Abstract
Background
To limit the spread of SARS-CoV-2, an evidence-based understanding of the symptoms is critical to inform guidelines for quarantining and testing. The most common features are purported to be fever and a new persistent cough, although the global prevalence of these symptoms remains unclear. The aim of this systematic review is to determine the prevalence of symptoms associated with COVID-19 worldwide.
Methods
We searched PubMed, Embase, CINAHL, AMED, medRxiv and bioRxiv on 5th April 2020 for studies of adults (>16 years) with laboratory test confirmed COVID-19. No language or publication status restrictions were applied. Data were independently extracted by two review authors into standardised forms. All datapoints were independently checked by three other review authors. A random-effects model for pooling of binomial data was applied to estimate the prevalence of symptoms, subgrouping estimates by country. I2 was used to assess inter-study heterogeneity.
Results
Of 851 unique citations, 148 articles were included which comprised 24,410 adults with confirmed COVID-19 from 9 countries. The most prevalent symptoms were fever (78% [95% CI 75%-81%]; 138 studies, 21,701 patients; I2 94%), a cough (57% [95% CI 54%-60%]; 138 studies, 21,682 patients; I2 94%) and fatigue (31% [95% CI 27%-35%]; 78 studies, 13,385 patients; I2 95%). Overall, 19% of hospitalised patients required non-invasive ventilation (44 studies, 6,513 patients), 17% required intensive care (33 studies, 7504 patients), 9% required invasive ventilation (45 studies, 6933 patients) and 2% required extra-corporeal membrane oxygenation (12 studies, 1,486 patients). The mortality rate was 7% (73 studies, 10,402 patients).
Conclusions
We confirm that fever and cough are the most prevalent symptoms of adults infected by SARS-CoV-2. However, there is a large proportion of infected adults which symptoms-alone do not identify.
Introduction
The novel coronavirus (SARS-CoV-2; 2019-nCoV; COVID-19) pandemic is a global crisis. As of April 10th 2020, there were over 1.5 million confirmed cases of whom over 92,000 have died [1]. In the absence of a vaccine or treatment with proven efficacy, limiting human-to-human transmission is critical [2–4]. Self-isolation (or self-quarantine) is an effective global strategy for limiting transmission following the emergence of symptoms [5] and equally, the manifestation of symptoms is used to guide testing.
Coronavirus is most infectious in the early phase of the illness [6,7] [8], so screening people with compatible symptoms [9] is fundamental to determining who should be quarantined and be tested [9]. Several systematic reviews have considered the symptoms of COVID-19 (amongst other parameters) [8,10–14] although all of them have major limitations. None systemically searched the grey literature (e.g. preprint archives such as medRxiv and bioRxiv) and in the context of a pandemic, the quality and quantity of the literature is evolving at speed [15]. Without incorporating all relevant preprints the findings of any systemic review will be weeks-months out-of-date at the time of publication [16]. Furthermore, with few included studies (30 in the largest and most recent [12]), the range of symptoms were limited and the estimates of prevalence are likely to be upwardly biased because only unwell patients (largely those admitted to hospital) were tested in the early phase of the outbreak.
To facilitate the rapid dissemination of high-quality open-science, there has been a surge of preprints related to COVID-19 manuscripts uploaded to the online archives medRxiv and bioRxiv [15]. The necessity to address deficiencies in current literature and potential to substantially improve the precision of estimates of symptom prevalence using both indexed and (the more voluminous and up-to-date) preprint literature from multiple geographical regions, represents the rational for this review.
The aim of this systematic review is to determine the prevalence of symptoms associated with COVID-19 worldwide.
Methods
This systematic review was designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews [17] and a pre-published protocol [18], and is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (see S1 Checklist) [18,19].
Participants and studies
Studies reporting the prevalence of patient-reported symptoms or clinician observed features in adults (>16 years) with laboratory confirmed novel coronavirus (SARS-CoV-2; covid-19) derived from oro- or naso-pharyngeal swabs. We excluded case reports, articles which failed to disaggregate symptoms in adult and paediatric cohorts, studies of patients with prior respiratory infections (e.g. tuberculosis) or co-infections with other viruses (e.g. similar viruses SARS-CoV-1 or HCoV-EMC/2012, etc) and articles which we are unable to translate to English in a timely fashion.
Target condition
The incubation period of COVID-19 is typically 5 days, but reported to last a maximum of 7 days [20–24]. The illness typically lasts 8 days [21]. Therefore, we will include any symptom(s) described up to 15 days before laboratory confirmed infection and during the illness.
Search strategy
PubMed, Embase, AMED and CINAHL, medRxiv [25] and bioRxiv were interrogated according to our search strategy (S1 Appendix). Searches were limited to 1st January onwards. No language restrictions were applied.
Study selection process
After de-duplication, all unique citations were independently screened by three review authors (MG, LG and RGW). The full texts of all potentially relevant articles were obtained. The reference lists for included articles and other systematic reviews were also scrutinised. Final lists of included articles were compared and disagreements resolved by consensus discussion between five authors (MG, LG, ZK, ELC and RGW).
Data extraction
Two authors (MG and LG) independently extracted data and three authors (ZM, ELC and RGW) checked the accuracy of the extracted data using a standardised spreadsheet. Disagreements were resolved by discussion. We combined the following symptoms: “chest tightness” into the more prevalent symptom of wheeze; “shivers” and “chills” into rigors; malaise and “generalised weakness” (in the absence of any objective neurological deficit) into the more widely reported symptom of fatigue; conjunctivitis, conjunctival congestion and conjunctivital secretions into conjunctivitis. Where studies reported one symptom “or” another (e.g. nausea or vomiting) we did not extract this information as it was impossible to disaggregate. Where studies reported one symptom “and” another (e.g. nausea and vomiting) we extracted the prevalence of both. When studies grouped symptoms together (e.g. “respiratory symptoms”) without further description or definition we were not able to extract this information.
Methodological quality assessment
The risk of bias for included studies was not assessed for two main reasons: firstly, there is no consensus on ideal tool, nor one designed specifically for studies of prevelance [26] and secondly, such assessments would not change the approach to the modelling or presentation of the data, as per our protocol. Given such assessments are also time-intensive, we have taken the pragmatic decision to not perform risk of bias assessments.
Analysis
The pooled prevalence of symptoms were estimated using the metaprop package [27] in Stata/MP v15. Dersimonian and Laird random-effects were used given the geographical and study-level heterogeneity. A random-effects model including Freeman-Tukey arcsine transformation of the prevalence was used to normalise variance. 95% confidence intervals (CIs) were computed around the study-specific and pooled prevalence based on the score-test statistic. [28]. The variation in prevalence by country was assessed by subgroup meta-analyses and meta-regression. Statistical heterogeneity is assessed by I2 which corresponds with the proportion of total variation due to inter-study heterogeneity and by p-values for inter-study heterogeneity within countries, between countries and overall [29]. Given the use of a random-effects model, inter-study heterogeneity within countries was only assessable when at least three studies were available. A z-test (and the corresponding p-values) assessed whether the observed prevalence was different from zero percent. Publication bias was not assessed.
Results
Study selection
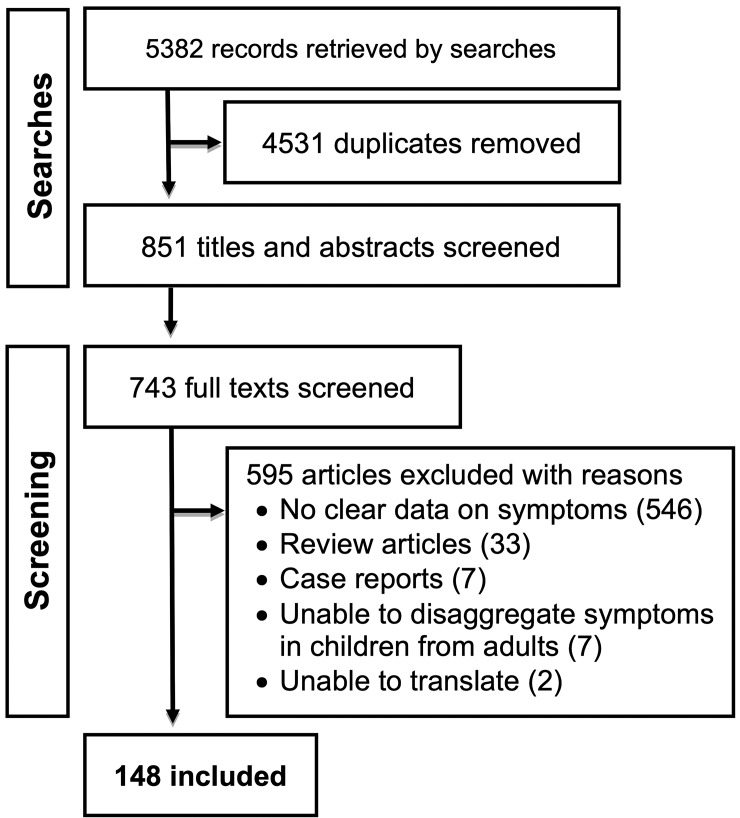
Our search returned 2403 hits in PubMed, 2234 in Embase, 310 in CINAHL, 1 in AMED, and 434 preprints in medRxiv and bioRxiv on 5th April 2020. Following deduplication, the titles and abstracts of 851 unique records were assessed against the inclusion criteria. 743 of these were deemed to be potentially eligble. Full text screening then resulted in 148 included articles (Fig 1).
Fig 1. Study flow chart.
Study characteristics
This review describes 24,410 adults with laboratory confirmed COVID-19 from 9 countries, including China [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44–49,50–59,60–69,70–79,80–89,90–99,100–109,110–119,120–129,130–139,140–149,150–159,160–165], the UK [166,167], the USA [168,169], Singapore [170,171], Italy [172,173], Australia [174], Japan [175], Korea [176] and the Netherlands [177]. The mean age of patients was 49 years (SD 11) and where sex data were available, the ratio of males:females was 1.2:1 (10,306:8593). The characteristics of the included studies are shown in S1 Table.
Thirty-four studies reported that 845 of 7519 patients required non-invasive ventilation (pooled prevalence 17% [95% CI 11%-24%]; I2 98%). Forty-four studies (6513 patients) reported that 970 patients were admitted to an intensive care unit (pooled prevalence 19% [95% CI 13%-26%]; I2 97%). Forty-five studies (6933 patients) reported that 495 required invasive mechanical ventilation (pooled prevalence 9% [95% CI 6%-13%]; I2 95%). Twelve studies (1486 patients) reported that 2% of patients (36) required extra-corporeal membrane oxygenation (95% CI 0%-5%; I2 95%). Of the 73 studies that reported survival in 10,402 patients, there were 938 deaths (pooled prevalence 7% [95% CI 4%-11%]; I2 98%) which were attributable to COVID-19.
Evidence synthesis
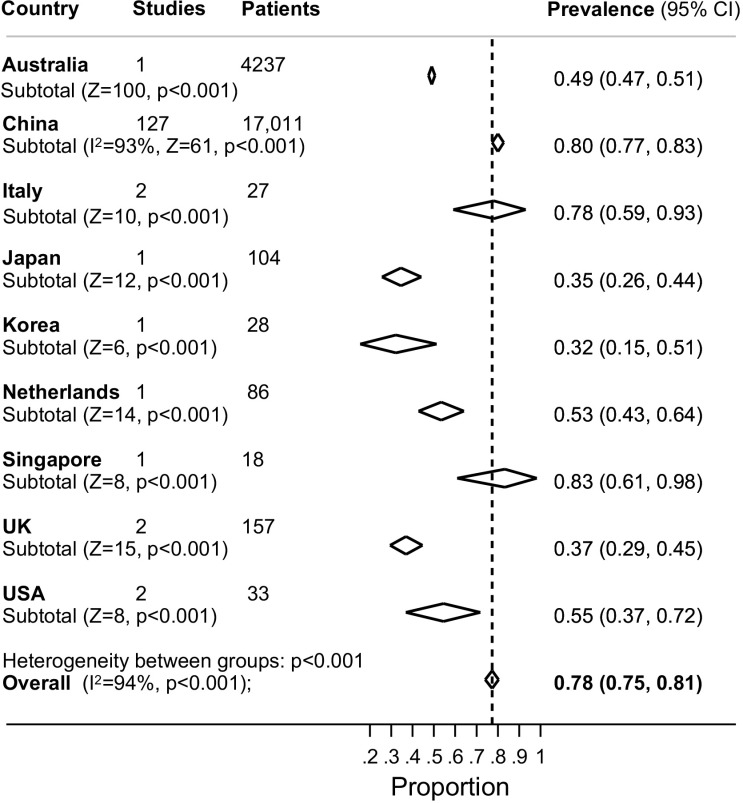
Table 1 shows the meta-analysed prevalence of symptoms, group by bodily system, and S2 Table shows the meta-analytical prevalence estimates from studies grouped by geographical region. The most prevalent symptom in patients with laboratory confirmed COVID-19 was a fever, experienced by 78% of patients (99% CI 75%-81%; Fig 2 and S2–S5 Figs). Whilst, there was substantial heterogeneity between countries (I2 94%) with estimates ranging from 83% in Singapore (99% CI 61%-98%) to 32% in Korea (99% CI 15%-51%), there was no evidence of a statistically significant difference between countries (S2 Table). A cough was the second most prevalent symptom, reported by 57% of test-positive patients (95% CI 54%-60%; Fig 3 and S6–S10 Figs). Whilst there was substantial heterogeneity between countries (I2 94%) with estimates ranging from 18% in Korea (99% CI 8%-36%) to 76% in the Netherlands (95% CI 66%- 83%), there was no evidence of a statistically significant difference.
Table 1. Meta-analysis of the prevalence of symptoms in adults with laboratory test confirmed COVID-19.
| System | Symptom | Number of studies | Number of people | Prevalence (95% CI) | I2 |
|---|---|---|---|---|---|
| Systemic | Fever | 138 | 21,701 | 78 (75, 81) | 94% |
| Fatigue | 78 | 13,385 | 31 (27, 35) | 95% | |
| Myalgia | 72 | 11,389 | 17 (14, 19) | 88% | |
| Rigors | 17 | 2834 | 18 (13, 22) | 88% | |
| Arthralgia | 2 | 401 | 11 (8, 14) | / | |
| Rash | 1 | 1099 | 0 (0, 1) | / | |
| Respiratory | Any cough (dry or productive) | 138 | 21,682 | 57 (54, 60) | 94% |
| Dry (non-productive) cough | 136 | 17,380 | 58 (54, 61) | 93% | |
| Productive cough | 70 | 10,017 | 25 (22, 28) | 90% | |
| Dyspnoea | 94 | 12,713 | 23 (19, 28) | 97% | |
| Chest pain | 30 | 3510 | 7, (4, 10) | 92% | |
| Haemoptysis | 21 | 4698 | 2 (1, 2) | 42% | |
| Wheeze | 16 | 2013 | 17 (9, 26) | 96% | |
| Ear, nose and throat | Sore throat | 78 | 11,721 | 12 (10, 14) | 88% |
| Rhinorrhoea | 36 | 10,656 | 8 (5, 12) | 97% | |
| Vertigo / dizziness | 16 | 1972 | 11 (6, 16) | 90% | |
| Nasal congestion | 10 | 2584 | 5 (3, 7) | 78% | |
| Hyposmia | 3 | 317 | 25 (4, 55) | / | |
| Hypogeusia | 2 | 220 | 4 (1, 8) | / | |
| Otalgia | 1 | 68 | 4 (1, 11) | / | |
| Gastrointestinal | Diarrhoea | 93 | 11,707 | 10 (8, 12) | 93% |
| Nausea | 27 | 4584 | 6 (3, 10) | 95% | |
| Vomiting | 26 | 4959 | 4 (2, 8) | 94% | |
| Abdominal pain | 19 | 3331 | 4 (2, 7) | 88% | |
| Central nervous system | Headache | 65 | 15,958 | 13 (10, 16) | 97% |
| Confusion | 6 | 869 | 11 (7, 15) | 67% | |
| Ataxia | 1 | 214 | 0 (0, 2) | / | |
| Eyes | Conjunctivitis | 9 | 2715 | 2 (1, 4) | 80% |
| Ophthalmalgia | 1 | 534 | 4 (3, 6) | / | |
| Photophobia | 1 | 534 | 3 (2, 4) | / |
Fig 2. Forest plot of the prevalence of fever in adults with laboratory test confirmed COVID-19.
The diamonds are summary estimates from each country.
Fig 3. Forest plot of the prevalence of cough (dry or productive) in adults with laboratory test confirmed COVID-19.
The diamonds are summary estimates from each country.
Discussion
This review describes 24,410 adults with laboratory test confirmed COVID-19 from 9 countries. We confirm that the purported cardinal symptoms of fever and a new persistent cough are indeed the most prevalent symptoms of COVID-19 worldwide. However, we also show that at approximately 1 in 5 test-positive adults were never febrile and fewer than 3 in 5 developed a cough. Since the patients in the included studies are likely to have moderate-severe disease warranting hospitalisation and thus testing, it is likely that we over-estimate the true prevalence of symptoms in the population. Consequently, the use of symptoms alone to screening adults for SARS-CoV-2 infection is likely to miss a substantial number of infected individuals.
Our point estimates of the prevalence of fever (78%) and cough (57%) are approximately 10% lower than the estimates from prior reviews [8,10–13] which we feel might explained by two specific factors. Firstly, prior reviews [8,10–13] did not systematically search (or search at all [8,10,11,13]) for preprints uploaded to online repositories such as medRxiv or bioRxiv [15], both of which have seen a surge in uploads related to the COVID-19 pandemic [15]. This explains why the largest and most recent other review (posted on March 25th 2020 in medRxiv [12]) included just 30 studies. Secondly, several weeks have passed since the other reviews [8,10–13] were performed and the delay from searching to posting a preprint in medRxiv was between 11 days [11] and 5 weeks [12,13,178]. For those articles not uploading a preprint, the delay from searching to publication was 3 weeks [10]. Therefore, it is likely that the prior reviews [8,10–13] had a higher proportion of adults with more severe disease (given that testing was limited to those admitted to hospital in the early phase of the outbreak) whereas more recent studies are likely to include adults with mild symptoms due to the wider availability of testing alongside natural progression of the disease. Conversely, more-recent studies of real-time population-wide tracking of self-reported symptoms in subsequently test-positive patients are essentially identical to our point estimates for cough [179]; however, data concerning fever [180] are less concordant but given the variability of core body temperature, methods of measurement and the definition of this symptom, variability is expected.
Limitations
We acknowledge that there is both within-country and between-country differences in the estimated prevalence of different symptoms, which presents issues with regards to generalising the findings. However, the unique strength of a meta-analysis is the ability to compare datasets from difference sources, identify patterns and discrepancies [181], and no statistical technique is able to correct for weaknesses or idiosyncrasies of the original data. Differences in the study designs, settings and what types of patients (mild, moderate or critically unwell) were sampled are all likely to be responsible for the observed heterogeneity. The sampling strategy is known to bias the prevalence of conditions and ideally, prevalence studies recruit a (non-probabilistic) consecutive sample because they are more likely to represent the target population. In comparison, convenience sampling (i.e. those with available data) and purposive sampling (e.g. reports of individuals with specific clinical features) which are common in the included studies, introduce selection bias and tend to upwardly bias estimates of symptom prevalence. Equally, enrolling patients from hospital settings rather than the community is more likely to upwardly bias the estimates of prevalence. Overall, we suspect that our results over-estimate the true prevalence of symptoms amongst test-positive adults.
In some instances, it was impossible to ascertain whether different publications which originated from the same hospital or region included (some of) the same subjects because the recruitment timeframes and sampling strategies were not sufficiently described in the study methods. We recommend that future publications detail (where possible) if their sample is also represented in other works and describe their methods in accordance with relevant reporting guidelines.
The way in which we extracted some of the data might bias the findings. We dichotomised fever (based on the definition in the parent study) and thresholds differed study-to-study (between 37°C and 38°C, S2 Table) which limits the transferability of the findings to clinical practice. We also did not extract data on combinations of symptoms (such as fever and cough together, or diarrhoea and vomiting for example) which was on oversight in the protocol development phase though equally, this was poorly reported in the literature. Future researchers who wish to build upon this dataset might consider extracting combinations of symptoms alongside isolated symptoms from the few studies were this is reported [174].
Conclusions
We confirm that fever and cough remain the most prevalent symptoms of adults infected by SARS-CoV-2. However, there is a large proportion of infected adults which symptoms-alone do not identify. To expedite future iterations of this work, our data is freely available in the Open Science Framework repository.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
See the bibliography at the end of the supplementary materials. RT = reverse transcriptase; PCR = polymerase chain reaction.
(DOCX)
(DOCX)
Data Availability
The raw extracted data and additional relevant files are available via an Open Science Framework repository (https://doi.org/10.17605/OSF.IO/9VX6U).
Funding Statement
Ryckie G Wade is a Doctoral Research Fellow funded by the National Institute for Health Research (NIHR, DRF-2018-11-ST2-028). Luke McGuinness is supported by an NIHR Doctoral Research Fellowship (DRF-2018-11-ST2-048). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Marc Arbyn was supported by the VALCOR project (Sciensano, Brussels, Begium). The funders had no role in the initiation, conduct or publication of this project.
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report– 81. World Heal. Organ. 2020.
- 2.Jefferson T, Del Mar C, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:b3675–b3675. 10.1136/bmj.b3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F, Zviedrite N, Uzicanin A. Effectiveness of workplace social distancing measures in reducing influenza transmission: A systematic review. BMC Public Health. BMC Public Health; 2018;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston E, Bucher K, Rekito A. Coronavirus Disease 2019 and Influenza 2019–2020. JAMA. 2020;323:1122 10.1001/jama.2020.2633 [DOI] [PubMed] [Google Scholar]
- 6.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;1–10. [DOI] [PubMed] [Google Scholar]
- 7.Woelfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Mueller MA, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. [Google Scholar]
- 8.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gostic K, Gomez AC, Mummah RO, Kucharski AJ, Lloyd-Smith JO. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain V, Yuan J-M. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. [DOI] [PMC free article] [PubMed]
- 12.Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. [Google Scholar]
- 13.Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, Diagnosis, and Treatment of COVID-19: A systematic review of case reports and case series. 10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebrahimi M, Saki A, Rahim F. Laboratory findings, signs and symptoms, clinical outcomes of Patients with COVID-19 Infection: an updated systematic review and meta-analysis. [Google Scholar]
- 15.Sumner J, Haynes L, Nathan S, Hudson-Vitale C, McIntosh LD. Reproducibility and reporting practices in COVID-19 preprint manuscripts. medRxiv Prepr. 2020; [Google Scholar]
- 16.Huisman J, Smits J. Duration and quality of the peer review process: the author’s perspective. Scientometrics. Springer Netherlands; 2017;113:633–50. 10.1007/s11192-017-2310-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collab. 2011;
- 18.Grant MC, McGuinness L, Geoghegan L, Clarke EL, Wade RG. The prevalence of different symptoms in adults infected by the novel coronavirus (SARS-CoV-2; covid-19): A systematic review and meta-analysis protocol. FigShare. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies. JAMA. 2018;319:388 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 20.X. J, M.-H. L, S. R. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. (Jiang, Luo) The Joint Center of Translational Precision Medicine Guangzhou Institute of Pediatrics, Guangzhou Women and Children Medical Center, Guangzhou, China: John Wiley and Sons Inc. (P.O.Box 18667, Newark NJ 07191–8667, United States); 2020;92:476–8. [DOI] [PMC free article] [PubMed]
- 21.Chang, G. M, X. Y, Y. T, X. P, F. W, et al. Time Kinetics of Viral Clearance and Resolution of Symptoms in Novel Coronavirus Infection. Am J Respir Crit Care Med. (Chang, Mo, Yuan, Tao, Peng, Wang, Xie, Qin) Chinese PLA General Hospital, Beijing, China: NLM (Medline); 2020;1–12. [DOI] [PMC free article] [PubMed]
- 22.MA L. What we know so far: COVID-19 current clinical knowledge and research. Clin Med. England; 2020;20:124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M. W, A. D-S, A. A, R. O, S. Z, S. P. COVID-19—what should anaethesiologists and intensivists know about it? Anaesthesiol Intensive Ther. (Wujtewicz, Dylczyk-Sommer, Aszkielowicz, Owczuk) Department of Anaesthesiology and Intensive Therapy, Medical University of Gdansk, Gdansk, Poland: NLM (Medline); 2020;52:34–41. [DOI] [PMC free article] [PubMed]
- 24.Y. W, Q. Q, Y. C. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. (Wang, Wang, Qin) Laboratory of Human Virology and Oncology, Shantou University Medical College, Guangdong Province, Shantou 515041, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed]
- 25.McGuinness L, Schmidt L. medRxivr: Accessing medRxiv data in R [Internet]. 2020. Available from: https://github.com/mcguinlu/medrxivr [Google Scholar]
- 26.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. Elsevier Inc; 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 27.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Heal. 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller JJ. The Inverse of the Freeman–Tukey Double Arcsine Transformation. Am Stat. 1978;32:138–138. [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.武汉市卫生委员会对新型冠状病毒感染的肺炎. 武汉市卫生健康委员会关于新型冠状 病毒感染的肺炎情况通报.
- 31.Lancet The. Emerging understandings of 2019-nCoV. Lancet. 2020;395:311 10.1016/S0140-6736(20)30186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020;382:1199–207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J et al. [Analysis of clinical features of Huxi, 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jiehe He Zazhi. 2020;43:E005. [DOI] [PubMed] [Google Scholar]
- 35.D. W, B. H, C. H, F. Z, X. L, J. Z, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA—J Am Med Assoc. (Wang, Hu, Hu, Zhu, Liu, Zhang, Wang, Xiang, Peng) Department of Critical Care Medicine, Zhongnan Hospital of Wuhan University, Wuhan, Hubei 430071, China: American Medical Association (E-mail: smcleod@itsa.ucsf.edu); 2020;323:1061–9. [DOI] [PMC free article] [PubMed]
- 36.Chang D, Lin M, Wei L, Xie L, Zhu G, Dela Cruz CS, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To KK-W, Tsang OT-Y, Yip CC-Y, Chan K-H, Wu T-C, Chan JM-C, et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YSL, Zhang D, Tang S, Chen H, Chen L, He X, et al. The Epidemiological and Clinical Characteristics of 2019 Novel Coronavirus Infection in Changsha, China. SSRN Electron J. 2020; [Google Scholar]
- 40.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020;200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang MQ, Wang XH, Chen YL, Zhao KL, Cai YQ, An CL et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E013 10.3760/cma.j.issn.1001-0939.2020.0013 [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (Yang, Yu, Xu, Shu, Liu, Wu, Wang, Pan, Zou, Yuan, Shang) Department of Critical Care Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China: Lancet Publishing Group (E-mail: cususerv@lancet.com); 2020; [DOI] [PMC free article] [PubMed]
- 44.D. Z, L. Z, H. Z, F. Y, F. G, L. W, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. (Zhao, Zheng, Zhao) Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Anhui Medical University, Anhui Province, Hefei, China: NLM (Medline); 2020;
- 45.P. Y, J. Z, Z. Z, Y. H, L. H. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. (Yu) Jingan District Center for Disease Control and Prevention, Shanghai, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed]
- 46.Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. Philadelphia, Pennsylvania: Lancet; 2020;395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.S. L, J. L, Z. Z, Z. J, J. C, C. H, et al. Alert for non-respiratory symptoms of Coronavirus Disease 2019 (COVID-19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients. J Med Virol. (Lu, Lin, Zhang, Jiang, Chen, Hu) Department of Internal Medicine, Affiliated Chencun Hospital of Shunde Hospital, Southern Medical University, Shunde District, Guangdong, China: NLM (Medline); 2020;
- 48.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74 10.1186/s12931-020-01338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, et al. Analysis of Epidemiological and Clinical features in older patients with Corona Virus Disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. (Lian, Hao, Cai, Zhang, Zheng, Jia, Hu, Zhang, Zhang, Yu, Wang, Gu, Ye, Jin, Lu, Yu, Yu, Qiu, Li, Sheng, Yang) State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborativ: NLM (Medline); 2020;
- 50.Liu W, Tao Z-W, Lei W, Ming-Li Y, Kui L, Ling Z, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). (Liu, Lei, Ming-Li, Yi) Department of Respiratory and Critical Care Medicine, Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430014, China: NLM (Medline); 2020;1.
- 51.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. (Shi, Shen, Liu, Liang, Zhao, Huang, Yang, Huang) Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.S. C, Y. S, E. L. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. (Chen, Shao) Department of Obstetrics and Gynecology, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed]
- 53.Xu Y-H, Dong J-H, An W-M, Lv X-Y, Yin X-P, Zhang J-Z, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. (Xu, Dong, An, Lv, Zhang, Gao) Department of Radiology, The Fifth Medical Center of Chinese PLA General Hospital, 100 West Fourth Ring Road, Fengtai District, Beijing 100039, China: W.B. Saunders Ltd; 2020;80:394–400. [DOI] [PMC free article] [PubMed]
- 54.Yang S, Shi Y, Lu H, Xu J, Li F, Qian Z, et al. Clinical and CT features of early-stage patients with COVID-19: a retrospective analysis of imported cases in Shanghai, China. Eur Respir J. (Yang, Shi, Hua, Ding, Song, Shen, Lu, Shan, Zhang) Department of Radiology, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China: NLM (Medline); 2020;2000407. [DOI] [PMC free article] [PubMed]
- 55.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. (Liu, Li, Zhang, Wang) Department of Radiology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, No. 1665 Kongjiang Road, Yangpu District, Shanghai 200092, China: W.B. Saunders Ltd; 2020; [DOI] [PMC free article] [PubMed]
- 56.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. Elsevier Ltd; 2020;80:388–93. 10.1016/j.jinf.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (Chen, Guo, Yu, Li, Xu, Gong, Liao, Zhang) Department of Gynaecology and Obstetrics, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China: Lancet; 2020;395:809–15. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.W Z., C X., L Y., C F., Z W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. (Wang) Department of Respiratory Disease, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China: NLM (Medline); 2020;14:64–8. 10.5582/bst.2020.01030 [DOI] [PubMed] [Google Scholar]
- 59.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;2:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Dong X, Cao Y, Yuan Y, Yang Y, Yan Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. (Zhang, Dong, Gao) Department of Allergology, Zhongnan Hospital of Wuhan University, Wuhan, China: Blackwell Publishing Ltd; 2020;all.14238. [DOI] [PubMed] [Google Scholar]
- 61.Chu J, Yang N, Wei Y, Yue H, Zhang F, Zhao J, et al. Clinical characteristics of 54 medical staff with COVID-19: A retrospective study in a single center in Wuhan, China. J Med Virol. (Chu, Wei, Yue, Zhang, Zhao, Zhang) Department of Respiratory and Critical Care Medicine, Tongji Medical College, Huazhong University of Sciences and Technology, Wuhan, HuBei 430030, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (Guan, Ni, Hu, Liang, Ou, He, Liu, Shan, Lei, Hui, Du, Li, Zeng, Yuen, Chen, Tang, Wang, Chen, Xiang, Li, Wang, Liang, Peng, Wei, Liu, Hu, Peng, Wang, Liu, Chen, Li, Zheng, Qiu, Luo, Ye, Zhu, Zhong) From the State Key Laboratory of Respiratory Disease, Na: NLM (Medline); 2020;NEJMoa2002032. [Google Scholar]
- 63.Deng Y, Liu W, Liu K, Fang Y-Y, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China. Chin Med J (Engl). (Deng, Liu, Fang, Shang, Zhou, Wang, Leng, Wei, Chen, Liu) Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China: NLM (Medline); 2020;1. [Google Scholar]
- 64.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. (Wu, Yu, Cao, Li) State Key Laboratory for the Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis. (Huang, Wang, Chen, Zhou, Chen, Zhou, Wang, Liu, Guo) Department of Plastic Surgery, Zhongnan Hospital of Wuhan University, Wuhan 430071, China: Elsevier USA; 2020;101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu K, Fang Y-Y, Deng Y, Liu W, Wang M-F, Ma J-P, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). (Kui, Fang, Deng, Liu) Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China: NLM (Medline); 2020;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. (Mo, Deng, Xiong, Gao, Liang, Luo, Chen, Song, Ma, Chen, Zheng, Cao, Zhang) Department of Infectious Disease, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China: NLM (Medline); 2020; [Google Scholar]
- 68.Y G., P Y., D Q., C L., L J., L Y., et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. (Ye, Pan, Deng, Chen, Li, Li) Department of Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan, Hubei, China: W.B. Saunders Ltd; 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (Zhou, Fan, Liu, Wang, Gu, Li, Zhang, Cao) Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, Institute of Respiratory Medicine, Chinese Academy of Medical Scienc: Lancet Publishing Group (E-mail: cususerv@lancet.com); 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical Features and Chest CT Manifestations of Coronavirus Disease 2019 (COVID-19) in a Single-Center Study in Shanghai, China. Am J Roentgenol. (Cheng, Lu, Cao, Qin, Pan, Yan, Yang) Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, No. 197, Ruijin Er Rd, Shanghai 200025, China: NLM (Medline); 2020;1–6. [DOI] [PubMed] [Google Scholar]
- 71.Xu T, Chen C, Zhu Z, Cui M, Chen C, Dai H, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. (Xu, Cui) Institute of Hepatology, the Third People’s Hospital of Changzhou, Changzhou, China; Department of Infectious Diseases, the Third People’s Hospital of Changzhou, Changzhou, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. (Yu, Li, Kang, Xiong, Wang, Lin, Liu, Xiao, Liu, Deng, Chen, Zeng, Feng, Wu) Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Li X, Zhang W, Shi Z-L, Zheng Z, Wang T. Clinical Features and Treatment of 2019-nCov Pneumonia Patients in Wuhan: Report of A Couple Cases. Virol Sin. (Zhang, Li, Zheng, Wang) Department of Respiratory Disease and Intensive Care, Renmin Hospital of Wuhan University, Wuhan 430060, China: Science Press (E-mail: rfchen@issas.ac.cn); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. (Wan, Xiang, Fang, Li, Hu, Lang, Huang, Sun, Xiong, Huang, Lv, Shen, Yang, Huang, Yang) Pharmaceutical Department of Chongqing Three Gorges Central Hospital, Chongqing University Three Gorges Hospital, Chongqing 404100, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. (Wang, Li, Wen, Zhang) Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. (Liu) Hainan General Hospital, Geriatric center, China: W.B. Saunders Ltd; 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (Huang, Zhang, Yu, Xia, Wei, Wu, Xie) Jin Yin-tan Hospital, Wuhan, China: Lancet; 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. (Xu, Wu, Xu, Sheng, Cai, Qiu, Li) State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Centre for Infectious Diseases, Collaborative Innovation Centre for Diagnosis and Treatment of Infectious Diseases, First: BMJ Publishing Group (E-mail: subscriptions@bmjgroup.com); 2020;368:m606 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (Chen, Qi, Liu, Xu, Shen) Department of Infectious Diseases and Immunology, Shanghai Public Health Clinical Center, Shanghai 201508, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo S, Zhang X, Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol. (Luo, Xu) Department of Radiology, Zhongnan Hospital of Wuhan University, Hubei Province, Wuhan 430071, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong X, Cao Y, Lu X, Zhang J, Du H, Yan Y, et al. Eleven faces of coronavirus disease 2019. Allergy. (Dong, Zhang, Gao) Department of Allergology, Zhongnan Hospital of Wuhan University, Donghu Road 169, Wuhan, Hubei 430071, China: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. (Song, Shi, Shan, Zhang, Shen, Shi) Department of Radiology, Shanghai Public Health Clinical Center, No. 2501 Caolang Road Jinshan District, Shanghai 201508, China: Radiological Society of North America Inc. (820 Jorie Boulevard, Oak Brook IL 60523–2251, United States); 2020;295:210–7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. Elsevier Ltd; 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin X, Lian J-S, Hu J-H, Gao J, Zheng L, Zhang Y-M, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. (Jin, Gao, Ren) Department of Gastroenterology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China: BMJ Publishing Group (E-mail: subscriptions@bmjgroup.com); 2020;gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Cai H, Hu J, Lian J, Gu J, Zhang S, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. (Zhang, Cai, Hu, Lian, Gu, Zhang, Ye, Lu, Jin, Yu, Jia, Zhang, Sheng, Li, Yang) State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnos: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S. Initial clinical features of suspected Coronavirus Disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. (Zhu, Xie, Lu, Xu, Zhou, Fang) Department of Emergency, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China: John Wiley and Sons Inc. (P.O.Box 18667, Newark NJ 07191–8667, United States); 2020;jmv.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am J Roentgenol. (Zhao, Xie, Liu) Department of Radiology, Second Xiangya Hospital, Central South University, No. 139 Middle Remin Rd, Changsha, Hunan 410011, China: NLM (Medline); 2020;1–6. [Google Scholar]
- 88.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. (Wu, Du, Zhou, Song) Department of Pulmonary Medicine, QingPu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai, China: American Medical Association (E-mail: smcleod@itsa.ucsf.edu); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Invest Radiol. (Li, Guo, Chen, Fang, Li) Department of Radiology, Second Affiliated Hospital of Chongqing Medical University, Chongqing, China: NLM (Medline); 2020;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, et al. The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emerg Microbes Infect. (Su, Zhang, Bian, Geng, Zhang) Department of infectious diseases, Jinan Infectious diseases Hospital of Shandong University, Jinan, China: NLM (Medline); 2020;9:707–13. 10.1080/22221751.2020.1744483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study.
- 92.Qi D, Yan X, Tang X, Peng J, Yu Q, Feng L, et al. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study.
- 93.Qi S, Guo H, Shao H, Lan S, He Y, Tiheiran M, et al. Computed Tomography Findings and Short-term follow-up with Novel Coronavirus Pneumonia. [Google Scholar]
- 94.Shi Q, Zhao K, Yu J, Feng J, Zhao K, Zhang X, et al. Clinical characteristics of 101 non-surviving hospitalized patients with COVID-19: A single center, retrospective study. [Google Scholar]
- 95.Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. [Google Scholar]
- 96.Sun X, Zhang X, Chen X, Chen L, Deng C, Zou X, et al. The infection evidence of SARS-COV-2 in ocular surface: a single-center cross-sectional study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tao Y, Cheng P, Chen W, Wan P, Chen Y, Yuan G, et al. High incidence of asymptomatic SARS-CoV-2 infection, Chongqing, China. [Google Scholar]
- 98.Tian S, Chang Z, Wang Y, Wu M, Zhang W, Zhou G, et al. Clinical characteristics and reasons of different duration from onset to release from quarantine for patients with COVID-19 Outside Hubei province, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang W, He J, Lie P, Huang L, Wu S, Lin Y, et al. The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: Disease Characteristics and Retrospective Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Zhou Y, Yang Z, Xia D, Geng S. Clinical Characteristics of Patients with Severe Pneumonia Caused by the 2019 Novel Coronavirus in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu H, Hou K, Xu H, Li Z, Chen H, Zhang N, et al. Acute Myocardial Injury of Patients with Coronavirus Disease 2019. [Google Scholar]
- 102.Xu Y, Li Y -z. Y -r. z r, Zeng Q, Lu Z -b. b, Li Y -z. Y -r. z r, Wu W, et al. Clinical Characteristics of SARS-CoV-2 Pneumonia Compared to Controls in Chinese Han Population.
- 103.Xu Y, Xu Z, Liu X, Cai L, Zheng H, Huang Y, et al. Clinical findings in critical ill patients infected with SARS-Cov-2 in Guangdong Province, China: a multi-center, retrospective, observational study. [Google Scholar]
- 104.Zeng L, Li J, Liao M, Hua R, Huang P, Zhang M, et al. Risk assessment of progression to severe conditions for patients with COVID-19 pneumonia: a single-center retrospective study. [Google Scholar]
- 105.Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H-YY, Wang L-WW, Chen Y-YY, Shen X-KK, Wang Q, Yan Y-QQ, et al. A Multicentre Study of 2019 Novel Coronavirus Disease Outcomes of Cancer Patients in Wuhan, China. [Google Scholar]
- 108.Zhang Y. Gastrointestinal tract symptoms in coronavirus disease 2019: Analysis of clinical symptoms in adult patients. [Google Scholar]
- 109.Zhao Z, Xie J, Yin M, Yang Y, He H, Jin T, et al. Clinical and Laboratory Profiles of 75 Hospitalized Patients with Novel Coronavirus Disease 2019 in Hefei, China. [Google Scholar]
- 110.Zhou F, Yu X, Tong X, Zhang R. Clinical features and outcomes of 197 adult discharged patients with COIVD-19 in Yichang, Hubei. [Google Scholar]
- 111.Ai J-W, Zhang H-C, Xu T, Wu J, Zhu M, Yu Y-Q, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. [Google Scholar]
- 112.Qifang B, Yongsheng W, Shujiang M, Chenfei Y, Xuan Z, Zhen Z, et al. Epidemiology and Transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv. 2020; [Google Scholar]
- 113.Bian H, Zheng ZH, Wei D, Zhang Z, Kang WZ, Hao CQ, et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. [Google Scholar]
- 114.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a Designated Infectious Diseases HospitalOutside Hubei Province,China. [DOI] [PubMed] [Google Scholar]
- 115.Cao W. Clinical features and laboratory inspection of novelcoronavirus pneumonia (COVID-19) in Xiangyang, Hubei. [Google Scholar]
- 116.Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. [Google Scholar]
- 117.chen d., Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019. (COVID-19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019. [Google Scholar]
- 119.Chen H, Zhang Z, Wang L, Huang Z, Gong F, Li X, et al. First Clinical Study Using HCV Protease Inhibitor Danoprevir to Treat Naive and Experienced COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen J, Fan H, Zhang L, Huang B, Zhu M, Zhou Y, et al. Retrospective Analysis of Clinical Features in 101 Death Cases with COVID-19. [Google Scholar]
- 121.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, et al. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: A cross-sectional study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, et al. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B virus Co-infection Xiaoping Chen 1*#, Qunqun Jiang 1*, Zhiyong Ma 1*,. medRxiv Prepr. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen X, Ling J, Mo P, Zhang Y, Jiang Q, Ma Z, et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. [Google Scholar]
- 124.Chen X, Zheng F, Qing Y, Ding S, Yang D, Lei C, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019. in areas adjacent to Hubei, China: a double-center observational study. [Google Scholar]
- 125.Chen Z, Hu J, Zhang Z, Jiang S, Wang T, Shi Z, et al. Caution: The clinical characteristics of COVID-19 patients at admission are changing. [Google Scholar]
- 126.Chen Z, Hu J, Zhang Z, Shan Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. [Google Scholar]
- 127.Cui P, Chen Z, Wang T, Dai J, Zhang J, Ding T, et al. Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: a descriptive study in Wuhan, China. [Google Scholar]
- 128.dong l., Zhou J, Niu C, Wang Q, Pan Y, Wang X, et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. [Google Scholar]
- 130.Fan L, Liu C, Li N, Liu H, Gu Y, Liu Y, et al. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan Z, Chen L, Li J, Tian C, Zhang Y, Huang S, et al. Clinical Features of COVID-19 Related Liver Damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fei J, Fu L, Li Y, Xiang H-X, Xiang Y, Li M-D, et al. Reduction of lymphocyte at early stage elevates severity and death risk of COVID-19 patients: a hospital-based case-cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng C, Huang Z, Wang L, Chen X, Zhai Y, Zhu F, et al. A Novel Triage Tool of Artificial Intelligence Assisted Diagnosis Aid System for Suspected COVID-19 pneumonia In Fever Clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feng Z, Yu Q, Yao S, Luo L, Duan J, Yan Z, et al. Early Prediction of Disease Progression in 2019. Novel Coronavirus Pneumonia Patients Outside Wuhan with CT and Clinical Characteristics. [Google Scholar]
- 135.Fu H, Xu H, Zhang N, Xu H, Li Z, Chen H, et al. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-up of COVID-19 patients. [Google Scholar]
- 136.Fu L, Fei J, Xiang HX, Xiang Y, Tan ZX, Li MD, et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. [Google Scholar]
- 137.Fu S, Fu X, Song Y, Li M, Pan P h, Tang T, et al. Virologic and clinical characteristics for prognosis of severe COVID-19: a retrospective observational study in Wuhan, China. [Google Scholar]
- 138.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, et al. A Tool to Early Predict Severe 2019-Novel Coronavirus Pneumonia (COVID-19): A Multicenter Study using the Risk Nomogram in Wuhan and Guangdong, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Han Y, Zhang H, Mu S, Wei W, Jin C, Xue Y, et al. Lactate dehydrogenase, a Risk Factor of Severe COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. [Google Scholar]
- 141.Ji W, Bishnu G, Cai Z, Shen X. Analysis clinical features of COVID-19 infection in secondary epidemic area and report potential biomarkers in evaluation. [Google Scholar]
- 142.Kang Y. Critical Care for Patients with Severe Covid-2019. in Sichuan Province, China: Provincial Cohort Study. [Google Scholar]
- 143.lei l., Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing,China. [Google Scholar]
- 144.Lei Y, lan y., lu j., huang x., silang b., zeng f. Clinical features of imported cases of coronavirus disease 2019. in Tibetan patients in the Plateau area. [Google Scholar]
- 145.Leung KS-S, Ng TT-L, Wu AK-L, Yau MC-Y, Lao H-Y, Choi M-P, et al. A Territory-wide study of COVID-19 cases and clusters with unknown source in Hong Kong community: A clinical, epidemiological and phylogenomic investigation. [Google Scholar]
- 146.Li J, Li S, Cai Y, Liu Q, Li X, Zeng Z, et al. Epidemiological and Clinical Characteristics of 17 Hospitalized Patients with 2019. Novel Coronavirus Infections Outside Wuhan, China. [Google Scholar]
- 147.Li J, Zhang Y, Wang F, Liu B, Li H, Tang G, et al. Sex differences in clinical findings among patients with coronavirus disease 2019. (COVID-19) and severe condition. [Google Scholar]
- 148.Li K, Chen D, Chen S, Feng Y, Chang C, Wang Z, et al. Radiographic Findings and other Predictors in Adults with Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Q, Ding X, Xia G, Geng Z, Chen F, Wang L, et al. A simple laboratory parameter facilitates early identification of COVID-19 patients. [Google Scholar]
- 151.Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019. novel coronavirus disease (COVID-19) in the Fever Clinic of a teaching hospital in Beijing: a single-center, retrospective study. [Google Scholar]
- 152.Liao J, Fan S, Chen J, Wu J, Xu S, Guo Y, et al. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin Y, Lv S, Wang J, Kang J, Zhang Y, Feng Z. Ultra-High-Resolution CT Follow-Up in Patients with Imported Early-Stage Coronavirus Disease 2019. (COVID-19) Related Pneumonia. [Google Scholar]
- 154.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J, Ouyang L, Guo P, Wu H s., Fu P, Chen Y l., et al. Epidemiological, Clinical Characteristics and Outcome of Medical Staff Infected with COVID-19 in Wuhan, China: A Retrospective Case Series Analysis. [Google Scholar]
- 156.Liu L, Liu W, Wang S, Zheng S. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu R, Ming X, Zhu H, Song L, Gao Z, Gao L, et al. Association of Cardiovascular Manifestations with In-hospital Outcomes in Patients with COVID-19: A Hospital Staff Data. [Google Scholar]
- 158.Liu T, Zhang J, Yang Y, Zhang L, Ma H, Li Z, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. [Google Scholar]
- 159.Liu Y, Sun W, Chen L, Wang Y, Zhang L, Yu L. Clinical Characteristics and Progression of 2019. Novel Coronavirus-Infected Patients Concurrent Acute Respiratory Distress Syndrome. [Google Scholar]
- 160.Lu H, Ai J, Shen Y, Li Y, Li T, Zhou X, et al. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. [Google Scholar]
- 161.Lu J, Hu S, Fan R, Liu Z, Yin X, Wang Q, et al. ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. [Google Scholar]
- 162.Luo L, Liu D, Liao X -l., Wu X -b., Jing Q -l., Zheng J -z., et al. Modes of contact and risk of transmission in COVID-19 among close contacts. [Google Scholar]
- 163.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. [Google Scholar]
- 164.Miao C, Zhuang J, Jin M, Xiong H, Huang P, Zhao Q, et al. A comparative multi-centre study on the clinical and imaging features of comfirmed and uncomfirmed patients with COVID-19. [Google Scholar]
- 165.Nie R, Wang S -s., Yang Q, Fan C -f., Liu Y -l., He W -c., et al. Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019. [Google Scholar]
- 166.Zhang Y, Chen R, Wang J, Gong Y, Zhou Q, Cheng H -h. h, et al. Anaesthetic managment and clinical outcomes of parturients with COVID-19: a multicentre, retrospective, propensity score matched cohort study. [Google Scholar]
- 167.Easom N, Moss P, Barlow G, Samson A, Taynton T, Adams K, et al. 68 Consecutive patients assessed for COVID-19 infection; experience from a UK regional infectious disease unit. Influenza Other Respi Viruses. (Easom, Moss, Barlow, Samson, Taynton, Adams, Ivan, Burns, Gajee, Eastick, Lillie) Department of Infection, Hull University Teaching Hospitals NHS Trust, Castle Hill Hospital, East Yorkshire HU16 5JQ, United Kingdom: NLM (Medline); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kujawski SA, Wong KK, Collins JP, Epstein L, Killerby ME, Midgley CM, et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv Prepr. 2020; [DOI] [PubMed] [Google Scholar]
- 169.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. (Arentz) Department of Global Health, University of Washington, 325 Ninth Ave, Seattle, WA 98104, United States: American Medical Association (E-mail: smcleod@itsa.ucsf.edu); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun Y, Koh V, Marimuthu K, Ng OT, Young B, Vasoo S, et al. Epidemiological and Clinical Predictors of COVID-19. Clin Infect Dis. (Sun, Lee, Cook, Leo) Saw Swee Hock School of Public Health, National University of Singapore and National University Health System, Science Drive 2, Singapore: NLM (Medline); 2020; [Google Scholar]
- 171.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. (Young, Ong, Ng, Marimuthu, Ang, Mak, Cui, Chen, Chan, Vasoo, Lin, Leo, Lye) National Centre for Infectious Diseases, Singapore, Singapore: American Medical Association (E-mail: smcleod@itsa.ucsf.edu); 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marchese-Ragona R, Ottaviano G, Nicolai P, Vianello A, Carecchio M. Sudden hyposmia as a prevalent symptom of COVID-19 infection. [Google Scholar]
- 173.Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. [Google Scholar]
- 174.Tsang T. COVID-19, Australia: Epidemiology Report 10: Reporting week ending 23:59 AEST 5 April 2020. Commun Dis Intell. 2020;44. [DOI] [PubMed] [Google Scholar]
- 175.Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, et al. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise ship “Diamond Princess” in Japan. [Google Scholar]
- 176.I. K, Y. P, Y. W, J. L, J. C, J. C, et al. Early Epidemiological and Clinical Characteristics of 28 Cases of Coronavirus Disease in South Korea. Osong Public Heal Res Perspect. (Kong, Park, Woo, Lee, Cha, Choi, Kim, Kim, Park, Yum, Kim, Jo) Center for Disease Prevention, KCDC, South Korea: Korea Centers for Disease Control and Prevention (E-mail: sidus@korea.kr); 2020;11:8–14. [DOI] [PMC free article] [PubMed]
- 177.Kluytmans M, Buiting A, Pas S, Bentvelsen R, van den Bijllaardt W, van Oudheusden A, et al. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. [Google Scholar]
- 178.Sun P, Qie S, Liu Z, Ren J, Jianing Xi J. Clinical characteristics of 50404 patients with 2019-nCoV infection. [Google Scholar]
- 179.Menni C, Valdes A, Freidein M, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Medicine 2020: In press. 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rossman H, Keshet A, Shilo S, et al. A framework for identifying regional outbreak and spread of COVID-19 from one-minute population-wide surveys. Nature Medicine 2020;26(5):634–638. 10.1038/s41591-020-0857-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Greenland S. Can Meta-analysis Be Salvaged? Am J Epidemiol. 1994;140:783–7. 10.1093/oxfordjournals.aje.a117326 [DOI] [PubMed] [Google Scholar]