the mean adenosine transient concentrations and inter-event times of males and females. The distribution of concentrations and inter-event times of adenosine transients were analyzed using a Kolmogorov–Smirnov (K-S) test. The mean adenosine transient concentrations and inter-event times of the various stages of estrous were analyzed by one-way ANOVA. The distributions of the adenosine transient concentrations and inter-event times were analyzed using a Kruskal-Wallace test. Outlier tests were conducted using the ROUT method and eight rats scattered throughout the different groups were excluded due to these tests. With the addition of the eight animals removed due to outlier testing to the five animals that died during testing, the 82 removed due to exclusion criteria, and the 64 included in the study, 159 total animals were utilized in this study. Statistical significance was designated at p < 0.05 and all data are presented as mean ± standard error of the mean (SEM).
Results
Spontaneous Adenosine Detection in vivo
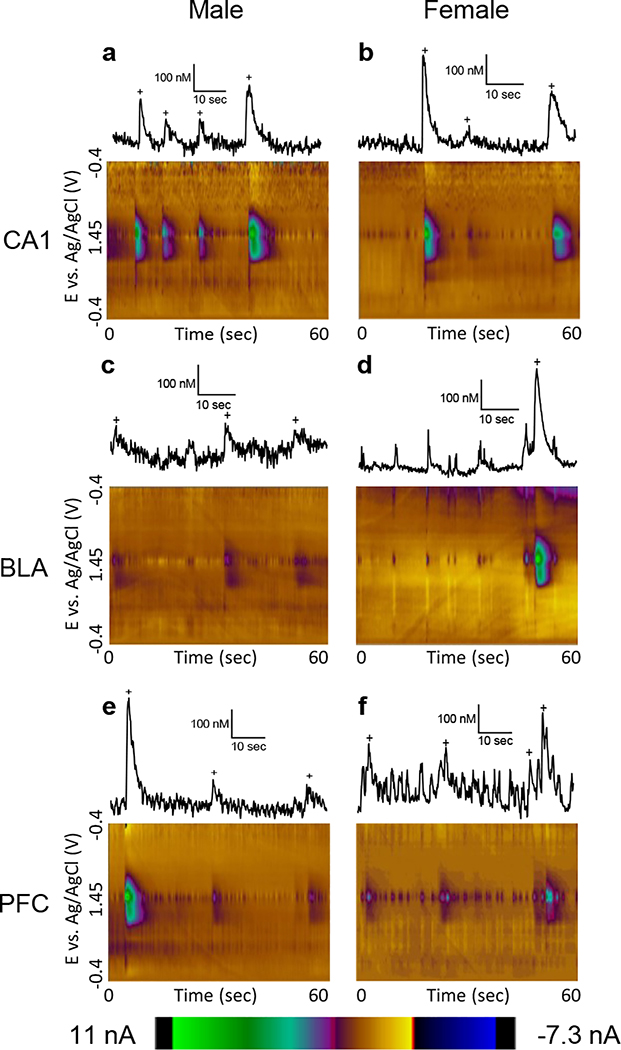
Adenosine was detected using fast-scan cyclic voltammetry at a carbon-fiber microelectrode, which allows real-time measurement of adenosine changes with subsecond temporal resolution (Shin et al. 2019; Swamy and Venton 2007; Nguyen and Venton 2015; Ganesana et al. 2017). This technique is best at identifying fast changes, due to background subtraction of data, and basal levels are not determined. Fig. 2 shows representative color plot data for each sex, by respective brain region as well as an extracted current vs. time trace for each color plot at the peak potential for adenosine oxidation (1.3 V on the back scan). Adenosine is identified from background subtracted cyclic voltammograms and false color plots by the two unique peaks produced by two sequential, two electron oxidation peaks characteristic of this compound (Swamy and Venton 2007). Automated programs are used to identify adenosine transients from the data (see Methods) (Borman et al. 2017). Peaks identified as adenosine are marked with + signs. Because there can be noise fluctuations at the primary oxidation voltage, an important part of the identification of a transient as adenosine is the presence of a peak at the secondary oxidation potential (1.0 V on the forward scan) that starts after the primary peak.
Figure 2. Example data from male and female rats from each brain region.
Concentration traces (top) and 3D color plots (bottom) compare adenosine release in the CA1 region of the hippocampus in (a) male) and (b) female rats, the basolateral amygdala in (c) male and (d) female rats, and the prefrontal cortex in (e) male and (f) female rats. Adenosine transients are marked with plus (+) signs in the concentration traces, which are all scaled the same to highlight the varying concentrations.
Comparison of Spontaneous Adenosine Transients in Males and Females
Three brain regions were chosen to examine the effect of sex on spontaneous adenosine: the hippocampus, prefrontal cortex, and basolateral amygdala. Our lab has previously studied transient adenosine release in the hippocampus and the cortex (Ganesana and Venton 2018; Lee and Venton 2018), but not in the amygdala. The amygdala was chosen because previous studies saw sex differences in neurochemistry and because it is also a part of the limbic system, controlling the formation of fear and emotional memory (Canli et al. 2002; Juraska et al. 2013; Barker et al. 2009; Garris and Wightman 1994; Ramikie and Ressler 2018). As shown in Fig. 2, the frequency of transients is highest in the hippocampus and lowest in the amygdala, with higher concentration transients in the cortex and amygdala, and lower in the hippocampus. Transients in the hippocampus tended to be larger and less frequent in females, compared to males (Fig. 2a, b). This relationship was also seen in the amygdala (Fig. 2c, d), although the transient frequency was greatly reduced compared to the other regions in females (Fig. 2d). In the cortex, sex differences were reversed, with males showing larger, less frequent transients and females having smaller, more frequent transients (Fig. 2e, f).
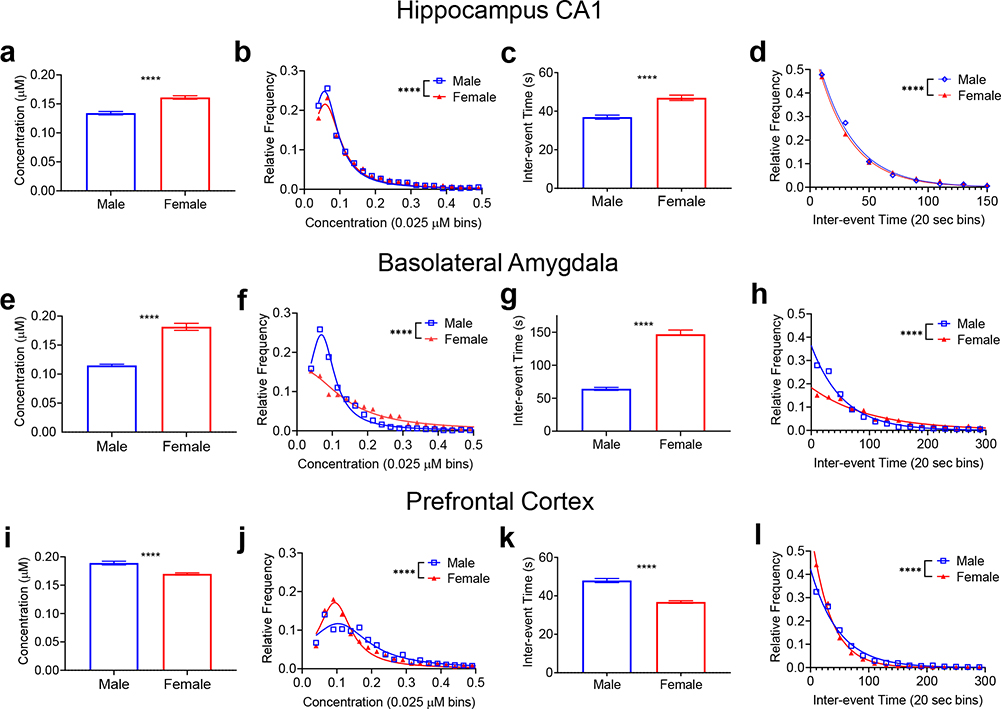
Figure 3 compares the average concentration and frequency of spontaneous adenosine transients in each brain region. Fig. 3a shows the average concentration of each event in the hippocampus, which was 0.134 ±0.003 μM for males and 0.161 ±0.003 μM for females, a significant difference (n=3080 and 4639, respectively Welch’s t-test, p<0.0001). A histogram of the distribution of the concentration values is shown in Figure 3b. The male rats had significantly more small concentration transients compared to females (K-S test, p<0.0001), which had a wider distribution of concentrations. The frequency of the transient events was also evaluated using inter-event time, the time in between two consecutive transients. The average inter-event time was significantly greater in females, 47±1 s, compared to males, 36±1 s (Fig. 3c, Welch’s t-test, n=3080 m/4639 f, p<0.0001). The distribution of inter-event times was also significantly different (K-S test, p<0.0001) (Fig. 3d). Thus, in the hippocampus, female rats had larger concentration adenosine transients, but these transients occurred less frequently than in males.
Figure 3. Comparison of spontaneous adenosine between males and females in the hippocampus, basolateral amygdala, and prefrontal cortex.
Mean transient concentrations (a) in females were significantly larger in the hippocampus (Welch’s t-test, n=3080 M/4639 F transients, p<0.0001, n is same for panels a-d) and (b) the distribution of transient concentrations was wider in the females (K-S test, p<0.0001). (c) Mean inter-event time was significantly longer in females in the hippocampus (Welch’s t-test, p<0.0001) (d) and the distribution of inter-event times was significantly wider in females (K-S test, p<0.0001). In the basolateral amygdala, the same trends are observed where (e) mean concentration (Welch’s t-test, n=1934 M/1057 F transients, p<0.0001, n is same for panels e-h) is significantly larger in females and the (f) frequency distribution is wider in females (K-S test, p<0.0001). The BLA (g) inter-event time is also significant longer in females (Welch’s t-test, p<0.0001) and (h) the distribution of inter-event times is wider in females (K-S test, p<0.0001). In the prefrontal cortex, the trend reverses. (i) Mean transient concentrations were significantly larger in males (Welch’s t-test, n=2393 M/6233 F transients, p<0.0001, n is same for panels i to l) and (j) the distribution was wider for males (K-S test, p<0.0001), while (k) inter-event time (Welch’s t-test, p<0.0001) was significantly longer in males with (l) the distribution was wider for males (K-S test, p<0.0001). All error bars are SEM. **** p<0.0001
In the basolateral amygdala, the average transient concentration was 0.115±0.002 μM for males, significantly less than 0.182±0.006 μM for females (n=1934 and 1057 transients, Welch’s t-test, p<0.0001) (Fig. 3e). The distribution of the concentration values (Fig. 3f) shows that males had significantly more smaller concentration transients (K-S test, p<0.0001). The inter-event times in females were longer than those in males with a mean of 147±6 s for females and 64±2 s for males (Fig. 3g, Welch’s t-test, p<0.0001). The distributions of interevent times were also significantly different (K-S test, p<0.0001) with males having more frequent transients (Fig. 3h). These trends are similar to those observed in the hippocampus, with the female rats showing larger, but less frequent transients, than the males.
The prefrontal cortex shows the opposite relationship between adenosine concentration and frequency for males and females. In males, the average transient concentration of 0.189±0.003 μM was significantly larger than the concentration of 0.170±0.002 μM in females (n=2393 and 6258 transients, respectively, Welch’s t-test, p<0.0001) (Fig. 3i). The concentration distributions confirm this trend, with a wider distribution in males than in females (K-S test, p<0.0001) (Fig 3j). For frequency, mean inter-event time was significantly longer in males (48±1 s) than in females (37±1 s) (Welch’s t-test, p<0.0001) (Fig. 3k). For the inter-event time distributions in Figure 2l, the distribution is wider in males (K-S test, p<0.0001). Thus, in the amygdala, transient concentration in the males was higher but the events were less frequent, and the trends were opposite those observed in the other two regions.
Comparison of Spontaneous Adenosine Transients among Brain regions
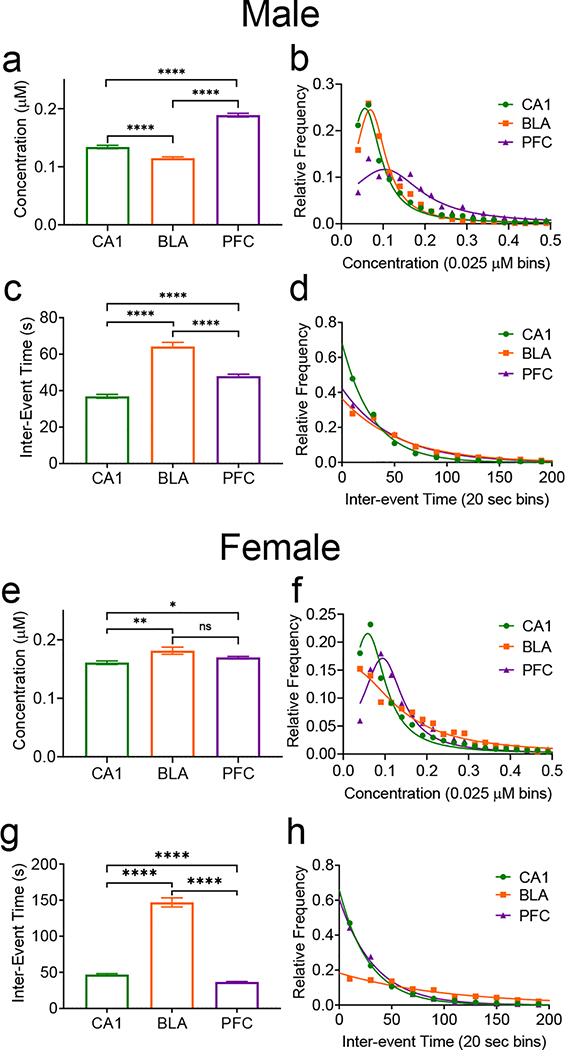
While Fig. 3 compared males and females for adenosine release per region, the trends in males and females were also compared among the three regions (Fig. 4). In males, the mean transient concentration was significantly different among all brain regions studied (n=3080/CA1, 1934/BLA, 2393/PFC transients, One-way ANOVA with Tukey’s multiple comparisons test, p<0.0001). The average concentration is highest in the prefrontal cortex (0.189±0.003 μM) followed by the hippocampus (0.134 ±0.003 μM) and finally the basolateral amygdala (0.115±0.002 μM) (Fig. 4a). The distributions for the transient concentrations were significantly different overall. The hippocampus and cortex were significantly different as well as the cortex and amygdala (Kruskal-Wallace test, p<0.0001), but not the hippocampus and amygdala (p>0.9999) (Fig. 4b). For inter-event time, the longest mean inter-event time was in the amygdala at 64±2 s, followed by the cortex at 48±1 s, and the hippocampus at 37±1 s, all of which were significantly different from each other (One-way ANOVA with Tukey’s multiple comparisons test, p<0.0001) (Fig. 4c). The inter-event time distributions were all significantly different with the amygdala having the widest distribution (Kruskal-Wallace test, p<0.0001) (Fig. 4d). In males, the highest concentrations were in the cortex, but the lowest frequency was in the amygdala.
Figure 4. Comparison of spontaneous adenosine transients in the hippocampus, basolateral amygdala, and prefrontal cortex in male (top, a-d) and female (bottom, e-h) rats.
(a) In males, there was an overall effect of region on the mean transient concentrations and all pairs of columns were significantly different from each other (One-way ANOVA, p<0.0001 Tukey’s multiple comparisons test, significance marked, n=3080 CA1/2393 PFC/1934 BLA transients, n is the same for all statistics, panels a-d). (b) There was a main effect of region on distribution of concentrations, and comparisons between individual regions were significant except between the CA1 and the BLA (Kruskal-Wallace test, p<0.0001, CA1-PFC & PFC-BLA p<0.0001). (c) There was a significant effect of region on inter-event time in males, with significant differences between all columns (One-way ANOVA, p<0.0001, Tukey’s multiple comparisons test, p<0.0001). (d) There was a significant main effect of frequency distribution in males, with all regions being significantly different (Kruskal-Wallace test, p<0.0001). (e) In females, there is a significant main effect of region on transient concentration and the concentration differed between the CA1-BLA and CA1-PFC, but not the PFC-BLA (One-way ANOVA, p=0.0009, with Tukey’s multiple comparisons test, n=4639 CA1/6258 PFC/1057 BLA transients, CA1-PFC p=0.0321, CA1-BLA p=0.0076, n is same for all female statistics, panels e-h). (f) In females, there is a main effect of region on concentration distribution and the CA1 is significantly different from both the PFC and the BLA, but the PFC and the BLA were not different (Kruskal-Wallace test, p<0.0001, CA1-PFC & CA1-BLA p<0.0001). (g) There is a significant main effect of region on inter-event time in females and comparison between all regions are significantly different (One-way ANOVA, p<0.0001, Tukey’s multiple comparisons test, p<0.0001). (h) Overall, there was a main effect of region on inter-event time distribution. However, the frequency distributions (Kruskal Wallace test, p<0.0001, CA1-BLA/PFC-BLA p<0.0001, CA1-PFC) were not significantly different between the CA1 and the PFC, but were significantly different between all other regions and overall. All error bars are SEM. **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05
In females, the amygdala has the highest concentration (0.182±0.006 μM), followed by the cortex (0.170±0.002 μM) and the hippocampus (0.161±0.003 μM) (Fig. 4e). Overall, the concentrations had a main effect of region (n=4639/CA1, 1057/BLA, 6258/PFC transients, One-way ANOVA with Tukey’s multiple comparisons test, p=0.0009). Individually, the hippocampus was significantly different from both other regions, but the amygdala and the cortex were not significantly different. For transient concentration distribution (Fig. 4f), there was also a main effect of region (Kruskal-Wallace test, p<0.0001). By region, the distributions followed the same pattern as the concentration with the hippocampus being significantly different from both other regions, but the cortex and amygdala were not. For inter-event time, the means (Fig. 4g) were significantly different among all regions, with the amygdala having the longest inter-event time at 147±6 s, followed by the hippocampus at 47±1 s, and the cortex at 37±1 s (One-way ANOVA with Tukey’s multiple comparisons test, p<0.0001). There was an overall effect of region of the distributions of the inter-event times (Fig. 4h), but the distributions of the cortex and the hippocampus were not significantly different from each other, while the amygdala was significantly wider than both (Kruskal-Wallace test, p<0.0001). In females, the highest concentration transients were in the amygdala, which also had the lowest frequency.
Both males and females had a much lower frequency in the amygdala than the other two regions but they differed greatly in the transient concentration in the amygdala, with males having a much smaller concentration compared to females. The relationship between the average concentration in the cortex and the hippocampus are similar between males and females, however the trends for inter-event times for those regions are reversed. Thus, the patterns in concentration and inter-event time were not the same between sexes for the different regions.
Estrous Cycle Comparison of Spontaneous Adenosine Transients
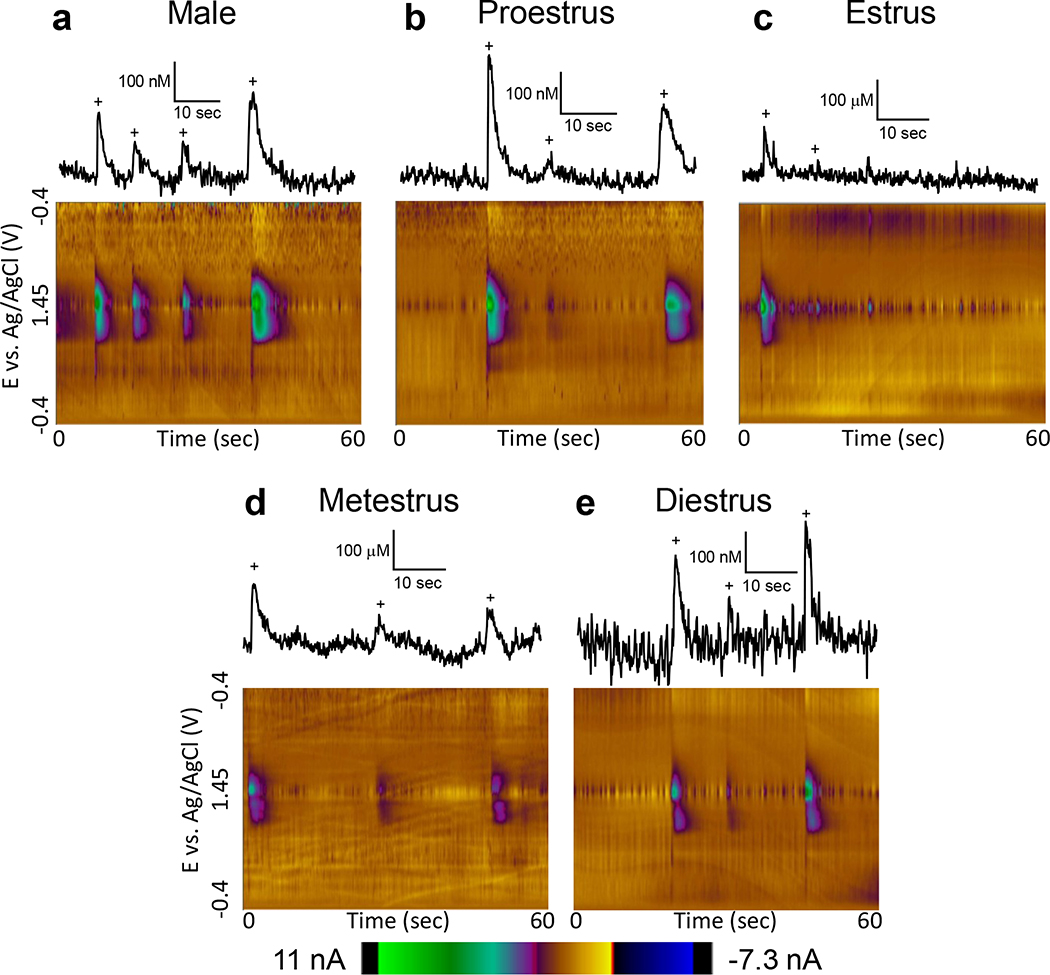
Female hormones vary throughout the estrous cycle, and these fluctuations in hormones could cause differences in neurochemistry (Yoshinaga et al. 1969; Gundlah et al. 1998). Thus, we examined how adenosine transients vary with the estrous cycle. Two regions were compared: the hippocampus, which had a high frequency of transients, and the cortex, because it had a different pattern of frequency and concentration sex differences from the hippocampus and amygdala. Fig. 5 shows representative color plots in the hippocampus from a male and from females in the proestrus, estrus, metestrus, and diestrus stages. An extracted concentration vs. time trace is shown with adenosine transients marked with a + sign. The frequency of transients was generally higher in males than any of the individual stages of estrous in females (Fig 5a). The concentration of the proestrus stage was the highest and proestrus and diestrus had greater concentrations than males (Fig. 5b, d). The estrus stage showed both the lowest frequency and the lowest concentration (Fig. 5e).
Figure 5. Example male data and female data by estrous cycle in the hippocampus.
Concentration traces (top) and 3D color plots (bottom) for adenosine transients in the CA1 region of the hippocampus in (a) male and female rats during (b) proestrus, (c) estrus, (d) metestrus, and (e) diestrus highlighting the differences in transient concentration and frequency between estrous stages. Adenosine transients are marked with plus (+) signs on the concentration traces, which are all scaled the same to highlight the variety of concentrations.
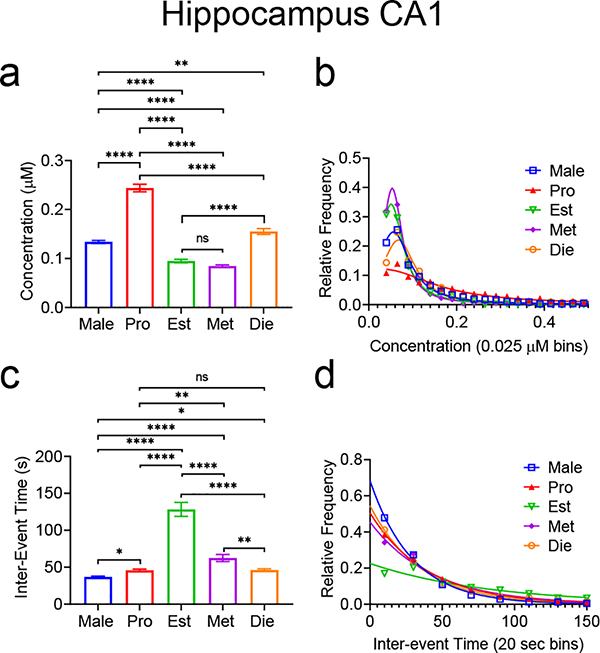
Figure 6a summarizes the hippocampus data. Overall, there was a significant main effect of estrous cycle on mean adenosine transient concentration (n=3080/Male, 1237/Pro, 558/Est, 858/Met, 1232/Die transients, One-way ANOVA with Tukey’s multiple comparisons test, p<0.0001). The highest mean concentration was during the proestrus stage at 0.243±0.008 μM, followed by diestrus at 0.155±0.006 μM, then males at 0.134±0.003 μM. Transient adenosine concentration in the hippocampus is lowest during estrus at 0.095±0.003 μM and metestrus at 0.085±0.002 μM, which were not significantly different from each other. Estrus and metestrus were, however, significantly smaller than all other stages and males. There were a variety of differences between the stages, which are categorized and summarized in Figure 6. The distributions of transient concentrations overall were significantly different, but individually, estrus and metestrus distributions were not significantly different, while all other stages were significantly different from each other (Fig. 6b, Kruskal-Wallace test, n=3080 Male/1237 Pro/558 Est/858 Met/1232 Di, p<0.0001). In Fig. 6c, there was a significant main effect of estrous cycle on average inter-event time (One-way ANOVA with Tukey’s multiple comparisons test, n=3080 Male/1237 Pro/558 Est/858 Met/1232 Die, p<0.0001). Males had the shortest inter-event time at 37±1 s, followed by proestrus and diestrus at 46±2 s, metestrus at 63±5 s, and estrus at 128±9 s. Proestrus and diestrus were not significantly different from each other, but all other comparisons were (One-way ANOVA with Tukey’s multiple comparisons test, p<0.0001, Pro-Met p=0.0010, Met-Die p=0.0014, Male-Pro p=0.0425, Male-Die p=0.0313). For the distribution of the inter-event times, there was a significant main effect of estrous stage/sex on inter-event time distribution and the distributions of proestrus, diestrus, and metestrus were not significantly different, while estrus and males were significantly different from each other and all other stages (Kruskal-Wallace test, p<0.0001) (Fig. 6d).
Figure 6. Spontaneous adenosine transient comparison in the hippocampus between male rats and female rats in various stages of estrous.
(a) Overall, there was a main effect of stages of estrous/sex on mean transient concentration (One-way ANOVA with Tukey’s multiple comparisons test, n=3080 Male/1237 Pro/558 Est/858 Met/1232 Die transients, p<0.0001, n is the same for all statistics in this figure). Differences between groups are marked (Tukey’s multiple comparison). (b) There is a main effect of stage of estrous/sex on the concentration distribution (Kruskal-Wallace, p<0.0001). Pairwise comparisons of different stages were also significantly different, except between estrus and metestrus, where there was no significance (Kruskal-Wallace test, Male-Pro/Male-Est/Male-Met/Male-Die/Pro-Est/Pro-Met/Pro-Die/Est-Die/Met-Die p<0.0001). (c) There was a significant main effect of estrous cycle/sex on mean inter-event time (One-way ANOVA, p<0.0001), with the mean inter-event time of the stages being significantly different, except between proestrus and diestrus (Tukey’s multiple comparisons test, p<0.0001, Pro-Met p=0.0010, Met-Die p=0.0014, Male-Pro p=0.0425, Male-Die p=0.0313). (d) There was a significant main effect of estrous stage/sex on inter-event time distribution (Kruskal-Wallace, p<0.0001) and pairwise comparisons were significantly different except between proestrus and metestrus, proestrus and diestrus, and metestrus and diestrus (Male-Pro/MaleEst/Male-Met/Male-Die/Pro-Est/Est-Male, Est-Die p<0.0001). All error bars are SEM. **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05
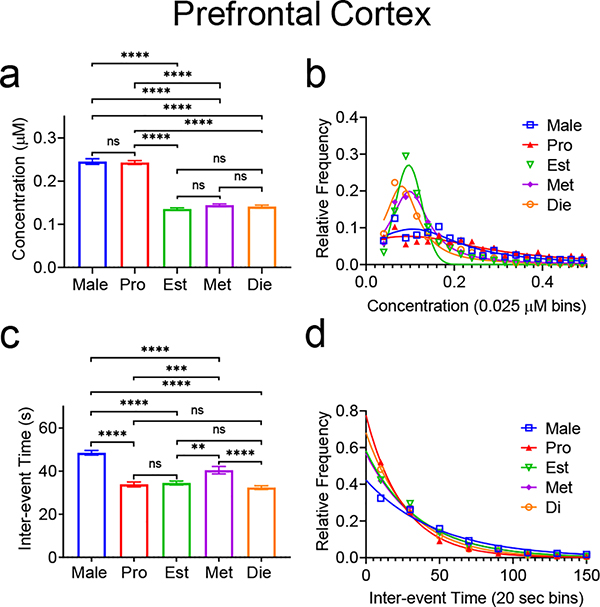
Due to the differences in patterns between the cortex and the other two regions, we also examined how stage of estrous affects adenosine in the cortex. Overall, there was a significant effect of estrous stage/sex for both mean concentration (Fig. 7a, One-way ANOVA with Tukey’s multiple comparisons test, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die, p<0.0001) and for concentration distribution (Fig. 7b, Kruskal-Wallace test, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die, p<0.0001). As shown in Figure 7a, the mean transient concentration for males (0.245±0.006 μM) and for proestrus (0.243±0.004 μM) are not significantly different. The mean concentrations for estrus (0.136±0.003 μM), metestrus (0.144±0.003 μM), and diestrus (0.141±0.003 μM) are also not significantly different from each other, but each stage is significantly different from the other groups (One-way ANOVA with Tukey’s multiple comparisons test, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die, p<0.0001). For the transient distributions, estrus and diestrus are not significantly different, and estrus is not significantly different from metestrus. All other distributions were significantly different from each other (Kruskal-Wallace test, p<0.0001, Fig. 7b). There was also a significant main effect of estrous cycle on both the mean inter-event time and the frequency distribution (Fig. 7c, One-way ANOVA with Tukey’s multiple comparisons test, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die, p<0.0001, Fig. 7d, Kruskal-Wallace test, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die p<0.0001). Average inter-event times (Fig. 7c) in proestrus (34±1 s), estrus at (34±1 s), and diestrus (32±1 s) were not significantly different from each other, but were significantly shorter than both males at 49±1 s and metestrus at 40±2 s (One-way ANOVA p<0.0001 with Tukey’s multiple comparisons test, Male-Pro/Male-Est/Male-Met/Male-Die/Met-Die p<0.0001, Pro-Met p=0.0015, Est-Met p=0.0062). For the inter-event time distributions, there was an overall significant effect of stage of estrous/sex (Kruskal-Wallace test, p<0.0001, Fig. 7d). By sex/region, there was no significance between proestrus and diestrus or between estrus and metestrus. All female stages were significantly different than males (Kruskal-Wallace test, Male-Pro/Male-Est/Male-Met/Male-Die/Pro-Est/Pro-Met p<0.0001, Met-Di p=0.0001, Est-Di p=0.0101).
Figure 7. Spontaneous adenosine transient comparison in the prefrontal cortex between male rats and female rats in various stages of estrous.
(a) There is a main effect of stage of estrous and sex on mean transient concentrations (One-way ANOVA, p<0.0001, n=2367 Male/1699 Pro/1668 Est/1765 Met/1776 Die transients, n is same for all statistics in this figure). Pairwise comparisons are marked (Tukey’s multiple comparisons test). (b) Overall, there was a main effect of stage of estrous/sex on transient concentration distribution (Kruskal-Wallace, p<0.0001). Pairwise comparisons of distributions were also significantly different, except between estrus and metestrus, and estrus and diestrus where there was no significant difference (Kruskal-Wallace test, Male-Est/Male-Met/Male-Die/ProEst/Pro-Met/Pro-Die p<0.0001, Male-Pro p=0.0001, Male-Die p=0.0453). (c) There was an overall main effect of stage of estrous/sex on inter-event time (One-way ANOVA, p<0.0001). Individual comparisons are marked (Tukey’s multiple comparison test). (d) There was a significant main effect of estrous stage/sex on the distribution of inter-event times (Kruskal-Wallace, p<0.0001). Pairwise comparisons of distributions were significantly different except between proestrus and diestrus, and estrus and metestrus (Kruskal-Wallace test, Male-Pro/Male-Est/Male-Met/Male-Die/Pro-Est/Pro-Met p<0.0001, Met-Die p=0.0001, Est-Die p=0.0101). All error is in SEM. **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05
The results of estrous cycle data were compared to see if any patterns emerged between regions. In both regions, the mean concentration of transients in the proestrus stage was significantly higher than that of the other stages. Otherwise, the patterns in the regions were different and not predictable, with variations in both concentration and inter-event time across the remainder of the stages.
Discussion
This study shows that the concentration and frequency of spontaneous adenosine release differs between male and female rats but that the patterns of sex differences is not the same in all brain regions. For example, females have larger, but fewer transients, in the hippocampus and amygdala, but in the cortex, females have smaller, but more frequent adenosine transients than males. Changes during the female estrous cycle were examined, and in the hippocampus, the concentration and frequency were high during estrus and proestrus. In the cortex, the pattern was different, with a high frequency release for all stages, but lower concentrations for estrus, diestrus, and metestrus. Thus, we demonstrate that spontaneous adenosine release varies across the estrous cycle in females. These results show that there are complex sex differences for adenosine which may lead to differences in neuromodulation, and that sex as a biological variable should be considered in studies of developing treatments targeting the adenosine system.
Spontaneous Adenosine Transient Varies by Sex
Sex differences have been observed in many neurochemicals; for example, dopamine release is larger and uptake is faster in females (Walker et al. 2000). Adenosine regulates phasic dopamine (Ross and Venton 2015), and so we hypothesized that adenosine might also have sex differences. Table 1 summarizes the major findings for sex differences in adenosine. We studied different brain regions because our previous studies showed that adenosine release varied greatly among regions (Nguyen et al. 2014; Lee and Venton 2018; Pajski and Venton 2013). In the hippocampus and amygdala, males had smaller concentration, more frequent transients while, in females, the transients were larger but at a lower frequency. In the cortex, the pattern was the opposite with larger, less frequent transients for males and smaller, more frequent transients for females. Thus, sex differences were not the same in every region.
Table 1.
Relative comparison of transient adenosine concentration and frequency between males and females in the hippocampus, basolateral amygdala, and prefrontal cortex
| Concentration | Frequency | |||||
|---|---|---|---|---|---|---|
| CA1 | BLA | PFC | CA1 | BLA | PFC | |
| Male | Low | Low | High | High | High | Low |
| Female | High | High | Low | Low | Low | High |
One trend evident from Table 1 is a trend towards higher concentrations of adenosine associated with lower frequencies of release. While the mechanism of release is not yet fully elucidated, adenosine is likely formed from extracellular metabolism of released ATP (White and Hoehn 1991; Cunha et al. 1996), and it is possible that more ATP builds up and is available for release when the frequency is lower. Changes in frequency of ATP release could affect the quantal size of the vesicles allowing them more time to accumulate ATP from within the cell. Exocytotically released ATP is packaged together with other neurotransmitters, such as acetylcholine or glutamate, or on its own (Pankratov et al. 2006), and release from different types of vesicles could also account for some of the observed discrepancies in concentration. However, the observed trend between high concentration and low frequency was not found when the stages of estrous were compared, and was not apparent in previous research (Wang and Venton 2019a; Nguyen et al. 2014). Thus, higher frequency leading to low concentrations is a trend that could be investigated further to determine if there is a clear mechanism to link frequency and concentration.
Multiple brain regions were investigated to determine if sex differences were consistent throughout the brain, and Fig. 4 shows that the patterns between regions were not the same in males and females. The concentrations varied significantly from region to region in males, while in females, the mean concentrations in the cortex and amygdala were similar. One of the biggest differences is that the amygdala has the lowest concentration in males but the highest concentration in females. The frequency was lowest (i.e. inter-event time was highest) in the amygdala for both sexes. Comparing the hippocampus and cortex, the frequency for males was higher in the hippocampus than the cortex, while the trend was flipped in females, which had a higher frequency in the cortex. Thus, the trends in frequency and concentration are complex and not consistent between the sexes.
The differences in patterns for females from males might be caused by different levels of estradiol. Progesterone and estrogen modulate neurological functions including oxidative stress, glial function, and mitochondrial function (Brinton et al. 2008). Barker and Galea showed that the estradiol concentrations in the hippocampus and the amygdala are much larger than in the cortex (Barker et al. 2009). Estradiol also increases cyclic adenosine monophosphate (cAMP) concentrations through the adenylate cyclase pathway in some estrogen dependent cells, although the brain was not studied (Aronica et al. 1994). Increased cAMP is generally excitatory and might increase the concentration of the adenosine transients in the amygdala and hippocampus, which have higher levels.
While hormone levels could affect receptor sensitivity, differences in spontaneous adenosine might also simply be due to differences in receptor density between males and females. Both A1 and A2A receptors regulate spontaneous adenosine release (Wang and Venton 2019b; Wang and Venton 2017; Nguyen et al. 2014), with A1 receptors antagonists increasing the frequency of adenosine release (Dunwiddie and Fredholm 1984) and A2A receptor antagonists decreasing the frequency (Ishikawa et al. 1997). For example, if there were more A1 receptors in one sex or a brain region, more inhibition would be expected to lower frequency of adenosine release. The opposite would be true if there were more A2A receptors. Receptor activation is complicated because the two main types of adenosine receptors work against each other. There is currently no published information on A1 and A2A receptor densities in females, so it is not possible to match the neurochemistry data with receptor density and future studies could examine sex differences in receptors.
Spontaneous Adenosine Transients vary during the estrous cycle
Female hormone levels change over the stages of estrous, with concentrations of estrogen and progesterone peaking during proestrus, then dropping during estrus and the beginning of metestrus, and rising again during late metestrus and diestrus (Ramikie and Ressler 2018). We examined the patterns of spontaneous adenosine release in the two regions with higher frequencies (hippocampus and cortex) that had opposite patterns in terms of concentration and frequency in males and females. The overall trends for these findings are summarized in Table 2. In the hippocampus, the highest concentration is during the proestrus stage, followed by diestrus, with estrus and metestrus the lowest. The concentration in the hippocampus correlates with the fluctuation of estrous cycle hormones (Ramikie and Ressler 2018), as adenosine transients are higher concentration when estrogen and progesterone levels are high. Thus, estradiol might have a stimulatory effect, activating the same pathway as excitatory A2A receptors (Aronica et al. 1994; Wang and Venton 2017), that affects the concentration of the transients. The frequency of adenosine events is also affected as the frequency is higher when estradiol and progesterone are higher and lower when the hormones drop during estrus. Therefore, in the hippocampus, frequency and concentration of adenosine transients are both elevated when estrogen and progesterone levels are high.
Table 2.
Relative comparison of transient adenosine concentration and frequency between males and stages of estrus in the hippocampus and prefrontal cortex
| Concentration | Frequency | |||
|---|---|---|---|---|
| CA1 | PFC | CA1 | PFC | |
| Proestrus | High | High | High | High |
| Estrus | Low | Low | Low | High |
| Metestrus | Low | Low | Med | High |
| Diestrus | Med | Low | High | High |
In the cortex, the highest concentration adenosine events also occur during proestrus, a similar trend as in the hippocampus. The frequency of events in the cortex is higher in females for all stages of estrous than in males. Thus, in the cortex, the effect of estrogen on the concentration and frequency is not as pronounced as in the hippocampus. Previous studies showed low levels of estrogen in the cortex (Barker et al. 2009) and thus estrogen might change less and cause fewer changes during the estrous cycle. Future experiments should examine the role of estrogen in the brain to regulate spontaneous adenosine concentration and the frequency of release events. If estrogen does regulate spontaneous adenosine release, this has implications for its role as a neuromodulator and shows that it might vary during the estrous cycle for regions that have more estrogen.
Implications of Sex Differences in Adenosine Release
Adenosine is a neuromodulator that can modulate other neurotransmitters and blood flow. For example, rapid adenosine release can modulate phasic dopamine release via the A1 receptor (Ross and Venton 2015); when adenosine is present, phasic dopamine decreases by about 50%. Serotonin release is inhibited by A1 receptors or stimulated by A2A receptors (Okada et al. 2001) and similar observations have been made for adenosine regulation of glutamate (Quarta et al. 2004), acetylcholine (Jin and Fredholm 1996), and norepinephrine (Sperlágh and Vizi 2011). Thus, a change in either the frequency or concentration of adenosine in males vs females might lead to variety of downstream effects on neurotransmitter signaling. Changes in concentration are important because larger adenosine signals would be able to activate lower affinity receptors, diffuse further, and have a longer lasting effect. Targeting adenosine as a treatment for disease has been proposed for schizophrenia and Parkinson’s disease (Akhondzadeh et al. 2000; Ferré et al. 2018), as it would have downstream effects on the dopamine system, and these studies show it is important to consider sex and estrus cycle in any treatment strategy, as they affect adenosine levels. Previous work has shown that changes in frequency are the most common regulation of spontaneous adenosine release (Nguyen et al. 2017; Nguyen et al. 2014). The higher the frequency of spontaneous adenosine release, the tighter the regulatory control over the neurotransmitters in that area. The inverse correlation between frequency and concentration observed shows that while release may be more frequent, each event is typically smaller and that might mitigate some of the effects of higher frequency. Larger adenosine events also affect blood flow, causing vasodilation and increases in brain oxygen (Wang and Venton 2017). Thus, larger but low frequency transients might affect blood flow more. Adenosine plays a role in neuromodulation and neuroprotection during ischemia (Ganesana and Venton 2018; Wang and Venton 2019b), and sex differences in adenosine might cause less neuroprotection in a given area if the frequency is low. Understanding these complex differences in spontaneous adenosine between the sexes and during different stages of estrous is important for understanding variations in the endogenous neuromodulation by adenosine and designing effective treatments targeting adenosine as a neuromodulator.
Conclusions
The concentration and frequency of spontaneous adenosine transients varies with sex and by region. In the hippocampus and amygdala, females had higher concentration but lower frequency adenosine release but the pattern was the opposite in the cortex, where females had lower concentrations and higher frequencies. In addition, spontaneous adenosine release also varies with different stages of the estrous cycle. While the patterns were not the same for the hippocampus and the cortex, higher concentrations were observed during proestrus, where estrogen and progesterone concentrations are high. Thus, it is important that sex and estrous cycle differences are closely examined in future studies in order to better understand adenosine neuromodulation. In the future, it would be beneficial to examine how sex affects both the fast and slow methods of adenosine release or how basal levels of extracellular adenosine may vary between the sexes. It would also be valuable to examine gonadectomized males and females to do a more in-depth study of how hormones, such as estradiol, testosterone, and progesterone affect spontaneous adenosine release.
Acknowledgments
This research was supported by grants from NIH (R01NS076875 & R01EB026497) to BJV.
Abbreviations
- CA1
CA1 region of the hippocampus
- PFC
Prefrontal cortex
- BLA
Basolateral amygdala
- SEM
Standard error of the Mean
- RRID
Research Resource Identifier (see scicrunch.org)
Footnotes
conflict of interest disclosure
The authors declare no competing financial interests.
References
- Abel KM, Drake R, Goldstein JM (2010) Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Shasavand E, Jamilian HR, Shabestari O, Kamalipour A (2000) Dipyridamole in the treatment of schizophrenia: Adenosine-dopamine receptor interactions. J. Clin. Pharm. Ther 25, 131–137. [DOI] [PubMed] [Google Scholar]
- Aronica SM, Kraus WL, Katzenellenbogen BS (1994) Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription (estrogens/antlesrgens/breast cancer/uterus). Proc. Nati. Acad. Sci. USA 91, 8517–8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LAM, Eb D- (2009) General and Comparative Endocrinology Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen. Comp. Endocrinol 164, 77–84. [DOI] [PubMed] [Google Scholar]
- Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST, Venton BJ (2017) Automated Algorithm for Detection of Transient Adenosine Release. ACS Chem. Neurosci 8, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, et al. (2008) Progesterone receptors : Form and function in brain. 29, 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JDE (2002) Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci. U. S. A 99, 10789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti F, Cellai L, Melani A, Donati C, Bruni P, Pedata F (2013) Adenosine is present in rat brain synaptic vesicles. Neuroreport 24, 982–7. [DOI] [PubMed] [Google Scholar]
- Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem 139, 1019–1055. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Vizi ES, Ribeiro JA, Sebastião AM (1996) Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J. Neurochem 67, 2180–2187. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Breslin FJ, Rieger JM, Beauglehole A, Lynch WJ (2012) Time and sex-dependent effects of an adenosine A2A/A1 receptor antagonist on motivation to self-administer cocaine in rats. Pharmacol. Biochem. Behav 102, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM (2009) Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog. Neuro-Psychopharmacology Biol. Psychiatry 33, 251–261. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Fredholm BB (1984) Adenosine receptors mediating inhibitory electrophysiological responses in rat hippocampus are different from receptors mediating cyclic AMP accumulation. Naunyn. Schmiedebergs. Arch. Pharmacol 326, 294–301. [DOI] [PubMed] [Google Scholar]
- Etherington LV, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli BG (2009) Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology 56, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Díaz-Ríos M, Salamone JD, Prediger RD (2018) New Developments on the Adenosine Mechanisms of the Central Effects of Caffeine and Their Implications for Neuropsychiatric Disorders. J. Caffeine Adenosine Res 8, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM (2005) Adenosine and Brain Function. Int. Rev. Neurobiol 63, 191–270. [DOI] [PubMed] [Google Scholar]
- Ganesana M, Lee ST, Wang Y, Venton BJ (2017) Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem 89, 314–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesana M, Venton BJ (2018) Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS One 13, e0196932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM (1994) Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. J. Neurosci 14, 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z (2014) Sex differences in Parkinson ‘ s disease. Front. Neuroendocrinol 35, 370–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM (1978) Evidence for a morphological sex difference within the medial preoptic area of the rat brain. 148, 333–346. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Simon LD, Auerbach SB (1998) Differences in hypothalamic serotonin between estrous phases and gender: An in vivo microdialysis study. Brain Res. 785, 91–96. [DOI] [PubMed] [Google Scholar]
- Huffman ML, Venton BJ (2008) Electrochemical properties of different carbon-fiber microelectrodes using fast-scan cyclic voltammetry. Electroanalysis 20, 2422–2428. [Google Scholar]
- Ishikawa S, Saijoh K, Okada Y (1997) Endogenous adenosine facilitates neurotransmission via A(2A) adenosine receptors in the rat superior colliculus in vivo. Brain Res. 757, 268–275. [DOI] [PubMed] [Google Scholar]
- Jin S, Fredholm BB (1996) Adenosine A2A receptor stimulation increases release of acetylcholine from rat hippocampus but not striatum, and does not affect catecholamine release. Naunyn. Schmiedebergs. Arch. Pharmacol 355, 48–56. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Sisk CL, DonCarlos LL (2013) Sexual differentiation of the adolescent rodent brain: Hormonal influences and developmental mechanisms. Horm. Behav 64, 203–210. [DOI] [PubMed] [Google Scholar]
- Klyuch BP, Dale N, Wall MJ (2012) Neuropharmacology Receptor-mediated modulation of activity-dependent adenosine release in rat cerebellum. Neuropharmacology 62, 815–824. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F (2001) Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem 79, 463–484. [DOI] [PubMed] [Google Scholar]
- Lee ST, Venton BJ (2018) Regional Variations of Spontaneous, Transient Adenosine Release in Brain Slices. ACS Chem. Neurosci 9, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F (2006) Sex differences in the basolateral amygdala: The extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur. J. Neurosci 24, 3245–3254. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ (2014) Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One 9, e87165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Venton BJ (2015) Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Comput. Struct. Biotechnol. J 13, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Wang Y, Ganesana M, Venton BJ (2017) Transient adenosine release is modulated by NMDA and GABA B receptors. ACS Chem. Neurosci 8, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2008) Part 5 : Preparation, Reading and Reporting of Vaginal Smears. OECD Guidel. Test. Chem, 116–125. [Google Scholar]
- Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S (2001) Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J. Neurosci 21, 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH (2004) Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn. 55, 134–147. [DOI] [PubMed] [Google Scholar]
- Pajski MLML, Venton BJJ (2013) The mechanism of electrically stimulated adenosine release varies by brain region. Purinergic Signal. 9, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA (2006) Vesicular release of ATP at central synapses. Pflugers Arch. Eur. J. Physiol 452, 589–597. [DOI] [PubMed] [Google Scholar]
- Park TS, Gidday JM (1990) Effect of dipyridamole on cerebral extracellular adenosine level in vivo. J. Cereb. Blood Flow Metab 10, 424–427. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam-Boston. [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RDS, Takahashi RN (2009) Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: A rodent model of attention-deficit hyperactivity disorder. Behav. Pharmacol 20, 134–145. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR (2004) Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J. Neurochem 88, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Ramikie TS, Ressler KJ (2018) Mechanisms of Sex Differences in Fear and Posttraumatic Stress Disorder. Biol. Psychiatry 83, 876–885. [DOI] [PubMed] [Google Scholar]
- Ross AE, Nguyen MD, Privman E, Venton BJ (2014) Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J. Neurochem 130, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AE, Venton BJ (2015) Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J. Neurochem 132, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. J. Neurochem 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M (2006) Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 29, 647–654. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT (2004) Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatry 9, 531–538. [DOI] [PubMed] [Google Scholar]
- Shin M, Wang Y, Borgus JR, Venton BJ (2019) Electrochemistry at the Synapse. Annu. Rev. Anal. Chem 12, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J (2001) Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci 21, 6292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD (2010) Sex differences in cerebral ischemia: Possible molecular mechanisms. J. Neurosci. Res 88, 2765–2774. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES (2011) The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr. Top. Med. Chem 11, 1034–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BEK, Venton BJ (2007) Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal. Chem 79, 744–750. [DOI] [PubMed] [Google Scholar]
- Tavilani H, Sheikh N, Vaisi-raygani A, Setarehbadi R (2008) Sex differences in adenosine deaminase activity of stroke patients. Clin. Chem. Lab. Med 46, 506–9. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Cao Q (2020) Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst. in press. DOI: 10.1039/C9AN01586H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal F, Zimmermann S, Makhsudova L, Montag AC, Erion MD, Bullough DA, Ito BR (2003) Modulation of cardiac remodeling by adenosine: in vitro and in vivo effects. Mol Cell Biochem 251, 17–26. [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM (2000) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95, 1061–70. [DOI] [PubMed] [Google Scholar]
- Wall M, Dale N (2008) Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr. Neuropharmacol 6, 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Venton BJ (2017) Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J. Neurochem 140, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Venton BJ (2019a) Comparison of spontaneous and mechanically-stimulated adenosine release in mice. Neurochem. Int 124, 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Venton BJ (2019b) Caffeine Modulates Spontaneous Adenosine and Oxygen Changes during Ischemia and Reperfusion. ACS Chem. Neurosci 10, 1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TD, Hoehn K (1991) Release of adenosine and ATP from nervous tissue, in Adenosine Nerv. Syst, pp. 173–195. Academic Press, Inc., London. [Google Scholar]
- Yang JN, Tiselius C, Daré E, Johansson B, Valen G, Fredholm BB (2007) Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiol. 190, 63–75. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Hawkins RA, Stocker JF (1969) Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology 85, 103–112. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR (1999) Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: A possible role of testosterone. Neuroscience 94, 251–259. [DOI] [PubMed] [Google Scholar]