Abstract
Background
Leukocyte alterations are a common hematological alteration among malaria patients.
Objectives
This systematic review and meta-analysis aimed to provide data and evidence comparing alterations in total leukocyte counts in malaria patients compared to febrile/healthy subjects at baseline before treatment. A systematic review was conducted by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses.
Data sources
Web of Science (ISI), Scopus, and Medline.
Study eligibility criteria, participants, and interventions
All published articles reporting a total leukocyte count of patients infected with malaria, non-malaria (febrile or healthy group) at baseline before treatment before August 27, 2019, were retrieved, and data were extracted by two main reviewers independently.
Study appraisal and synthesis methods
We used a forest plot, heterogeneity test (Cochran’s Q), and the degree of heterogeneity (I2) to test whether the included studies were heterogeneous. The quality of the included studies was determined by a quality assessment guide based on the quality assessment tool developed by the Newcastle-Ottawa Scale (NOS). Cochran’s Q (Chi-square) and Moran's I2 were used to evaluate heterogeneity. Meta-regression using STATA software was conducted to find the source of heterogeneity. A funnel plot with Egger’s test was used to examine the significance of publication bias among the included studies. The mean differences were estimated using a random-effects model.
Results
Out of the 2,261 articles screened, 29 articles were included in this systematic review and meta-analysis. The heterogeneity test indicated that there was heterogeneity among the included studies with no publication bias. The meta-analysis demonstrated that the total leukocyte count was significantly lower in patients with malaria (n = 4,619) than in those without malaria (n = 10,056) (Z = 4.0, P-value < 0.00001, mean difference = -1.38, 95% CI = -2.06-(-0.71)). Leukocyte differential alterations, low lymphocyte counts (P-value <0.0001, mean difference = -1.03, 95% CI = -1.53-(-0.53)) and a high NL ratio were found in the malaria group (n = 1,579) compared to the non-malaria group (n = 4,991) (P-value <0.0001, mean difference = 0.6, 95% CI = 0.32–0.88). The subgroup analysis indicated that there was a significantly lower total leukocyte count in the malaria group (n = 3,545) than in the febrile group (n = 8,947) (Z = 1.33, P-value < 0.0001, mean difference = -1.76, 95% CI = -2.56-(-0.96)), but no significant difference was found between the malaria group (n = 1,232) and the healthy group (n = 1,679) (P-value > 0.05).
Limitations
As the specific diagnoses in the febrile groups were not reported in the included studies so that the results of the present study need to be carefully interpreted.
Conclusions and implications of key findings
This systematic review demonstrated that the total leukocyte count was affected by malarial infection at baseline despite the heterogeneity of the included studies. Future work must aim to understand the treatment-related total leukocyte reduction during follow-up or post-treatment outcomes in malaria-endemic settings.
Introduction
Malaria is a major public health problem worldwide, especially in sub-Saharan Africa, with estimated 228 million cases and 405,000 deaths worldwide in 2018 [1]. The clinical manifestations of malaria patients can be divided into uncomplicated malaria and severe malaria. Severe malaria is characterized by the presence of one of the following: bleeding or disseminated intravascular coagulation (DIC), metabolic acidosis, prostration, severe anemia, hypoglycemia, shock, jaundice, impaired consciousness, multiple convulsions, acute kidney injury, or pulmonary edema [2]. Uncomplicated malaria is characterized by nonspecific symptoms, with fever as a hallmark and other nonspecific signs, such as malaise, anorexia, headache, myalgia, nausea, vomiting or chills [3]. Laboratory findings of uncomplicated and severe malaria show some degree of anemia and thrombocytopenia [4, 5–7, 8, 9], which are the two most recognized laboratory findings among most literature reviews. However, the overall understanding of leukocyte alterations in uncomplicated and severe malaria is still incomplete, and this is the first gap addressed in the present study.
Leukocyte alterations are a common hematological alteration among malaria patients [7, 10–18]. Previous studies have described leukopenia during malarial infection [7, 11–15, 18]. However, some studies observed leukocytosis during malarial infection [10, 16, 17]. A previous study by Zahorec et al. introduced the neutrophil-lymphocyte ratio (NLR) as a better indicator of systemic inflammation and stress than C-reactive protein (CRP) level [19]. Our previous study also demonstrated that neutrophil and lymphocyte counts were the most important leukocytic changes associated with malaria infection as NLR in malaria infected patients was higher in comparison to non-malaria infected patients [13]. To date, there have been no systematic reviews or meta-analyses focusing on leukocyte alterations during malarial infection. The association between leukocyte counts during malarial infection is limited. Previous studies demonstrated that during early malarial infection, the leukocyte count decrease was related to fever outcomes [20, 21]. The alteration of leukocyte counts in combination with routine malaria diagnosis, such as microscopy techniques, in malaria-endemic areas may prove beneficial for laboratory technicians or physicians, especially in patients with very low parasitemia. Alterations of leukocyte counts might be used in combination with other markers to help diagnose malaria and could be useful for the management of malarial patients.
The second gap in previous studies is that most of the studies reported a significant difference in hematological parameters in febrile patients who were negative for the malaria parasite as a non-malaria group. However, these patients might have other infections and therefore do not represent a healthy population. These bacterial or viral infections might affect hematological variables in different ways. This systematic review and meta-analysis aimed to provide data and evidence comparing total leukocyte alterations among malaria patients and febrile/healthy subjects at baseline before treatment.
Materials and methods
A systematic review was conducted by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions [22] (see S1 Checklist).
Definitions
The malaria group included patients who were infected with at least one of five Plasmodium species, which included P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. The febrile group included patients who were recruited for the assessment of malaria parasites, but no malaria parasites were found. The healthy group was the group of patients who were assessed as healthy in the same area of study.
Eligibility criteria
Searches for this study were limited to human studies but were not limited by year, country or language. Only original research studies with quantitative analysis were considered, thereby excluding animal studies, clinical drug trials, in vitro and in vivo studies, reviews, systematic reviews, short reports, letters to the editor, quizzes, and articles for which the full text was unavailable. Further, studies which involve the analysis of baseline leukocyte count for both malaria groups and non-malaria groups, and. studies of enrolled patients with malarial infection and non-malaria patients (febrile or healthy) with a report on the total leukocyte count at baseline before treatment were included. Studies involving patients with hematological diseases (sickle cell anemia, thalassemia, and hemoglobinopathies), hematological malignancies (lymphoma, leukemia, and multiple myeloma), chronic liver disease (hepatitis B and C), or other diseases/conditions such as human immunodeficiency virus infection, acquired immune deficiency syndrome (HIV/AIDS), organ transplantation, pregnant women, or were exhibiting mixed infections were excluded from this study.
Search strategy
Published studies were identified using keywords in combination with truncations. AND with OR was used to combine terms “(malaria OR plasmodium) AND (leukocyte OR white blood cell)” (see S1 Table). The searches for articles from all three databases started on 27 August 2019 and finished on 28 August 2019. The searches were conducted in the following three main research databases: MEDLINE (1947–2019, 27 August), SCOPUS (1921–2019, 27 August), and ISI Web of Science (2002–2019, 27 August). The papers were imported into EndNote X9 (Thomson Reuters, USA) for reference management. Two main reviewers (MK and KK) independently examined all papers and performed the study selection. The first step of reviewing the papers was the identification of relevant articles based on titles and abstracts. If a paper was potentially related or if it was unclear if it was related, a full-text review of the paper was performed before a decision was made to include or exclude it from this study. The second step of reviewing the papers was reading the text of the articles, which was conducted by each reviewer independently. For discordances between the two reviewers regarding full article reviews, a third reviewer participated and decided whether the study should be included or excluded.
Data extraction
Data extraction was conducted for each selected article, and the following data were extracted: author, references, study area, year of study, mean age, age range, sex ratio, type of Plasmodium sp., and severe complications. The detection of malaria parasites were based on any of these methods: rapid diagnostic test (RDT), microscopy, polymerase chain reaction (PCR), or any test combination. The number of participants in malaria and non-malaria groups, characteristics of controls, and diagnostic techniques were also extracted. Extracted data were entered into an Excel sheet.
Quality of included studies
Quality assessment was performed with the quality assessment tool developed by the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses [23]. The quality assessment tool was used to evaluate the validity of the included studies, which is also shown in Table 2.
Table 2. Quality of the included studies.
| No. | Reference | Selection | Compatibility | Exposure | Quality of included studies | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Non-malaria | Definition of Non-malaria | Ascertainment of Exposure | Same method of ascertainment for cases and non-malaria | Nonresponse Rate | ||||
| 1. | Adam et al. [41] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 2. | Ansart et al.[37] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 3. | Anwar et al. [42] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 4. | Awoke N and Arota A [5] |
✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 5. | Chaves et al. [15] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 6. | Erhart et al [6] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 7. | Frimpong et al. [26] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 8. | Goncalves et al. [39] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 9. | González et al. [34] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 10. | Hänscheid et al. [7] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 11. | Hasona et al. [43] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 12. | Hojo-Souza et al. [32] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 13. | Igbeneghu et al. [8] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 14. | Jeremiah et al. [28] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 15. | Kayode et al. [29] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 16. | Kim et al. [4] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 17. | Kimbi et al. [9] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 18. | Koltas et al. [35] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 19. | Kotepui et al. [13] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 20. | Maghendji-Nzondo et al. [44] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 21. | Maghendji-Nzondo et al. [27]] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 22. | Okafor et al. [38] | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Medium | |
| 23. | Ourives et al. [33] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 24. | Philipose CS and Umashankar T [40] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 25. | Rodrigues-da-Silva et al. [45] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 26. | Salih et al. [31] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 27. | Squire et al. [36] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 28. | Sumbele et al. [30] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
| 29. | Worku et al. [46] | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | Good |
Meta-analysis
A meta-analysis was conducted using Review Manager (RevMan) 5.3 software (Version 5.3, London, UK). Heterogeneity was assessed using Cochran’s Q (Chi-square) and Moran's I2. In cases where heterogeneity existed, meta-regression was performed using STATA software (StataCorp, USA), and subgroup analyses were conducted to explore the source(s) of heterogeneity. For studies that reported the median and range, we estimated the mean and standard deviation according to the method devised by Hozo et al. [24]. Missing standard deviations were calculated by the imputation of average standard deviations borrowed from other studies according to the method devised by Furukawa et al. [25]. Mean differences and the 95% confidence interval were the effect measures for the mean differences in the total leukocyte count among malaria and non-malaria groups and were calculated by using the generic inverse variance method and random-effects model. Moreover, mean differences in leukocyte differential count for neutrophils, lymphocytes and the neutrophil/lymphocyte ratio (NL ratio) were also analyzed.
Assessment of publication bias
Publication bias was evaluated using a funnel plot. Egger’s test was used to test for funnel plot asymmetry.
Results
General characteristics of included studies
A total of 2,261 potentially relevant articles were identified for this systematic review after duplicate citations were removed. After reviewing the title and abstract, 623 articles were selected for the full text review. Among the 623 articles, 594 were removed because they did not report the leukocyte count or because of any of the inclusion and exclusion criteria in this study. Out of 2,261 potentially relevant articles, 29 met the inclusion criteria and were subsequently included in this review (Table 1). The majority of the studies were conducted in African countries (15 of 29 studies). Seven studies were conducted in Asian countries, and six studies were conducted in South America. Eight of the articles from five African countries–Ghana [26], Gabon [7, 27], Nigeria [28, 29], Cameroon [9, 30], and Sudan [31]–reported only P. falciparum infection, while seven of the articles in Brazil [15, 32, 33], Venezuela [34], Republic of Korea [4], Turkey [35], and Ghana [36] reported only P. vivax infection. P. ovale infection was described only in three studies conducted in France [37], Thailand [6], and South Africa [38]. P. malariae infection was described only in two studies conducted in Thailand [6] and Nigeria [8]. Mixed infections of P. falciparum and P. vivax were found only in Ethiopia [5], Thailand [6], Brazil [39], and India [40], whereas mixed infections of P. vivax/P. malariae and P. falciparum/P. ovale were found in Nigeria [8] and South Africa [38], respectively. The mean age of the participants was 23±13.1 years, while the sex ratio of males/females was 1.15:1. All articles were published between 1997 and 2019 (Fig 1). Six studies reported 224 cases of severe malaria in their publications [7, 27, 30, 31, 36, 38]. Most of the severe complications in the studies were severe anemia (21.9%, 49/224), cerebral malaria (13.8%, 31/224), hyperparasitemia (9.8%, 22/224), repeated convulsions (8%, 18/224), more than one complication (3.6%, 8/224), hypotension (3.1%, 7/224), jaundice (2.7%, 6/224), hypoglycemia (1.8%, 4/224), and prostration (0.4%, 1/224). The non-malaria groups were divided into febrile and healthy groups based on the descriptions by the authors. The febrile group included patients who were suspected of having malaria, but blood parasitemia was negative by any of these techniques, including microscopy, rapid diagnostic tests (RDTs), and polymerase chain reaction (PCR). The healthy group included healthy individuals in the same endemic area as the malarial patients in their studies.
Table 1. Characteristics of the included studies.
| References | Study area (years of the survey) | Mean age of the malaria group (years) | Mean age of the non-malaria (years) | Male sex | Plasmodium sp. | Total leukocyte count (103/μL) (mean ± SD) | Severity | Source of leukocyte count | All participants | Cases of P.f | Cases of P.v | Cases of P.o | Cases of P.m | All cases | Non-malaria | Characteristics of non-malaria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adam et al., 2017 [41] | Sudan (2014–2015) | 20.7 ±19.6 | 20±19 | All groups = 50%; Malaria = 50%; Non-malaria = 50% |
P. falciparum and P. vivax | Mean leukocyte count in malaria 6.7± 0.9; -P.f 6.6±0.9; -P.v 6.9±1.3; Mean leukocyte count in non-malaria 8.1± 1.4 |
UM | Sysmex XN-9000; Hyogo, Japan |
324 | 107 | 55 | 162 | 162 | Febrile | ||
| Ansart et al., 2010 [37] | France (2002–2003) | 1.1–55 | 1.1–42 | All groups = 57%; Malaria = 57%; Non-malaria = 57% |
P. falciparum, P. vivax, and P. ovale |
Mean leukocyte count in malaria 9.25; Mean leukocyte count in non-malaria 11.2 |
NA | NA | 272 | 36 | 14 | 4 | 34 | 218 | Febrile | |
| Anwar et al., 2016 [42] | Pakistan (2015–2016) | 15–20 | 21–60 | All groups = 100%; Malaria = 100%; Non-malaria = 100% |
Did not define species | Mean leukocyte count in malaria 5.03±13.8; Mean leukocyte count in non-malaria 9.5±13.8 |
NA | NA | 65 | Species not defined 60 | 60 | 5 | Healthy | |||
| Awoke N and Arota A, 2019 [5] | Ethiopia (2016) | 27.6 | 27.6 | All groups = 70%; Malaria = 70%; Non-malaria = 70% |
P. falciparum, P. vivax, and mixed infection (4 cases) |
Mean leukocyte count in malaria 5.3±2.2 (including mixed infections); Mean neutrophil count 3.52±1.8; Mean lymphocyte count 1.17±0.65; NL ratio = 3±2.77 Mean leukocyte count in non-malaria 5.8±1.8; Mean neutrophil count 3.30±1.4; Mean lymphocyte count 1.78±0.58; NL ratio = 1.85±2.41 |
NA | CELL-DYN 1800 | 340 | 105 | 61 | - | 166 | 170 | Febrile | |
| Chaves et al., 2016 [15] | Brazil (NA) | 35.4 ±14.3 | 27.1 ±7.4 | All groups = 54.5% Malaria = 45% Non-malaria = 64% |
P. vivax | Median leukocyte count in malaria 5.7 (4.0–7.3); Mean neutrophil count 3.45±0.89; Mean lymphocyte count 1.28±0.38; NL ratio = 2.7±2.34 Median leukocyte count in non-malaria 6.7 (6.1–7.8); Mean neutrophil count 4.05±0.53; Mean lymphocyte count 2.05±0.35; NL ratio = 1.98±1.51 |
UM | Sysmex KX-21 N® | 56 | - | 36 | - | 36 | 20 | Healthy | |
| Erhart et al., 2004 [6] | Thailand (2001) | All groups = 28 | All groups = 63% |
P. falciparum, P. vivax, P. malariae, P. ovale, and mixed infections (23 cases) |
Mean leukocyte count in malaria 6.4; -P.f 6.3; -P.v 6.6 (excluding mixed infections) Mean leukocyte count in non-malaria 8.4 |
UM | Beckman-Coulter, Inc, Fullerton, CA |
2149 | 414 | 646 | 2 | 15 | 1060 | 979 | Febrile | |
| Frimpong et al., 2018 [26] | Ghana (NA) | 6.5 (4.7–8) | 9 (8–11) | All groups = 50%; Malaria = 47%; Non-malaria = 53% |
P. falciparum | Mean leukocyte count in malaria 6.4 (3.2–9.0); Median leukocyte count in non-malaria 7 (5.7–8.0) |
UM | NA | 57 | 40 | 40 | 17 | Healthy | |||
| Goncalves et al., 2010 [39] | Brazil (NA) | 35 (23.3–42.3) | 32 (28–42) | All groups = 57.1%; Malaria = 55.5%; Non-malaria = 58.7% |
P. falciparum, P. vivax, and mixed infections (14 cases) |
Mean leukocyte count in malaria 5.6±0.7; -P.f 5.3±0.8; -P.v 5.7±0.6 (excluding mixed infections); Mean leukocyte count in non-malaria 7.8±1.1 |
UM | ABX Micro 60, Horiba, Montpellier, France | 80 | 19 | 43 | 62 | 18 | Healthy | ||
| González et al., 2009 [34] | Venezuela (NA) | 3–67 | NA | All groups = 64.4; Malaria = 64.4%; Non-malaria = NA |
P. vivax | Mean leukocyte count in malaria 7,01±2,34; Mean neutrophil count 4.96±2.0; Mean lymphocyte count 2.98±1.28; NL ratio = 1.66±1.56 Mean leukocyte count in non-malaria 8,01 ± 2,03; Mean neutrophil count 4.27±1.75 Mean lymphocyte count 4.89±1.41; NL ratio = 0.96±1.24 |
NA | NA | 69 | 59 | 59 | 30 | Healthy | |||
| Hänscheid et al., 2008 [7] | Gabon (2003–2004) | 3.7 | 0.6 | All cases = 46% |
P. falciparum | Mean leukocyte count in malaria 8.7±9.6; Mean leukocyte count in severe malaria 10±3.92; Mean leukocyte count in uncomplicated malaria 8.1±2.6; Mean neutrophil count 3.8±1.49; Mean lymphocyte count 3±1.73; NL ratio = 1.27±1.1; Mean leukocyte count in non-malaria 9.5±1.2; Mean neutrophil count 2.7±1.11; Mean lymphocyte count 5.5±1.42; NL ratio = 0.49±0.78 |
UM = 104 SM = 48 SA = 15 HP = 13 HG = 3 CM = 17 |
Cell- Dyn 3000® (CD3000) instrument (Abbott, Santa Clara, California |
368 | 152 | 30 | 216 | Febrile | |||
| Hasona et al., 2016 [43] | Saudi Arabia (2014–2015) | All groups = 20–60 | NA | P. falciparum and P. vivax | Mean leukocyte count in malaria - P.f. 4.21 ± 0.35; - P.v. 3.45 ± 0.12; Mean neutrophil count 2.9±0.04; Mean lymphocyte count 2.63±0.02; NL ratio = 1.1±2.0; Mean leukocyte count in non-malaria 6.94 ± 0.13; Mean neutrophil count 3.47±0.03; Mean lymphocyte count 2.78±0.02; NL ratio = 1.25±1.5 |
NA | SYSMX.KX-21n | 120 | 6 | 24 | 20 | 90 | Healthy | |||
| Hojo-Souza et al., 2015 [32] | Brazil (NA) | 38.5 (19–61) | 34.0 (22–37) | All groups = 48.7; Malaria = 27.3%; Non-malaria = 70% |
P. vivax | Mean leukocyte count in malaria 5.5± 0.4; Mean neutrophil count 3.69±2.58; Mean lymphocyte count 1.51±1.23; NL ratio = 2.44±2.1; Mean leukocyte count in non-malaria 8.1 ± 0.5; Mean neutrophil count 5.27± 3.97; Mean lymphocyte count 2.65±2.22; NL ratio = 1.99±1.79 |
UM | ABX Pentra 90; Horiba Diagnostics, Kyoto, Japan |
31 | 20 | 152 | 11 | Healthy | |||
| Igbeneghu et al., 2011 [8] | Nigeria (NA) | 31.9± 11.1 | 34.0± 12.1 | All groups = 80.9; Malaria = 90.8%; Non-malaria = 70.7% |
P. vivax, P. malariae, and mixed infections (3 cases) | Mean leukocyte count in malaria 4.68±1.4; Mean leukocyte count in non-malaria 5.38±2.1 |
UM | Coulter counter (STKS model) | 668 | 136 | 2 | 138 | 527 | Healthy | ||
| Jeremiah et al., 2007 [28] | Nigeria (2005–2006) | All groups = 1–8 | All groups = 48.8% |
P. falciparum | Mean leukocyte count in malaria 5.4±2.3; Mean leukocyte count in non-malaria 5.3±2.3 |
UM | Turk’s method | 240 | 66 | 66 | 174 | Healthy | ||||
| Kayode et al., 2011 [29] | Nigeria (2010–2011) | All groups = 14–30 | NA | P. falciparum | Mean leukocyte count in malaria 6.5±0.1; Mean neutrophil count 4.24±0.02; Mean lymphocyte count 2.26±0.02; NL ratio = 1.88±1.0; Mean leukocyte count in non-malaria 5.1±0.2; Mean neutrophil count 1.76±0.09; Mean lymphocyte count 3.93±0.06; NL ratio = 0.45±1.5 |
NA | WBC diluting fluid | 40 | 30 | 30 | 10 | Healthy | ||||
| Kim et al., 2008 [4] | Republic of Korea (2000–2006) | 26.1±11.1 | 24.5±3.7 | All groups = 79.4; Malaria = 81.8%; Non-malaria = 76.9% |
P. vivax | Mean leukocyte count in malaria 4.9±1.4; Mean leukocyte count in non-malaria 5.9±1.4 |
UM | Cell-Dyn 4000, Abbott diagnostics, USA | 141 | 55 | 55 | 52 | Healthy | |||
| Kimbi et al., 2013 [9] | Cameroon (2011) | All groups = 8.26±2.2 | All groups = 47.8% |
P. falciparum | Mean leukocyte count in malaria 5.1 ±2.5; Mean leukocyte count in non-malaria 6.3± 1.9 |
UM | Beckman Coulter counter (URIT 3000) | 728 | 158 | 158 | 570 | Febrile and healthy | ||||
| Koltas et al., 2007 [35] | Turkey (2002–2004) | 33.8±18.6 | 39±15 | All groups = 61.5% |
P. vivax | Mean leukocyte count in malaria 6.2 ±1.9; Mean leukocyte count in non-malaria 7.6± 2.2 |
NA | NA | 142 | 90 | 90 | 52 | Healthy | |||
| Kotepui et al., 2014 [13] | Thailand (2009) | 24.5 (17–38) | 16 (7–35) | All groups = 55.7%; Malaria = 60.3%; Non-malaria = 51.1% |
P. falciparum and P. vivax | Mean leukocyte count in malaria 5.9±0.9; -P.f 6.0±1.0; -P.v 5.7±0.8; Mean neutrophil count 3.71±0.66; Mean lymphocyte count 1.35±0.37; NL ratio = 2.75±1.78; Mean leukocyte count in non-malaria 9.0±1.8; Mean neutrophil count 5.36±1.41; Mean lymphocyte count 2.39±0.57; NL ratio = 2.24±2.47 |
NA | BC-5200 Haematology Analyzer (Mindray, Nanshan, Shenzhen, China |
4985 | 352 | 351 | 703 | 4282 | Febrile | ||
| Maghendji-Nzondo et al., 2016 [44] | Gabon (2013–2014) | 51.6±39.2 | 45.2±39 | All groups = 52.3%; Malaria = 50%; Non-malaria = 54.5% |
P. falciparum | Mean leukocyte count in malaria 5.6±4.1; Mean leukocyte count in non-malaria 11.4± 7.5 |
NA | Coulter STKS (STKS®, Coulter Corp, USA). |
1129 | 530 | 530 | 1079 | Febrile | |||
| Maghendji-Nzondo et al., 2016 [27] | Gabon (2011–2012) | 63.4 ± 39.4 | 40.3 ± 37.1 | All groups = 47%; Malaria = 50%; Non-malaria = 44% |
P. falciparum and P. malariae |
Mean leukocyte count in malaria 8.6 ± 6.4 Mean leukocyte count in non-malaria 10.8 ± 6.3 |
UM = 145 SM = 17 SA = 12 CM = 4 PT = 1 |
Coulter STKS (STKS®, Coulter Corp, USA). |
940 | 158 | 4 | 162 | 778 | Febrile | ||
| Okafor et al., 2016 [38] | South Africa (2012–2013) | NA | All groups = = 61.8% |
P. falciparum and mixed infections (6 cases) |
Mean leukocyte count in malaria 4.1 ± 0.46 (including mixed infections); Mean leukocyte count in non-malaria 5.6 ± 1.89 |
UM = 82 SM = 10 SA = 10 |
Sysmex XE 5000 Automated Haematology Analyser, (Sysmex, Canada) |
92 | 6 | 6 | 86 | Febrile | ||||
| Ourives et al., 2015 [33] | Brazil (NA) | >18 | 35–55 | All groups = = 44%; Malaria = NA; Non-malaria = 44% |
P. vivax | Mean leukocyte count in malaria 7.6; Mean leukocyte count in control 6.0 |
NA | ABX PENTRA 90, (Horiba Diagnostic, Kyoto, Japan) | 173 | 148 | 148 | 25 | Healthy | |||
| Philipose CS and Umashankar T, 2016 [40] | India (2014) | 36.1±17.1 | 48.3±19.2 | NA | P. falciparum, P. vivax and mixed infections (18 cases) | Mean leukocyte count in malaria 6.3±3.1 (including mixed infections); Mean neutrophil count 3.96±2.98; Mean lymphocyte count 1.59±1.23; NL ratio = 2.49±2.42; Mean leukocyte count in control 9.5±5.7; Mean neutrophil count 6.56±5.52; Mean lymphocyte count 1.98±1.26; NL ratio = 3.33±4.38 |
NA | Beckmann Coulter® hematological analyzer |
300 | 180 | 2 | 182 | 100 | Febrile | ||
| Rodrigues-da-Silva et al., 2014 [45] | Brazil (2010) | All groups = 28.3 (22.5–40) | All groups = = 27%; Malaria = 27%; Non-malaria = NA |
P. falciparum and P. vivax | Mean leukocyte count in malaria 5.1±0.8; - P.f = 4.9±0.8; - P.v = 5.2±0.8; Mean neutrophil count 3.35±0.63; Mean lymphocyte count 2.1±0.31; NL ratio = 1.6±2.2; Mean leukocyte count in controls; 6.5±0.6 Mean neutrophil count 3.85±0.5; Mean lymphocyte count 2.24±0.29; NL ratio = 1.72±1.72 |
UM | ABX PENTRA 90, (Horiba Diagnostic, Kyoto, Japan) | 83 | 24 | 47 | 71 | 12 | Healthy | |||
| Salih et al., 2018 [31] | Sudan (2015) | 5.3±3.9 | 5.7±3 | All groups = 59.4% |
P. falciparum | Median leukocyte count in malaria 7.4 (5.2−9.5); Mean leukocyte count in severe malaria 8.98±2.06; Mean leukocyte count in uncomplicated malaria 7.33±1.09; Mean neutrophil count 4.63±1.17; Mean lymphocyte count 2.45±0.72 NL ratio = 1.89±1.63; Median leukocyte count in control 9.1 (5.3−12.4); Mean neutrophil count 3.45±1.15; Mean lymphocyte count 3.8±0.89; NL ratio = 0.91±1.29 |
UM = 63 SM = 67 CM = 10 CV = 18 SA = 9 HG = 1 HT = 7 JD = 6 HP = 9 MT1 = 8 |
Sysmex XN-9000; Hyogo, Japan | 180 | 130 | 130 | 50 | Healthy | |||
| Squire et al., 2016 [36] | Ghana (2012–2013) | 4.9 (3.7–6.4) | 3.96 (3.0–4.9) | All groups = 57.7%; Malaria = 64%; Non-malaria = 51.4% |
P. vivax | Mean leukocyte count in malaria 9.5±1.6; Mean leukocyte count in severe malaria 8.91±0.8; Mean leukocyte count in uncomplicated malaria 10.1±2.8; Mean leukocyte count in control 8.9±0.9 |
UM = 24 SM = 81 SA = 2 |
Sysmex KX- 21 N, Japan |
150 | 105 | 105 | 45 | Febrile | |||
| Sumbele et al., 2017 [30] | Cameroon (2014) | All groups = 25.5 | All groups = 49.6% | P. falciparum | Mean leukocyte count in malaria 8.9±3.3; Mean leukocyte count in control 8.4±3.4 |
UM = 124 SM = 1 SA = 1 |
URIT-3300 Automated Hematology Analyzer (Guilin Botest Medical Electronic Co. Ltd, PR China) |
387 | 125 | 125 | 262 | Febrile | ||||
| Worku et al., 1997 [46] | Ethiopia (NA) | All groups = 27 (22–32) | All groups = 79.5% | P. falciparum and P. vivax | Mean leukocyte count in malaria 5.5±0.7; - P.f. = 4.9±0.6; - P.v. = 6.0±0.8; Mean leukocyte count in control -7.4±1.3 |
NA | NA | 55 | 19 | 20 | 39 | 16 | Healthy | |||
UM = uncomplicated malaria, SM = severe malaria, SA = severe anemia, HP = hyperparasitemia, HG = hypoglycemia, CM = cerebral malaria, PT = prostration, HT = hypotension, CV = convulsion, JD = jaundice, MT1 = more than 1 complication.
Fig 1. PRISMA diagram.

Flow chart for study selection.
Malaria indicators
Twenty included studies (20/29, 69%) used only the microscopic method for detection of malaria parasites. Five studies used both microscopy and PCR [15, 32, 34, 39, 46]. Two studies used both microscopy and RDT [35, 38]. Two studies used three methods for malaria detection including microscopy, RDT, and PCR methods (S2 Table).
Leukocyte indicators
Most of the leukocyte counts in the 29 studies were obtained using hematology analyzers from different manufacturers. Six studies used a Sysmex [15, 31, 36, 38, 41, 43]. Three studies used a Cell-Dyn [4, 5, 7]. Three studies used an ABX [32, 39, 45]. Three studies used a Beckman-Coulter [6, 9, 40]. Two studies used a Coulter STKS [27, 44]. Other studies used a Coulter counter [8], BC-5200 Haematology Analyzer [13], and URIT-3300 [30]. Two studies used Turk’s method, which is a traditional protocol for leukocyte counting using a hemocytometer [28, 29].
Quality of included studies
All 29 studies included in the present study were rated with a score according to the NOS guidelines. Overall, twenty-eight studies were rated “good” with a maximum of 9 stars, and one study was rated “medium” with 8 stars because there was no clear definition of the non-malaria group reported by the authors. The rating details are provided in Table 2.
Meta-analysis
A meta-analysis of the leukocyte count in the malaria and non-malaria groups was conducted to examine the statistical significance and mean difference across the 29 studies. The analysis demonstrated that there were significantly lower leukocyte counts in patients in the malaria group than in those in the non-malaria group (Z = 4.0, P-value < 0.00001, mean difference = -1.38, 95% CI = -2.06-(-0.71)). Only 2 of the 29 included studies presented a significantly higher total leukocyte count in the malaria group than in the non-malaria group [29, 33] (Fig 2). Five out of twenty-nine studies presented total leukocyte counts that were not significantly different in the malaria group and the non-malaria group [7, 26, 28, 30, 42].
Fig 2. Forest plot of the total leukocyte count among included studies.
Forest plot showing the total leukocyte count in the malaria and non-malaria groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Data heterogeneity
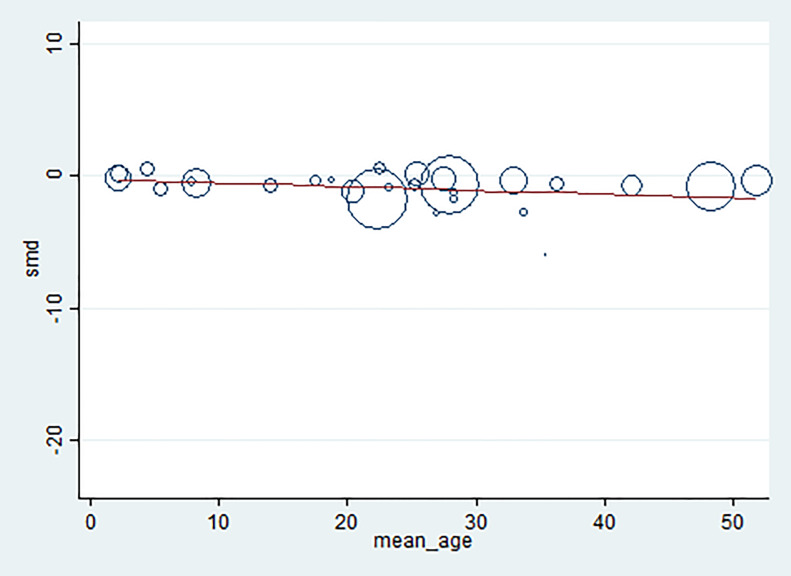
The present study used a forest plot, heterogeneity test (Cochran’s Q), and the degree of heterogeneity (I2) to test whether the included studies had heterogeneity. The results from the forest plot indicated that the standard deviations overlapped among the included studies. Cochran’s Q indicated that the results were significant (P-value < 0.000001, Chi2 = 4794.7, df = 28, Tau2 = 3.23) with an I2 of 99%. A meta-regression with mean age as a covariate was performed to determine if age was the source of heterogeneity or whether it modified the outcome. Meta-regression using STATA software indicated that mean age was not the source of heterogeneity and it did not modify the outcome (P-value = 0.48, percent of residual variation (I2) = 97.9% (Table 3, Fig 3).
Table 3. Meta-regression analysis of mean age.
| SMD | Coefficient | Standard error | t-statistic | P-value | 95% CI |
|---|---|---|---|---|---|
| Mean age | -0.03 | 0.04 | -0.72 | 0.48 | -0.11–0.05 |
| Constant | -0.25 | 1.02 | -0.25 | 0.81 | -2.35–1.84 |
* SMD: The standardized mean difference.
Fig 3. Meta-regression graph of mean age as a covariate.
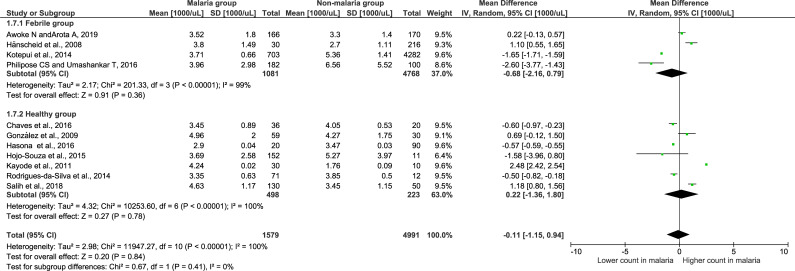
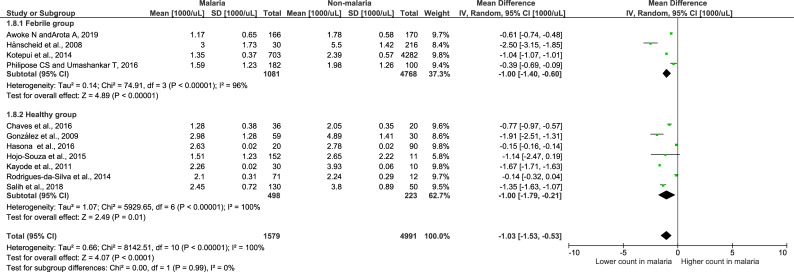
Meta-analysis of leukocyte differential counts
Meta-analysis of leukocyte differential counts was available in 11 studies and was also analyzed. For neutrophil counts, the meta-analysis showed no difference in neutrophil counts between the malaria and non-malaria groups (P value = 0.84, mean difference = -0.11, 95% CI = -1.15–0.94) (Fig 4). For lymphocyte counts, the results showed that the malaria group had a significantly lower lymphocyte count than the non-malaria group (P value <0.0001, mean difference = -1.03, 95% CI = -1.53-(-0.53)) (Fig 5). For the NLR, the results showed that the NLR was higher in the malaria group than in the non-malaria group (P value <0.0001, mean difference = 0.6, 95% CI = 0.32–0.88) (Fig 6). The difference of leukocyte count between severe and non-severe malaria group was also analyzed. The results demonstrated no significant difference of the total leukocyte count between severe and non-severe group (P value = 0.37).
Fig 4. Forest plot of the neutrophil count among included studies.
Forest plot showing the neutrophil count in the malaria and non-malaria groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Fig 5. Forest plot of the lymphocyte count among included studies.
Forest plot showing the lymphocyte count in the malaria and non-malaria groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Fig 6. Forest plot of the NL ratio among included studies.
Forest plot showing the NL ratio in the malaria and non-malaria groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
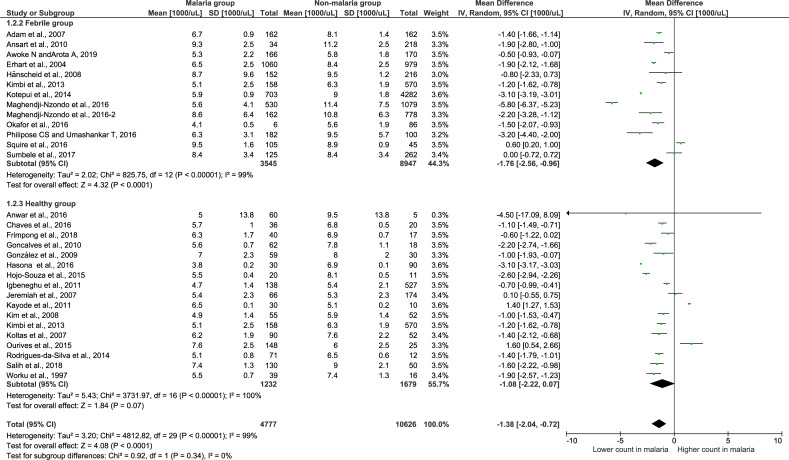
Subgroup analysis of the febrile and healthy groups
To determine whether using febrile and healthy controls impacts the differences in total leukocyte counts, a subgroup analysis of the non-malaria groups was conducted (Fig 7). The results showed that 13 studies using febrile controls demonstrated a significantly lower leukocyte count in the malaria group than in the non-malaria group (Z = 1.33, P-value < 0.0001, mean difference = -1.76, 95% CI = -2.56-(-0.96), I2 = 99%). Interestingly, seventeen studies using healthy participants as a control group demonstrated no significance in the leukocyte count among the malaria and healthy groups (Z = 1.33, P-value = 0.07, mean difference = -1.07, 95% CI = -2.22–0.07, I2 = 100%). The subgroup analysis showed no significant difference between subgroups (P-value = 0.34), demonstrating that the subgroup (febrile or healthy) was not the source of the heterogeneity or that it could not explain the heterogeneity among the 29 included studies.
Fig 7. Subgroup analysis of the total leukocyte count.
Forest plot of the subgroup analysis showing the total leukocyte count in malaria compared with the febrile and healthy groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Analysis of P. falciparum using febrile and healthy groups
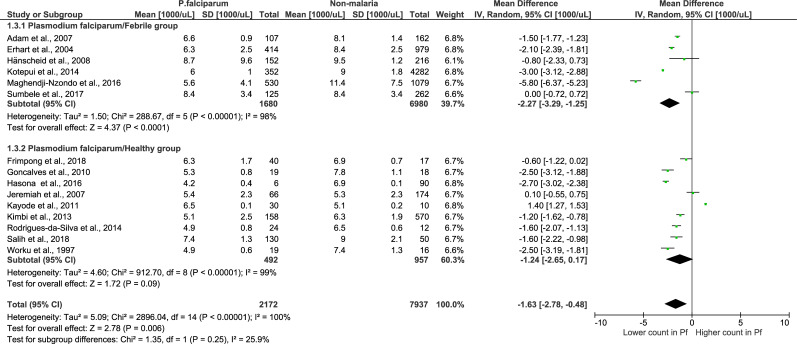
When using febrile groups, the analysis of 6 studies with P. falciparum monoinfection demonstrated that there was a significantly lower total leukocyte count in the P. falciparum monoinfection group than in the febrile group (Z = 4.37, P-value < 0.0001, mean difference = -2.27, 95% CI = -3.29-(-1.25), I2 = 98%) (Fig 8). Whereas, in studies using healthy groups, the analysis of 9 studies with P. falciparum monoinfection demonstrated that there was no significant difference in leukocyte counts in the P. falciparum monoinfection group and the healthy group (Z = 1.72, P-value = 0.09, mean difference = -1.24, 95% CI = -2.65–0.17, I2 = 99%). The subgroup analysis showed that there was no significant difference between subgroups (P-value = 0.25), demonstrating that the subgroup (febrile or healthy) was not the source of the heterogeneity or that it could not explain the heterogeneity among the 9 included studies.
Fig 8. Subgroup analysis of P. falciparum.
Forest plot of subgroup analysis showing the total leukocyte count in the P. falciparum group compared to the febrile and healthy groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Analysis of P. vivax using febrile and healthy controls
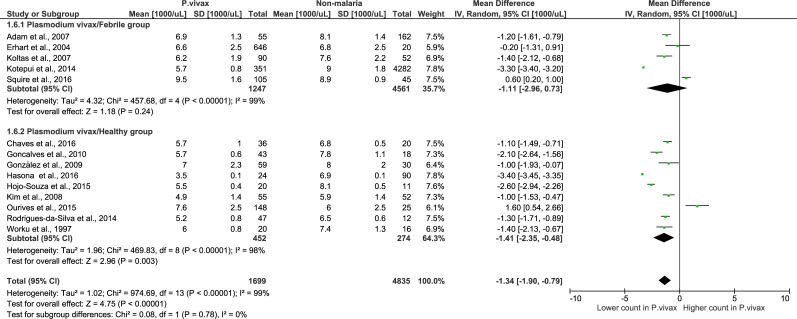
For P. vivax monoinfection, when using a febrile group, the analysis using 5 studies with P. vivax monoinfection demonstrated that there was no significant difference in total leukocyte counts in the P. vivax monoinfection group compared to the febrile group (Z = 1.18, P-value = 0.24, mean difference = -1.11, 95% CI = -2.96–0.73, I2 = 99%). When using the healthy group as a control, the analysis using 9 studies with P. vivax monoinfection demonstrated that there was a significantly lower total leukocyte count in the P. vivax monoinfection group than in the healthy group (Z = 2.96, P-value = 0.003, mean difference = -1.14, 95% CI = -2.35–0.48, I2 = 98%). The subgroup analysis showed that there was no significant difference between the subgroups (P-value = = 0.78) (Fig 9), demonstrating that the subgroup (febrile or healthy) was not the source of the heterogeneity or that it could not explain the heterogeneity among the 5 included studies.
Fig 9. Subgroup analysis of P. vivax.
Forest plot of subgroup analysis showing the total leukocyte count in P. vivax compared to febrile and healthy groups. [1000/μL] refers to 1000 per microliter; “IV" in "IV, Random” refers to Inverse variance; "Total" in top row refers to number of patients included; A green square in the horizontal line refers to the mean difference for each of included study.
Publication bias
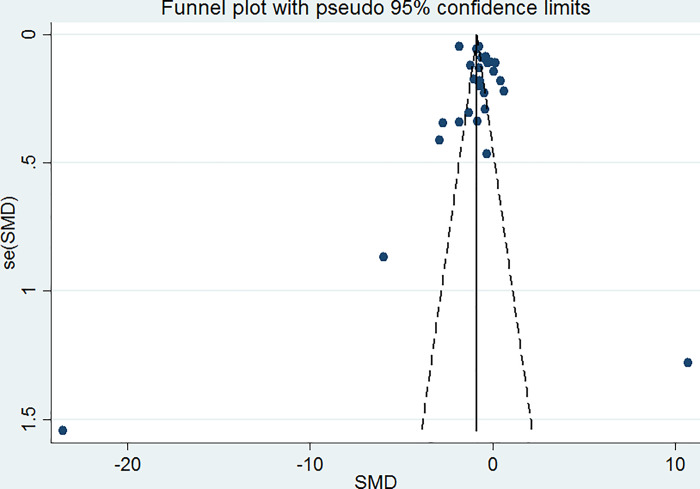
Funnel plot analysis generated a symmetrical funnel plot (Fig 10). The symmetry of the funnel plot was assessed by Egger’s test, which indicated that no small-study effects were found by using linear regression analysis (P-value = 0.497, slope coefficient = -0.99). The symmetrical funnel plot indicated that there was no publication bias.
Fig 10. Funnel plot.
Funnel plot showing publication bias among the included studies.
Discussion
This systematic review and meta-analysis described the pooled mean difference of the total leukocyte count of our study. The results demonstrated that there was a lower total leukocyte count in the malaria group than in the non-malaria groups. The lower total leukocyte count in this meta-analysis might be explained by the localization of leukocytes away from the peripheral circulation, such as in the spleen, at the sites of infection, or in other peripheral pools, resulting in a low number of total leukocytes detected in the circulation [12, 47]. A previous study suggested that the alteration of immune cells in the peripheral blood was also the cause of leukopenia [48]. Immunity against malaria parasite invasion and the infection of red blood cells is very high during the liver stage or exoerythrocytic stage compared to the erythrocytic stage, and major immune responses for these two stages involve CD8+ T cells and antibodies, respectively [49]. Another possible immune mechanism against malaria infection involves interleukin 12 (IL-12), which is involved in the pathogenesis of malarial pancytopenia, the pathogenesis of low total leukocytes, red blood cells, and platelet production from the bone marrow [50]. One study suggested that the glycosylphosphatidylinositol antigen of malaria induces monocyte and macrophage activation, resulting in the release of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1α, and the phagocytosis of both infected red blood cells and leukocytes [51]. Several studies have reported that TNF, IL-12, IL-10, and other cytokines can suppress the production of leukocytes from bone marrow by inhibiting hemopoietic growth factors or stimulating macrophages to release cytotoxic chemicals, causing damage to hemopoietic cells [52–55].
The differences in leukocyte differential counts were also assessed in the present study. Among the 11 included studies that reported differential counts, the results demonstrated that the absolute lymphocyte count and NLR were significantly altered during malaria infection. The absolute lymphocyte counts of the malaria group were low, and the NLR was high. These results were consistent with our previous study demonstrating that neutrophil and lymphocyte counts were the most important leukocytic changes associated with malaria infection [13]. Our previous study also demonstrated that there was a significantly higher NLR in the malaria-infected group than in the noninfected group [13]. Moreover, the NLR was found to be correlated with malaria parasitemia, and it was inferior to CRP as a marker for severe imported malaria [56]. The reduction in lymphocyte counts in the present study may be due to the redistribution of lymphocytes, with sequestration in the spleen of malaria-infected patients [50].
Heterogeneity was observed in the 29 articles included in the present study. Although we used covariates or confounders such as age groups and subgroup analyses of Plasmodium species and febrile/healthy groups, they did not reduce the degree of heterogeneity (I2) in the included studies. Regarding the test for publication bias by Egger’s test (which might determine publication bias as the source of heterogeneity), no publication bias was found among the 29 studies. It could be concluded that publication bias was not the underlying cause of data heterogeneity in the articles included in the present study.
In our systematic review and meta-analysis, the non-malaria groups included both febrile patients who were negative for malaria parasites and healthy individuals who were located in the same areas as the malaria group. The results demonstrated that there was a lower total leukocyte count in the malaria group than in the non-malaria group. However, a subgroup analysis of the febrile and healthy groups indicated that there was a significantly lower total leukocyte count in the malaria group than in the febrile group, while a subgroup analysis of the seventeen studies using the healthy group as a control demonstrated that there was no significant decrease in the total leukocyte count in the malaria group. The total leukocyte count in the malaria group was significantly different from that in the febrile group. Therefore, it is crucial for researchers to report the total leukocyte count in malaria patients compared to that in febrile control individuals rather than compared with healthy individuals; a low leukocyte count is likely to be a useful indicator to aid in malaria diagnosis in malaria-endemic areas.
To confirm whether each Plasmodium species differentially impacted the total leukocyte count, a subgroup analysis of the total leukocyte count in each Plasmodium spp. was conducted. The mean difference indicated that there was a significant reduction in the total leukocyte count in patients with P. falciparum and P. vivax monoinfection compared to that in patients in the non-malaria group. Compared with the febrile group, the P. falciparum monoinfection group had a lower total leukocyte count, while the P. vivax monoinfection group showed no difference in the total leukocyte count. Compared with the healthy group, the P. falciparum monoinfection group did not show a difference in the total leukocyte count, while the P. vivax monoinfection group had a lower total leukocyte count. We did not analyze the difference in the total leukocyte count between P. falciparum and P. vivax because the included studies did not report total leukocyte counts separately for each species, but some previous studies indicated that there was a significantly lower total leukocyte count in patients with P. falciparum monoinfection than in those with P. vivax monoinfection [6, 39, 45, 46].
The meta-analysis demonstrated that two studies in Nigeria (2010–2011) and Brazil [29, 33] presented an increase in the total leukocyte count in malaria patients compared to non-malaria patients. The study by Kayode et al. suggested that the increased total leukocyte count was due to the increased mobilization of leukocytes from the bone marrow to the bloodstream to fight against malarial parasites [29]. Another study indicated that there was no significant difference in the total leukocyte count [33]. Both studies used healthy control groups for the comparison of the total leukocyte count in the malaria group. Interestingly, the latter study had results consistent with the present study, indicating that there was no significant difference in the total leukocyte count in malaria and healthy groups [33]. All of these reports did not indicate the number of days of fever before admission. Our previous study indicated that there were lower neutrophil/monocyte and higher lymphocyte counts when comparing patients with fever ≤ 3 days and patients with fever > 3 days (P-value < 0.05). However, the total leukocyte count did not change during the 3 days before admission [57]. The association of the number of days of fever with the leukocyte count might be explained by a study among native Dutch volunteers who were bitten by infected mosquitoes in a Controlled Human Malaria Infection (CHMI) model, which demonstrated an increase in the peripheral total leukocyte count during malarial infection in the liver stage; however, a subsequent decrease occurred when the parasites appeared in the peripheral blood [10]. The mechanism of leukocytosis might involve the infection by malaria parasites in the bone marrow (which would inhibit the release of leukocytes), increases in proinflammatory cytokines in the peripheral blood, or febrile paroxysms [50].
A study among children in Africa indicated that a high leukocyte count was associated with a great risk for death [17]. The cause of death might be delayed diagnosis, as a previous study indicated that there was an initial increase in peripheral total leukocyte count during liver-stage infection and a significantly lower total leukocyte count during the blood stage [10]. Here, we present gathered data that support evidence of total leukocyte count as an early detection tool to screen malarial infection in conjunction with other malarial confirmation tests, such as in routine malaria diagnosis in malaria-endemic areas. Further, these meta-analytic results provide clinicians with a rationale for instituting specific initiatives and early interventions in malaria-endemic areas, which may decrease mortality and provide less complicated and possibly more economical management of potential Plasmodium-related patients, even before the actual confirmation of Plasmodium infection. A higher total leukocyte count might indicate the initial stage of malarial infection in the liver, while a low total leukocyte count could be an indicator of the progression of malarial infection during the blood stage.
Summary of evidence
In summary, our recommendations for further work include using febrile individuals as a control group to provide data on the differences in the total leukocyte count between the malaria group and non-malaria groups. This would allow for a precise understanding of malarial infection-related outcomes and could identify important predictors of the prognosis of malaria patients. The standardization of both malaria detection techniques and total leukocyte indicators would ensure improved comparability between studies as well as a common approach for reporting results, which should minimize heterogeneity and allow the calculation of precise effect measures in a model.
Limitations
The present study had several limitations. First, the heterogeneity, which was not due to publication bias and other confounders that were already tested in this meta-regression analysis, was a limitation. Some of the published data could not be retrieved because the full-text manuscripts were not available. Second, specific leukocyte changes might vary with the level of malaria endemicity, background hemoglobinopathy, nutritional status, demographic factors, and malaria immunity [58], which could not be ruled out from this study based on the exclusion criteria. Third, two included studies measured the total leukocyte count using manual methods [28, 29], which might result in unreliable total leukocyte counts during meta-analysis and impact the mean difference. These two studies demonstrated that there was a higher mean leukocyte count in the malaria group than in the non-malaria groups. Fourth, the results of the present study need to be carefully interpreted, as the specific diagnoses in the febrile groups were not reported in the included studies. Such information may be highly relevant, such as bacterial or viral diseases that are likely to cause leukocytosis or dengue, which is likely to cause leukopenia.
Conclusion
The present systematic review demonstrated that the total leukocyte count was a hematological parameter that is affected by malarial infection before treatment. Despite the heterogeneity of the included studies, a significant reduction in the total leukocyte count in malaria patients is a concern. We recommend including febrile individuals in the non-malaria group to provide data on the differences in total leukocyte counts in the malaria groups and non-malaria groups. Future studies must aim to understand the effects of Plasmodium infection on each specific leukocyte subpopulation, as well as interventions that cause total leukocyte reductions during follow-up or post-treatment outcomes in malaria-endemic settings.
Supporting information
(DOC)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the authors of all of the published studies that contributed to the data included in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was partially supported by the new strategic research (P2P) project, Walailak University, Thailand. The funders had a role in the collection, analysis, and interpretation of the data. There was no additional external funding received for this study.
References
- 1.WHO. World malaria report 2019 [Available from: https://www.who.int/publications-detail/world-malaria-report-2019
- 2.WHO. Guidelines for the treatment of malaria2010.
- 3.Warrell DA. Clinical features of malaria. London: A Hodder Arnold Publication; 1993. [Google Scholar]
- 4.Kim JS, Oh JS, Chang EA, Bae SY, Nam DH, Lee CH, et al. Alteration of platelet counts and lipid profiles after treatment of acute Plasmodium vivax. Acta Tropica. 2008;106(1):39–43. 10.1016/j.actatropica.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 5.Awoke N, Arota A. Profiles of hematological parameters in plasmodium falciparum and plasmodium vivax malaria patients attending tercha general hospital, Dawuro zone, south Ethiopia. Infection and Drug Resistance. 2019;12:521–7. 10.2147/IDR.S184489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, et al. Hematologic and clinical indices of malaria in a semi-immune population of Western Thailand. American Journal of Tropical Medicine and Hygiene. 2004;70(1):8–14. [PubMed] [Google Scholar]
- 7.Hänscheid T, Längin M, Lell B, Pötschke M, Oyakhirome S, Kremsner PG, et al. Full blood count and haemozoin-containing leukocytes in children with malaria: Diagnostic value and association with disease severity. Malaria Journal. 2008;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igbeneghu C, Odaibo AB, Olaleye DO. Impact of asymptomatic malaria on some hematological parameters in the iwo community in southwestern nigeria. Medical Principles and Practice. 2011;20(5):459–63. 10.1159/000327673 [DOI] [PubMed] [Google Scholar]
- 9.Kimbi HK, Sumbele IU, Nweboh M, Anchang-Kimbi JK, Lum E, Nana Y, et al. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: A cross-sectional study. Malaria Journal. 2013;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Wolfswinkel ME, Langenberg MCC, Wammes LJ, Sauerwein RW, Koelewijn R, Hermsen CC, et al. Changes in total and differential leukocyte counts during the clinically silent liver phase in a controlled human malaria infection in malaria-naive Dutch volunteers. Malar J. 2017;16(1):457 10.1186/s12936-017-2108-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor WR, Widjaja H, Basri H, Ohrt C, Taufik T, Tjitra E, et al. Changes in the total leukocyte and platelet counts in Papuan and non Papuan adults from northeast Papua infected with acute Plasmodium vivax or uncomplicated Plasmodium falciparum malaria. Malar J. 2008;7:259 10.1186/1475-2875-7-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, et al. White blood cell counts and malaria. J Infect Dis. 2005;192(2):323–30. 10.1086/431152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand-Myanmar border. Malaria Journal. 2014;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kini RG, Chandrashekhar J. Parasite and the Circulating Pool- Characterisation of Leukocyte Number and Morphology in Malaria. J Clin Diagn Res. 2016;10(5):EC44–8. 10.7860/JCDR/2016/16425.7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaves YO, Da Costa AG, Pereira MLM, De Lacerda MVG, Coelho-Dos-Reis JG, Martins-Filho OA, et al. Immune response pattern in recurrent Plasmodium vivax malaria. Malaria Journal. 2016;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modiano D, Sirima BS, Konate A, Sanou I, Sawadogo A. Leucocytosis in severe malaria. Trans R Soc Trop Med Hyg. 2001;95(2):175–6. 10.1016/s0035-9203(01)90152-x [DOI] [PubMed] [Google Scholar]
- 17.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119(3):839–47. 10.1046/j.1365-2141.2002.03904.x [DOI] [PubMed] [Google Scholar]
- 18.Tangpukdee N, Yew HS, Krudsood S, Punyapradit N, Somwong W, Looareesuwan S, et al. Dynamic changes in white blood cell counts in uncomplicated Plasmodium falciparum and P. vivax malaria. Parasitol Int. 2008;57(4):490–4. 10.1016/j.parint.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 20.Rzepczyk CM, Stamatiou S, Anderson K, Stowers A, Cheng Q, Saul A, et al. Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to gamma delta T cells. Scand J Immunol. 1996;43(2):219–27. 10.1046/j.1365-3083.1996.d01-24.x [DOI] [PubMed] [Google Scholar]
- 21.Church LW, Le TP, Bryan JP, Gordon DM, Edelman R, Fries L, et al. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis. 1997;175(4):915–20. 10.1086/513990 [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Canada: Ottawa Hospital Research Institut; [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59(1):7–10. 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Frimpong A, Kusi KA, Tornyigah B, Ofori MF, Ndifon W. Characterization of T cell activation and regulation in children with asymptomatic Plasmodium falciparum infection. Malaria Journal. 2018;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maghendji-Nzondo S, Nzoughe H, Lemamy GJ, Kouna LC, Pegha-Moukandja I, Lekoulou F, et al. Prevalence of malaria, prevention measures, and main clinical features in febrile children admitted to the Franceville Regional Hospital, Gabon. Parasite. 2016;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeremiah ZA, Uko EK, Buseri FI, Jeremiah TA. Malarial iron-deficiency anaemia among asymptomatic Nigerian children. Journal of Nutritional and Environmental Medicine. 2007;16(3–4):232–41. [Google Scholar]
- 29.Kayode OT, Kayode AAA, Awonuga OO. Status of selected hematological and biochemical parameters in malaria and malaria-typhoid co-infection. Journal of Biological Sciences. 2011;11(5):367–73. [Google Scholar]
- 30.Sumbele IUN, Nkemnji GB, Kimbi HK. Soil-transmitted helminths and plasmodium falciparum malaria among individuals living in different agroecosystems in two rural communities in the mount Cameroon area: A cross-sectional study. Infectious Diseases of Poverty. 2017;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salih MM, Eltahir HG, Abdallah TM, Elmahdi T, Khamis AH, Malik EM, et al. Haematological parameters, haemozoin-containing leukocytes in Sudanese children with severe plasmodium falciparum malaria. Journal of Infection in Developing Countries. 2018;12(4):273–8. 10.3855/jidc.9906 [DOI] [PubMed] [Google Scholar]
- 32.Hojo-Souza NS, Pereira DB, Mendes TAO, Passos LSA, Gazzinelli-Guimarães AC, Gazzinelli-Guimarães PH, et al. CD4 + T cells apoptosis in Plasmodium vivax infection is mediated by activation of both intrinsic and extrinsic pathways. Malaria Journal. 2015;14(1). 10.1186/s12936-014-0529-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ourives SS, Dos Santos DSA, Além LR, Rios-Santos F, Fontes C, Damazo AS. Analysis of parasitological and haematological parameters and of CD4+ and CD8+ cell number in patients with Plasmodium vivax malaria. Research Journal of Parasitology. 2015;10(1):1–14. [Google Scholar]
- 34.González B, Rodulfo H, De Donato M, Berrizbeitia M, Gómez C, González L. Hematologic variations in patient with malaria caused by Plasmodium vivax before, during and after treatment. Investigacion Clinica. 2009;50(2):187–201. [PubMed] [Google Scholar]
- 35.Koltas IS, Demirhindi H, Hazar S, Ozcan K. Supportive presumptive diagnosis of Plasmodium vivax malaria: Thrombocytopenia and red cell distribution width. Saudi Medical Journal. 2007;28(4):535–9. [PubMed] [Google Scholar]
- 36.Squire DS, Asmah RH, Brown CA, Adjei DN, Obeng-Nkrumah N, Ayeh-Kumi PF. Effect of Plasmodium falciparum malaria parasites on haematological parameters in Ghanaian children. Journal of Parasitic Diseases. 2016;40(2):303–11. 10.1007/s12639-014-0501-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansart S, Perez L, Thellier M, Danis M, Bricaire F, Caumes E. Predictive factors of imported malaria in 272 febrile returning travelers seen as outpatients. Journal of Travel Medicine. 2010;17(2):124–9. 10.1111/j.1708-8305.2009.00382.x [DOI] [PubMed] [Google Scholar]
- 38.Okafor UE, Tsoka-Gwegweni JM, Bibirigea A, Irimie A, Tomuleasa C. Parasitaemia and haematological changes in malaria-infected refugees in South Africa. South African Medical Journal. 2016;106(4):413–6. [Google Scholar]
- 39.Gonçalves RM, Salmazi KC, Santos BAN, Bastos MS, Rocha SC, Boscardin SB, et al. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: Do different parasite species elicit similar host responses? Infection and Immunity. 2010;78(11):4763–72. 10.1128/IAI.00578-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philipose CS, Umashankar T. The role of haematological parameters in predicting malaria with special emphasis on neutrophil lymphocyte count ratio and monocyte lymphocyte ratio: A single Institutional experience. Tropical Parasitology. 2016;6(2):147–50. 10.4103/2229-5070.190833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adam I, Ali EA, Abdalla TM. Platelet distribution width, mean platelet volume and haematological parameters in patients with uncomplicated plasmodium falciparum and P. vivax malaria. F1000Research. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anwar A, Shaukat H, Khan HA, Nadeem A, Firdous K. Frequency of pancytopenia in malarial patients, a clinical study. Pakistan Journal of Medical and Health Sciences. 2016;10(1):294–5. [Google Scholar]
- 43.Hasona N, Amer O, Raef A. Hematological alterations and parasitological studies among infected patients with Plasmodium vivax and Plasmodium falciparum in Hail, Kingdom of Saudi Arabia. Asian Pacific Journal of Tropical Disease. 2016;6(9):695–8. [Google Scholar]
- 44.Maghendji-Nzondo S, Kouna LC, Mourembou G, Boundenga L, Imboumy-Limoukou RK, Matsiegui PB, et al. Malaria in urban, semi-urban and rural areas of southern of Gabon: Comparison of the Pfmdr 1 and Pfcrt genotypes from symptomatic children. Malaria Journal. 2016;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Rodrigues-da-Silva RN, Lima-Junior JC, Fonseca e Fonseca BP, Zuquim Antas PR, Baldez A, Storer FL, et al. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Memorias do Instituto Oswaldo Cruz. 2014;109(2):154–62. 10.1590/0074-0276140275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worku S, Bjorkman A, Troye-Blomberg M, Jemaneh L, Farnert A, Christensson B. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clinical and experimental immunology. 1997;108(1):34–41. 10.1046/j.1365-2249.1997.d01-981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leoratti FM, Trevelin SC, Cunha FQ, Rocha BC, Costa PA, Gravina HD, et al. Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis. 2012;6(6):e1710 10.1371/journal.pntd.0001710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grobusch MP, Kremsner PG. Uncomplicated malaria. Curr Top Microbiol Immunol. 2005;295:83–104. [PubMed] [Google Scholar]
- 49.Hisaeda H, Yasutomo K, Himeno K. Malaria: immune evasion by parasites. Int J Biochem Cell Biol. 2005;37(4):700–6. 10.1016/j.biocel.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 50.Wickramasinghe SN, Abdalla SH. Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol. 2000;13(2):277–99. 10.1053/beha.1999.0072 [DOI] [PubMed] [Google Scholar]
- 51.Schofield L, Vivas L, Hackett F, Gerold P, Schwarz RT, Tachado S. Neutralizing monoclonal antibodies to glycosylphosphatidylinositol, the dominant TNF-alpha-inducing toxin of Plasmodium falciparum: prospects for the immunotherapy of severe malaria. Ann Trop Med Parasitol. 1993;87(6):617–26. 10.1080/00034983.1993.11812820 [DOI] [PubMed] [Google Scholar]
- 52.Simms HH, Gaither TA, Fries LF, Frank MM. Monokines released during short-term Fc gamma receptor phagocytosis up-regulate polymorphonuclear leukocytes and monocyte-phagocytic function. J Immunol. 1991;147(1):265–72. [PubMed] [Google Scholar]
- 53.Means RT Jr., Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. J Cell Physiol. 1992;150(1):59–64. 10.1002/jcp.1041500109 [DOI] [PubMed] [Google Scholar]
- 54.Miller AR, Suttles J, Stout RD. Cytokine priming reduces dependence on TNF-R2 for TNF-alpha-mediated induction of macrophage nitric oxide generation. J Interferon Cytokine Res. 1996;16(12):1055–63. 10.1089/jir.1996.16.1055 [DOI] [PubMed] [Google Scholar]
- 55.Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, Weinberg JB. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am J Trop Med Hyg. 1999;61(2):249–52. 10.4269/ajtmh.1999.61.249 [DOI] [PubMed] [Google Scholar]
- 56.van Wolfswinkel ME, Vliegenthart-Jongbloed K, de Mendonca Melo M, Wever PC, McCall MB, Koelewijn R, et al. Predictive value of lymphocytopenia and the neutrophil-lymphocyte count ratio for severe imported malaria. Malar J. 2013;12:101 10.1186/1475-2875-12-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manas Kotepui CR, Kwuntida Uthaisar. Clinical characteristics, parasitediagnosis and hematological parameters of malaria in Surat Thani province, Thailand. J Health Res 31(4):281–8. [Google Scholar]
- 58.Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65(5):614–22. 10.4269/ajtmh.2001.65.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.