Abstract
Cancer-associated fibroblasts (CAFs) are activated fibroblasts that constitute the major components of tumor microenvironment (TME) and play crucial roles in tumor development and metastasis. Here, we generated fibroblast-specific inducible focal adhesion kinase (FAK) knockout (cKO) mice in a breast cancer model to study potential role and mechanisms of FAK signaling in CAF to promote breast cancer metastasis in vivo. While not affecting primary tumor development and growth, FAK deletion significantly suppressed breast cancer metastasis in vivo. Analyses of CAFs derived from cKO mice as well as human CAFs showed that FAK is required for their activity to promote mammary tumor cell migration. We further showed that FAK ablation in CAFs decreased their exosome amount and functions to promote tumor cell migration and other activities, which could contribute to the reduced metastasis observed in cKO mice. Lastly, profiling of miRs from CAF exosomes showed alterations of several exosomal miRs in FAK-null CAFs, and further analysis suggested that miR-16 and miR-148a enriched in exosomes from FAK-null CAFs contribute to the reduced tumor cell activities and metastasis. Together, these results identify a new role for FAK signaling in CAFs that regulate their intercellular communication with tumor cells to promote breast cancer metastasis.
Keywords: Cancer-associated fibroblasts, FAK signaling, breast cancer metastasis, exosomal miRNAs, mouse models
Introduction
Cancer metastasis is an inefficient multi-step process requiring the disseminated tumor cells to adapt and survive at a foreign microenvironment in distant tissues. During this process, intercellular communication is critical, especially between tumor cells and the surrounding tumor microenvironment (TME), to create a tumor-favoring niche that allows tumor growth and colonization. Cancer-associated fibroblasts (CAFs) are activated fibroblasts that constitute the major components of TME and play crucial roles in tumor development and metastasis 17, 24. In human breast cancer, increased CAFs has been shown to associate with aggressive disease, recurrence and drug resistance 4. However, the role and mechanisms of CAF contribution to breast cancer metastasis is still poorly understood. It remains as a significant challenge to elucidate intracellular signaling pathways in CAFs that regulate intercellular communication between CAF and tumor cells critical for different stages of cancer metastasis.
Exosomes are a class of extracellular vesicles generated in multi-vesicular bodies and released from different cells 30, 38. They range from 50–150 nm in size and contain bioactive molecules such as proteins, lipids, and nucleic acids, including microRNAs (miRNA). Previous studies showed that cancer cells secrete exosomes to regulate recipient cell functions in TME to facilitate cancer metastasis and progression 20, 45. Exosomal miRNAs derived from cancer cells contribute to increased tumor angiogenesis and metastasis by affecting both local TME and distal organs via circulation 13. A recent study suggested that CAF-derived exosomes can promote stemness properties, EMT phenotype, and anchorage-independent growth of tumor cells 7. However, in contrast to extensive studies of exosomes and miRNAs from cancer cells, less is known about the functions of CAF-derived exosomes through delivering miRNA contents to recipient cancer cells to promote cancer metastasis.
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that mediates signal transduction by integrins and other cell surface receptors to regulate cell adhesion, migration, survival, proliferation and differentiation in a variety of cells 25, 31, 32, 37, 48. FAK overexpression in breast cancer correlates with poor prognosis. Previous studies have strongly implicated FAK in the development and progression of breast and other cancers 10, 23. Several groups, including us, have shown that deletion of FAK in mammary epithelial cells suppresses tumor formation and progression in mouse models of breast cancer 18, 21, 26, 27. We and others have also shown that FAK signaling play important roles in endothelial cells to promote embryonic and cancer angiogenesis and drug resistance 43, 44, 49. However, in contrast to the wealth of knowledge on FAK in tumor cells, much less is known about its potential role in tumor stroma, other than a role for endothelial FAK in tumor angiogenesis.
In this study, we generated and analyzed fibroblast-specific inducible FAK knockout mice in a breast cancer model to investigate the potential role and mechanisms of FAK in CAF to promote breast cancer metastasis in vivo. Our studies showed that FAK ablation in fibroblasts significantly reduced breast cancer metastasis by impairing the abilities of CAF-derived exosomes to promote cancer cell migration and other activities at least in part due to altered exosomal miR-148a and miR-16. These results provide significant insights into the multiple mechanisms of FAK signaling in TME besides their essential role in mammary tumor cells, which may contribute to the future development of novel therapies for breast cancer targeting FAK.
Materials and Methods
Tumor mice
MMTV-PyMT;FAKf/f mice have been described previously 21, and Col1a2-creERT mice obtained from Jackson Lab. The tumor cohorts were maintained on a FVB/N genetic background after extensive back-crossing (7x or more). Mice were housed and handled according to local, state, and federal regulations, and all experimental procedures were carried out as per the guidelines of the Institutional Animal Care and Use Committee at the University of Cincinnati. Tumors were measured by calipers and volume was calculated as (½)(length)(width)2. To induce deletion, mice were treated with 2mg tamoxifen for three times every other day, intra-peritoneally.
Cell culture and treatment
MDA-MB-231, MCF-7 and WI-38 cells were obtained from ATCC. These cells were cultured in DMEM with 10% fetal bovine serum. Cells were routinely tested for mycoplasma contamination. Murine fibroblasts were prepared from primary tumors and lung tissues, as described previously 5, 7. Isolated fibroblasts were cultured in DMEM/F12 supplemented with 10% FBS, insulin (10ng/ml) and EGF (10ng/ml). Wound healing, trans-well invasion and sphere-forming assays were carried out as described previously 21. For trans-well assays, 10% serum media was used as a chemoattractant in the lower chamber while tumor cells were seeded in the upper chamber with 0.1% FBS and tumor cells were assayed for 16 hours. For co-culture trans-well assays (Figures 2C-D), tumor conditioned media pre-educated WI-38 cells were seeded in the lower chamber with respective tumor conditioned media before plating tumor cells in the upper chambers 2 hours later. For conditioned media, cells were cultured in 10% serum media and media was collected after 72 hours. For treatment with miRNA inhibitors (synthesized from IDT), cells were treated at the indicated concentrations for 48 hours prior to starting an assay.
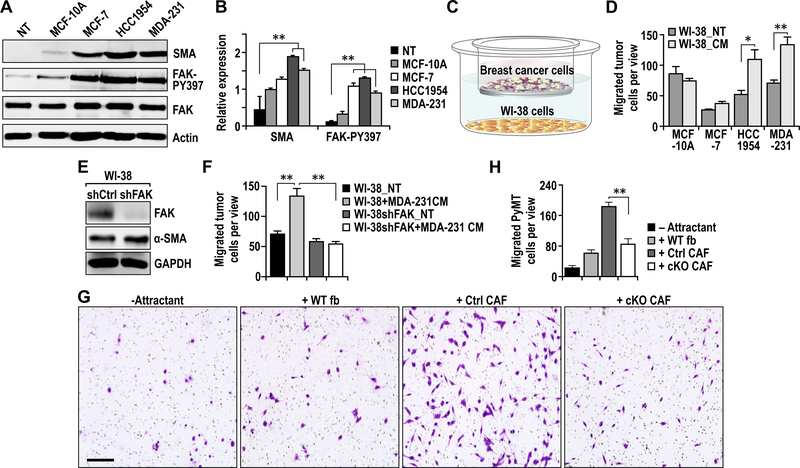
Fig. 2.
FAK signaling in CAFs is required for their promotion of tumor cell migration. A. Immuno-blots showing levels of SMA, FAK-pY397, FAK and Actin in WI-38 human fibroblasts that are not treated (NT) or treated with conditioned media from respective human breast cancer cells. B. Quantification of respective protein levels normalized against total FAK in WI-38 fibroblasts described in A. C. Illustration of co-culture trans-well migration assay. D. Quantification of migrated cells in trans-well migration assays of human breast cancer cells that were educated by fibroblasts (not treated [NT] or treated with respective conditioned media from tumor cells). E. Immuno-blots showing levels of FAK, SMA and GAPDH in WI-38 cells treated with shCtrl or shFAK to knockdown FAK. F. Quantification of trans-well migration assays of MDA-MB-231 tumor cells to WI-38 cells treated with shFAK and/or MDA-231 conditioned medium for 72 hours. Levels of FAK knockdown in WI-38 cells are indicated by immune-blots. G-H. Representative images (G) and quantification (H) of migrated cells in trans-well migration assays of PyMT tumor cells to primary fibroblasts purified from lungs of WT, Ctrl or cKO mice. Scale bar, 50μm. Error bars indicate mean ±SEM. *p<0.05, **P<0.01.
EDU incorporation assay
Cells were cultured with 5-ethynyl-2′-deoxyuridine (EdU) treatment (100 nM) for 4 hrs. The formalin-fixed cells were stained with Tris (100 mM), CuSO4 (1 mM), fluorescent-488 azide (100 μM), ascorbic acid (50 mM) and DAPI (1 μg/ml) for 10 min. Three independent field of views for stained cells were imaged by fluorescence microscopy and counted for statistical analysis.
Antibodies
Antibodies used in this study were Integrin β4 (Thermo Fisher, MA180984), Pan-cytokeratin (Cell Signaling, 4545), Cyclin E (Santa Cruz, sc-481), beta-Actin (Sigma-Aldrich, A5441), FAK (Santa Cruz, sc558), pY397-FAK (Abcam, 39967), SMA (Abcam, 7817), CD63 (System Biosciences, EXOAB-CD63A-1), CD81 (Cell Signaling, 10037), ALIX (Cell Signaling, 2171) and GAPDH (Cell Signaling, 2118).
Histology and immunohistochemistry
For histological analysis of tissues, samples were fixed overnight in 10% phosphate-buffered formalin, dehydrated in alcohol gradients, xylene and paraffin before being embedded. Then, they were sectioned (5-μm) and subjected to immuno-histochemistry as previously described 21.
Immunoblotting
Cell lysates were prepared with modified radio immunoprecipitation assay (RIPA) buffer as described previously 21 with the addition of Halt protease and phosphatase inhibitors (Thermo Scientific, 78425; 78428). Protein concentrations were quantified by the bicinchoninic acid method, subjected to SDS-PAGE and analyzed by immunoblotting as described previously 21.
Exosome isolation and characterization
Exosomes were isolated by ultracentrifugation as described previously 11, 14. Exosome size were determined using Nanosight and purity by CD63 flow cytometry. Scanning electron microscopy was used to validate the size distribution of exosomes.
Quantitative PCR
Total RNA was isolated from cells using an RNAeasy kit (Qiagen, 74004) per the manufacturer’s instructions. miRNAs were prepared from isolated exosomes using mirVana miRNA Isolation Kit (Thermo Scientific) according to manufacturer’s instructions. Equal amounts of RNA were then reverse-transcribed using SuperScript III first-strand synthesis kit (Invitrogen, 18080–044) using random hexamers as primers. cDNA samples were then subjected to qRT-PCR analysis with SYBR Green (BioRad, 1725121) in a BioRad CFXConnect (Bio-Rad, Hercules, CA, USA) thermo-cycler. For miRNA analysis, snRNA RNU6B was used to normalize expression levels. List of primers used are available upon request.
miRNA sequencing analysis
MiRNA sequencing was performed at the Genomics, Epigenomics and Sequencing Core in University of Cincinnati. Briefly, NEBNext small RNA sample library preparation kit (NEB, Ipswich, MA) was used to prepare the library for sequencing, with the following modification for precise library size selection. First, RNA 3’ adaptor is specifically modified to target miRNAs and other small RNAs that have a 3’ hydroxyl group resulting from enzymatic cleavage by Dicer or other RNA processing enzymes. After ligation, the excess 3’ adaptor is removed by hybridization to prevent adaptor-dimer formation. Then, the 5’ ends of miRNAs that have a 5’-phosphate are ligated to the 5’ adaptor. Following 3’ and 5’ ligation, reverse transcription is performed to convert the ligated small RNAs into cDNA, and then uniquely indexed by PCR to generate the sequencing library. The libraries containing DNAs ranging from 135–146 bp were purified on 2.75% agarose gels (using a custom-designed DNA ladder for 135–146 bp mixed with library DNA) and eluted in 20 μl. Two μl of the libraries were then diluted (1:104) and analyzed by NEBNext Library Quant kit (NEB) using QuantStudio 5 Real-Time PCR System (Thermofisher, Waltham, MA). The quantified libraries were clustered onto a flow cell at the concentration of 15 pM using Illumina’s TruSeq SR Cluster Kit v3, and sequenced for 51 cycles using TruSeq SBS kit on Illumina HiSeq system. Sequence reads were aligned and analyzed for differential levels of individual miRNAs by The Laboratory for Statistical Genomics and Systems Biology in the University of Cincinnati.
Statistical analysis
Data were plotted as means ± SEM and statistical significance was determined using a two-tailed t-test.
Results
FAK ablation in fibroblasts inhibits breast cancer metastasis in vivo
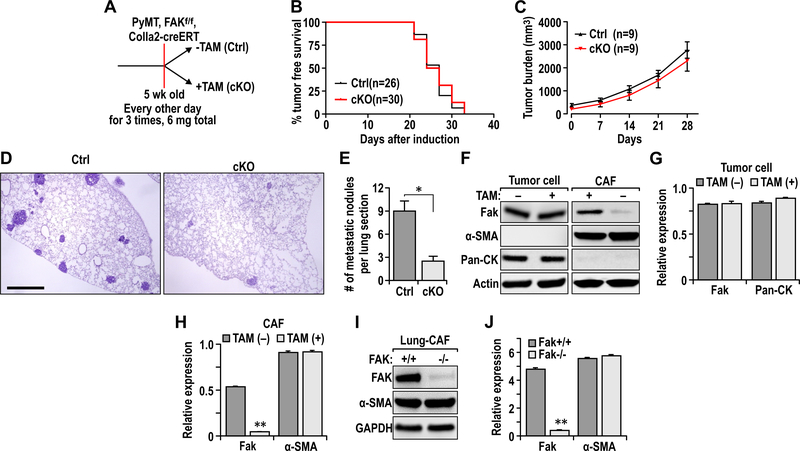
To study the potential role of FAK signaling in CAFs to promote breast cancer development and metastasis in vivo, we crossed MMTV-PyMT;FAKf/f mice 21 with Col1a2-CreER transgenic mice that express tamoxifen (TAM)-activated Cre recombinase in CAFs 2, 15, 50 to produce MMTV-PyMT;FAKf/f;Cre mice. They (and all strains used in this study) were backcrossed to FVB/N genetic background (syngeneic to the MMTV-PyMT mice used throughout the study) for seven or more times (>99%, or virtually pure FVB/N) to eliminate potential influence of genetic background on phenotypes. Cohorts of female MMTV-PyMT;FAKf/f;Cre mice were treated with vehicle (designated as Ctrl mice) or TAM (designated as cKO mice after induced FAK deletion) at 5 weeks of age every other day for 3 times [2 mg each time] (Fig. 1A). Unlike previous studies deleting FAK in tumor cells 21, ablation of FAK in CAFs did not affect endogenous mammary tumor development in cKO mice relative to Ctrl mice (Fig. 1B). Further, primary tumor growth was comparable in cKO and Ctrl mice (Fig. 1C). Interestingly, however, we observed that cKO mice developed metastasis at a significantly reduced frequency than Ctrl mice at 4 weeks after TAM treatments (Figs. 1D-1E).
Fig. 1.
FAK ablation in fibroblasts inhibits breast cancer metastasis. A. Experimental design scheme for induction of FAK deletion in transgenic mice. B. Kaplan-Meier tumor free curve of Ctrl (n=26) and cKO (n=30) mice. C. Endogenous primary tumor growth curves of Ctrl (n=9) and cKO (n=9) mice. D. H&E staining of lungs from Ctrl and cKO mice at end point (4 weeks after Tamoxifen induction). Scale bar, 2mm E. Quantification of metastatic nodules per lung section in Ctrl (n=9) and cKO mice (n=9). F. Immuno-blots showing levels of FAK, SMA, Pan-CK and Actin in primary tumor cells and fibroblasts. G-H. Quantification of respective protein levels normalized against Actin in (G) tumor cells and (H) CAFs described in F. I. Immuno-blots showing levels of FAK, SMA and GAPDH in lung CAFs isolated from Ctrl and cKO mice. Error bars represent mean ± s.e.m. *P≤0.05. J. Quantification of respective protein levels normalized against GAPDH in lung CAFs described in I.
To verify FAK deletion in CAFs, tumor cells and CAFs were prepared from mammary tumors in cKO and Ctrl mice, as described previously 5, 7, 21. Western blot analysis of lysates showed FAK deletion specifically in CAFs, but not mammary tumor cells, from cKO mice (Figs. 1F-1H). We next isolated CAFs from lungs of these mice by FACS using PDGFRβ as a marker 35, and showed that FAK was deleted in cKO CAFs compared to CAFs from Ctrl mice (Figs. 1I-1J). Lung epithelial cells from cKO and Ctrl mice showed comparable levels of FAK (data not shown). Western blotting analysis using alpha-smooth muscle actin (a-SMA) as a marker for fibroblasts activation (i.e. CAFs) showed comparable levels of a-SMA in fibroblasts from cKO and Ctrl mice, suggesting that FAK deletion did not affect CAF formation in cKO mice. Together, these results suggest that FAK deletion in CAF suppressed breast cancer metastasis, although it did not affect primary mammary tumor development and growth.
FAK signaling in CAFs is required for their promotion of tumor cell migration in vitro
To further evaluate a role of FAK signaling in CAFs to promote human breast cancer metastasis, we prepared human CAFs and examined their effects on human breast cancer cell migration in vitro. First, WI-38 human fibroblasts were incubated with conditioned media (CM) from several human breast cancer cells, and examined by western blotting using antibody for a-SMA (Figs. 2A-2B). We found that CM from MCF-7, HCC-1954 and MDA-231 breast cancer cells, but not MCF10A (a normal mammary epithelial cell line), induced WI-38 cells to a CAF-like state with increased expression of a-SMA. FAK phosphorylation was also increased in WI-38 cells treated with CM from these breast cancer cells, supporting a potential role for FAK signaling in CAFs. We next analyzed the ability of the treated WI-38 cells to promote migration of breast cancer cells, which is an important characteristic of metastasis. We found that WI-38 cells treated with HCC-1954 and MDA-231 CM promoted migration of these two breast cancers relative to WI-38 cells not treated (NT) with the CM, while those treated with MCF7 CM increased its migration slightly (Figs. 2C and 2D).
To examine directly the role of FAK signaling in CAF, we generated WI-38 cells with stable FAK knockdown using lentiviral vectors for FAK shRNA and examined their ability to promote migration of MDA-231 cells following treatment with MDA-231 CM. We found that FAK knockdown did not change activation of WI-38 cells by MDA-231 CM, as measured by a-SMA expression (Fig. 2E). However, FAK knockdown abolished the ability of MDA-231 CM treated-WI-38 cells to promote migration of breast cancer cells (Fig. 2F). Consistent with these results using WI-38 cells, CAFs from Ctrl mice, but not those from cKO mice, showed greater ability to promote PyMT tumor cell migration, relative to lung fibroblasts from WT mice (Figs. 2G and 2H). Collectively, these results suggest that FAK signaling in CAFs is important for their ability to stimulate tumor cell migration, which were consistent with the decreased metastasis in cKO mice in vivo.
FAK deletion in CAFs results in the reduced amount and defective exosomes for promoting tumor cell migration and other functions
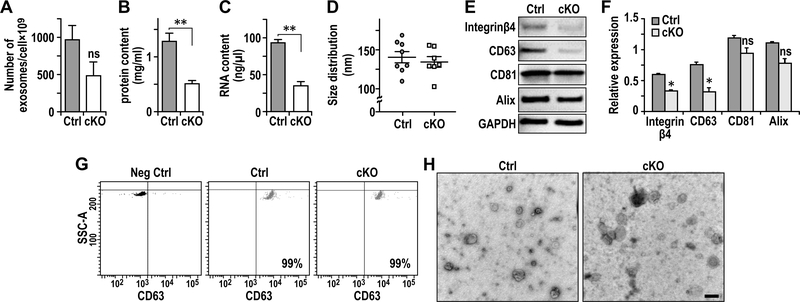
Exosomes are small micro vesicles containing functional biomolecules like proteins and RNAs, which can transfer between the donor cells and the recipient cells 30, 38. To explore whether FAK signaling in CAFs regulates tumor cells through exosomes, we isolated and examined exosomes from Ctrl and cKO CAFs by ultracentrifugation of the CM, as described previously 11, 14. We found comparable number of exosomes in CAFs from Ctrl and cKO mice (Fig. 3A). However, both protein and RNA contents were decreased in exosomes from cKO CAFs relative to those in Ctrl CAFs (Figs. 3B and 3C), suggesting that FAK deletion in CAFs reduced the amount of protein or RNA in exosomes. On the other hand, size distributions of exosomes isolated from CAFs of cKO and Ctrl mice were similar (Fig. 3D). Analyses of the exosome preparations by Western blotting showed that both Ctrl and cKO exosomes are enriched in various exosome proteins; including CD81 and Alix at comparable levels in both exosomes, and CD63 and integrin β4 at reduced levels in exosomes from cKO CAFs relative to exosomes from Ctrl CAFs (Figs. 3E and 3F). FACS analysis using CD63 as a marker verified purities of greater than 99% in both exosomes from Ctrl and cKO CAFs (Fig. 3G). Electron microscopy further confirmed purity of exosome preparations, showing exosomes ranging between 70 to 170 nm (Fig. 3H), which is comparable to estimates by Nanosight (see Fig. 3D).
Fig. 3.
FAK deletion in CAFs results in defective exosomes. A-C. Quantification of (A) numbers of exosome generated per million of cells (volume of preparation), (B) protein content (mg/ml) per volume of preparation and (C) RNA content per volume of preparation of exosomes from purified primary lung fibroblasts. D. Quantification of exosome size distribution by nanosight. E. Immuno-blots showing levels of exosome markers Integrinβ4, CD63, CD81 and Alix using purified exosomes from primary lung fibroblasts of Ctrl or cKO mice. F. Quantification of respective protein levels from purified exosomes normalized against GAPDH as described in E. G. CD63 FACS analysis of purified exosomes. Exosomes without CD63 primary antibody incubation were utilized as negative control. H. Representatives images of electron microscopic analysis of exosomes derived from the primary lung fibroblasts. Scale bar, 100nm. Error bars indicate mean ±SEM. **P<0.01.
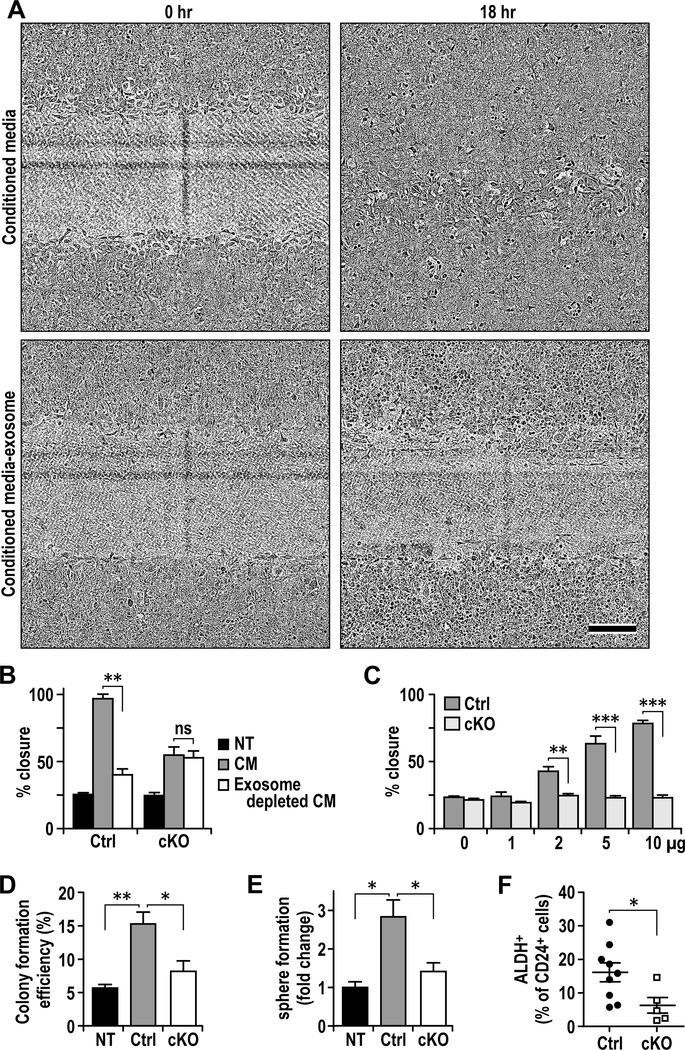
We next examined the effect of CM from Ctrl CAFs with or without exosomes on PyMT tumor cell migration using wound closure assay. We found that while the Ctrl CM significantly increased tumor cell migration, this activity was much lower after removal of exosomes (Figs. 4A and 4B). CM from cKO CAFs also promoted tumor cell migration but to a less extent, and removal of exosomes from the media did not affect this activity (Fig. 4B). Interestingly, CM from cKO CAFs with exosome depletion showed greater activity to promote tumor cell migration than CM from Ctrl CAFs without exosomes (Fig. 4B), suggesting Ctrl exosomes are primarily responsible for the activity in CM from Ctrl CAFs whereas the activity over NT in CM of cKO CAFs are likely due to other factors (e.g. altered chemokine compositions). Indeed, adding back Ctrl CAF exosomes, but not cKO CAF exosomes, to the exosome-depleted CM promoted tumor cell migration in a dose-dependent manner (Fig. 4C). Purified exosomes from Ctrl CAFs, but not those from cKO CAFs, also increased tumor cell colony formation in soft agar (Fig. 4D). Analysis of human CAFs also showed that knockdown of FAK by shRNA reduced the ability of CM from these cells to promote tumor cell migration and proliferation (Fig. S1). Previous studies suggested important roles of breast cancer stem cells (BCSCs) in metastasis 6, 29, 39, 47, we therefore also examined the effect of CAF exosomes on BCSCs. We found that exosomes from Ctrl CAF increased tumor sphere formation of PyMT primary tumor cells compared to cKO exosomes or no addition (Fig. 4E). Consistent with these in vitro data, cKO mice showed decreased amount of BCSCs than Ctrl mice, as measured by ALDH assays (Fig. 4F). Taken together, these results suggest that FAK signaling in CAFs regulates the generation of exosomes and their functions to promote breast cancer metastasis.
Fig. 4.
Exosomes from FAK deficient CAFs are deficient in promoting tumorigenic phenotypes. A. Representative images of wound healing assay of PyMT cells treated with Ctrl or cKO CAF conditioned media with or without exosome removal for 18 hrs. Scale bar represents 200μm. B. Quantification of wound healing assay as described in A (n=3). C. Wound healing assay of PyMT cells treated with exosome at various doses (0–10 μg) for 18 hours (n=3). D-F Colony formation (D), sphere formation (E) and quantification of ALDH+ cells in PyMT cells that are not treated (NT) or treated with 10μg of purified exosomes from Ctrl or cKO CAFs. Error bars indicate mean ±SEM. *p<0.05, **p<0.01, ***p<0.001.
Regulation of miR-16 and miR-148a in CAF exosomes by FAK contributes to altered ability of CAFs to affect tumor cell activity and metastasis
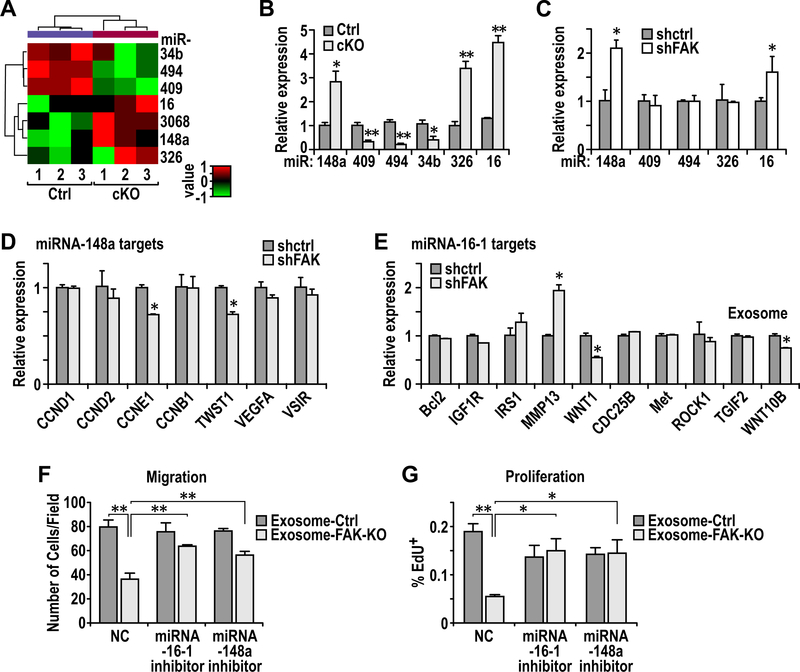
MiRNAs encapsulated in exosomes are abundant and play important roles in inter-cellular communications 13, 45. We therefore hypothesized that FAK deletion in CAFs alter miRNAs in exosomes to abolish their activity to promote tumor cell functions and metastasis. To identify the specific miRNAs involved, we performed miRNA-sequencing of CAF-derived exosomes to generate miRNA profiles from Ctrl and cKO mice (n= 3 for each). Comparative analysis of miRNA profiles identified 3 decreased miRNAs and 4 increased miRNAs in cKO CAF-derived exosomes relative to those from Ctrl mice (Fig. 5A). Using additional preparations of CAF-derived exosomes of Ctrl and cKO mice, qRT-PCR further confirmed reduced levels of miR-34b, miR-409 and miR-494 as well as increased amount of miR-16, miR-148a and miR-326 in cKO CAF-derived exosomes (Fig. 5B), suggesting that these exosomal miRs may mediate CAF regulation of mammary tumor metastasis in cKO mice.
Fig. 5.
Regulation of miR-16 and miR-148a in CAF exosomes by FAK contributes to altered ability of CAFs to affect tumor cell activity and metastasis. A. Heatmap showing differentially expressed miRs in exosomes from Ctrl or cKO CAFs (n=3 each). B. Quantitative-PCR analysis of miR levels in exosomes from Ctrl and cKO CAFs (n=3 each). C. Quantitative-PCR analysis of miR levels in exosomes from WI-38 fibroblasts transduced with shCtrl or shFAK and educated by MDA-MB-231 cells. D-E. Quantitative-PCR analysis of (D) miR148a target genes and (E) miR16 target genes in MDA-MB-231 cells that were treated with exosomes from shCtrl or shFAK transduced WI-38 fibroblasts. F. Trans-well migration assays for PyMT cells treated with exosomes from Ctrl or cKO lung CAFs, along with specified miRNA inhibitors. NC denotes scrambled control oligo. G. EdU incorporation assay for PyMT cells treated with exosomes from Ctrl or cKO lung CAFs, along with specified miRNA inhibitors. Error bars indicate mean ±SEM. *p<0.05, **p<0.01.
We next prepared exosomes from human WI-38 cells with or without FAK knockdown (see Fig. 2E) that had been treated MDA-231 CM and examined the levels of these miRs. MiR-16 and miR-148a showed increased expression in WI-38 cells with FAK knockdown, consistent with results in mouse CAFs, although miR-326 was not increased after FAK knockdown (Fig. 5C). Surprisingly, we did not find the decreased expression of miR-409 or miR-494 in WI-38 cells after FAK knockdown, and miR-34b was not detected in WI-38 cells with or without FAK knockdown. Similar analysis in WI-38 cells treated with MCF-7 CM showed that FAK knockdown did not change the levels of any of these miRs (Fig. S2), which is consistent with the observation that WI-38 cells treated with MCF-7 CM did not affect migration of these cells (see Fig. 2D). These results further support that exosomal miR-16 and/or miR-148a play a role in mediating CAF regulation of recipient tumor cells. Indeed, both miR-16 and miR-148a have been reported to act as tumor suppressive miRs in different cancers including breast cancer 3, 19, 28. Thus, it is possible that exosomes from CAFs lacking FAK (either from cKO mice, or human WI-38 cells with FAK knockdown) and enriched with miR-16 and/or miR-148a inhibit various tumor cell activities compared to exosomes from Ctrl CAFs (see Figs. 4C-4F and S1).
To further evaluate this notion, we examined expression of a series of putative targets of miR-16 and miR-148a in the recipient MDA-231 cells treated by exosomes from human CAFs with or without FAK knockdown. We found that miR-16 targets CCNE1 and TWIST1 as well as miR-148a targets WNT1 and WNT10B were significantly decreased in tumor cells treated with exosomes from CAFs with FAK knockdown compared to those treated with exosomes from Ctrl CAFs (Figs. 5D and 5E). Moreover, decreased levels of Twist1 and Ccne1 proteins were found in tumor cells treated with exosomes from cKO CAFs vs those from Ctrl CAFs (Fig. S3). We next used inhibitors against miR-16 and miR-148a to further validate their roles in decreasing mammary tumor cell activities. As expected, transfection of miR-148a and miR-16 inhibitors restored the levels of miR-148a targets CCNE1 and Twist1 and miR-16 target Wnt10b, respectively, in tumor cells treated with exosomes from cKO CAFs (Fig. S4). Importantly, transfection of either of these inhibitors reversed inhibition of tumor cell migration and proliferation by exosomes from cKO CAFs (Figs. 5F and 5G). Moreover, transfection of these two inhibitors into cKO CAFs also reduced the levels of corresponding miR-148a and miR-16 in exosomes derived from these cells (Fig. S5A), as expected, and these exosomes induced more tumor cell migration compared to exosomes from cKO CAFs treated with scrambled control (Fig. S5B). These results confirmed efficacy of the inhibitors, and are consistent with the idea that the increased amount of miR-16 and miR-148 in exosome from cKO CAF is responsible for the lower migration of tumor cells compared to those treated with Ctrl CAF exosomes. Lastly, miR-148a and miR-16 inhibitors did not affect the base line proliferation of tumor cells (i.e. those without exosome treatment)(Fig. S6), supporting that they acted on reserving the effect of corresponding miRNAs enriched in exosomes from cKO CAFs which were delivered to tumor cells. Together, these results suggested that miR-16 and miR-148a enriched in exosomes from FAK-null CAFs play a key role in decreasing mammary tumor cell functions and metastasis (Fig. S7).
Discussion
Cancer is increasingly recognized as a disease involving abnormalities in both tumor cells and other surrounding cells within TME as well as altered communications between the cells 12. It is well established that malignant cancer cells can activate multiple mechanisms to “educate” both local and distant fibroblasts into CAFs, which in turn influence various cancer cell behavior to promote cancer metastasis to distant organs. By creating a fibroblast-specific inducible FAK knockout in a mouse model of breast cancer as well as knocking down FAK in human CAFs, we showed a critical role for FAK signaling in CAFs for metastasis. Therefore, unlike some other key signaling molecules that could play opposing roles in cancer vs stromal cells for cancer progression and metastasis 8, 46, FAK’s function in CAFs as well as its pro-tumorigenesis role in tumor and endothelial cells 18, 21, 26, 27, 43, 44, 49 highlight significant potential for FAK inhibitors for systemic cancer therapy by attacking both tumor cells and multiple cells in TME crucial for cancer metastasis.
Besides well-established roles of FAK in mediating intracellular signaling in tumor and other cells, recent studies suggested new functions for FAK in the regulation of TME through affecting intercellular communications. FAK in tumor cells has been implicated in creating an immune suppressive TME through inflammatory chemokine production and tumor-associated regulatory T cells (Tregs) recruitment 33, 34. Other studies suggested a role for FAK in regulating the composition of the fibrotic and immune-suppressive niche in pancreatic tumor model 16, 42. Our results reveal new FAK functions in fibroblasts required for their activation to CAFs in response to tumor cells, and more importantly the ability of CAFs to promote breast tumor cell migration. FAK can regulate diverse signaling pathways through its phosphorylation of and interaction with multiple other molecules. Using FAK mutant knock-in alleles in breast cancer models, we showed previously that FAK kinase activity was required for some, but not other, subtypes of breast cancer 22, and that FAK interaction with endophilin A2 specifically affect breast cancer stem cell pool but not bulk tumor cells 9. It will be interesting to determine the role of FAK kinase activity and specific downstream pathways in its regulation of CAF-derived exosomal miRNAs to promote breast cancer metastasis using similar strategies.
Exosomes have emerged as an important mediator of inter-cellular communication between various cells. Our findings are consistent with a number of previous studies showing promotion of breast cancer cell migration and metastasis by CAF-derived exosomes 20, 36.Moreover, these data provide strong support for a role of exosomal miRNAs in mediating the the reduced metastasis upon FAK ablation in CAFs of cKO mice, although we could not exclude potential contribution by changes in exosomal RNAs and/or proteins from cKO CAFs (e.g. decreased amount of integrin β4 and/or CD63, see Figs. 3E and 3F). We found that FAK ablation in CAFs increased levels of exosomal miR-148a, which decreased WNT1 and WNT10B expression in the recipient cancer cells. These data provided further support the previous observation that CAF exosomes stimulate Wnt-PCP signaling to drive breast cancer cell invasive activities 20. Our studies also showed increased levels of exosomal miR-16 from FAK-null CAFs that decreased other genes CCNE1 and TWIST1 to reduced cancer cell proliferation and EMT. Besides the increased tumor suppressive miR-148a and miR-16, we also found reduced levels of oncogenic miRs-34b, 409 and 494 in CAF-derived exosomes of cKO mice. However, we did not detect similar decreases of these oncogenic miRs in human CAFs following FAK knockdown, which could be due to incomplete ablation of FAK in these cells by knockdown approach. Nevertheless, it is conceivable that inhibition of one or more of the oncogenic miRs will reduce the ability of CAF exosomes to promote metastasis in cKO mice, possibly in conjunction with the increased levels of tumor suppressive miR-16 and/or miR-148a, as discussed above. Indeed, one caveat in support of the roles of increased miR-16 and miR-148a in cKO CAFs is the reduced amount of cKO exosomes compared to that from Ctrl CAFs (thus cancelling out the net effect of the enriched miRs on per exosome basis). However, multiple miRs may act in an integrated manner to influence recipient tumor cell behavior, and in such a case, both the reduced oncogenic miRs and the enriched suppressive miRs from the overall reduced exosome amount in cKO CAFs may be involved.
Cancer metastasis is a complex, multiple stage process including tumor cell intravasation into the blood or lymphatic vasculature at the primary tumor site, survival in the blood/lymphatic vessels, and colonization and growth at the metastatic site such as the lung. Interestingly, we found that FAK ablation in CAFs inhibited breast cancer metastasis, but it had no apparent effects on the development and growth of primary tumors. Thus, it is possible that defective CAFs at the lung metastatic sites could be responsible for the reduced colonization and/or growth at the tumor cells to inhibit metastasis observed in cKO mice. Alternatively, our data showing that FAK ablation diminished the ability of CAFs to promote tumor cell migration are also consistent with the possibility that cKO CAFs affected metastasis by reduced support for tumor cell intravasation despite not affecting the primary tumor development or growth. Future studies will be directed towards examining these possibilities to further dissect the mechanism involved. We also noted that FAK-null CAF exosomes with enriched miR-16 and miR-148a inhibited proliferation of tumor cells in vitro, although primary tumor growth was not affected in cKO mice in vivo. It is possible that primary tumors developed compensatory mechanisms so that their growth was less dependent on possible effects of FAK-null CAFs in vivo. Another, not mutually exclusive possibility is that tumor cells metastasized to the lungs have a more stringent requirement for their microenvironment including CAFs to support their growth to form lung nodules (and FAK deletion in CAFs compromising such supportive function), which is reflected in the in vitro assays. Lastly, though less likely, it is also possible that FAK signaling is preferentially more important in lung CAFs (where they were isolated for the in vitro assays) than CAFs associated with primary tumors in mammary gland. Future studies will be necessary to gain additional mechanistic insights using our unique mouse models and reagents derived from them. Likewise, it will also be interesting to evaluate potential differential changes in specific subsets of CAFs upon FAK deletion and their contributions to the reduced metastasis in vivo, given recent studies showing multiple subsets of CAFs that could support or suppress tumor progression 1, 4, 40. Lastly, defective CAFs upon FAK ablation could modulate other TME components such as immune cells and ECs to impact indirectly on cancer metastasis. The availability of the fibroblast-specific cKO mice described here, along with FAK cKO mice in ECs 41 and mammary tumor cells 21 will offer appropriate models to fully understand the complex mechanism of FAK signaling in the regulation of metastasis in vivo.
In summary, our studies revealed a new function for FAK in intercellular communication between CAFs in TME and mammary tumor cells important for cancer cell migration and metastasis. FAK ablation in CAFs impaired their ability to promote cancer cell migration and other activities due to alterations in exosomal miRs. These findings provide new insights into the complex role of FAK signaling in TME and cross-talk with mammary tumor cells and further highlight the potential of targeting FAK in the development of systemic and effective therapy for advanced and metastatic breast cancer.
Supplementary Material
Acknowledgements
We would like to thank Glenn Doerman for his help in the preparation of figures. We are grateful to members of our laboratory for their critical comments and suggestions for the manuscript. The work in this study is funded by NIH grants R01CA163493, R01NS094144 and R01HL073394 to J.-L. Guan
References
- 1.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Frontiers in oncology 2014; 4: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bou-Gharios G, Garrett LA, Rossert J, Niederreither K, Eberspaecher H, Smith C et al. A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol 1996; 134: 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99: 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018; 33: 463–479 e410. [DOI] [PubMed] [Google Scholar]
- 5.Costea DE, Hills A, Osman AH, Thurlow J, Kalna G, Huang X et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res 2013; 73: 3888–3901. [DOI] [PubMed] [Google Scholar]
- 6.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med 2007; 58: 267–284. [DOI] [PubMed] [Google Scholar]
- 7.Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M et al. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 2017; 8: 19592–19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S et al. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell 2016; 30: 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan H, Zhao X, Sun S, Luo M, Guan JL. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial-mesenchymal transition and mammary cancer stem cell activities in vivo. J Biol Chem 2013; 288: 3322–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol 2007; 263: 103–153. [DOI] [PubMed] [Google Scholar]
- 11.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods in molecular biology 2015; 1295: 179–209. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 13.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Molecular cancer 2015; 14: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B, Wu Z, Nakashima T, Phan SH. Mesenchymal-specific deletion of C/EBPbeta suppresses pulmonary fibrosis. The American journal of pathology 2012; 180: 2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016; 22: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalluri R. The biology and function of fibroblasts in cancer. Nature reviews Cancer 2016; 16: 582–598. [DOI] [PubMed] [Google Scholar]
- 18.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A 2007; 104: 20302–20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Deng X, Zeng X, Peng X. The Role of Mir-148a in Cancer. Journal of Cancer 2016; 7: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012; 151: 1542–1556. [DOI] [PubMed] [Google Scholar]
- 21.Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res 2009; 69: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo M, Zhao X, Chen S, Liu S, Wicha MS, Guan JL. Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nature reviews Cancer 2005; 5: 505–515. [DOI] [PubMed] [Google Scholar]
- 24.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 2006; 5: 1597–1601. [DOI] [PubMed] [Google Scholar]
- 25.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 2003; 116: 1409–1416. [DOI] [PubMed] [Google Scholar]
- 26.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. The American journal of pathology 2008; 173: 1551–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest 2009; 119: 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas MA, Venturutti L, Huang YW, Schillaci R, Huang TH, Elizalde PV. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res 2012; 14: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 2009; 324: 1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruivo CF, Adem B, Silva M, Melo SA. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res 2017; 77: 6480–6488. [DOI] [PubMed] [Google Scholar]
- 31.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 2001; 1540: 1–21. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 2004; 14: 92–101. [DOI] [PubMed] [Google Scholar]
- 33.Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A et al. Nuclear FAK Controls Chemokine Transcription, Tregs, and Evasion of Anti-tumor Immunity. Cell 2015; 163: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrels B, McGivern N, Canel M, Byron A, Johnson SC, McSorley HJ et al. IL-33 and ST2 mediate FAK-dependent antitumor immune evasion through transcriptional networks. Sci Signal 2017; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015; 7: 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD et al. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol 2014; 16: 889–901. [DOI] [PubMed] [Google Scholar]
- 37.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res 2006; 12: 3233–3237. [DOI] [PubMed] [Google Scholar]
- 38.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Current opinion in cell biology 2009; 21: 575–581. [DOI] [PubMed] [Google Scholar]
- 39.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nature reviews Cancer 2007; 7: 791–799. [DOI] [PubMed] [Google Scholar]
- 40.Su S, Chen J, Yao H, Liu J, Yu S, Lao L et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018; 172: 841–856 e816. [DOI] [PubMed] [Google Scholar]
- 41.Sun S, Wu HJ, Guan JL. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci Rep 2018; 8: 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Symeonides SN, Anderton SM, Serrels A. FAK-inhibition opens the door to checkpoint immunotherapy in Pancreatic Cancer. J Immunother Cancer 2017; 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavora B, Batista S, Reynolds LE, Jadeja S, Robinson S, Kostourou V et al. Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med 2010; 2: 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavora B, Reynolds LE, Batista S, Demircioglu F, Fernandez I, Lechertier T et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 2014; 514: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016; 164: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 46.Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell 2016; 29: 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res 2006; 66: 1883–1890; discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 2009; 28: 35–49. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol 2010; 189: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts : a potentially powerful technique for investigating gene function in fibrosis. The American journal of pathology 2002; 160: 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.