Abstract
The clinical effect of the geriatric nutritional risk index (GNRI) on patients with acute respiratory distress syndrome (ARDS) remains unclear. The aim of this study was to evaluate the association between the GNRI on admission and 30-day mortality in patients with ARDS. From January 2014 to May 2019, we retrospectively reviewed medical records for patients with ARDS admitted to a medical intensive care unit, who met for the Berlin definition. The GNRI was calculated as follows: 1.519 × serum albumin, (g/L) + (41.7 × present weight, kg/ideal body weight, kg). Clinical data of 224 patients were analyzed. Median age was 72 years old and 71.4% was men. ARDS was mostly of pulmonary origin (94.2%). 30-day mortality was 61.6% (138/224). APACHE II and SOFA scores and the frequency of septic shock and acute kidney injury, were significantly higher in non-survivors. The median GNRI score was higher in survivors than in non-survivors (86.9 vs 79.8, P = .001). In multivariate analysis, GNRI scores were associated with 30-day mortality (hazard ratio, 0.978; 95% confidence interval 0.966–0.990, P = .001). The GNRI on admission was associated with 30-day mortality and may be useful index to assess mortality in patients with ARDS.
Keywords: acute respiratory distress syndrome, geriatric nutritional risk index, mortality
1. Introduction
Acute respiratory distress syndrome (ARDS) accounts for one of the common reasons to admit in intensive care unit (ICU) and receive mechanical ventilation. It also contribute substantially to high mortality.[1] Adequate nutrition is essential to improve clinical outcomes of critically ill patients.[2] In addition to mechanical ventilation and other adjunctive strategies, nutritional support has also been shown to be important in the management of patients with ARDS,[3] and initial nutritional assessment is a key step to provide adequate nutrition.[4] The nutritional risk index (NRI) is a nutritional screening method that was primarily developed for elderly people.[5] It consists of real, usual body weight and serum albumin levels. In difficulty in identifying the usual body weight of elderly patients, geriatric nutritional risk index (GNRI) using the ideal body weight was made to predict the risk of mortality in hospitalized elderly patients.[6] Using this simple calculation, it might be feasible to evaluate the nutritional status of critically ill patients in ICU. The clinical implication of GNRI on clinical outcomes in patients with ARDS has not been elucidated. The aim of this study was to evaluate the association between NRI and 30-day morality in patients with ARDS.
2. Patients and methods
2.1. Patients
From January 2014 to May 2019, the electronic medical records of patients older than 18 years, who had been admitted to a medical ICU (MICU) at a tertiary hospital and had received invasive mechanical ventilation were retrospectively reviewed. Of these, clinical data concerning patients with ARDS who met the Berlin definition criteria were retrospectively analyzed.[7]
This study was approved by the Institutional Review Board of Gyeongsang National University Hospital (IRB No. 2019–02–022). Given the retrospective nature of the study design, informed consent was waived. This study was undertaken in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration and its later amendments or comparable ethical standards.
2.2. Data collection
Baseline (age, gender, body weight, height, comorbidities) and clinical characteristics (acute physiology and chronic health evaluation [APACHE] II score, sequential organ failure assessment [SOFA] score, septic shock, and treatment modalities), in addition to laboratory test results (white cell count, hemoglobin concentration, platelet counts, C-reactive protein levels, total protein levels, serum albumin levels, and arterial blood gas analysis), and clinical outcomes (30-day mortality) were analyzed.
2.3. Calculation of the GNRI
The NRI was originally derived from serum albumin concentration and the ratio of present to usual body weight.[5] The Geriatric NRI (GNRI) was a modified form of NRI because usual weight is often impossible to obtain in elderly patients.[6] GNRI was calculated as follows; (1.519 × serum albumin, g/dL) + {41.7 × present weight (kg)/ideal body weight (kg)}.
2.4. Statistical analysis
Categorical variables were compared using the chi-squared test and the Mann–Whitney test was used for continuous variables. Univariate and multivariate Cox regression analyses were performed to evaluate factors associated with 30-day mortality. The area under the receiver operating characteristic curve (AUROC) was calculated to assess discrimination of 30-day mortality for the GNRI in relation to APACHE II and SOFA score, which are known to be prognostic factors in the critically ill patients. The AUROC curves for these parameters were compared using MedCalc Software (version 15; Ostend, Belgium). All other statistical analyses were conducted using SPSS software (version 18.0; SPSS Inc., Chicago, IL). All significance tests were 2-sided, and, a P value < .05 was considered significant.
3. Results
3.1. Characteristics of the patients
The cause of ARDS was infection in 97.8% (219/224) patients. Pneumonia was the most common source in 94.5% (207/219) patients. The distribution of organism was as follows;
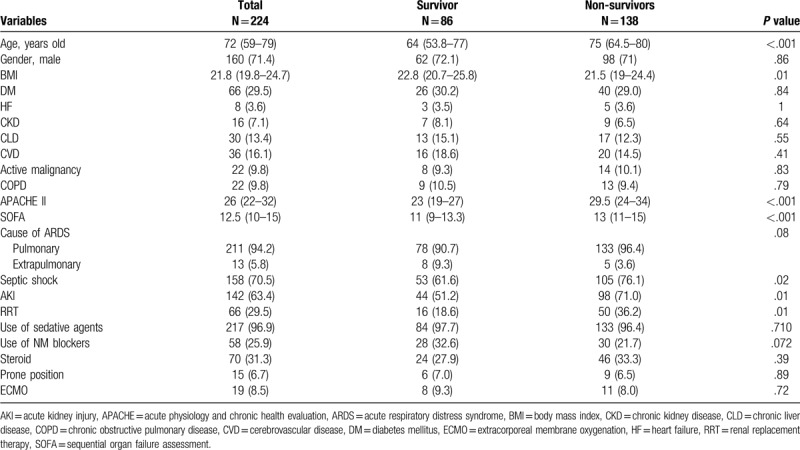
17.8% gram positive cocci, 18.8% gram negative bacilli, 4.9% mycobacteria, 12.1% respiratory viruses and 4.5% fungi. The characteristics of the patients with ARDS are shown in Table 1. The median patient age was 72 years, 160 patients were male, and 138 patients died at 30-day. The BMI was higher in survivors than in non-survivors. There was no significant difference regarding comorbidities. Severity of illness scores, such as APACHE II and SOFA, were significantly higher in non-survivors than in survivors. The frequencies of acute kidney injury and renal replacement therapy were significantly higher in non-survivors than in survivors. There was no difference in use of sedative agents and neuromuscular blocking agents. In terms of steroid, hydrocortisone and methylprednisolone were administered in 34.3% (24/70) and 65.7% (46/70), respectively. There was no significant difference of types of steroid between survivors and non-survivors. In 24 patients receiving hydrocortisone, hydrocortisone was administered within 24 hours in 19 patients and within 3 days in 5 others. Median duration was 2.5 days (Interquartile range: 2–4.8 days) and median dose was 200 mg/day. In 46 those receiving methylprednisolone, methylprednisolone was administered within 48 hours in 30 patients, 3 to 7 days in 12 those and over 7 days in 4 others. Median duration was 6 days (IQR: 2.8–11.3 days). The median dosage of methylprednisolone per day was 50 mg/day (IQR: 38.8 mg–68.9 mg)
Table 1.
Baseline characteristics of total patients, survivor and non-survivors at 30 days.
3.2. Laboratory and ventilator values of the patients
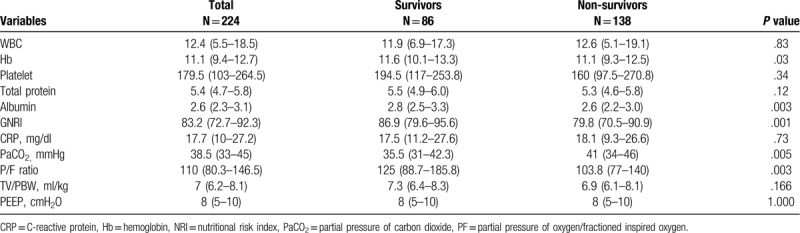
Laboratory and ventilator data are presented in Table 2. Hemoglobin and serum albumin levels were significantly lower in non-survivors than those of survivors. Higher partial pressure of arterial carbon dioxide and lower partial pressure of oxygen in arterial blood/fraction of inspired oxygen ratios were observed in non-survivors compared to survivors. Tidal volume divided by predicted body weight and positive end expiratory pressure did not differ.
Table 2.
Laboratory and ventilator parameters at admission.
3.3. The GNRI score and prognostic factors
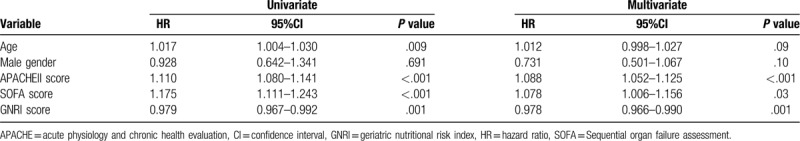
The median GNRI score was 83.2 (interquartile range, 72.7–92.3). The GNRI score was significantly lower in non-survivors than in survivors [79.8 (70.5–90.9) vs 86.9 (79.6–95.6), P = .001]. Univariate and multivariate analyses for factors associated with 30-day mortality in patients with ARDS were performed, and are shown in Table 3. In multivariate analysis, APACHE II, SOFA, and GNRI scores were associated with 30-day mortality in patients with ARDS.
Table 3.
Univariate and multivariate analysis for factor associated with 30-day mortality.
3.4. Comparisons regarding the AUROC curves for GNRI, BMI and serum albumin
The AUROC curves for the GNRI score, APACHE II score, and SOFA score, when compared to predict 30-day mortality, were 0.686 [(95% confidence interval (CI): 0.621–0.747, P < .001]; 0.777 [95% CI: 0.717–0.830, P < .001] and; 0.708 [95% CI 0.644–0.767, P < .003], respectively, (Fig. 1). There was a higher trend towards APACHE II score compared to GNRI score (APACHE II vs GNRI score, P = .07) but There was no significant difference between GNRI and SOFA (GNRI vs SOFA score, P = .679).
Figure 1.
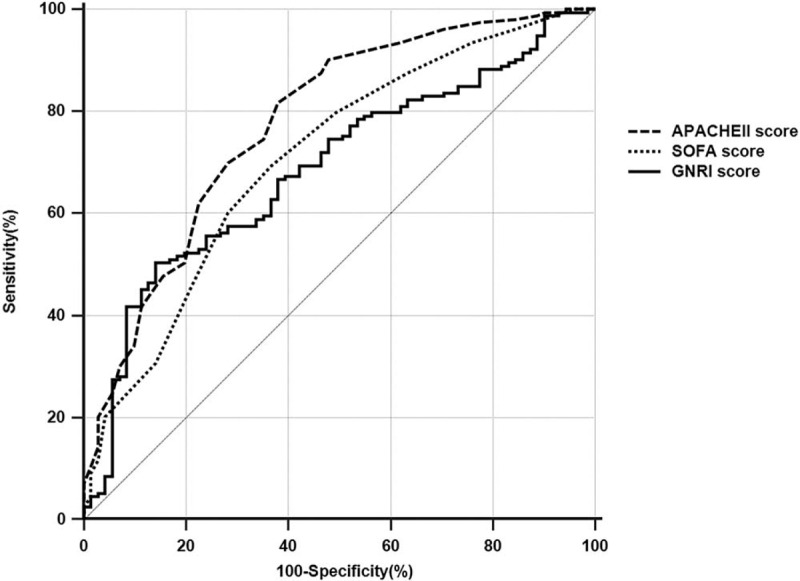
The area under the receiver operating characteristic curves to predict 30-daymortality. APACHE = acute physiology and chronic health evaluation, GNRI = geriatrtic nutritional risk index, SOFA = sequential organ failure assessment.
4. Discussion
The present study showed that the GNRI score was significantly lower in non-survivors with ARDS at 30-day and was associated with 30-day mortality. The GNRI score was similar to SOFA score to assess 30-day mortality in patients with ARDS.
ARDS is frequent clinical condition in the critically ill patients admitted to ICUs who have received invasive mechanical ventilation.[1] The cause of ARDS is heterogeneous and it has a high mortality.[7] A protective ventilation strategy and other adjunctive therapies have been shown to reduced mortality of ARDS.[8] Determining the nutritional status of critically ill patients admitted to ICUs has been emphasized,[9] and is also essential for patients with ARDS.[3] The assessment of nutritional status is a key step to support adequate nutrition. NUTRIC score and the modified NUTRIC score are well-known scores to evaluate the nutrition status of critically ill patients. These scores have been associated with clinical outcomes in critically ill patients.[10,11] However, the usefulness of relatively simple index scores has not been fully elucidated among critically ill patients with ARDS.
NRI score originally developed to screen nutritional status in the elderly patients.[5] Geriatric NRI (GNRI) was derived from NRI, which replace the usual weight with ideal body weight due to obtaining the usual weight from the elderly patients.[6]
Several studies have reported the usefulness of the GNRI to evaluate outcomes for various clinical conditions including abdominal surgery,[12] chronic kidney disease,[13] heart failure.[14,15]
The GNRI score was simple to calculate and it was feasible to evaluate the nutritional status of the patients in the ICU. The clinical impact of the GNRI on critically ill patients had previously been unclear, and the association between the GNRI and clinical outcomes has not been elucidated in patients with ARDS. In the present study, GNRI score was calculated at the time of admission to the MICU. The GNRI score was significantly lower in patients with ARDS who died than in those who survived at 30-day. In the multivariate analysis, the GNRI was associated with 30-day mortality as well as with APACHE II and SOFA scores. In the present study, the GNRI was similar to SOFA score to predict the 30-day mortality. The findings in the present study suggest that the GNRI score might be a feasible index to use when assessing clinical outcomes for patients with ARDS. Patients with ARDS and with a low GNRI score at the time of ICU admission may require more attention to enhance nutritional support.
There are several limitations in the current study. First, selection bias could not be excluded, given this was a retrospective study. Second, the usual body weight in a stable status could not be obtained; instead, the ideal body weight was calculated and was used alternatively in the formula, which might be less accurate in predicting clinical outcomes for patients with ARDS. Third, we did not evaluate the sequential GNRI score and the monitoring of adequate nutritional support according to GNRI score. Further prospective studies are required to overcome these limitations.
In conclusion, the GNRI was significantly lower in non-survivors at 30-day and was associated with 30-day mortality. The GNRI might be a feasible method to predict outcomes in patients with ARDS.
Author contributions
Conceptualization: Jung-Wan Yoo, Ho Cheol Kim. Data curation: Sunmi Ju, Seung Jung Lee, Yu Ji Cho, Jong Deog Lee. Methodology: Sunmi Ju, Seung Jung Lee, Yu Ji Cho, Jong Deog Lee. Writing – original draft: Jung-Wan Yoo, Ho Cheol Kim.
Footnotes
Abbreviations: APACHE = acute physiology and chronic health evaluation, ARDS = Acute respiratory distress syndrome, AUROC = The area under the receiver operating characteristic curve, BMI = body mass index, CI = confidence interval, GNRI = geriatric nutritional risk index, ICU = intensive care unit, PaCO2 = partial pressure of carbon dioxide, PF = partial pressure of oxygen/fractioned inspired oxygen, SOFA = sequential organ failure assessment.
How to cite this article: Yoo JW, Ju S, Lee SJ, Cho YJ, Lee JD, Kim HC. Geriatric nutritional risk index is associated with 30-day mortality in patients with acute respiratory distress syndrome. Medicine. 2020;99:25(e20671).
JWY and HCK contributed equally to this work.
The authors have no conflicts of interest to declare.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. We have no funding for this work.
References
- [1].Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- [2].Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48–79. [DOI] [PubMed] [Google Scholar]
- [3].Krzak A, Pleva M, Napolitano LM. Nutrition therapy for ALI and ARDS. Crit Care Clin 2011;27:647–59. [DOI] [PubMed] [Google Scholar]
- [4].Reber E, Gomes F, Vasiloglou MF, et al. Nutritional risk screening and assessment. J Clin Med 2019;8(7.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buzby GP, Knox LS, Crosby LO, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr 1988;47:366–81. [DOI] [PubMed] [Google Scholar]
- [6].Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005;82:777–83. [DOI] [PubMed] [Google Scholar]
- [7].Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [8].Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:562–72. [DOI] [PubMed] [Google Scholar]
- [9].Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. New Engl J Med 2014;370:1227–36. [DOI] [PubMed] [Google Scholar]
- [10].Jeong DH, Hong SB, Lim CM, et al. Comparison of accuracy of NUTRIC and modified NUTRIC scores in predicting 28-Day mortality in patients with sepsis: a single center retrospective study. Nutrients 2018;10:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsai MH, Huang HC, Peng YS, et al. Nutrition risk assessment using the modified NUTRIC score in cirrhotic patients with acute gastroesophageal variceal bleeding: prevalence of high nutrition risk and its independent prognostic value. Nutrients 2019;11:2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hanada M, Yamauchi K, Miyazaki S, et al. Geriatric Nutritional Risk Index, a predictive assessment tool, for postoperative complications after abdominal surgery: a prospective multicenter cohort study. Geriatr Gerontol Int 2019;19:924–9. [DOI] [PubMed] [Google Scholar]
- [13].Kuo IC, Huang JC, Wu PY, et al. A low geriatric nutrition risk index is associated with progression to dialysis in patients with chronic kidney disease. Nutrients 2017;9:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoshihisa A, Kanno Y, Watanabe S, et al. Impact of nutritional indices on mortality in patients with heart failure. Open heart 2018;5:e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nishi I, Seo Y, Hamada-Harimura Y, et al. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail 2019;6:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


