Abstract
The complement cascade was first recognized as a downstream effector system of antibody-mediated cytotoxicity. Consistent with this view, it was discovered in the 1960s that complement is activated in the glomeruli of patients with immune-complex glomerulonephritis. More recently, research has shown that complement system has many additional functions relating to regulation of the immune response, homeostasis, and metabolism. In parallel with these discoveries, it has also become clear that the complement system is important to the pathogenesis of many non-immune-complex mediated kidney diseases. In fact, in atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy (C3G), uncontrolled complement activation is the primary driver of disease. Complement activation generates multiple pro-inflammatory fragments, and if not properly controlled it can cause fulminant tissue injury. Furthermore, the mechanisms of complement activation and complement-mediated injury vary from disease to disease. Many new drugs that target the complement cascade are in clinical development, so it is important to fully understand the biology of the complement system and its role in disease.
Keywords: complement, inflammation, glomerulonephritis, antibody mediated allograft rejection, therapeutics, diagnostics
Introduction
The complement cascade is a phylogenetically ancient system.1 Complement research also has a long history, as described in recent reviews.2 In the 1800s, researchers first observed that components within normal serum are bactericidal. In 1895 Jules Bordet discovered that a heat stable component of immune serum (later identified as antibodies) agglutinated live vibrios, and that a heat labile fraction of the serum (later identified as complement) mediated lysis of the bacteria. The complement system was originally called “alexin”, from the Greek word for “to defend”, but the name “complement” was later coined to describe the role of this system in complementing the function of antibodies. These experiments shaped the original understanding of the complement cascade as a system of extracellular proteins that are a downstream effector of antibodies. Although this description is accurate, more recent research has expanded our understanding of complement biology.
Early studies of the role of complement in kidney disease were influenced, perforce, by understanding of the complement system at that time. Early in the 20th century, for example, it was discovered that immune-complex glomerulonephritis is associated with low levels of circulating complement proteins.3 After methods were developed to detect C3 within tissues by immunofluorescence, it was also found that C3 is deposited in the glomeruli of patients with lupus nephritis.4 These findings fit well with the perception of the complement cascade as a downstream effector of antibody-mediated responses. Clinical evaluation of complement levels and tissue deposits became a routine part of the assessment of patients with suspected glomerulonephritis, and a large body of data was subsequently collected regarding complement in the various forms of glomerulonephritis.5,6
Although these clinical tests strengthened the link between immune-complex diseases and complement activation, they also revealed that the complement system is activated in some diseases not associated with immune-complex deposition. It was found that C3 levels fall in some patients with hemolytic uremic syndrome7,8, for example, and that C3 can be deposited in the absence of immunoglobulin.9 These observations did not fit into the paradigm of complement as simply an effector system for antibodies, and they initially remained unexplained.
Over the past 20 years, several developments have considerably advanced our understanding of complement in kidney disease. Genomics studies have revealed multiple strong associations of genetic variants of complement proteins with disease.10–13 In addition, genetically modified mice have demonstrated a functional role for the complement cascade in different models of kidney disease.14,15 Furthermore, these two types of research have intersected, as investigators have identified mutations in human patients and then generated transgenic mouse models to prove that a given mutation is causal of disease.16,17 Experimental work in mice has confirmed a pathogenic role for complement in antibody mediated diseases, such as lupus nephritis and antibody mediated transplant rejection (AMR). However, research has also revealed a role for complement in unexpected diseases, such as anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis.18 Thus, in spite of a long history of research into the role of complement in kidney disease, this is still a rapidly evolving field.
Overview of the complement cascade
The complement cascade is comprised of circulating and cell surface proteins. Similar to the coagulation cascade, complement proteins circulate as zymogens that are activated upon cleavage. Once this process starts, the early activated proteins form proteolytic enzymes that then cleave downstream proteins. This cascade structure allows rapid amplification of the cleavage process, and activation can generate millions of downstream fragments. Because the activation products can have such potent effects, the process is checked by a family of inactivating proteins, or complement regulators.19,20
Initiation.
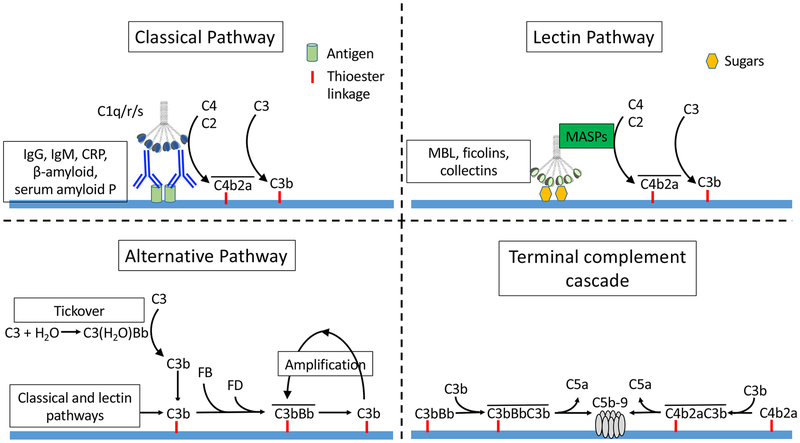
Complement activation can be initiated through three different pathways – the classical pathway (CP), lectin pathway (LP), and alternative pathway (AP). Each pathway generates protein complexes that can cleave the C3 protein, referred to as “C3 convertases” (Figure 1). C3 is generally regarded as the central protein of the complement cascade.
Figure 1. Complement activation pathways.
Complement activation can be initiated through three pathways. The classical pathway is activated when immunoglobulins or other activating proteins bind to ligands and engage C1q. The C1qrs complex cleaves C4 and C2, generating the classical pathway C3 convertase (C4b2a). The lectin pathway is activated when mannose binding lectin (MBL) or other activating pathways bind to sugars and engage the mannose associated serine proteases (MASPs). The MASPs cleave C4 and C2, generating C3 convertase (C4b2a). The alternative pathway is activated spontaneously when water binds hydrolyses C3, forming a molecule with C3 convertase activity [C3(H2O)]. C3b generated by C3(H2O) or the other activation pathways then combines with factor B. Factor D then cleaves factor B to form a C3 convertase (C3bBb), which cleaves additional C3. C3b generated by this convertase can, itself, form more C3bBb, providing further amplification of this process. Either of the C3 convertases (C3bBb and C4b2a) can combine with an additional C3b to form C5 convertase. C5 convertases catalyze the cleavage of C5, and formation of C5b-9 (the terminal complement complex or membrane attack complex).
Classical pathway.
The CP is activated when C1q binds to the Fc domains of IgM and IgG. C1q is a hexameric protein, and it binds to immunoglobulin with the greatest affinity when it engages multiple Fc regions on polymeric IgM or to the Fc regions of six IgG molecules simultaneously. Consequentloy, IgG activates the CP most efficiently when multiple immunoglobulin molecules are clustered together in close proximity. IgG isotypes have a varying ability to activate the CP (IgG3 > IgG1 > IgG2>> IgG4). In vitro experiments have shown that IgA immune-complexes, on the other hand, activate the AP but are poor activators of the CP.21 Binding of C1q to IgM or IgG containing immune-complexes is the first stept in CP activation, and C1q can be detected in biopsies of patients with immune-complex glomerulonephritis, such as those with lupus nephritis. Interestingly, autoantibodies to C1q are a hallmark of lupus, and are associated with kidney involvement.21 Autoantibodies to C1q within immune deposits may amplify activation and contribute to tissue injury.
The link between immune-complexes and complement activation is not always straightforward, however. In primary membranous nephropathy (MN), for example, antibodies to the phospholipase A2 receptor are predominantly IgG4, a weak classical pathway activator. The mechanism of glomerular complement activation may involve the LP, the AP, and/or involvement of other antibody isotypes early in the disease.22 Similarly, IgA does not activate the classical pathway, and complement activation in IgA nephropathy may involve codeposition of other antibody isotypes or activation through the AP and LP.23
Once the CP is activated, C4 from the plasma is cleaved into C4a and C4b. C4b, can bind to nearby tissues and also forms part of the CP C3-convertase. CP regulators control this process by cleaving C4b into iC4b and C4d (Figure 2). C4d is catalytically inactive, but it remains bound to involved tissues and can serve as a marker of CP activation within the tissue. C4d is seen, for example in the peritubular capillaries of patients with AMR24 and in the glomeruli of patients with immune-complex-mediated glomerulonephritis.25 There are cases of AMR in which C4d deposits are not seen. It is not known whether antibody causes injury by non-complement mechanisms in this setting, or whether the endothelial cells are able to clear the deposited C4d.
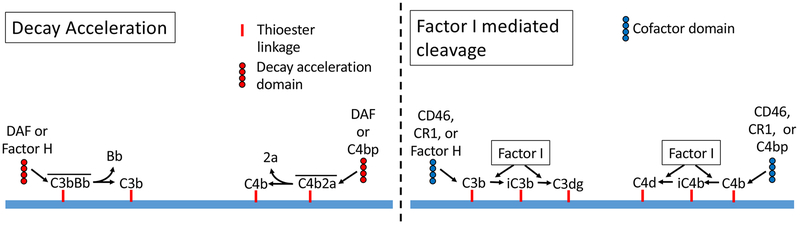
Figure 2. Inhibition of C3 convertases.
The C3 convertases are inactivated by two mechanisms: decay acceleration and factor I mediated cleavage. Decay acceleration shortens the half-life of the convertase. Once the components are separated they cannot recombine. Factor I cleaves C3b and C4b, generating the catalytically inactive iC3b and iC4b fragments, respectively. Factor I requires the presence of proteins that can act as “cofactors”. CD46 (also called membrane cofactor protein, or MCP) and complement receptor-1 (CR1) can serve as cofactors for cleavage of either C3b or C4b. Factor H is a decay accelerator and a cofactor for the alternative pathway. C4 binding protein (C4bp) is a decay accelerator and cofactor for the classical and lectin pathway convertases.
Lectin pathway.
The LP is activated by a group of proteins that bind to certain sugars expressed on the surface of bacteria.26 Proteins that can activate this pathway include mannose binding lectin (MBL), ficolins A, B, or C, and collectins 10 and 11.27 When bound to target sugar motifs, these proteins engage serine proteases that can cleave C4, forming the same C3-convertase generated by the CP (C4b2a). LP activating proteins can also bind to certain endogenous proteins. For example, collectin-11 binds to L-fucose expressed on the surface of damaged kidney tubules, activating the LP and causing further inflammatory injury.28 MBL deposits have also been detected in glomerular diseases, including IgA nephropathy, membranous nephropathy , and lupus nephritis.29,30 This indicates that the LP can be activated within the glomerulus in these diseases. Although specific molecular triggers of the LP have not been identified in these diseases, in the case of IgA nephropathy, the IgA containing immune-complexes may activate the LP through the interaction of MBL with glycan side-chains on the IgA molecule.31
Alternative pathway.
Unlike the other two pathways, AP activation does not require specific protein activators. The AP is continually activated at a low level in plasma through a process called “tickover,” and can also be secondarily activated by the CP and LP (Figure 1). Intact C3 is the second most abundant plasma protein, with a normal concentration of greater than 1 g/L. Tickover is a process by which plasma water spontaneously hydrolyzes circulating C3 to form a molecule referred to as C3(H2O). C3(H2O) can bind the AP protein factor B. The attached factor B is then cleaved by factor D (a circulating serine protease), generating a weak C3 convertase [C3(H2O)Bb]. Although this convertase does not attach to surfaces, it slowly cleaves additional C3 in in the circulation, generating C3b. C3b contains a thioester bond that can react with amines and hydroxyl groups on nearby surfaces, forming a covalent attachment. C3b that is fixed to target surfaces can then combine with factor B to generate an immobilized form of C3bB. Factor D cleaves the factor B in this complex, generating the AP convertase (C3bBb), which catalyzes the deposition of additional C3b on target surfaces. Surface-bound C3b generated by the CP and LP can also combine with factor B to form C3bBb. This too can trigger amplification through the AP unless the C3b is inactivated (Figure 2).
As outlined above, AP convertases can cleave C3 in solution (“fluid phase”) and on the surface of cells and tissues (“solid phase”). It is believed that fluid phase activation and activation directly on the glomerular capillary wall may each contribute to glomerular injury. Although it can be difficult to distinguish the respective roles of fluid phase and solid phase AP activation in kidney injury, it is more intuitive that activation directly on glomerular surfaces would be injurious. Nevertheless, there are animal models and disease associated mutations that support the contribution of dysregulated fluid phase activation to disease.32,33
Given the important role that the AP plays in kidney disease, there are drugs in development that specifically factor B and factor D, components of the AP. Factor D circulates in plasma at a concentration of ~2 μg/mL. Among the complement proteins this is a relatively low concentration, making it a potentially advantageous target. A small molecule inhibitor of factor D is in clinical development for AP-mediated diseases, such as C3G. Interestingly, mannose-associated serine protease (MASP)-3, a protease that is associated with the LP, cleaves the precursor form of factor D (pro-FD) into its active form.34 Thus, the LP and AP are interconnected, and drugs that block MASP-3 could potentially block AP activation.
The AP, therefore: 1) spontaneously and indiscriminately deposits a low level of C3b on nearby surfaces, 2) self-amplifies on surfaces unless inhibited by regulatory proteins, and 3) amplifies activation after it is initiated through the CP and LP. Because AP activation does not require the specific recognition events that trigger the CP and LP, proper regulation of the AP is even more important than for the other two pathways, explaining why genetic mutations in complement regulatory proteins are most commonly associated with dysregulation of the AP.
Cross-talk between complement and the coagulation cascade.
The complement and coagulation cascades are both systems of circulating proteins that are activated by proteases. Research has revealed multiple points of cross-talk between these two systems, where activation of proteases in one of cascades can activate proteins of the other system.35 Interactions between the cascades are bi-directional. For example, the coagulation enzymes thrombin36 and kallikrein can activate C3 and C5.37 Experiments have shown activating and inhibitory functions for plasmin.37 Complement activation can induce pro-thrombotic effects through its interactions with myeloid cells, platelets, and endothelial cells.35 In some contexts, these interactions are probably physiologically beneficial. For example, thrombosis is an important part of the immune response to pathogens. However, these interactions may also contribute to the pathogenesis of diseases such as aHUS, which are characterized by intravascular complement activation and thrombosis. These interactions are also relevant to anti-complement therapeutics, as activation of complement by coagulation enzymes may bypass some inhibitory strategies. Interestingly, heparin has both anti-coagulant and anti-complement functions.38
Terminal complement activation.
The three activation pathways converge at the level of C3, creating an area of C3b fixation on target surfaces. An additional C3b can react with either of the C3 convertases to form trimolecular complexes: C4b2a3b or (C3b)2Bb. These complexes lose the ability to cleave C3 but gain the ability to cleave C5 (“C5 convertase”), and cleave C5 into C5a and C5b fragments. C5b can bind to C6 and C7 to form an amphophilic trimer that contains a lipid binding site, allowing the complex to attach to nearby cell membranes where it can next attach a C8 molecule. This C5b-8 complex then receives C9 proteins, which polymerize on the cell surface and form a pore through the lipid membrane. Surface C5b-9 allows the free flow of ions into and out of cells, and can cause osmotic lysis. Even in sublytic quantities C5b-9 deposition can cause activation. In podocytes, for example, C5b-9 insertion causes production of reactive oxygen species, proteases, extracellular matrix, and secretion of TGF-β.39 Deposition of C5b-9 on mesangial cells induces secretion of prostaglandin and IL-1.40 These may be important causes of podocyte injury in membranous nephropathy, and deficiency of terminal pathway proteins prevent proteinuria in models of the disease.41
Anaphylatoxins.
C3a is generated during C3 cleavage, and C5a is generated during C5 cleavage. These soluble protein fragments, referred to collectively as the anaphylatoxins, have molecular weights of ~5 kD. Transmembrane receptors for the anaphylatoxins (C3aR, C5aR1, and C5aR2) are primarily expressed on myeloid cells, although they can also be expressed on some other cell types.42 The anaphylatoxins are potent chemoattractants. They primarily have pro-inflammatory effects43, although in some settings they also have anti-inflammatory effects.44 C3aR and C5aR are expressed on renal tubular epithelial cells, and C3a induces these cells to produce chemokines.45 C3a and C5a are rapidly inactivated by carboxypeptidases in plasma, which removes arginine from the carboxy terminus of the peptides. These fragments probably contribute to the pathogenesis of glomerular disease, and detection of increased levels in plasma can also be useful as a biomarker of inflammation and injury.46
Complement regulation.
Like all components of the immune system, a critical feature of the complement cascade is the ability to discriminate “self” from “non-self” and healthy cells from damaged and transformed cells. If not adequately controlled, the same inflammatory effects that eliminate infections can cause tissue damage. Complement activation within a tissue is controlled by the balance of activating and regulatory proteins. The activating proteins of the CP are highly specific (e.g. antibodies), those of the LP are less specific, and the AP is largely indiscriminate. Consequently, proper regulation is probably most important for the AP, and the mere absence of proper regulation is sufficient to permit AP mediated inflammation.
The complement system is regulated by a family of cell surface and soluble proteins that block activation at several different points in the cascade.47 There are specific inhibitors of each of the activation pathways. Factor H and CRIg block the AP, C1-INH and C4 binding protein block the CP, and MAp44 blocks the LP. Decay accelerating factor shortens the half-life of the C3 convertases, thereby inhibiting complement activation at the level of C3 and C5. Similarly, CD46 (also called membrane cofactor protein, MCP) blocks activation at the level of C3 and C5 by serving as a necessary co-factor for the factor I-mediated cleavage of C3. Finally, CD59 inhibits the formation of C5b-9 on cell membranes.
Complement activation is a dynamic process, as the proteins that promote activation (e.g. C3 and/or antibodies) can be upregulated, and regulatory proteins can be downregulated.48 Activating factors, such as antibodies, can overwhelm complement inhibitors expressed on a target cell. A loss of complement regulatory function can be caused by a number of different events. Congenital defects in complement regulators have been identified in various disease states. Patients can also develop autoantibodies to factor H which block it from functioning properly. Up to 5% of patients with childhood aHUS may have anti-factor H antibodies49, and cases of anti-factor H antibodies were recently reported in three patients with membranous nephropathy.50 C3 nephritic factors (C3Nefs) do not bind to C3 or factor B individually, but they bind to the C3 convertase and stabilize it, making it resistant to regulation. Antibodies that do bind to C3 or factor B have also been identified in patients.51 All of these different defects can have the same function effect – to shift the balance of complement activation/inhibition.
The factor H related proteins (FHRs), are a group of five proteins that also function to impair factor H. These proteins arose through reduplication of the factor H gene, but they do not contain the inhibitory region of the protein. Mutations and overexpression of these proteins have been associated with aHUS, C3G, and IgA nephropathy.52 It is believed that the FHRs are competitive antagonists of factor H, blocking it from binding to tissue surfaces. By blocking factor H they can cause AP “dysregulation” within tissues, exacerbating disease. Annexin A2 is another protein that blocks factor H from controlling the AP on kidney cells53, although a specific role of this protein in disease has not been demonstrated.
The local nature of activation
Most of the proteins in the complement cascade circulate in plasma, and most clinical complement measurements are performed on proteins in the plasma. Activation of the cascade can occur in the fluid phase, consuming C3 and/or C4 and generating soluble C3a, C3b and C5a. Nevertheless, it is noteworthy that disease-associated activation usually occurs locally at specific tissue sites, such as within the mesangium or glomerular capillary wall. Measurement of changes in plasma complement proteins, such as C3 and C4, will not always reflect activation within tissues. Not surprisingly, kidney biopsies show complement activation in diseases in which the circulating complement levels of complement proteins may not change, such as membranous nephropathy, anti-glomerular basement membrane disease, and IgA nephropathy.5
Experimental work has shown that complement activation can have key signaling effects at the level of cell-cell interactions. An important example of this is activation of T cells by dendritic cells. Dendritic cells interact directly with T cells to activate or inhibit them in an antigen-specific manner. Experiments in rodent models demonstrated that AP proteins produced by the dendritic cell are exported and can activate near the cell-cell interface.54,55 The degree of activation is regulated by complement regulators expressed on the dendritic cell, and modulates the T cell response to antigen. These findings illustrate localized effects of complement that can influence the immune response to kidney antigens.
Complement in inflammation
Even during an appropriate response to pathogens, complement activation can cause damage to nearby tissues. This is particularly true when the response is overwhelming, such as in the setting of sepsis. The same downstream complement fragments that help to eliminate pathogens during infections are also causes of tissue injury in autoimmune diseases.22 Complement activation has been implicated in the pathogenesis of a large number of diseases, affecting essentially every organ in the body.56 Robust complement activation contributes to the pathogenesis of fulminant diseases, such as sepsis57 and aHUS.58 More subtle imbalances of complement regulation are associated with chronically progressive diseases, such as IgA nephropathy59 and macular degeneration.60
In patients with an impaired ability to regulate complement, a disease flare can be triggered by physiologic or appropriate complement activation. Transient infections or minor stressors can lead to chronic complement-mediated disease. In women with congenital mutations in factor H, for example, pregnancy is one of the most common triggers of TMA.61 The exact mechanisms linking these events are incompletely understood, but pregnancy probably causes vascular changes or releases AP activators that cause intravascular complement activation. In most patients this may have only limited effects, whereas in susceptible patients it can lead to life-threatening disease. Similarly, C3G can be triggered by infection-associated complement activation that would ordinarily be self-limited. Once complement-mediated disease becomes active it can be very difficult to control. For example, patients with heterozygous factor H mutations have only a partial deficiency of the protein. Yet once they develop active aHUS, they usually do not respond to plasma infusions, even though plasma therapy should replace the defective factor H.58
IgA nephropathy is an example of chronic AP dysregulation. Our current understanding of this disease is that it is caused by immune-complexes composed of immunoglobulins bound to galactose deficient IgA1.23 These immune-complexes deposit in the mesangium and cause disease. Genetic variations or differences in expression levels of factor H and the FHRs can influence the disease.59 In these patients, the differences in the ability to regulate the AP are probably fairly minor. Over time, however, these small differences probably affect the rate of disease progression.
Evidence that complement is involved in kidney disease
As mentioned above, complement proteins have been analyzed in kidney disease for decades, and we have accumulated a great deal of data regarding deposition of complement proteins in kidney biopsies and perturbations in circulating C3 and C4 levels in patients with kidney disease. Many additional lines of evidence have also implicated complement in kidney disease.
Genomics studies.
A large number of genomic studies have identified strong correlations between genetic variants in complement genes and the risk of disease. Genetic analysis of complement genes has been very comprehensive in aHUS and C3G, implicating defects in several complement regulatory proteins with these diseases.11,62–65 Gene sequencing frequently reveals genetic variants, and the challenge can be to predict which of the variants are causative of disease. In aHUS and C3G, genetic variants have been identified that track with disease penetrance in families, supporting a critical role of the variant in disease risk.66–70 Investigators have also generated recombinant forms of the mutated proteins and confirmed that the mutations have functional effects on complement activation or regulation.16
The identification of mutations in multiple different complement proteins provides further support regarding the functional significance of the variants. The association of inactivating mutations in different AP regulators (factor H, factor I, and MCP) with aHUS, for example, overwhelmingly implicate AP dysregulation in disease pathogenesis. The identification of gain of function mutations in C3 and factor B (AP activating proteins) in some patients 71,72, provides further evidence that imbalances in the AP are causative of disease. Similarly, the discovery of autoantibodies to complement regulatory proteins in patients with the same diseases illustrates that different molecular defects can have the same pathologic effect.
Animal models.
The complement system in rodents is largely similar to that in humans. This has allowed investigators to test the role of the complement cascade in a broad range of models, including immune-complex disease73, ANCA vasculitis18, C3G15, aHUS74, ischemia/reperfusion75, focal segmental glomerulosclerosis76, and allograft rejection.77
Therapeutics.
The most compelling evidence for an important role of complement in disease is the effective use of complement inhibitory drugs in human patients. Eculizumab, an FDA approved inhibitory antibody against C5, can rapidly and effectively treat aHUS. There are also case reports and small case series in which eculizumab has been effectively used in C3G, lupus nephritis, IgA nephropathy, and antibody mediated transplant rejection.78 Many new complement inhibitory drugs are in development.79 These may provide effective new treaments for kidney diseases, and may also reveal new functions of complement in disease.
Recent Discoveries
Recent studies have revealed several unexpected functions of complement in health and disease. Studies have also shown that cells other than hepatocytes can generate complement proteins, and that activation at the cell-cell interface can generate important signals. Work has also shown that complement fragments have important physiologic functions inside of cells.80 These findings highlight many localized effects for this system, sometimes at the level of the single cell. These are functions that may contribute to disease, but that will not be reflected in tissue staining or plasma complement measurements.
Research has also revealed that the complement system has an important role in shaping the adaptive immune response, rather than simply being a downstream effector system for antibodies. The complement cascade has a strong adjuvant effect on the response of B cells to antigens, and it also controls T cell activation and polarization.81 This suggests that complement inhibition could have effects on autoimmunity, and that complement inhibition could have multiple effects on disease.
Why the kidney?
The involvement of complement in so many different diseases points to a unique susceptibility of the kidney to complement-mediated injury. For example, systemic defects in complement regulation often manifest in isolated diseases of the kidney. In addition, multiple different causes of kidney disease engage the complement system. Several features of the kidney may contribute to this. Fenestrations in glomerular endothelial cells may increase the access of large complement proteins in plasma to basement membrane. In the case of the kidney, the glomerular basement membrane is a large surface that does not express intrinsic complement regulators, and it may be particularly dependent on factor H. Complement proteins may also become concentrated in the glomerular capillaries as plasma water is filtered, exposing the GBM to high concentrations of AP proteins.
There may be other specific characteristics of the kidney that favor complement activation. For example, ammonia is generated in the kidney and also causes AP activation by nucleophilic interaction with C3.82 In a small clinical study, administration of bicarbonate, which reduces ammonia production, reduced levels of complement fragments in the urine.83 Such approaches could be useful for blocking maladaptive complement activation in the kidney while leaving the other functions of complement intact.
Future directions.
The complement cascade is activated in many different kidney diseases, and research during the past 20 years has improved our understanding of the molecular mechanisms that engage the complement system within the kidney. Greater insight into the role of complement in disease will provide us with a better understanding of the risks and benefits of complement inhibition. Many new complement inhibitory drugs are in development, so detailed understanding of the role of complement in different kidney diseases will also help researchers and clinicians to choose the optimal agent for a given disease. Small molecule inhibitors of C5a, for example, block the effects of C5a while leaving other complement functions intact. These agents may be well-suited for some diseases, but inappropriate for others.
An important feature of the complement system is that it generates multiple different soluble and tissue bound biomarkers. Clinicians have utilized some of these biomarkers for decades, such as measurement of C3 and C4 levels. New tools for non-invasively detecting complement activation in tissues are also in development.84,85 As better methods of monitoring complement activation enter the clinic, they may provide clinicians with more accurate methods for identifying those patients in whom complement inhibitory drugs are indicated.
Clinical summary:
The complement system is an important part of the immune system and helps protect the host from infection.
It is well known that complement is activated by immunoglobulins and immune complexes, but activation can also be triggered by several other molecular patterns and proteins.
Uncontrolled or inappropriate complement activation causes inflammation, and the complement system has been implicated in the pathogenesis of a wide range of kidney diseases.
Therapeutic complement inhibitors have been approved for the treatment of atypical hemolytic uremic syndrome, and may also be effective in other kidney diseases.
Acknowledgments
Financial disclosure:
The author’s research is supported by National Institutes of Health grants DK113586 and DK076690. The author receives royalties from Alexion Pharmaceuticals, Inc. He is also a consultant for AdMIRx, Inc., a company developing complement inhibitors. He holds stocks and will receive royalty income from AdMIRx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Dodds AW, Matsushita M. The phylogeny of the complement system and the origins of the classical pathway. Immunobiology. 2007;212(4-5):233–243. [DOI] [PubMed] [Google Scholar]
- 2.Sim RB, Schwaeble W, Fujita T. Complement research in the 18th-21st centuries: Progress comes with new technology. Immunobiology. 2016;221(10):1037–1045. [DOI] [PubMed] [Google Scholar]
- 3.Kellett CE. COMPLEMENT TITRE IN ACUTE NEPHRITIS WITH SPECIAL REFERENCE TO CAUSATION BY REVERSED ANAPHYLAXIS. The Lancet. 1936;228(5909):1262–1265. [Google Scholar]
- 4.Lachmann PJ, Muller-Eberhard HJ, Kunkel HG, Paronetto F. The localization of in vivo bound complement in tissue section. J Exp Med. 1962;115:63–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert LA, Cosio FG, Neff JC. Diagnostic significance of hypocomplementemia. Kidney Int. 1991;39(5):811–821. [DOI] [PubMed] [Google Scholar]
- 6.di Belgiojoso GB, Tarantino A, Durante A, Guerra L. Complement deposition in glomerular diseases. Proceedings of the European Dialysis and Transplant Association European Dialysis and Transplant Association. 1975;11:515–521. [PubMed] [Google Scholar]
- 7.Cameron JS, Vick R. Letter: Plasma-C3 in haemolytic-uraemic syndrome and thrombotic thrombocytopenic purpura. Lancet. 1973;2(7835):975. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan BS, Thomson PD, MacNab GM. Letter: Serum-complement levels in haemolytic-uraemic syndrome. Lancet. 1973;2(7844):1505–1506. [DOI] [PubMed] [Google Scholar]
- 9.Verroust PJ, Wilson CB, Cooper NR, Edgington TS, Dixon FJ. Glomerular complement components in human glomerulonephritis. J Clin Invest. 1974;53(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu F, Maga T, Meyer NC, et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X, Pickering MC, Smith RJ. C3 glomerulopathy: the genetic and clinical findings in dense deposit disease and C3 glomerulonephritis. Seminars in thrombosis and hemostasis. 2014;40(4):465–471. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Zhai YL, Wang FM, et al. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J Am Soc Nephrol. 2015;26(5):1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigg RJ, Lim A, Haas M, Alexander JJ, He C, Carroll MC. Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int. 1998;53(2):320–330. [DOI] [PubMed] [Google Scholar]
- 15.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. In. Nat Genet. Vol 312002:424–428. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Jackson K, Yang Y, Denton H, et al. Hyperfunctional complement C3 promotes C5-dependent atypical hemolytic uremic syndrome in mice. J Clin Invest. 2019;129(3):1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda Y, Mohammed I, Song D, et al. Murine systemic thrombophilia and hemolytic uremic syndrome from a factor H point mutation. Blood. 2017;129(9):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–740. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt CQ, Lambris JD, Ricklin D. Protection of host cells by complement regulators. Immunol Rev. 2016;274(1):152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiemstra PS, Gorter A, Stuurman ME, Van Es LA, Daha MR. Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol. 1987;17(3):321–326. [DOI] [PubMed] [Google Scholar]
- 22.Thurman JM, Yapa R. Complement Therapeutics in Autoimmune Disease. Frontiers in immunology. 2019;10:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maillard N, Wyatt RJ, Julian BA, et al. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26(7):1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10(10):2208–2214. [DOI] [PubMed] [Google Scholar]
- 25.Sethi S, Nasr SH, De Vriese AS, Fervenza FC. C4d as a Diagnostic Tool in Proliferative GN. J Am Soc Nephrol. 2015;26(11):2852–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaya da Costa M, Poppelaars F, Berger SP, Daha MR, Seelen MA. The lectin pathway in renal disease: old concept and new insights. Nephrol Dial Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Garred P, Genster N, Pilely K, et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev. 2016;274(1):74–97. [DOI] [PubMed] [Google Scholar]
- 28.Farrar CA, Tran D, Li K, et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest. 2016;126(5):1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17(6):1724–1734. [DOI] [PubMed] [Google Scholar]
- 30.Lhotta K, Wurzner R, Konig P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol Dial Transplant. 1999;14(4):881–886. [DOI] [PubMed] [Google Scholar]
- 31.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001;167(5):2861–2868. [DOI] [PubMed] [Google Scholar]
- 32.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118(2):608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Barricarte R, Heurich M, Valdes-Canedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120(10):3702–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobo J, Szakacs D, Oroszlan G, et al. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Scientific reports. 2016;6:31877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL. Haemostasis and innate immunity - a complementary relationship: A review of the intricate relationship between coagulation and complement pathways. Br J Haematol. 2018;180(6):782–798. [DOI] [PubMed] [Google Scholar]
- 36.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. [DOI] [PubMed] [Google Scholar]
- 37.Foley JH, Conway EM. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016;118(9):1392–1408. [DOI] [PubMed] [Google Scholar]
- 38.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–1226. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham PN, Quigg RJ. Contrasting roles of complement activation and its regulation in membranous nephropathy. J Am Soc Nephrol. 2005;16(5):1214–1222. [DOI] [PubMed] [Google Scholar]
- 40.Lovett DH, Haensch GM, Goppelt M, Resch K, Gemsa D. Activation of glomerular mesangial cells by the terminal membrane attack complex of complement. J Immunol. 1987;138(8):2473–2480. [PubMed] [Google Scholar]
- 41.Baker PJ, Ochi RF, Schulze M, Johnson RJ, Campbell C, Couser WG. Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in rats. Am J Pathol. 1989;135(1):185–194. [PMC free article] [PubMed] [Google Scholar]
- 42.Laumonnier Y, Karsten CM, Kohl J. Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol Immunol. 2017. [DOI] [PubMed] [Google Scholar]
- 43.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46(14):2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coulthard LG, Woodruff TM. Is the Complement Activation Product C3a a Proinflammatory Molecule? Re-evaluating the Evidence and the Myth. J Immunol. 2015;194(8):3542–3548. [DOI] [PubMed] [Google Scholar]
- 45.Thurman JM, Lenderink AM, Royer PA, et al. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178(3):1819–1828. [DOI] [PubMed] [Google Scholar]
- 46.Schroppel B, Heeger PS, Thiessen-Philbrook H, et al. Donor Urinary C5a Levels Independently Correlate With Posttransplant Delayed Graft Function. Transplantation. 2019;103(1):e29–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thurman JM, Ljubanovic D, Royer PA, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116(2):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durey MA, Sinha A, Togarsimalemath SK, Bagga A. Anti-complement-factor H-associated glomerulopathies. Nature reviews Nephrology. 2016;12(9):563–578. [DOI] [PubMed] [Google Scholar]
- 50.Seikrit C, Ronco P, Debiec H. Factor H Autoantibodies and Membranous Nephropathy. New England Journal of Medicine (NEJM). 2018;379(25):2479–2481. [DOI] [PubMed] [Google Scholar]
- 51.Marinozzi MC, Roumenina LT, Chauvet S, et al. Anti-Factor B and Anti-C3b Autoantibodies in C3 Glomerulopathy and Ig-Associated Membranoproliferative GN. Journal of the American Society of Nephrology (JASN). 2017;28(5):1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT. Complement factor H related proteins (CFHRs). Mol Immunol. 2013;56(3):170–180. [DOI] [PubMed] [Google Scholar]
- 53.Renner B, Tong HH, Laskowski J, et al. Annexin A2 Enhances Complement Activation by Inhibiting Factor H. J Immunol. 2016;196(3):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heeger PS, Lalli PN, Lin F, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017;18(12):1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czermak BJ, Sarma V, Pierson CL, et al. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5(7):788–792. [DOI] [PubMed] [Google Scholar]
- 58.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676–1687. [DOI] [PubMed] [Google Scholar]
- 59.Thurman JM, Laskowski J. Complement factor H-related proteins in IgA nephropathy-sometimes a gentle nudge does the trick. Kidney Int. 2017;92(4):790–793. [DOI] [PubMed] [Google Scholar]
- 60.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61(2):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurman JM. A Patient with Hemolytic Uremic Syndrome and Kidney Failure. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(6):933–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaefer F, Ardissino G, Ariceta G, et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94(2):408–418. [DOI] [PubMed] [Google Scholar]
- 63.Fakhouri F, Fila M, Provot F, et al. Pathogenic Variants in Complement Genes and Risk of Atypical Hemolytic Uremic Syndrome Relapse after Eculizumab Discontinuation. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iatropoulos P, Noris M, Mele C, et al. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol. 2016;71:131–142. [DOI] [PubMed] [Google Scholar]
- 65.Bu F, Borsa NG, Jones MB, et al. High-Throughput Genetic Testing for Thrombotic Microangiopathies and C3 Glomerulopathies. Journal of the American Society of Nephrology. 2015;27(4):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imamura H, Konomoto T, Tanaka E, et al. Familial C3 glomerulonephritis associated with mutations in the gene for complement factor B. Nephrol Dial Transplant. 2015;30(5):862–864. [DOI] [PubMed] [Google Scholar]
- 67.Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23(7):1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376(9743):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lhotta K, Janecke AR, Scheiring J, et al. A large family with a gain-of-function mutation of complement C3 predisposing to atypical hemolytic uremic syndrome, microhematuria, hypertension and chronic renal failure. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(8):1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005;16(5):1177–1183. [DOI] [PubMed] [Google Scholar]
- 71.Roumenina LT, Frimat M, Miller EC, et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood. 2012;119(18):4182–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heurich M, Martinez-Barricarte R, Francis NJ, et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci U S A. 2011;108(21):8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexander JJ, Pickering MC, Haas M, Osawe I, Quigg RJ. Complement factor h limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol. 2005;16(1):52–57. [DOI] [PubMed] [Google Scholar]
- 74.Pickering MC, de Jorge EG, Martinez-Barricarte R, et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204(6):1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170(3):1517–1523. [DOI] [PubMed] [Google Scholar]
- 76.Lenderink AM, Liegel K, Ljubanovic D, et al. The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am J Physiol Renal Physiol. 2007;293(2):F555–564. [DOI] [PubMed] [Google Scholar]
- 77.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–587. [DOI] [PubMed] [Google Scholar]
- 78.Thurman JM, Le Quintrec M. Targeting the complement cascade: novel treatments coming down the pike. Kidney Int. 2016;90(4):746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mastellos DC, Ricklin D, Lambris JD. Clinical promise of next-generation complement therapeutics. Nat Rev Drug Discov. 2019;18(9):707–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arbore G, Kemper C, Kolev M. Intracellular complement - the complosome - in immune cell regulation. Mol Immunol. 2017;89:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160(11):5273–5279. [PubMed] [Google Scholar]
- 82.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76(2):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morita Y, Ikeguchi H, Nakamura J, Hotta N, Yuzawa Y, Matsuo S. Complement activation products in the urine from proteinuric patients. J Am Soc Nephrol. 2000;11(4):700–707. [DOI] [PubMed] [Google Scholar]
- 84.Foss CA, Kulik L, Ordonez AA, et al. SPECT/CT Imaging of Mycobacterium tuberculosis Infection with [(125)I]anti-C3d mAb. Mol Imaging Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao T, Zhang Y, Ren J, et al. Noninvasive quantification of intrarenal allograft C4d deposition with targeted ultrasound imaging. Am J Transplant. 2019;19(1):259–268. [DOI] [PubMed] [Google Scholar]