Abstract
Cell death can occur through numerous regulated mechanisms that are categorized by their molecular machineries and differing effects on physiology. Apoptosis and necrosis, for example, have opposite effects on tissue inflammation due to their different modes of execution. Another feature that can distinguish different forms of cell death is that they have distinct intrinsic effects on the cell populations in which they occur. For example, a regulated mechanism of necrosis called ferroptosis has the unusual ability to spread between cells in a wave-like manner, thereby eliminating entire cell populations. Here we discuss the ways in which cell death can propagate between cells in normal physiology and disease, as well as the potential exploitation of cell death propagation for cancer therapy.
Keywords: cell death, apoptosis, ferroptosis, entosis, propagation, competition
Regulated Mechanisms of Cell Death.
Death is the inevitable fate of most cells in metazoan organisms. Some cells, such as skin cells, intestinal epithelial cells and red blood cells, are short-lived and undergo death continuously as part of their normal life cycle, whereas others, such as endothelial cells and neurons, are long-lived and undergo death when they become aged, damaged or infected. In the adult human body, three hundred million cells are estimated to undergo cell death every minute [1]. Death also plays important roles in embryonic development, for example to eliminate supernumerary cells and to sculpt tissue structures [2]. Cell death is therefore involved in nearly every aspect of physiology, and is a highly regulated and coordinated cell activity.
Dysfunctional regulation of cell death is known to contribute to the development of diseases. Too much cell death can eliminate healthy cells, leading to the development of degenerative conditions. Too little cell death, on the other hand, allows damaged or dysfunctional cells to persist, which can lead to aging phenotypes including the development of cancers. While regulation of the amount of cell death is critical to maintaining healthy tissues, recent evidence suggests that the choice of cell death pathway may be just as important [3, 4]. It was once thought that apoptosis, a form of cell suicide, was the only regulated form of cell death, but recent findings have uncovered numerous additional regulated death mechanisms, including several forms of necrosis [3], a program called entosis [5], and others. In total, at least twelve major types of cell death are now proposed to exist that, like apoptosis, can induce the death of cells that are damaged, infected, or no longer needed in development [4].
Cell death mechanisms are typically categorized by their different morphological appearances and by the distinct machinery that controls their execution [4]. For example, apoptotic cells undergo shrinkage, blebbing and fragmentation in response to the activation of caspase proteases. Necrosis, on the other hand, involves cell swelling and plasma membrane rupture, which is in at least some cases mediated by the formation of pore structures in the plasma membrane [4]. Most necrotic death forms are caspase-independent, although two necrotic programs, pyroptosis and secondary necrosis, are known to require caspase activation [6–8]. While apoptosis is typically immunologically silent, and known to occur under normal physiological conditions, necrotic death by contrast is mainly induced in response to infection or other forms of cell stress, and is usually pro-inflammatory [3].
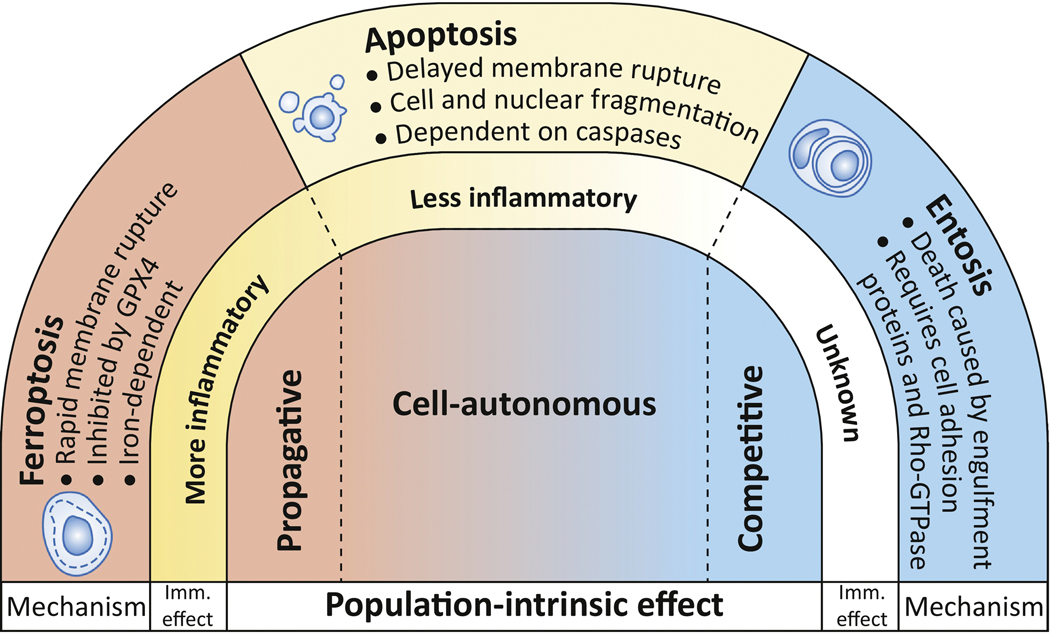
The distinct mechanisms and inflammatory effects of different cell death programs are becoming increasingly well-characterized, and have been reviewed extensively elsewhere [3, 4, 9, 10]. A less well-studied feature is the potential for different types of cell death to have distinct effects on the dynamics of the cell populations in which they occur. The demise of an individual cell can be a completely autonomous event, having no effect on neighboring cells; but death can also affect neighboring cell viability, either negatively, through what is called a bystander effect, or positively, by providing a survival advantage. Among the known forms of cell death, three examples best illustrate these extremes (Figure 1). First, apoptosis is generally thought to be a cell-autonomous suicide that does not impact neighboring cells, although in some contexts the execution of apoptotic cell death can be associated with secreted factors that either induce death or support survival in neighboring cells [11]. Entosis, on the other hand, is competitive by nature, as death execution requires the ingestion and killing of one cell by its neighbor [12]. And at the other end of the spectrum, we and others have shown that a regulated form of necrosis called ferroptosis has the ability to spread between cells in a wave-like manner, suggesting potent non-cell-autonomous killing activity [13, 14]. Induction of each of these different mechanisms has very different effects on cell populations (Figure 1). Ferroptosis can eliminate large groups of cells, which is predicted to be of therapeutic benefit for cancer treatment [13], while entosis can support the long-term survival of stressed populations, thereby potentially promoting cancer progression [15]. In this review we focus on one particular type of dynamic: the elimination of neighboring cells through death propagation. Despite widespread occurrence of population-scale deaths in biological systems [16], little is known about how different forms of cell death can affect population dynamics in this manner. Here we will discuss mechanisms of death synchronization and propagation in cell populations, and the potential influence of cell death population dynamics on cancer therapy.
Figure 1. Cell death mechanisms have different population-intrinsic effects.
Three regulated mechanisms of cell death are shown with their molecular mechanisms, effects on inflammation, and population-intrinsic effects. Apoptosis may be mostly cell-autonomous in its execution, but it can be involved in cell competition, or spread to kill neighboring cells through propagation, in a context-dependent manner. Entosis involves the killing of one cell by a neighboring cell, and is an innately competitive process. Ferroptosis has the ability to spread through cell populations and may be an intrinsically propagative mechanism.
The Death of Cells in Groups.
The death of cells in large groups is a common occurrence in biology. From bacterial populations, where large numbers of individual cells die in biofilms [17], to the slime mold Dictyostelium, where a significant proportion of cells undergoes death to form a spore-supporting stalk structure [18], and plants, where thousands of cells die synchronously to form the water and nutrient channeling vasculature, population-scale cell death is a common feature across evolutionary kingdoms [19]. In metazoans, entire organs are eliminated by cell death to remove developmental structures, such as the salivary gland in Drosophila, or the tails of developing tadpoles [2], and in mammals, groups of cells die to hollow the amniotic cavity in early development [20], to sculpt digits during the development of fingers and toes [21], and to hollow luminal structures in ducts of the developing mammary gland [22].
How are deaths that occur in large groups of cells coordinated? In some contexts, population-scale death is systemically controlled by signaling events that cause individual cell deaths to occur in a synchronous manner. For example, tail resorption in amphibians is induced by signaling from increased levels of thyroid hormone in the blood stream that induces individual cells to undergo apoptosis [2]. In other cases, the death of individual cells can trigger the spreading of trans factors that affect neighboring cells, through what is commonly referred to as a bystander effect. Deaths in this context often occur in a successive manner, with an expanding, wave-like appearance. In plants for example, the spreading of death signals between cells is a common activity that synchronizes differentiation of the vasculature [23], or promotes expansion of cell death zones as part of an innate immune response that limits pathogen infection (Figure 2a) [24, 25]. In metazoan organisms as well, some mechanisms of cell death exhibit context-specific, or in some cases intrinsic, abilities to spread between cells and synchronize death across cell populations.
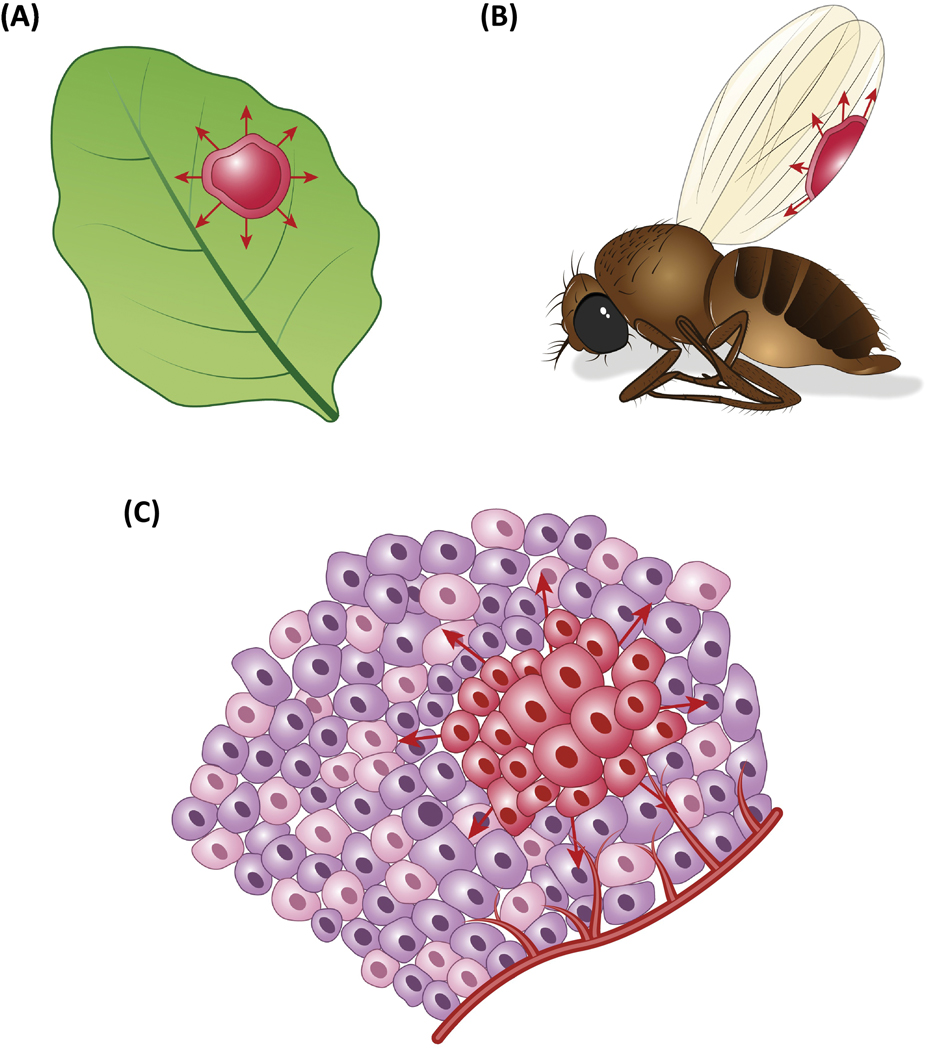
Figure 2. Spreading forms of cell death in biology.
(a) Plants exhibit cell death that can propagate between cells, forming expanding zones of dead tissue in response to oxidative damage or pathogen infection (red, arrows). (b) A wave of apoptosis is observed in developing flies that eliminates entire epithelial cell sheets to promote wing development. (c) In cancerous tissues, propagating forms of cell death such as ferroptosis might be effective mechanisms to eliminate large groups of malignant cells.
Mechanisms of Death Propagation in Metazoan Organisms.
Apoptosis.
Programmed cell death occurring in metazoan organisms is not generally thought to spread between cells. Apoptosis, for example, typically eliminates small numbers of individual cells in normal tissues, and is usually not observed to affect the viability of surrounding cells [26]. In C. elegans development, the majority of developmental cell deaths occur as isolated apoptotic events, and dying cells are engulfed by healthy adjacent cells. The fact that neighboring cells can function in the clearance of apoptotic cells through phagocytosis provides evidence that proximity to an apoptotic cell does not intrinsically inhibit viability. Epithelial cells also mediate engulfment of their apoptotic neighbors in mammalian tissues, for example in the hair follicle, lung and mammary gland [27–29].
In certain cases, however, the execution of apoptosis may be linked to diffusible signals that can lead to the death of adjacent cells. In Drosophila, apoptotic cell death during adult wing development occurs as a massive synchronous wave that successively kills the dorsal and ventral cuticle layers of epithelium, shortly after adults emerge from the puparium (Figure 2b) [30, 31]. Apoptosis in this context is initiated by upregulation of the pro-apoptotic gene hid in the wing epithelium, a common mechanism of apoptosis induction in Drosophila, and also requires the peptide hormone Bursicon, secreted by the nervous system, to eliminate cells primed by Hid expression [31]. That hid expression can induce a propagative mechanism in flies has been shown experimentally in the wing imaginal disc, where enforced hid overexpression in cells in the posterior portion induce the spread of cell death to anterior disc cells. This effect, called “apoptotis-induced-apoptosis”, results from the secretion of the death receptor ligand Eiger (a TNFα ortholog) by dying cells, which activates pro-apoptotic signaling in neighboring cells through activation of Jun-Kinase (JNK) [16]. While the execution of apoptosis may not have intrinsic spreadable properties, the additional secretion of paracrine factors can therefore endow apoptosis with propagative features that could play specialized roles in normal development. Intriguingly, TNFα secretion by apoptotic cells may also coordinate collective cell death in mammalian tissues, as epithelial cell death in the hair follicle in mice, which also involves groups of synchronously dying epithelial cells, was shown to involve a similar mechanism [16].
In developmental systems, communication between dying cells to coordinate the clearance of large structures may be a more commonly utilized strategy than is currently appreciated. Another example was recently discovered in the Drosophila salivary gland, which is removed during metamorphosis by simultaneous induction of apoptosis and the lysosomal degradative pathway autophagy [32]. The execution of death is timed by systemic signaling through the steroid hormone ecdysone, which controls upregulation of Hid [33] and the autophagy-initiating kinase Atg1 [32, 34, 35], thereby activating both pathways. Intriguingly, autophagy induction in this system is also synchronized between neighboring cells by the release of Macroglobulin complement-related (Mcr), a ligand that binds to the receptor Draper [36, 37]. Draper activation is required cell-autonomously for autophagy induction and the death of salivary gland cells [37], suggesting that the synchronous removal of an organ structure in this context may be partially enhanced by coordination of a death program between neighboring cells.
Necrosis.
Necrotic forms of cell death are often considered to be dangerous to surrounding tissue because they result in the release of toxic intracellular contents. Yet necrosis, like apoptosis, can also eliminate individual cells within tissues [26], and may spread to neighboring cells only under certain circumstances. In Drosophila, waves of death can be initiated by the expression of an activated glutamate receptor cation channel in a subset of developing neurons in the eye. This leads to necrotic death initiated by calcium influx, known as excitoxicity, that spreads to neighboring cells through secretion of Eiger and activation of JNK [38]. Waves of necrosis are also observed in response to excitotoxicity in mice, during neuronal cell death resulting from excessive stimulation of neurotransmitter receptors upon ischemic stroke or accumulation of extracellular glutamate. Receptor overstimulation leads to the pathological influx of calcium and causes necrosis that spreads from cell to cell through the transfer of calcium, or potentially other death-inducing signals, via gap junctions [39]. Interestingly, the propagation of calcium signals through gap junctions is also implicated in waves of necrotic cell death that occur in the gut epithelium of C. elegans upon aging-induced organismal death [40].
Among the recently identified forms of regulated necrosis, one particular mechanism called ferroptosis is thought to mediate a spreading effect that may be intrinsic to its execution. Ferroptosis was identified as the form of cell death induced by erastin, an inhibitor of the cystine/glutamate antiporter system xc-[41]. Treatment with erastin depletes intracellular cysteine and thereby inhibits generation of the major antioxidant glutathione. The resulting loss of antioxidant capacity renders cells susceptible to the detrimental effects of reactive oxygen species (ROS). Lipid ROS in particular, derived from polyunsaturated fatty acids (PUFAs), are thought to accumulate due to loss of function of the lipid peroxide-reducing enzyme GPX4, which utilizes glutathione as a cofactor [42]. In the presence of intracellular iron these lipid ROS can set off a chain reaction causing the spread of lipid peroxides throughout cell membranes, leading to the induction of necrosis [43]. Certain cell types, such as kidney epithelium, have been shown to be particularly sensitive to ferroptosis induction, and indeed this mechanism may underlie the pathological cell death associated with renal ischemia reperfusion injury and acute kidney failure, during which renal tubules are observed to undergo extensive necrosis [44]. Intriguingly, treatment of renal tubules with erastin ex vivo was shown to lead to the elimination of entire tubules by a necrotic death that appeared to spread from cell to cell, suggesting that ferroptosis might have the ability to propagate [14, 45]. Cell death resulting from ischemia-reperfusion injury in other tissues including intestinal epithelium [46], heart tissue [47], and excitotoxicity in brain [48, 49], which can also result in the necrotic death of large regions of cells, has also been linked to ferroptosis. Indeed, two recent publications have shown ischemia-reperfusion injury following myocardial infarction is at least partially mediated by ferroptosis [50, 51]. Intriguingly, this process often results in the formation of large contiguous areas of necrotic cells, a phenomenon referred to as contraction band necrosis, which has been hypothesized to be due to the cell-to-cell spreading of death. Together, these findings suggest that ferroptosis contributes to pathological necrosis in multiple contexts and may have the ability to spread through tissues.
Death Propagation in Cancer.
Intrinsic Mechanisms.
Although the development of cancer is generally linked to lowered levels of cell death, in some contexts tumors are found to contain large regions of dead cells, an observation commonly referred to as tumor necrosis. Tumor necrosis is a poor prognostic indicator [52], and is thought to result from reduced nutrient availability due to proliferative expansion or high interstitial pressure that outpaces or disrupts the available vasculature, leading to chronic ischemia. One example called comedo necrosis is characterized by large regions of dead cells within the interior of cancer lesions, where cells fail to survive beyond the diffusion limit of key nutrients from blood vessels. Necrosis of large cell populations in this context may result from the apoptotic death of individual cells [53, 54] and a lack of phagocytic clearance, which allows apoptotic cells to eventually lyse [1]. Alternatively, apoptosis-inhibited cancer cells may undergo necrosis due to energy deprivation [55], or due to induction of a regulated necrotic mechanism called necroptosis, which has also been shown to be induced by ischemic conditions [56–58], but is not known to exhibit propagative activity. Whether ferroptosis could contribute to spreadable necrosis in this context is not clear, but the emerging links between ferroptosis induction and ischemia are suggestive of this possibility [43]. In a recent report, the detachment of breast epithelial cells from extracellular matrix, a condition that also affects cells in the interior regions of carcinomas [5], was observed to lead to ferroptotic death [59]. Moreover, tumor suppression mediated by p53 has also been linked to ferroptosis induction, as a mutant version of p53 unable to stimulate apoptosis, growth arrest, or senescence, but retaining the ability to induce ferroptosis, was still capable of protecting mice from developing spontaneous tumors [60]. Ferroptosis could therefore conceivably be induced as an intrinsic mechanism of tumor suppression during cancer initiation or progression, and contribute to tumor necrosis by inducing the propagation of death through large regions of cells. Further studies are needed to examine whether ferroptosis occurs during carcinogenesis in model systems and clinical specimens.
Radiation-Induced Bystander Effect
In a therapeutic context, mechanisms that could induce or promote the propagation of cell death through solid tumors may be of particular interest, as cancer recurrence typically results from a failure of therapy to target all of the cells in a lesion, or from the intrinsic resistance of a minority of cells. In either case, the propagation of cell death may contribute to the elimination of cells that escape treatment or are resistant to cell-autonomous induction of death but may be sensitive to propagative mechanisms. Death propagation is a known contributor to the effects of radiation therapy, where the irradiation of individual cells can lead to the death of non-irradiated neighboring cells in trans, through what is called the radiation-induced bystander effect. The bystander effect is thought to promote the propagation of cell death through two parallel mechanisms, involving either secreted factors or the movement of cytosolic signals through gap junctions, leading to both long- and short-range effects. Conditioned medium from irradiated human fibroblasts or keratinocytes, for example, has been shown to induce death in 15–25% of non-irradiated cells [61, 62], and to inhibit clonogenic survival by up to 40% [62]. Furthermore, in 3-dimensional models of skin tissue, apoptosis-inducing effects were observed at a distance of up to 1mm from alpha particle-irradiated cells, leading to cell death in 4% of cells in non-irradiated neighboring tissue [63]. Gap junctions have also been implicated in mediating radiation-induced bystander effects directly between neighboring cells. For example, in cultured human fibroblasts and epithelial cells, low confluency or treatment with gap junction inhibitors was shown to block a bystander effect that led to the induction of p53 in neighboring, non-irradiated cells [64, 65].
Therapeutic Induction of Ferroptosis.
While the bystander effect is well known to occur in response to radiation-induced apoptosis, the induction of ferroptosis may represent a new strategy to trigger propagation of death through cancer tissues. Like erastin-induced ferroptosis in kidney tubules, the induction of ferroptosis in cancer cells by treatment with specialized nanoparticles was recently shown to occur with wave-like spatiotemporal patterns that resulted in the elimination of all cells in a culture. Ferroptosis induction by intravenous nanoparticle administration in mice also resulted in the regression of xenograft tumors, suggesting that this form of cell death may have potent tumor suppressive activity [13]. Whereas the radiation-induced bystander effect leads to the death of a relatively small percentage of surrounding cells, ferroptosis may be able to eliminate all neighboring cells in a propagating wave (Figure 2c).
In addition to treatment with ferroptosis-inducing nanoparticles, which mediate the delivery of iron into cells following particle endocytosis, ferroptosis induction through other mechanisms may also hold therapeutic potential. For example, erastin has been found to enhance the efficacy of treatment with various chemotherapeutic agents, such as the DNA damaging agents cisplatin in head and neck cancers [66], doxorubicin in acute myelogenous leukemia (AML) [67], and temozolomide in glioblastoma [68], while treatment with sorafenib, a tyrosine kinase inhibitor, can induce ferroptosis as a monotherapy [69]. A recent finding also linked immunotherapy-induced activation of cytotoxic T cells to the induction of ferroptosis in cancer cells. In this study interferon-γ secretion by activated T cells led to reduced expression of SLC7A11, a component of system xc-, in melanoma cells, resulting in increased lipid peroxidation and ultimately ferroptotic cell death, thus contributing to an immunotherapy-based anti-cancer response in mice [70]. Finally, some cancer types may be particularly susceptible to the induction of ferroptosis. In clear cell renal carcinoma, for example, ferroptosis sensitivity is linked to metabolic alterations arising from inactivation of the VHL tumor suppressor [71], whereas triple-negative breast cancer may be more susceptible due to elevated levels of PUFAs, a vulnerability that may also exist in other cancer types that have undergone epithelial-to-mesenchymal transition [72–74]. Elevated levels of intracellular iron may also result in increased sensitivity to ferroptosis in some cancers, for example as a result of the reduced ferroportin expression that is seen in acute myeloid leukemia [75].
As an increasing number of therapeutic options that can induce ferroptosis are now being developed, further studies are needed to investigate whether wave-like spatiotemporal patterns are a consistent feature of this type of cell death, and whether such waves can eliminate untreated cells in trans, similar to the radiation-induced bystander effect (see Outstanding Questions). The mechanism underlying the propagation of ferroptosis between cells also remains to be elucidated. Whereas radiation effects can in part spread through gap junctions, we have found in unpublished data that ferroptotic propagation does not require cell junctions, suggesting that secreted factors are more likely to play a role. Intriguingly, radiation and ferroptosis may share some regulatory features that could impinge upon propagation. For example, radiation and ferroptosis are both known to damage cells through the generation of ROS that are normally buffered by glutathione and may lead to lipid peroxidation [76, 77]. In addition, ferroptosis induction in tumors is known to lead to increased expression of PTGS2, the gene that encodes cyclooxygenase-2 (COX-2), a key arachidonic acid-metabolizing enzyme required for the generation of secreted inflammatory lipid signaling molecules called prostanoids. Intriguingly, the radiation-induced bystander effect has also been linked to increased expression of COX-2 in bystander cells both in culture and in irradiated mice, and COX-2 inhibition can block trans effects (Figure 3) [61, 78]. COX-2 inhibition did not affect ferroptotic cell death induced by treatment with erastin or the GPX4 inhibitor RSL3, but spatiotemporal patterns of cell death or potential trans effects were not examined [79].
Outstanding Questions:
Which cell death mechanisms have the ability to propagate between cells or participate in cell competition?
What are the population effects of other known forms of cell death such as necroptosis and pyroptosis?
By what mechanism does ferroptosis induce the death of neighboring cells, and what factors promote or limit the spread of death during this process?
Does cell death propagation contribute to more effective cancer therapy?
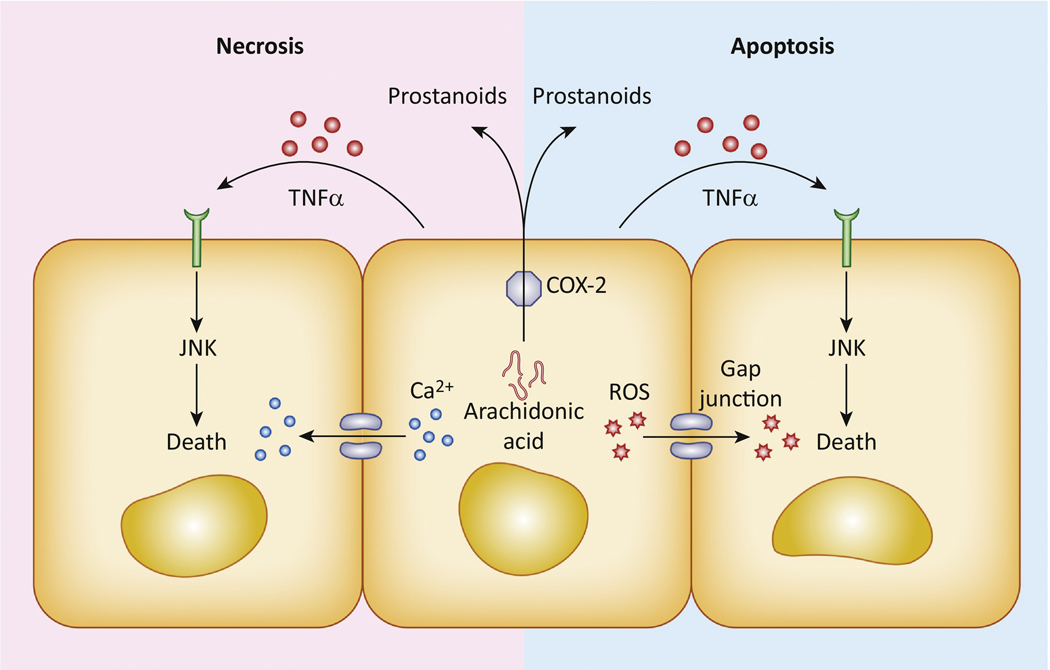
Figure 3. Mechanisms of cell death propagation.
Apoptotic and necrotic forms of cell death can propagate between cells by direct cell-to-cell transfer of death-inducing stimuli, such as calcium or reactive oxygen species, through gap junctions. Secreted factors such as TNFα or arachidonic acid-derived prostanoids can also play a role in killing neighboring cells or cells at larger distances.
Concluding Remarks.
Over the last fifteen years numerous new mechanisms of regulated cell death have been discovered that participate, along with apoptosis, in normal physiology or in cell responses to stress and infection. While the distinct regulatory features of different forms of cell death are becoming elucidated, death at the population scale is much less well understood (see Outstanding Questions). Here we have highlighted a form of regulated necrosis called ferroptosis that has the ability to spread through cell populations in a wave-like manner, and has also been shown to have therapeutic potential for the treatment of cancer. As we postulate that wave-like propagation in particular may be of therapeutic benefit, we have focused here on this property and other contexts where the synchronization of cell death at a population scale may occur.
In addition to propagation, several other population-intrinsic effects of cell death exist. Cell death also participates in competition between cells, where cells are not killed by, but rather benefit from, the deaths of their neighbors. Apoptosis, for example, can be initiated in developing tissues to eliminate cells that are deemed less fit than their neighbors. These deaths then support the proliferation of neighboring cells, an effect called compensatory proliferation or apoptosis-induced proliferation. This process can involve localized mechanical cues or secreted factors that, interestingly, include some of the same pathways implicated in the bystander effect, such as synthesis of prostaglandin E2 by COX-2, as well as the generation of ROS and JNK activation [80–83]. Another death mechanism that can mediate competitive interactions in cancer cell populations is entosis, through which cells compete to ingest and kill each other, and compensatory proliferation is mediated by direct nutrient transfer from loser to winner cells [15, 84]. Thus the types of cell death that occur in stressed tissues, and their effects on local cell interactions or the release of secreted factors, may dictate distinct population-scale effects.
We have shown previously that cell populations experiencing long-term stress (e.g. nutrient starvation) exhibit mixed cell responses involving different types of cell death, including apoptosis, necrosis and entosis, occurring simultaneously but at different frequencies in the population [15]. Cell death mixtures are also observed in clinical specimens [26] and during developmental cell death, for example during interdigitation [85]. In future studies, it may become increasingly important to quantify the relative frequencies of distinct cell death mechanisms within cell populations, particularly in the context of cancer therapy, where cell heterogeneity and extrinsic stresses are complex, and the induction of particular forms of cell death may be preferred for their potentially therapeutic, population-intrinsic effects.
In discussing mechanisms underlying the propagation of cell death, it may be interesting to also consider what factors could inhibit death spreading, and whether boundaries exist that may be able to limit tissue damage caused by cell death waves. It is conceivable that metabolic stress in tumors, resulting for example from nutrient deprivation and reduced glutathione synthesis, could render cancer cells more susceptible to ferroptosis than normal cells. In this case normal tissue could present a natural boundary to ferroptosis waves initiated in cancerous lesions. It is also possible that cells surrounding an expanding region of cell death could upregulate pathways that promote cell survival, as is known to occur in plants, where death spreading in response to pathogen infection is limited by the induction of autophagy as a protective mechanism in adjacent cells [86]. Understanding how death spreading can be inhibited may ultimately prove to be of therapeutic benefit to reduce toxicity to normal tissues during cancer therapy, or for the treatment of degenerative conditions where ferroptosis is thought to play a role in disease pathology, such as stroke or myocardial infarction.
While we have largely focused here on the discussion of ferroptosis, which exhibits clear wave-like patterns of propagation, it remains to be established whether other forms of cell death can also spread between cells (see Outstanding Questions). It has been shown that macrophages undergoing pyroptosis can release prion-like structures called ASC specks, which can then be taken up by neighboring cells and induce activation of the inflammasome, an important component of the pyroptotic machinery. It is unclear, however, whether this activity can induce cell death in neighboring macrophages [87, 88]. The development of methods to quantify spatiotemporal patterns of cell death will be important in future studies to establish which types of cell death exhibit non-random spatiotemporal patterns, and to examine whether ferroptosis induction in particular is linked invariably to propagative activity.
Highlights:
Different forms of cell death have different intrinsic effects on cell population dynamics.
Some mechanisms of regulated cell death, such as entosis, promote cell competition and provide a survival advantage to surrounding cells, whereas others, including ferroptosis, can negatively affect the viability of their neighbors.
Ferroptosis has the ability to propagate from cell to cell in a wave-like manner, allowing it to spread through and eliminate large cell populations.
Wave-like death propagation is observed in various biological contexts in normal physiology and disease, and induction of propagative cell death mechanisms may enhance cancer therapy.
Acknowledgements.
We thank members of the Overholtzer laboratory for helpful discussions, and apologize to authors whose work could not be discussed here due to space limitations. This work was supported by a grant from the National Cancer Institute (RO1CA154649, to M.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Arandjelovic S. and Ravichandran KS, Phagocytosis of apoptotic cells in homeostasis. Nat Immunol, 2015. 16(9): p. 907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson MD, Weil M, and Raff MC, Programmed cell death in animal development. Cell, 1997. 88(3): p. 347–54. [DOI] [PubMed] [Google Scholar]
- 3.Tait SW, Ichim G, and Green DR, Die another way--non-apoptotic mechanisms of cell death. J Cell Sci, 2014. 127(Pt 10): p. 2135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L, et al. , Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ, 2018. 25(3): p. 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overholtzer M, et al. , A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell, 2007. 131(5): p. 966–79. [DOI] [PubMed] [Google Scholar]
- 6.Brennan MA. and Cookson BT, Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol, 2000. 38(1): p. 31–40. [DOI] [PubMed] [Google Scholar]
- 7.Fink SL. and Cookson BT, Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol, 2006. 8(11): p. 1812–25. [DOI] [PubMed] [Google Scholar]
- 8.Rogers C, et al. , Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun, 2017. 8: p. 14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanden Berghe T, et al. , Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol, 2014. 15(2): p. 135–47. [DOI] [PubMed] [Google Scholar]
- 10.Gudipaty SA, et al. , Unconventional Ways to Live and Die: Cell Death and Survival in Development, Homeostasis, and Disease. Annu Rev Cell Dev Biol, 2018. 34: p. 311–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Garijo A. and Steller H, Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development, 2015. 142(19): p. 3253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Q, et al. , Competition between human cells by entosis. Cell Res, 2014. 24(11): p. 1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SE, et al. , Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol, 2016. 11(11): p. 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linkermann A, et al. , Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A, 2014. 111(47): p. 16836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamann JC, et al. , Entosis Is Induced by Glucose Starvation. Cell Rep, 2017. 20(1): p. 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Garijo A, Fuchs Y, and Steller H, Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. Elife, 2013. 2: p. e01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asally M, et al. , Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc Natl Acad Sci U S A, 2012. 109(46): p. 18891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levraud JP, et al. , Dictyostelium cell death: early emergence and demise of highly polarized paddle cells. J Cell Biol, 2003. 160(7): p. 1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg JT, Programmed cell death: a way of life for plants. Proc Natl Acad Sci U S A, 1996. 93(22): p. 12094–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coucouvanis E. and Martin GR, Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell, 1995. 83(2): p. 279–87. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S. and Shiota K, Sequential changes of programmed cell death in developing fetal mouse limbs and its possible roles in limb morphogenesis. J Morphol, 1996. 229(3): p. 337–46. [DOI] [PubMed] [Google Scholar]
- 22.Mailleux AA, et al. , BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell, 2007. 12(2): p. 221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabaldon C, et al. , Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol, 2005. 165(1): p. 121–30. [DOI] [PubMed] [Google Scholar]
- 24.Levine A, et al. , H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 1994. 79(4): p. 583–93. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Czymmek KJ, and Shapiro AD, Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the Arabidopsis hypersensitive response. Mol Plant Microbe Interact, 2003. 16(11): p. 962–72. [DOI] [PubMed] [Google Scholar]
- 26.Elmore SA, et al. , Recommendations from the INHAND Apoptosis/Necrosis Working Group. Toxicol Pathol, 2016. 44(2): p. 173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesa KR, et al. , Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature, 2015. 522(7554): p. 94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juncadella IJ, et al. , Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature, 2013. 493(7433): p. 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monks J, et al. , Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod, 2008. 78(4): p. 586–94. [DOI] [PubMed] [Google Scholar]
- 30.Link N, et al. , A collective form of cell death requires homeodomain interacting protein kinase. J Cell Biol, 2007. 178(4): p. 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Hughes G, et al. , Hid arbitrates collective cell death in the Drosophila wing. Mech Dev, 2015. 138 Pt 3: p. 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neufeld TP. and Baehrecke EH, Eating on the fly: function and regulation of autophagy during cell growth, survival and death in Drosophila. Autophagy, 2008. 4(5): p. 557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin VP. and Thummel CS, A balance between the diap1 death inhibitor and reaper and hid death inducers controls steroid-triggered cell death in Drosophila. Proc Natl Acad Sci U S A, 2004. 101(21): p. 8022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandy A, et al. , The NF-kappaB Factor Relish Regulates Atg1 Expression and Controls Autophagy. Cell Rep, 2018. 25(8): p. 2110–2120 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry DL. and Baehrecke EH, Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell, 2007. 131(6): p. 1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, et al. , Complement-Related Regulates Autophagy in Neighboring Cells. Cell, 2017. 170(1): p. 158–171 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPhee CK, et al. , Activation of autophagy during cell death requires the engulfment receptor Draper. Nature, 2010. 465(7301): p. 1093–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, et al. , Neuronal necrosis and spreading death in a Drosophila genetic model. Cell Death Dis, 2013. 4: p. e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. , Neuronal gap junctions are required for NMDA receptor-mediated excitotoxicity: implications in ischemic stroke. J Neurophysiol, 2010. 104(6): p. 3551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coburn C, et al. , Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLoS Biol, 2013. 11(7): p. e1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon SJ, et al. , Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 2012. 149(5): p. 1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seibt TM, Proneth B, and Conrad M, Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med, 2019. 133: p. 144–152. [DOI] [PubMed] [Google Scholar]
- 43.Stockwell BR, et al. , Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell, 2017. 171(2): p. 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarhan M, et al. , Immunological consequences of kidney cell death. Cell Death Dis, 2018. 9(2): p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skouta R, et al. , Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc, 2014. 136(12): p. 4551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, et al. , Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao M, et al. , Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell, 2015. 59(2): p. 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magtanong L. and Dixon SJ, Ferroptosis and Brain Injury. Dev Neurosci, 2019: p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeGregorio-Rocasolano N, Marti-Sistac O, and Gasull T, Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front Neurosci, 2019. 13: p. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, et al. , Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest, 2019. 130: p. 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang X, et al. , Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A, 2019. 116(7): p. 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vayrynen SA, et al. , Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer, 2016. 114(12): p. 1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipponen P, Apoptosis in breast cancer: relationship with other pathological parameters. Endocr Relat Cancer, 1999. 6(1): p. 13–6. [DOI] [PubMed] [Google Scholar]
- 54.Moinfar F, et al. , Mammary “comedo”-DCIS: apoptosis, oncosis, and necrosis: an electron microscopic examination of 8 cases. Ultrastruct Pathol, 2000. 24(3): p. 135–44. [DOI] [PubMed] [Google Scholar]
- 55.Steinbach JP, et al. , Hypoxia-induced cell death in human malignant glioma cells: energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell Death Differ, 2003. 10(7): p. 823–32. [DOI] [PubMed] [Google Scholar]
- 56.Huang CY, et al. , Resistance to hypoxia-induced necroptosis is conferred by glycolytic pyruvate scavenging of mitochondrial superoxide in colorectal cancer cells. Cell Death Dis, 2013. 4: p. e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang T, et al. , CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med, 2016. 22(2): p. 175–82. [DOI] [PubMed] [Google Scholar]
- 58.Zhou W. and Yuan J, Necroptosis in health and diseases. Semin Cell Dev Biol, 2014. 35: p. 14–23. [DOI] [PubMed] [Google Scholar]
- 59.Brown CW, Amante JJ, and Mercurio AM, Cell clustering mediated by the adhesion protein PVRL4 is necessary for alpha6beta4 integrin-promoted ferroptosis resistance in matrix-detached cells. J Biol Chem, 2018. 293(33): p. 12741–12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang L, et al. , Ferroptosis as a p53-mediated activity during tumour suppression. Nature, 2015. 520(7545): p. 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou H, et al. , Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A, 2005. 102(41): p. 14641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyng FM, Seymour CB, and Mothersill C, Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer, 2000. 83(9): p. 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belyakov OV, et al. , Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci U S A, 2005. 102(40): p. 14203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azzam EI, et al. , Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res, 1998. 150(5): p. 497–504. [PubMed] [Google Scholar]
- 65.Azzam EI, de Toledo SM, and Little JB, Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A, 2001. 98(2): p. 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roh JL, et al. , Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett, 2016. 381(1): p. 96–103. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, et al. , The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol, 2015. 2(4): p. e1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, et al. , Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-gamma-lyase function. Oncol Rep, 2015. 33(3): p. 1465–74. [DOI] [PubMed] [Google Scholar]
- 69.Dixon SJ, et al. , Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife, 2014. 3: p. e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, et al. , CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature, 2019. 569(7755): p. 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miess H, et al. , The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene, 2018. 37(40): p. 5435–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doll S, et al. , ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol, 2017. 13(1): p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Viswanathan VS, et al. , Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature, 2017. 547(7664): p. 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behan FM, et al. , Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature, 2019. 568(7753): p. 511–516. [DOI] [PubMed] [Google Scholar]
- 75.Trujillo-Alonso V, et al. , FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azzam EI, de Toledo SM, and Little JB, Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene, 2003. 22(45): p. 7050–7. [DOI] [PubMed] [Google Scholar]
- 77.Narayanan PK, Goodwin EH, and Lehnert BE, Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res, 1997. 57(18): p. 3963–71. [PubMed] [Google Scholar]
- 78.Chai Y, et al. , Radiation-induced non-targeted response in vivo: role of the TGFbeta-TGFBR1-COX-2 signalling pathway. Br J Cancer, 2013. 108(5): p. 1106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang WS, et al. , Regulation of ferroptotic cancer cell death by GPX4. Cell, 2014. 156(1–2): p. 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawamoto Y, Nakajima YI, and Kuranaga E, Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors. Int J Mol Sci, 2016. 17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madan E, Gogna R, and Moreno E, Cell competition in development: information from flies and vertebrates. Curr Opin Cell Biol, 2018. 55: p. 150–157. [DOI] [PubMed] [Google Scholar]
- 82.Bove A, et al. , Local cellular neighborhood controls proliferation in cell competition. Mol Biol Cell, 2017. 28(23): p. 3215–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gauron C, et al. , Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep, 2013. 3: p. 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamann JC. and Overholtzer M, Entosis enables a population response to starvation. Oncotarget, 2017. 8(35): p. 57934–57935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorda-Diez CI, et al. , Interdigital tissue regression in the developing limb of vertebrates. Int J Dev Biol, 2015. 59(1–3): p. 55–62. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, et al. , Autophagy regulates programmed cell death during the plant innate immune response. Cell, 2005. 121(4): p. 567–577. [DOI] [PubMed] [Google Scholar]
- 87.Franklin BS, et al. , The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol, 2014. 15(8): p. 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baroja-Mazo A, et al. , The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol, 2014. 15(8): p. 738–48. [DOI] [PubMed] [Google Scholar]