Abstract
Cadherin-based cell-cell junctions help metazoans form polarized sheets of cells, which are necessary for development of organs and compartmentalization of functions. The protein complexes making up cadherin-based junctions have ancient origins, with conserved elements shared between animals as diverse as sponges and vertebrates. In invertebrates, the formation and function of epithelial sheets depends on classical cadherin-containing adherens junctions, which link actin to the plasma membrane through α-, β- and p120 catenins. Next to adherens junctions, vertebrates also have a new type of cadherin-based intercellular junction called the desmosome, which allowed for creation of more complex and effective tissue barriers against environmental stress. While desmosomes have a molecular blueprint similar to adherens junctions, desmosomal cadherins, called desmogleins and desmocollins, link 10nm intermediate filaments (IF) instead of actin to the plasma membrane through protein complexes comprising relatives of β-catenin (plakoglobin) and p120 catenin (plakophilins). In turn, desmosomal catenins interact with members of the IF-binding plakin family to create the desmosomal-IF linking complex. In this essay, we discuss when and how desmosomal components evolved, and how their ability to anchor the highly elastic and tough IF cytoskeleton endowed vertebrates with robust tissues capable of not only resisting but also properly responding to environmental stress.
In Brief:
Green and colleagues discuss the evolution of desmosomal components and explore how their ability to anchor the intermediate filament cytoskeleton endowed vertebrates with tissues that can resist and also respond to environmental stress.
Introduction
Intercellular adhesion played a critical role in the evolution of multicellularity, which evolved independently over 20 times [1]. While the ability to form polarized, multicellular sheets of cells is found outside of metazoans in organisms such as Choanoflagellates, the social amoeba Dictyostelium and the ichthyosporean Sphaeroforma arctica [2–4], all metazoans have this ability. Here, construction of epithelial sheets consisting of many polarized cells held together by cell surface adhesion molecules provides the opportunity for compartmentalization of functions and organ development.
Of the five main classes of animal adhesion molecules – cadherins, Ig-superfamily molecules, selectins, mucins and integrins – cadherins stand out as critically important for metazoan epithelial cell-cell adhesion. The extracellular domains of cadherins associate homophilically or heterophilically to mediate cell-cell interactions, which are strengthened at their cytoplasmic interface by association with cytoskeletal elements through adaptor proteins called catenins and plakins (desmosomes)[5, 6]. These protein complexes organize into structurally distinct, macromolecular complexes called adhesive intercellular junctions [5] (Figure 1). In most animals, these intercellular cadherin-based junctions are limited to actin-associated adherens junctions (AJ). In vertebrates, AJs persist but we see the emergence of a new type of intercellular junction called the desmosome. Desmosomes still utilize cadherin receptors for attachment, but link to 10nm intermediate filaments (IF) instead of actin, through members of the desmosomal catenin (plakoglobin and plakophilins) and plakin families (Figure 1) [7]. As described in more detail below, in contrast to actin-containing microfilaments, IF can be stretched several times their length before rupturing and also exhibit the biophysical property of strain-stiffening, attributes that confer increased mechanical integrity to vertebrate tissues through linkage to desmosomes [8, 9].
Figure 1. Comparison of Desmosome and Adherens Junction Composition and Structure.
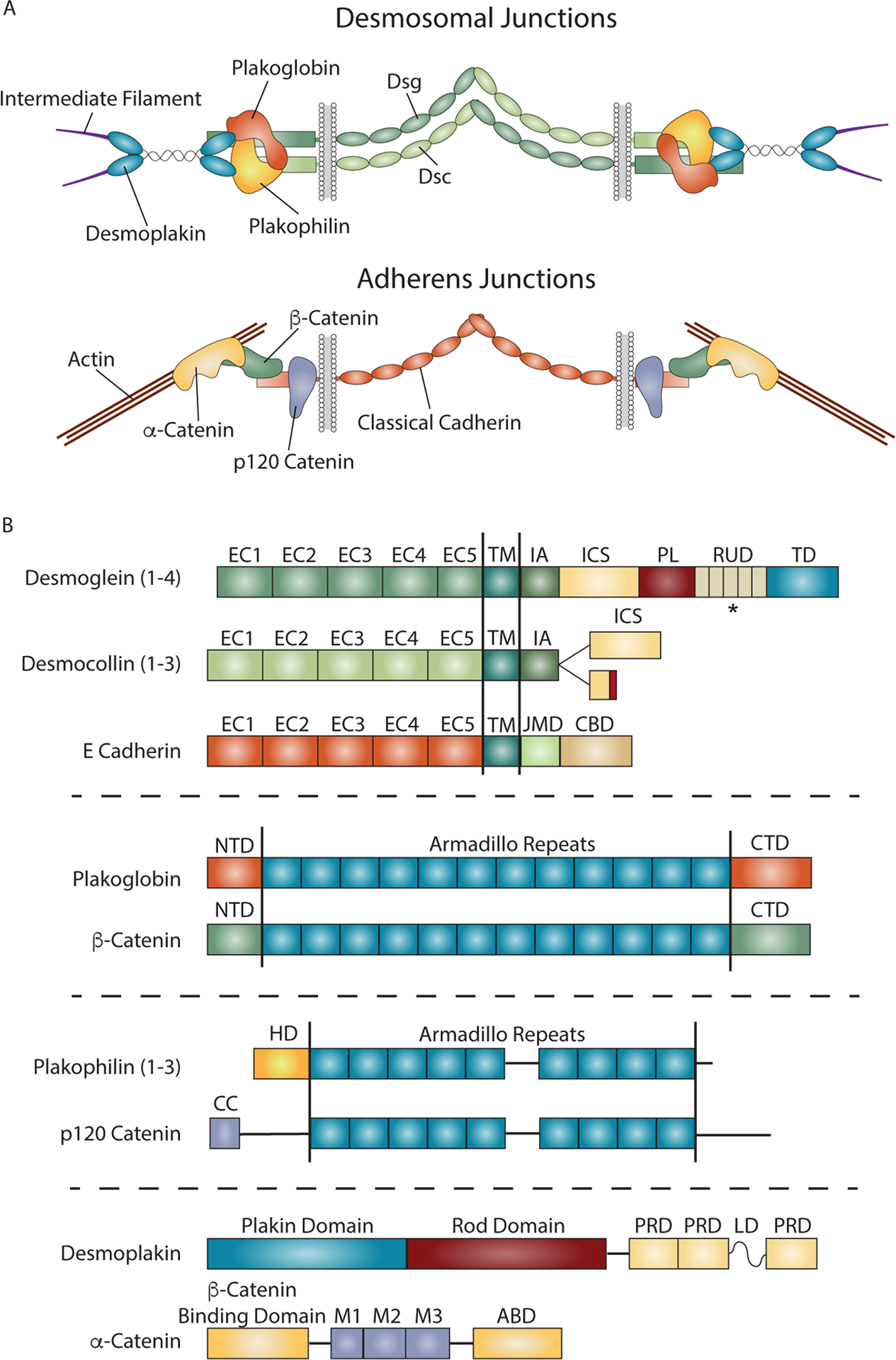
A) Both desmosomal and adherens junctions are built from cadherins and associated catenins that facilitate linkage to intermediate filaments (IF) and actin, respectively. In desmosomal junctions, adhesion is mediated by interactions between the ectodomains of desmosomal cadherins, desmogleins (Dsg) and desmocollins (Dsc). The desmosomal cadherin cytoplasmic domains anchor IF to the membrane through protein complexes containing plakoglobin and plakophilins, and the plakin desmoplakin. Adherens junctions mediate intercellular adhesion through homophilic interactions between cadherin ectodomains and link actin to the plasma membrane through α-, β- and p120 catenins that associate with the cadherin cytodomain. B) Domain structures for the major components of the desmosome and adherens junctions. The domain structures of desmoglein and desmocollin are compared to the classical cadherin E-cadherin. All three cadherins contain five extracellular cadherin (EC) domains responsible for adhesion, followed by a single pass transmembrane domain (TM), and a cytoplasmic tail domain that interacts with cytoskeletal linking complexes. The cytoplasmic domains contain an intracellular anchor (IA) and intracellular cadherin-like sequence (ICS) in desmosomal cadherins, and a juxtamembrane domain (JMD) and catenin binding domain (CBD) in classical cadherins. The ICS and CBD domains associate with plakoglobin or β-catenin/plakoglobin, in desmosomal and classical cadherins, respectively. Desmocollins exist as two splice variants, with the Dsc a form containing a full ICS domain, while Dsc b contains a truncated ICS domain and a short sequence of unique amino acids at the C-terminus. Desmogleins also contain an extended tail sequence with an intracellular proline rich linker (PL) domain, repeat unit domain (RUD) with varying number of repeats, and a desmoglein terminal domain (TD). Plakoglobin and β-catenin are armadillo proteins in desmosomes and adherens junctions respectively. Both contain 12 armadillo repeats flanked by N-terminal (NTD) and C-terminal (CTD) domains. Plakophilins and p120 catenin each contain 9 armadillo repeats, with plakophilins having an amino terminal head domain (HD), while p120 catenin contains an amino terminal coiled coil (CC) domain followed by a regulatory sequence. Both have multiple spliced forms not shown here, some which target these proteins to the nucleus. Desmoplakin and α-catenin are unrelated proteins that link these junctions to intermediate filaments or actin, respectively. Desmoplakin contains a plakin domain that interacts with desmosomal junctional proteins, and Plakin Repeat Domains (PRD) that link these junctions to intermediate filaments. α-catenin contains a β-catenin binding domain and an actin-binding domain (ABD) to link adherens junctions to actin. *Dsgs contain variable numbers of RUD repeats: Dsg1 has 5, Dsg2 has 6, Dsg3 has 2, and Dsg4 has 3.
Tracing the cadherin-catenin complex from pre-metazoans to early animals
Binding of cadherins to catenins through a cytoplasmic catenin-binding domain is a feature shared by most metazoans, but cadherin- and catenin-like molecules are not always found together in non-metazoan species, suggesting that they can have different functions [10]. In vertebrates, catenins consist of armadillo proteins β-catenin and p120 catenin and the actin-associated protein α-catenin [11](Figure 1). β-catenin and p120 catenin have the dual function of associating with either classical cadherin tails to regulate adhesion or with gene regulatory complexes to regulate transcription, both being ancient functions [12]. Choanoflagellates, close single-celled colony forming relatives of metazoans, have a number of non-classical cadherins. Choanoflagellate cadherins do not have a “classical” cadherin tail, however, suggesting that the “classical” core complex does not exist in this organism. Catenins are found in several non-metazoan eukaryotes that lack classical-like cadherins. Examples are Dictyostelium discoideum, where Aardvark (a possible β-catenin homolog) and Ddα-catenin (a vinculin/α-catenin-related protein) partner to organize cortical actomyosin and establish epithelial-like polarity in the transitional slug form [11](Figure 2). Catenin-like proteins are also found in a microeukaryotic relative of animals, the ichthyosporean S. arctica, during a transient epithelialization phase of life [4], and the choanoflagellate S. rosetta, where atypical cadherins and catenins are present in the same cell, but without evidence of interacting.
Figure 2. Innovations in Cadherin-based Adhesion and Junctions Across Species.
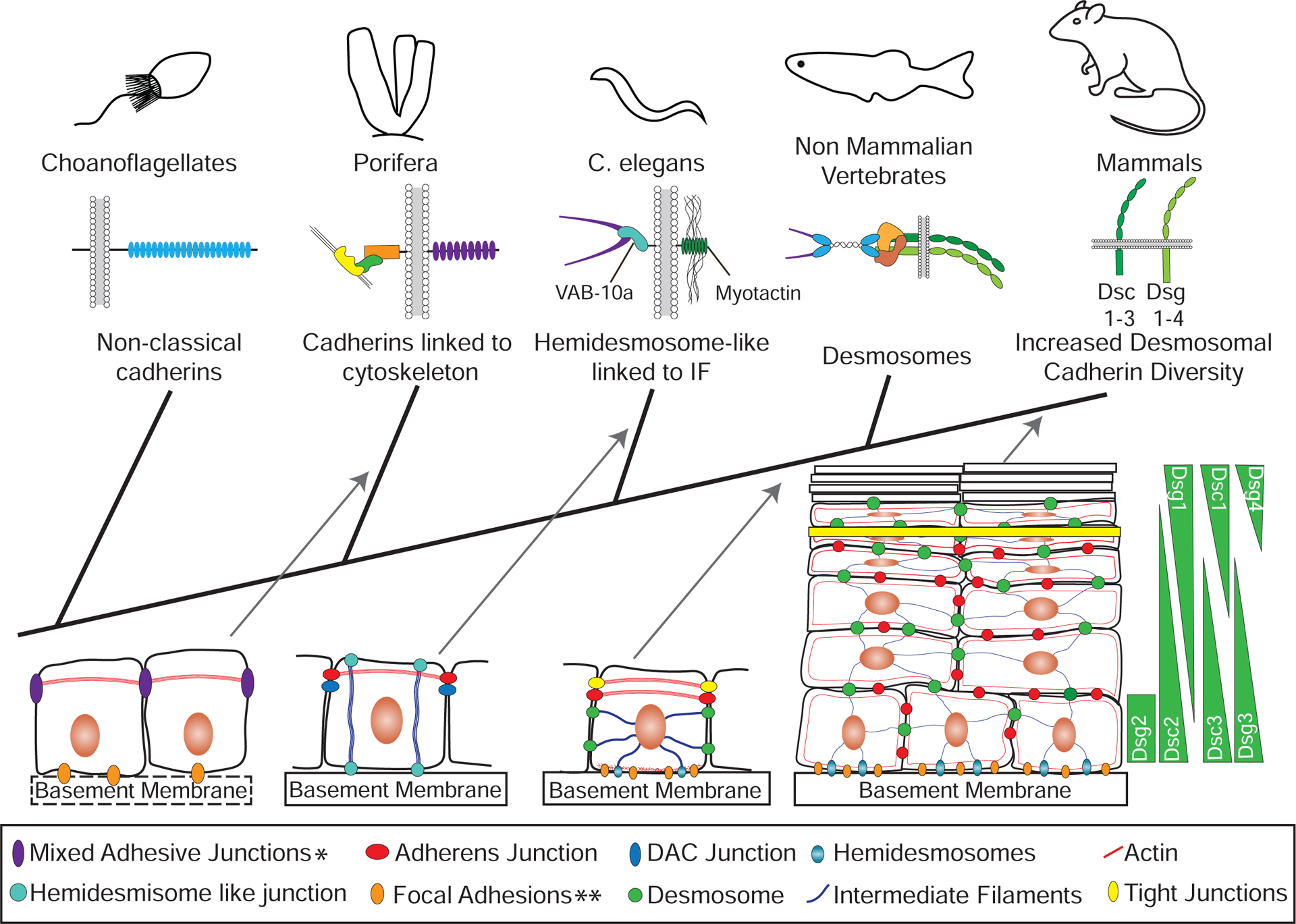
Some single celled organisms closely related to metazoans contain components similar to those found in adherens junctions, but there is no evidence that they interact with each other or mediate the formation of cell-cell junctions. For example, choanoflagellates have non-classical cadherins, which lack the intracellular domains able to interact with catenins. Porifera (sponges), metazoans distantly related to vertebrates, contain the major components of adherens junctions and there is evidence in some cases that these components interact to form junctional complexes. In addition, these cell-cell adhesive junctions can contain focal adhesion proteins including homologs for focal adhesion kinase, a β-integrin and vinculin. Only some porifera have a basement membrane, but even when absent focal adhesions can be present at the cell-substrate interface, which can contain β-catenin. Thus, the appearance of components of adherens junctions was an early event in metazoan evolution. Linking intermediate filaments to membrane junctions occurred later, for instance in C. elegans, where hemidesmosome-like structures in epithelial cells link intermediate filaments to the extracellular matrix through interactions between the spectraplakin VAB-10a and the transmembrane protein myotactin. There are two known cell-cell junction complexes in C. elegans epithelia: the most apical are vertebrate adherens junction homologs, and the basal are DLG-1/AJM-1 complex (DAC) junctions. Desmosomes appear in vertebrates concomitant with the appearance of desmosomal cadherins and the plakin protein desmoplakin, which links cell-cell junctions to intermediate filaments. In vertebrate simple epithelia, desmosomes are one of three intercellular junctions, which also include more apical tight junctions and adherens junctions. An expansion in the number of desmosomal cadherins occurred in mammals, where additional desmosomal cadherins are expressed in distinct patterns across different cell layers of complex epithelia. *Mixed adhesive junctions in porifera can contain adherens junction and focal adhesion proteins. **Focal adhesions in porifera can contain β-catenin.
When did the core elements of AJs first assemble as a complex linking the actin cytoskeleton to the plasma membrane? Genes encoding both the transmembrane and cytoplasmic components of the AJ are present in animals as diverse as sponges and vertebrates, indicating that this complex emerged during the earliest periods of animal evolution [1, 3, 10, 12](Figure 2). Direct experimental evidence that cadherins and catenins form a complex has been reported for a homoscleromorph sponge through a yeast two hybrid approach [13], and more recently for the demosponge Ephydatia muelleri through co-immunoprecipitation [12].
Intermediate filaments presage a new class of cell-cell membrane anchorage sites called desmosomes
Thus, the blueprint for intercellular junctions arose from puzzle pieces that appeared early in evolution and consolidated around a cadherin-catenin-actin core in early metazoans. However, additional structural elements were on the horizon: Intermediate filaments (IF) and their associated proteins and junctions [14–16]. IF are a large and diverse group of α-helical coiled coil proteins, which are structurally and functionally very different from actin or microtubules. From a biophysical standpoint, IF are notable for their ability to be stretched several times their length before rupturing and exhibit the non-linear trait of stiffening under strain. These properties make IF both flexible and tough, and thus well-suited for resisting mechanical stress in metazoan tissues [8, 9].
In addition to vimentin, desmin, keratins and neurofilaments, building blocks for cytoplasmic filaments, IF proteins also include nuclear lamins. These ancient structural components of inner nuclear membranes are present in most eukaryotes and help stabilize the nucleus. It has been proposed that lamins gave rise to cytoplasmic IF through gene duplication, which may have occurred multiple times [14, 17]. For instance, a lamin-derived cytoplasmic IF called cytotardin, found in the epithelia and foregut of microscopic invertebrates called tardigrades, appears to have evolved independently from other bilaterian cytoplasmic IF and may have allowed tardigrades to survive extreme environmental conditions [14].
Spectraplakins and plakins enabled the switch from actin binding in AJs to IF binding in desmosomes
When and how did IF begin to collaborate with linkers to the plasma membrane? IF attachment modules were first seen in the spectraplakins, a class of gigantic molecules that predates IF themselves. In the bilaterian versions, spectraplakins can harbor sequences encoding binding modules for all the major cytoskeletal elements including actin (calponin homology actin binding domains or ABDs), microtubules (GAS domain) and IF (plectin or plakin repeat domains or PRDs) as well as adhesive junctions (plakin domain) [18, 19] (Figure 3). Spectraplakins have been identified in animals as divergent as vertebrates and sponges. Different combinations of these domains are mixed and matched from two genes in mammals, ACF7/MCF1 and BPAG1, and a single gene in in C. elegans, Drosophila and zebrafish. By integrating cytoskeletal and adhesive elements, this modular strategy diversified tissue functions and helped coordinate cell architecture with cell dynamics and motility.
Figure 3. Spectraplakins and Plakins.
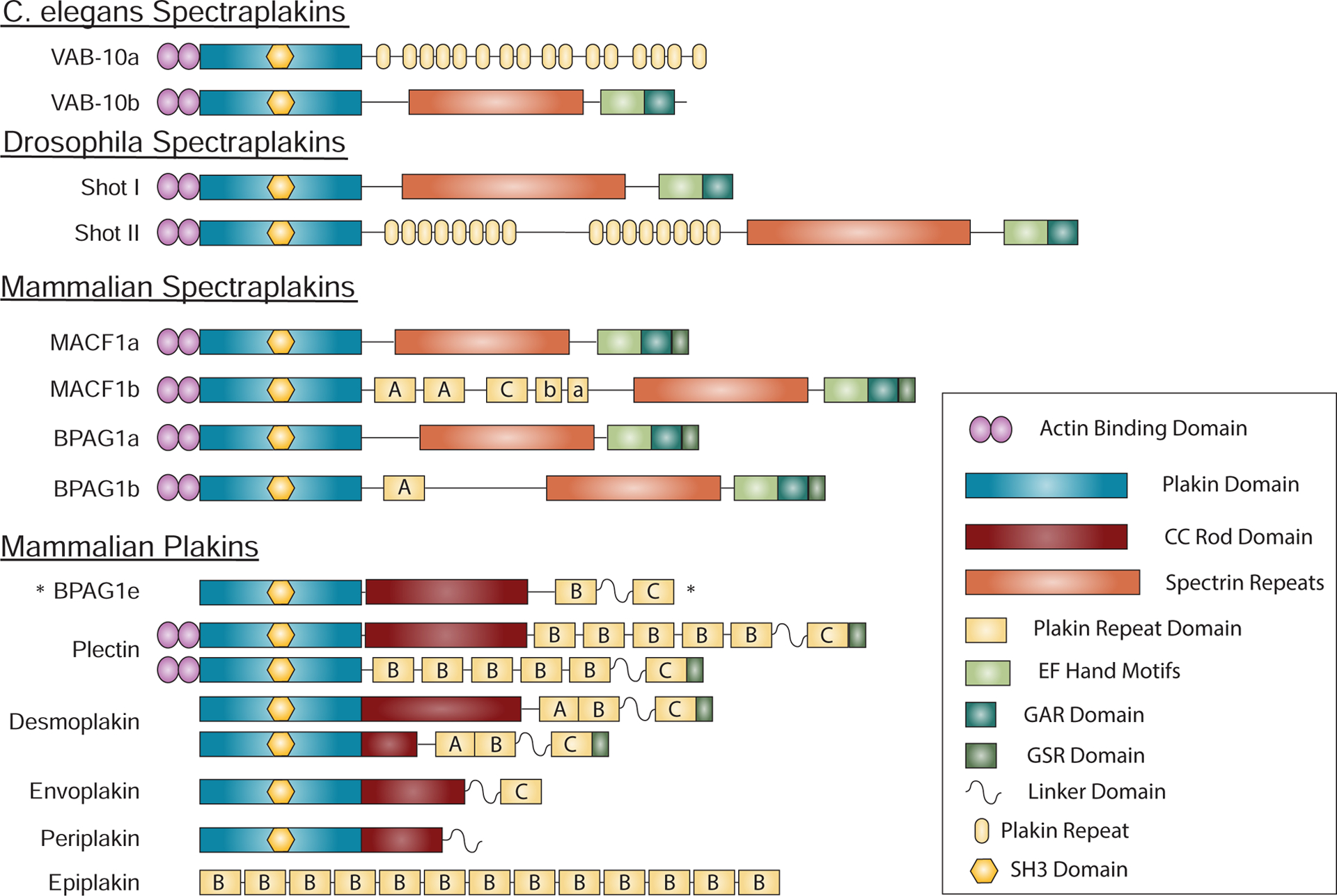
Spectraplakins are large proteins encompassing domains capable of interacting with all major cytoskeletal elements. These include actin binding domains (ABD), a plakin domain that targets proteins to junctions, plakin repeats that allow interactions with intermediate filaments, and EF hand, Gas2-related (GAR) and a regulatory glycine-serine-arginine rich (GSR) domains that harbor microtubule binding and regulatory functions. In mammalian spectraplakins and plakins, plakin repeats form plakin repeat domains (PRD) with three subtypes, A, B and C, and including a partial repeat (linker domain) between PRD B and C. The C. elegans and drosophila spectraplakins are encoded by the vab-10 and shot genes respectively, whereas mammalian spectraplakins are encoded by MACF1 and BPAG1/DST. The small number of spectraplakin genes give rise to a variety of spectraplakins by producing distinct splice variants. Plakins, present in vertebrates, likely arose from spectraplakins and allow linking of diverse junctions to intermediate filaments. Many plakins have lost the modules found in spectraplakins that allow interactions with actin and microtubules. BPAG1e, is unique in that it is classified as a plakin, while being a product of a spectraplakin gene. *This plakin is transcribed from from the bpag1 spectraplakin gene.
IF-binding PRD domains were first added to the mix of spectraplakin modules in bilaterians, preceding the appearance of desmosomes in vertebrates. In C. elegans, the spectraplakin VAB-10 anchors IF at membrane attachment sites connecting the epidermis to extracellular matrix underlying the cuticle and muscles [20, 21](Figure 2). These attachment sites are similar to mammalian hemidesmosomes, non-cadherin-based junctions that connect and stabilize epithelial cell attachments through an integrin receptor to the underlying extracellular matrix. Notably, in vertebrates, epidermal connections to hemidesmosomes are mediated through a product of the mammalian spectraplakin gene BPAG1. This molecule, called BPAG1e (“e” for epithelial) retains the N-terminal plakin domain, alpha-helical coiled rod and two of the IF-binding PRD repeats but dispenses with many of the spectrin repeats characterizing other spectraplakins.
These streamlined versions of spectraplakins are called “plakins”, vertebrate molecules that link IF to cell membranes and other cytoskeletal elements [22]. By retaining the PRD domains of their older relatives, most plakins share the ability to bind to IF [23]. In addition, spectrin repeats in the plakins form a highly conserved N-terminal “plakin” domain that allows plakins to bind to membrane adhesion complexes in hemidesmosomes (BPAG1 and plectin) and desmosomes (desmoplakin) [18]. Further trimming occurred in the plakins envoplakin, periplakin (which lost all of its PRDs) and epiplakin (which is a string of PRDs that lost its membrane binding plakin domain)[19]. Whereas most plakins are encoded by their own genes, which presumably arose by gene duplication from spectraplakins or an ancestral plakin, the BPAG1e example suggests that the first plakin is likely to have emerged directly from spectraplakin genes.
The appearance of desmoplakin in vertebrates allowed for attachment of IF to desmosomes, younger derivatives of AJs. Desmoplakin links keratin IF to desmosomes in epithelia, desmin IF to intercalated disc desmosomes in cardiac muscle, and vimentin IF to desmosomes in the pia and arachnoid meninges. While many plakins do not retain canonical microtubule binding domains, the plakin domain of desmoplakin harbors a region that associates with the microtubule plus tip protein EB1 to stabilize microtubules at desmosomes [24]. Further, desmoplakin is also required for the redistribution of microtubules during commitment to differentiation in the epidermis. Likewise, even though desmoplakin does not retain a bona fide actin binding domain, it nevertheless exhibits a close association with the actin cytoskeleton, possibly through plakophilin partners [24].
Some spectraplakins and plakins also share sequences that regulate cytoskeletal binding. An example of this is a GSR-rich segment found in several of the spectraplakins and plakins, including desmoplakin, plectin, and ACF7/MACF (Figure 3). In the former two, the virtually identical sequences regulate IF dynamics through GSK3-dependent phosphorylation of a serine kinase cascade [25]. The phosphorylated form is more dynamically associated with IF, whereas the hypophosphorylated form binds IF more tightly to promote strong desmosomal adhesion (see below discussion of “hyperadhesion”). In ACF7 a similar GSR sequence affects microtubule- instead of IF-binding, to regulate polarized cell movement [26]. In desmoplakin, arginine methylation was shown to cooperate with serine phosphorylation in this regulatory process. Whether arginine methylation contributes to ACF7-regulated microtubule dynamics is yet to be tested.
How did duplicated AJ paralogs change with the switch from actin binding to IF binding?
Bilaterians have everything necessary to link IF to the plasma membrane. The appearance of desmoplakin in vertebrates brought these connections to cell-cell interfaces. What else was required to take this step? Arguably, only a tweak of a connector with classical cadherins, as IF can associate with classical cadherins through a protein called plakoglobin. This armadillo protein arose from a gene duplication of β-catenin and is able to bind to both classical and desmosomal cadherins [27]. Desmoplakin couples VE-cadherin and N-cadherin to vimentin IF through plakoglobin in endothelial cells and lens fiber cells, respectively. Plakoglobin also mediates interactions with keratin IF and a Xenopus classical cadherin, C-cadherin, a connection that controls polarized cell protrusive behavior in migrating mesendoderm cells. In addition, desmosomal components can become intermixed with AJ components, most notably in the case of the “area composita” of the cardiac myocyte, where classical and desmosomal cadherins and plaque components are intermixed in some areas of the intercalated disc [24]. However, in none of these cases, do the “hybrid” structures exhibit the highly organized ultrastructure typical of desmosomes.
Plakoglobin expanded its functions by partnering with the “newest” cadherins, the desmogleins and desmocollins, which originated through duplications and divergence of the classic cadherins (Figures 1,4). In contrast to classical cadherin-containing AJs, desmosomes need members of both of these desmosomal cadherin families for their normal structure and function. Desmogleins and the longer “a” form of desmocollin both contain a conserved catenin binding domain to which plakoglobin binds [7, 28]. (A shorter spliced “b” form of desmocollin has a truncated catenin binding domain). Desmogleins also have an extended ~500 amino acid tail with unique regions downstream of the conserved catenin binding region that set them apart from classical cadherins and desmocollins [7]. This extended tail serves as a scaffold for new protein partners that enhance the scope of desmoglein’s functions in differentiation and morphogenesis [24].
Figure 4:
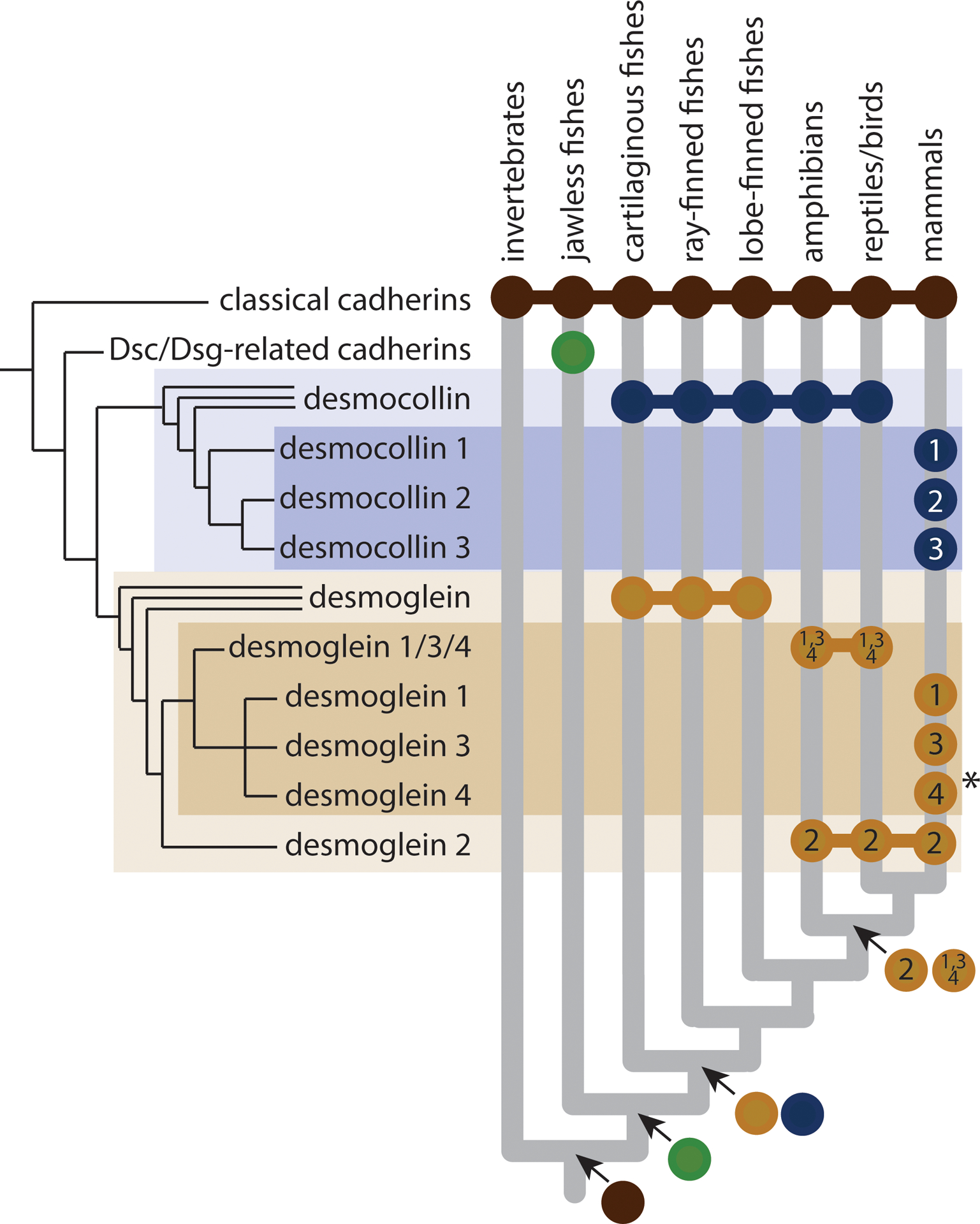
Origin and evolution of desmosomal cadherins. Nearly all animals have adherens junctions composed of classical cadherins, but desmosomes and desmosomal cadherins are restricted to vertebrates. Desmosomal cadherins in jawless vertebrates are uncharacterized, but desmoglein (Dsg) and desmocollin (Dsc) subfamilies appear to have diverged in the last common ancestor of jawed vertebrates. Within the desmoglein subfamily, Dsg2 and Dsg1/3/4 diverged in the last common ancestor of tetrapods. Mammals exhibit the greatest desmocollin and desmoglein diversity, with fully distinct paralogs of Dsc1,2,3 and Dsg1,2,3,4 in all major lineages, except cetaceans, which appear to have lost Dsg4 (indicated by asterisk).
The desmosomal cadherins associate with the IF cytoskeleton through plakoglobin and a new set of armadillo adaptor proteins, plakophilins 1–3, which are a vertebrate innovation coming from an ancestor common to p120/δ–catenin [27, 29]. Together the desmosomal cadherin-armadillo protein complex anchors IF at sites of cell-cell adhesion through desmoplakin. This, then, is the core desmosome “cadhesome” complex, analogous to the α-catenin/β-catenin/p120 catenin/classical cadherin complex that makes up the AJ (Figure 1). Thus, we can trace back each of the desmosome cadhesome components to classical cadhesome motifs, but only in vertebrates do they appear together.
Based on analysis of existing organisms, early vertebrate desmosomes likely contained a limited number of desmosomal cadherins and associated proteins, which expanded along with IF and concomitant with new environmental challenges. This expansion resulted in four desmoglein and three desmocollin genes in mammals: a single pair, desmoglein 2 and desmocollin 2, in simple epithelia and the remainder, desmogleins 1,3,4, and desmocollins 1,3 expressed in complex stratified tissues [30]. With the exception of desmoglein 4, these cadherins are also found in aquatic mammals, which evolved from multiple terrestrial ancestors (Figure 4). Desmogleins in fish are equally divergent from desmogleins 1–4 in mammals, whereas desmogleins similar to mammalian 1,3,4 appeared in amphibians and reptiles. Thus, the split between the Dsg2 and Dsg1/3/4 groups occurred in the last common ancestor of tetrapods, when the transition to land occurred. Desmocollins also exhibit increased diversity in mammals, where Dsc1/2/3 are all more closely related to each other than to those in fish, amphibians or reptiles/birds (Figure 4). In jawless aquatic vertebrates like lamprey and hagfish, ultrastructurally identifiable desmosomes are present [31], as are sequences distantly related to desmosomal cadherins (Figure 4). Distinct desmogleins and desmocollins are first evident in the jawed vertebrate lineage, after the divergence of lamprey and hagfish (Figure 4).
As mentioned above, desmosomal proteins appeared around the time that better barriers against the external environment appeared in vertebrates. A good example is the skin’s surface, where desmosomal cadherins are patterned in a differentiation-dependent manner and perform key roles in formation and function of the epidermal barrier [32]. For instance, desmoglein 1 comes on in concert with a commitment to stratify and differentiate in multi-layered tissues. Recent data suggest that when introduced ectopically into simple epithelial cells this cadherin was sufficient to force cells in the monolayer to stratify and form an additional layer, instead of extrusion and subsequent apoptosis [33]. On the other hand, desmoglein 3 is concentrated in the basal proliferating layer of complex epithelia and controls growth factor and proliferative pathways. When desmoglein 3 expression is forced suprabasally, the epidermis takes on attributes of mucosal (e.g. oral) epithelium [34]. Thus, vertebrate desmosomal cadherins are involved in the morphogenesis of robust multi-layered barriers.
Experimental evidence from amphibious killifish studies support that alterations in desmosomes occur as an adaptive response. When exposed to air, amphibious killifish exhibited a rapid increase in expression of desmosome molecules, including desmogleins, and an increase in desmosome size [35]. Dry conditions and heat are also associated with alterations in desmosomes, such as an increase in desmoglein 1 and superficial desmosomes called corneodesmosomes in mammalian epidermis [36]. These observations are consistent with the possibility that desmosomes helped organisms adjust to life on land. Indeed, as discussed in the next section, the desmosome-IF complex likely evolved to counter stress of all types.
The desmosome-IF complex: stress absorber and sensor.
Emerging evidence suggests that vertebrate desmosomes function in concert with IF to absorb, sense and respond to different types of stress, the best known being mechanical stress [16, 37, 38]. IFs in invertebrates that make these cytoskeletal fibers can also function in stress resistance. Other invertebrates lacking IF, like arthropods (e.g. Drosophila), resist stress by having a tough exoskeleton. However, the connection of IF to desmosomes at points of cell-cell contact in vertebrates augments the stress resisting function of IF to a supracellular level [39]. Further, in contrast to AJs, desmosomes can mature to become “hyperadhesive”, which is experimentally defined by their resistance to calcium chelation. Hyperadhesion is reversed in the case of injury by activation of PKC at the leading edge of a wound [32], which could work in part through modulating the IF binding capacity of desmoplakin [39].
Consistent with their role in countering mechanical stress, inherited and acquired diseases targeting IF and desmosomes manifest most obviously in the skin and heart—two organs that experience high level of mechanical stress [7, 24]. Further, IF and desmosomes respond to stress in an interdependent manner, as illustrated by the observed disassembly of desmosomes under stretch in cells from patients with EBS-type keratin mutations [39]. This observation is consistent with reported alterations in desmosome stability, structure and adhesion, and biochemical modifications such as desmoplakin phosphorylation in mouse keratin knock out cells and epidermis [40, 41].
The function of the desmosome-IF network goes beyond resisting mechanical stress in multiple tissues and organ systems [16, 36, 37]. Their role is particularly important in epidermis, which is constantly challenged by ultraviolet irradiation, dry air, heat, metabolic stress, pathogens and toxins. In addition to desmosomes’ response to dry air [35], desmosomes are also remodeled in response to heat, which drives the armadillo proteins called plakophilins to stress granules where they associate with RNA binding proteins and translational machinery [29]. Indeed, plakophilin 1 controls translation through its ability to interact with eIF4A1 and can switch between desmosomes and translation initiation complexes through its phosphorylation in response to growth factor signaling [29]. This phosphorylation induced switch potentially provides a means of regulating translation/growth in the context of stress. Phosphorylation may provide a more general adaptive mechanism to control stress-induced signaling and preserve tissue integrity in the case of both IF and desmosomes.
In contrast to the increased expression of desmogleins in response to dry air, desmoglein 1 is selectively turned over in response to several stressors including bacterial proteoglycans, ultraviolet radiation, and oxidative stress ([42, 43]; unpublished observations). In some cases, this may trigger a protective response, as normal human melanocytes treated with conditioned media from desmoglein 1-deficient keratinocytes, secrete more pigment and increase their dendricity, which augments transfer of DNA-protective pigment to surrounding keratinocytes in the epidermis [44]. While acute loss of desmoglein may be protective, chronic genetic depletion of desmoglein 1 as in the disorder SAM syndrome results in skin inflammation and allergies, possibly stimulated in part by pro-inflammatory and pro-allergic cytokine production in keratinocytes. The cadherin desmoglein 3, which is expressed in basal proliferating epidermal cells, has also been linked to stress protection by maintaining low levels of p53 expression in the face of ultraviolet radiation [45].
Desmosomes appeared around the time that adaptive immunity developed in jawless fish. Functional connections between stress-associated keratin and desmosome remodeling and the immune system are indeed emerging. For instance, in response to stress such as injury or in disorders like psoriasis, terrestrial epidermal keratins K1 and K10 switch to the “proliferation-associated” keratins K6, K16/17 [38, 46]. In addition to having distinct structural roles, these keratins contribute to keratinocyte alarmins that prompt an innate immune defense and adaptive immunity [46]. Recent studies show that some K17 is present in the nucleus, where it directly promotes inflammatory cytokine production by collaborating with the transcription factor Aire [47]. Interestingly, in cetaceans, the normal epidermal K1/10 pair are lost, but in their place, K6/16/17 are expressed, in this case as “normal” keratins. This evolutionary innovation is associated with structural features that provide an advantage for aquatic mammals, including a dramatically thickened epidermis [15]. Desmosomal cadherins in cetaceans, however, do not switch off their superficial desmoglein 1, possibly allowing cetaceans to continue to utilize desmoglein 1’s delamination functions. Just as keratins contribute to immune responses, loss of desmogleins triggers increased pro-inflammatory cytokine expression as well as anti-microbial peptides and S100 family members [44, 48] and unpublished observations). While information on how desmosomes interact with the immune system is in its infancy, collectively these observations suggest that the IF-desmosome network integrates mechanical and signaling changes that occur in response to environmental stress.
Future outlook
The appearance of desmosomes relatively late in evolution provided the opportunity for vertebrates to develop new mechanisms for generating complex tissues that effectively absorb and respond to mechanical and environmental stress. However, many questions remain. What were the driving factors that separated desmosomes from AJs along the evolutionary timeline? In considering this question, several features distinguish AJs and desmosomes, beyond anchoring actin versus IF to plasma membranes. First, the fact that desmosomes include representatives of two subclasses of cadherins provides opportunities for more complex pairing, utilizing homophilic and/or heterophilic adhesion. Also, the longer C-terminal domains of the desmogleins provide additional opportunities to extend cadherin functions in signaling. These features could provide an advantage when building complex tissues and diversifying tissue functions. Second, as mentioned above, desmosomes, but not AJs, can switch from calcium-dependent to calcium-independent status, a property known as “hyperadhesion”. The ability of desmosomes to convert between these adhesive states confers another level of flexibility and toughness to that conferred on tissues by IF. This property could help organisms to dynamically resist and respond to environmental stress: desmosomes could become more dynamic when remodeling is required, or tougher in response to mechanical or other types of stress. One could imagine that acquisition of regulatory modules that mediate this tunable switch could provide an advantage during evolution of the desmosome. Third, desmosomes, but not AJs, segregate into specialized lipid raft domains in the plasma membrane, providing a means of structural and functional compartmentalization [49]. One could envision that alterations in transmembrane domains or post-translational protein modifications that supported this compartmentalization could also provide an advantage.
The evolution of cadherins occurred in the context of changes in other cytoskeletal, extracellular matrix and junctional systems beyond cadherin-based junctions, raising another set of interesting questions. Did the arrival of desmosomes alter how keratins respond to stress, and how stress signals are propagated at a tissue level? Desmosomes are highly interconnected structurally and functionally with tight junctions and gap junctions [5, 24]; how were these relationships established in vertebrates upon the appearance of desmosomes? Equally important, extracellular matrix and specialized basement membranes, the thin continuous layers of matrix just beneath the basal plasma membrane of epithelial cells, were critical players in the appearance of multicellular epithelial cell sheets in early metazoans [50]. Like adhesive intercellular junctions, basement membranes and their associated integrin-based cell-substrate junctions, hemidesmosomes and focal contacts, were essential for tissue integrity and the success of multicellularity. To what extent did these junctional and adhesion systems co-evolve in vertebrates, and at the next level, to what extent did junctional networks co-evolve with other organ systems and the immune system? Further genetic and functional analysis in organisms beyond the accepted model systems promises to yield clues that could help unravel these relationships.
Acknowledgments
The authors thank all of the investigators whose work we refer to in this essay, and regret that due to limitations on reference citations we were unable to cite all relevant original papers. Work in the authors laboratories is funded by NIH R01 AR041836, R37 AR43380, R01 CA228196, and the JL Mayberry endowment to K.J.G., Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – CECAD, EXC 2030 – 390661388”, Project-ID 73111208 - SFB 829 A1, Z2”, and project ID NI1234/6–2 – SPP1782 to C.M.N, NASA Grant 16-EXO16_2–0041 to S.A.N. S.R.R-C. is supported by T32 CA070085 (Signal Transduction in Cancer). K.J.G. and C.M.N. thank the Alexander von Humboldt Foundation for supporting K.J.G. during her sabbatical at the University of Cologne, which helped inspire the conceptual basis for this essay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abedin M, and King N (2010). Diverse evolutionary paths to cell adhesion. Trends Cell Biol 20, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet T, Larson BT, Linden TA, Vermeij MJA, McDonald K, and King N (2019). Light-regulated collective contractility in a multicellular choanoflagellate. Science 366, 326–334. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson DJ, Nelson WJ, and Weis WI (2011). A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science 331, 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudin O, Ondracka A, Grau-Bove X, Haraldsen AA, Toyoda A, Suga H, Brate J, and Ruiz-Trillo I (2019). A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke WW (2009). Discovering the molecular components of intercellular junctions--a historical view. Cold Spring Harb Perspect Biol 1, a003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubsam M, Broussard JA, Wickstrom SA, Nekrasova O, Green KJ, and Niessen CM (2018). Adherens Junctions and Desmosomes Coordinate Mechanics and Signaling to Orchestrate Tissue Morphogenesis and Function: An Evolutionary Perspective. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomason HA, Scothern A, McHarg S, and Garrod DR (2010). Desmosomes: adhesive strength and signalling in health and disease. Biochem J 429, 419–433. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann H, and Aebi U (2016). Intermediate Filaments: Structure and Assembly. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzfeld M, Keil R, and Magin TM (2017). Desmosomes and Intermediate Filaments: Their Consequences for Tissue Mechanics. Cold Spring Harb Perspect Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray PS, and Zaidel-Bar R (2014). Pre-metazoan origins and evolution of the cadherin adhesome. Biol Open 3, 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller PW, Clarke DN, Weis WI, Lowe CJ, and Nelson WJ (2013). The evolutionary origin of epithelial cell-cell adhesion mechanisms. Curr Top Membr 72, 267–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schippers KJ, and Nichols SA (2018). Evidence of Signaling and Adhesion Roles for beta-Catenin in the Sponge Ephydatia muelleri. Mol Biol Evol 35, 1407–1421. [DOI] [PubMed] [Google Scholar]
- 13.Nichols SA, Roberts BW, Richter DJ, Fairclough SR, and King N (2012). Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/beta-catenin complex. Proc Natl Acad Sci U S A 109, 13046–13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hering L, Bouameur JE, Reichelt J, Magin TM, and Mayer G (2016). Novel origin of lamin-derived cytoplasmic intermediate filaments in tardigrades. Elife 5, e11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrlich F, Fischer H, Langbein L, Praetzel-Wunder S, Ebner B, Figlak K, Weissenbacher A, Sipos W, Tschachler E, and Eckhart L (2019). Differential Evolution of the Epidermal Keratin Cytoskeleton in Terrestrial and Aquatic Mammals. Mol Biol Evol 36, 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S (2018). Cytoplasmic Intermediate Filaments in Cell Biology. Annu Rev Cell Dev Biol 34, 1–28. [DOI] [PubMed] [Google Scholar]
- 17.Kollmar M (2015). Polyphyly of nuclear lamin genes indicates an early eukaryotic origin of the metazoan-type intermediate filament proteins. Sci Rep 5, 10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liem RK (2016). Cytoskeletal Integrators: The Spectrin Superfamily. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu L, Huang Z, Wu Z, Ali A, and Qian A (2018). Mammalian Plakins, Giant Cytolinkers: Versatile Biological Functions and Roles in Cancer. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gally C, Zhang H, and Labouesse M (2016). Functional and Genetic Analysis of VAB-10 Spectraplakin in Caenorhabditis elegans. Methods Enzymol 569, 407–430. [DOI] [PubMed] [Google Scholar]
- 21.Karabinos A (2019). Intermediate filament (IF) proteins IFA-1 and IFB-1 represent a basic heteropolymeric IF cytoskeleton of nematodes: A molecular phylogeny of nematode IFs. Gene 692, 44–53. [DOI] [PubMed] [Google Scholar]
- 22.Ruhrberg C, and Watt FM (1997). The plakin family: versatile organisers of cytoskeletal architecture. Curr. Opin. Genet. Devel 7, 392–397. [DOI] [PubMed] [Google Scholar]
- 23.Bouameur JE, Favre B, and Borradori L (2014). Plakins, a versatile family of cytolinkers: roles in skin integrity and in human diseases. J Invest Dermatol 134, 885–894. [DOI] [PubMed] [Google Scholar]
- 24.Green KJ, Jaiganesh A, and Broussard JA (2019). Desmosomes: Essential contributors to an integrated intercellular junction network. F1000Research 8 (F1000 Faculty Rev), 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albrecht LV, Zhang L, Shabanowitz J, Purevjav E, Towbin JA, Hunt DF, and Green KJ (2015). GSK3- and PRMT-1-dependent modifications of desmoplakin control desmoplakin- cytoskeleton dynamics. J Cell Biol 208, 597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, and Fuchs E (2011). Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell 144, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gul IS, Hulpiau P, Saeys Y, and van Roy F (2017). Metazoan evolution of the armadillo repeat superfamily. Cell Mol Life Sci 74, 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delva E, Tucker DK, and Kowalczyk AP (2009). The desmosome. Cold Spring Harb Perspect Biol 1, a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzfeld M, Wolf A, and Keil R (2014). Plakophilins in desmosomal adhesion and signaling. Cell Commun Adhes 21, 25–42. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney MG, Hu Y, Brennan D, Bazzi H, Christiano AM, and Wahl JK 3rd (2006). Delineation of diversified desmoglein distribution in stratified squamous epithelia: implications in diseases. Exp Dermatol 15, 101–109. [DOI] [PubMed] [Google Scholar]
- 31.Bartels H, and Potter IC (1998). Membrane structure of the cells of the lamprey notochord. Journal of Electron Microscopy 47, 627–636. [Google Scholar]
- 32.Berika M, and Garrod D (2014). Desmosomal adhesion in vivo. Cell Commun Adhes 21, 65–75. [DOI] [PubMed] [Google Scholar]
- 33.Nekrasova O, Harmon RM, Broussard JA, Koetsier JL, Godsel LM, Fitz GN, Gardel ML, and Green KJ (2018). Desmosomal cadherin association with Tctex-1 and cortactin-Arp2/3 drives perijunctional actin polymerization to promote keratinocyte delamination. Nat Commun 9, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias PM, Matsuyoshi N, Wu H, Lin C, Wang ZH, Brown BE, and Stanley JR (2001). Desmoglein isoform distribution affects stratum corneum structure and function. J Cell Biol 153, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y. w., Blanchard TS, Noll A, Vasquez P, Schmitz J, Kelly SP, Wright PA, and Whitehead A (2019). Life out of water: genomic and physiological mechanisms underlying skin phenotypic plasticity. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JL, Najor NA, and Green KJ (2014). Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harb Perspect Med 4, a015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toivola DM, Strnad P, Habtezion A, and Omary MB (2010). Intermediate filaments take the heat as stress proteins. Trends Cell Biol 20, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob JT, Coulombe PA, Kwan R, and Omary MB (2018). Types I and II Keratin Intermediate Filaments. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broussard JA, Jaiganesh A, Zarkoob H, Conway DE, Dunn AR, Espinosa HD, Janmey PA, and Green KJ Scaling up single cell mechanics to multicellular tissues: role of the intermediate filament-desmosome network. J. Cell. Sci, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loschke F, Homberg M, and Magin TM (2016). Keratin Isotypes Control Desmosome Stability and Dynamics through PKCalpha. J Invest Dermatol 136, 202–213. [DOI] [PubMed] [Google Scholar]
- 41.Vielmuth F, Wanuske MT, Radeva MY, Hiermaier M, Kugelmann D, Walter E, Buechau F, Magin TM, Waschke J, and Spindler V (2018). Keratins Regulate the Adhesive Properties of Desmosomal Cadherins through Signaling. J Invest Dermatol 138, 121–131. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JL, Koetsier JL, Sirico A, Agidi AT, Antonini D, Missero C, and Green KJ (2014). The desmosomal protein desmoglein 1 aids recovery of epidermal differentiation after acute UV light exposure. J Invest Dermatol 134, 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams MR, Nakatsuji T, Sanford JA, Vrbanac AF, and Gallo RL (2017). Staphylococcus aureus Induces Increased Serine Protease Activity in Keratinocytes. J Invest Dermatol 137, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnette CR, Roth-Carter QR, Koetsier JL, Broussard JA, Burks HE, Cheng K, Amadi C, Gerami P, Johnson JL, and Green KJ (2019). Keratinocyte cadherin desmoglein 1 controls melanocyte behavior through paracrine signaling. Pigment Cell Melanoma Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman A, Cai Y, Hunefeld C, Jedlickova H, Huang Y, Teck Teh M, Sharif Ahmad U, Uttagomol J, Wang Y, Kang A, et al. (2019). The desmosomal cadherin desmoglein-3 acts as a keratinocyte anti-stress protein via suppression of p53. Cell Death Dis 10, 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Yin M, and Zhang LJ (2019). Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hobbs RP, DePianto DJ, Jacob JT, Han MC, Chung BM, Batazzi AS, Poll BG, Guo Y, Han J, Ong S, et al. (2015). Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat Genet 47, 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, et al. (2013). Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 45, 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis JD, Caldara AL, Zimmer SE, Stahley SN, Seybold A, Strong NL, Frangakis AS, Levental I, Wahl JK 3rd, Mattheyses AL, et al. (2019). The desmosome is a mesoscale lipid raft-like membrane domain. Mol Biol Cell 30, 1390–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, Gray Jerome W, Hudson JK, Rokas A, and Hudson BG (2017). Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


