Supplemental Digital Content is available in the text
Keywords: birth weight, dichorionic diamniotic twins, miscarriage, multifetal pregnancy reduction, preterm birth
Abstract
Background:
Published findings on perinatal outcomes of multifetal pregnancy reduction (MPR) of dichorionic diamniotic (DCDA) twin pregnancy to singleton are controversial. We performed a meta-analysis to appraise the effects of MPR of DCDA twin pregnancy versus expectant management on perinatal outcomes.
Methods:
Four electronic databases were searched from their inception to June 15, 2019, to identify publications that appraised MPR before 15 weeks of gestation. Studies reporting perinatal outcomes of both MPR of DCDA twin pregnancy to singleton and expectant management were considered. The relative risks (RRs) and mean differences with 95% confidence intervals (CIs) were pooled using a random-effects model.
Results:
Six studies involving 7398 participants showed that MPR of DCDA twin pregnancy to singleton was associated with a lower risk of preterm birth (5 studies with 7297 participants; RR: 0.30, 95% CI: 0.22–0.40; P < .001) and higher birth weight (4 studies with 5763 participants; mean differences: 548.10 g, 95% CI: 424.04–672.15; P < .001) than expectant management; there was no difference in the occurrence of miscarriages (5 studies with 7355 participants; RR: 1.57, 95% CI: 0.90–2.75; P = .11). Sensitivity analysis showed that all the results were stable and reliable, with the omission of 2 studies with serious risk of bias.
Conclusion:
Compared to expectant management, MPR of DCDA twin pregnancy to singleton prevents preterm birth and low birth weight, without increasing the risk of miscarriages. Regarding perinatal morbidity related to preterm birth, MPR can be reserved as a remediation measure to improve the perinatal outcomes of DCDA twin pregnancies.
1. Introduction
With the prevalence of advanced maternal age and the wide use of assisted reproductive technology, the rate of twin pregnancies has increased globally over recent decades.[1,2] In the United States, the twin birth rate has risen by 76% from 1980 to 2009, with the highest peak of 33.9 per 1000 births in 2014.[3] Therefore, the guidelines of the American Society for Reproductive Medicine have been revised to advocate single-embryo transfer.[4] However, the economic burden associated with the technology is pushing both the patients and assisted reproductive technology providers to achieve a high pregnancy rate by transferring more than 1 embryo per cycle.[5] According to the National Vital Statistics Reports published in 2017, twin gestations demonstrated a steady high rate of 3.3% of the total births.[3]
The prevalence of twin gestations has become a serious medical and social problem, perplexing both patients as well as physicians. The rate of preterm births before 37 weeks was 59.43% for twins and 8.13% for singletons,[3] and this in turn led to high rates of perinatal morbidity and mortality in twins.[6–8] Moreover, women with twin gestations have a threefold higher risk of severe maternal complications than women with singletons.[9] Most patients opt to maximize their chance of having singletons given their awareness of the risks associated with twin gestations.[10]
Multifetal pregnancy reduction (MPR) is a technique developed to decrease the risks associated with multiple pregnancies. The commonly used methods are ultrasound-guided transabdominal injection of potassium chloride into the fetuses with a separate placenta[11,12] or ultrasound-guided transvaginal aspiration without potassium chloride injection.[13,14] Previous meta-analyses have demonstrated that MPR improves the perinatal outcomes of triplets,[15,16] but whether it is valuable in cases of dichorionic diamniotic (DCDA) twin pregnancy is unclear. Some studies have reported that MPR of DCDA twin pregnancy to singleton had better perinatal outcomes without increasing the occurrence of pregnancy loss,[11,14,17] while other studies did not confirm the improvement in the perinatal outcomes or, in contrast, observed a high risk of miscarriage after MPR.[12,13,18] Hence, the current meta-analysis aimed to critically appraise the role of MPR in the management of DCDA twin pregnancy before 15 weeks of gestation versus expectant management.
2. Materials and methods
This study was conducted in line with a protocol registered on PROSPERO (CRD 42019138869) and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.[19] This study was based on a retrospective review of publications, so it was not necessary to obtain informed consent from individual participants.
2.1. Search strategy
Relevant publications were identified by systematically searching PubMed, Embase, Web of Science, and Cochrane Library from inception to June 15, 2019. The search terms included “pregnancy reduction”, “twin pregnancy”, “dichorionic”, and their variants. There was no limitation with regard to the time or language of publication. The search strategy is presented in the Supplemental Content (Table S1). The references of the obtained studies were also checked for potentially eligible studies.
2.2. Study selection and eligibility criteria
Two independent authors (BHJ and QXH) selected the studies in a 2-stage method. First, the titles and abstracts of eligible citations were checked. Second, full-text evaluation was performed to identify eligible studies. Disagreements, if any, were settled by a third author (JS).
Studies were eligible if they:
-
(i)
involved women with DCDA pregnancies with 2 live fetuses of less than 15 weeks’ gestation;
-
(ii)
included data of both MPR and expectant management;
-
(iii)
described data regarding perinatal outcomes, for example, preterm birth rate, birth weight, miscarriage rate, and so on; and
-
(iv)
were prospective or retrospective studies of any design. When more than 1 paper with overlapping sample data from the same institute was published by the author, the recent publication or the publication with detailed information was included.
2.3. Data collection and quality assessment
Two reviewers (BHJ and QXH) extracted the data independently in duplicate. Disagreements, if any, were resolved by a third author (JS). The following information was extracted: first author, publication year, country, study design, mode of conception, indications for reduction, approach of reduction, gestational age at reduction, number of patients, definition of preterm birth and miscarriage, and covariates that were matched. For a few studies, only the data of the subgroups of interest were extracted. The primary outcomes of interest were preterm birth rate, birth weight, and miscarriage rate. The secondary outcomes were the rates of intrauterine growth retardation (IUGR), cesarean section, and gestational diabetes mellitus (GDM). The quality of included studies was assessed using the risk of bias In non-randomized studies of interventions tool.[20] The risk of bias was assessed by 2 independent reviewers based on 7 domains and ranked as “low”, “moderate”, “serious”, “critical”, or “no information”.
2.4. Statistical analysis
The Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK) was used to generate the relative risks (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes with 95% confidence intervals (CIs). All analyses were performed using a random-effects model, considering clinical and methodological heterogeneity. Heterogeneity among different studies was evaluated by the Q statistic with P-values and I2 statistics.[21] Heterogeneity was considered to be low when I2 < 50%. Sensitivity analysis was conducted by excluding studies with serious risk of bias. Potential publication bias was detected by the Egger test[22] using Stata version 15.1 (StataCorp, LLC). P < .05 was considered statistically significant. Power analyses were performed using Power Analysis and Sample Size 15.0.5 software (NCSS, LLC) with an α-error of 0.05; power > 80% was considered adequate.
3. Results
3.1. Search results
Figure 1 presents a flow diagram of the literature review. After removing the duplicate records, we screened 448 citations, and 12 full-text studies were found to be eligible for inclusion. After reviewing the full-texts of these studies, 5 studies with duplicated data[23–27] and 1 study that did not report MPR at a satisfactory gestational age were excluded.[28] Finally, 6 studies were included in this meta-analysis.[11–14,17,18]
Figure 1.
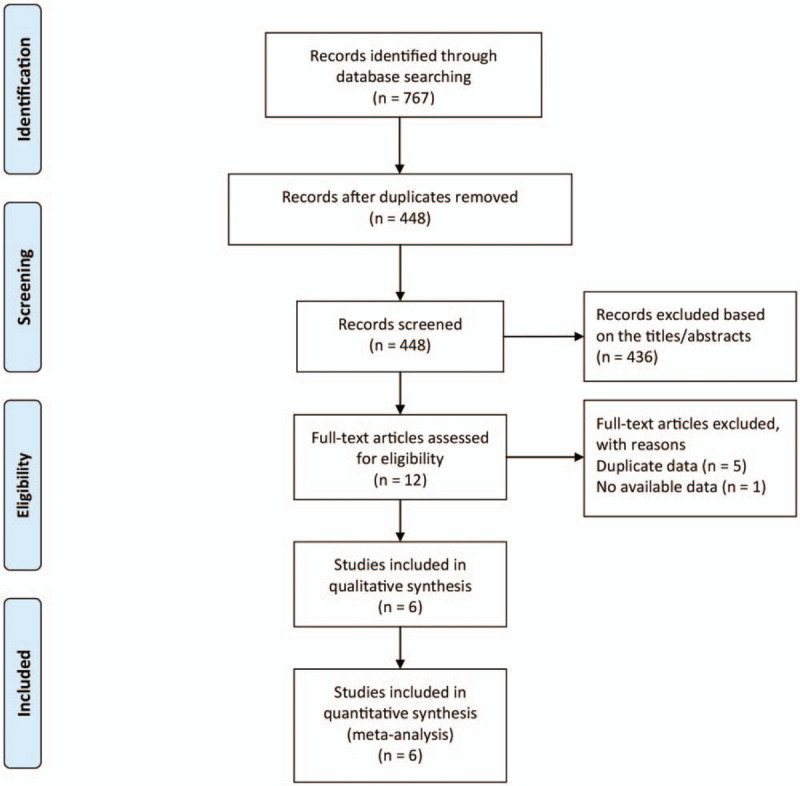
Flow chart depicting the literature screening process.
3.2. Study characteristics
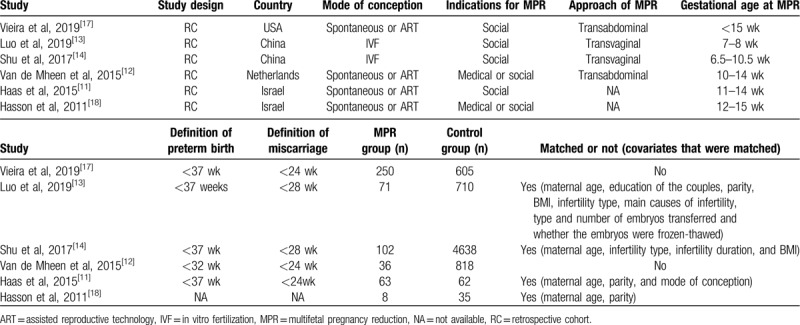
Six studies involving 7398 participants were included in the final analysis (Table 1). All 6 studies had a retrospective cohort design and were published between 2011 and 2019. Gestational age at reduction ranged from 6.5 to 15 weeks. The sample size of the MPR group ranged from 8 to 250, while the sample size of the expectant group ranged from 35 to 4638. A total of 530 twin gestations that underwent MPR and 6868 controls that underwent expectant management were included. Five studies reported the preterm birth rate: 4 studies defined it as delivery before 37 gestational weeks, and 1 study, as delivery before 32 gestational weeks. In the 5 studies that reported the miscarriage rate, 3 studies defined it as pregnancy loss before 24 gestational weeks, while the remaining 2 studies defined it as pregnancy loss before 28 gestational weeks. Due to the retrospective nature of the studies, the baseline values of maternal demographic characteristics showed wide variability, but some vital covariates were properly matched in 4 studies, thus increasing the comparability of the results.
Table 1.
Study features in the systematic review.
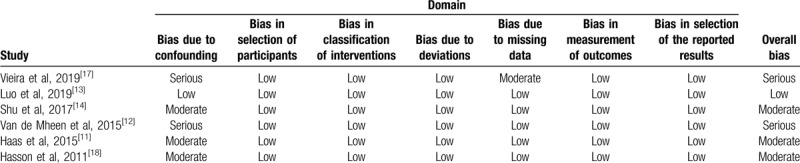
3.3. Risk of bias evaluation
Quality assessment using non-randomized studies of interventions tool revealed that most of the studies had moderate or serious risk based on the seven risk domains except for 1 study[13] which was well balanced with important covariates and had a low risk of bias (Table 2). The reasons for the ranking are presented in the Supplemental Content (Table S2).
Table 2.
Methodological quality assessment of included studies by the non-randomized studies of interventions tool.
3.4. Preterm birth rate
Five studies with 7297 participants described the effect of MPR of DCDA twin pregnancy on preterm birth rate. The pooled estimate of preterm birth rate for MPR of DCDA twin pregnancy to singleton was significantly lower than that for expectant management (RR: 0.30, 95% CI 0.22–0.40, P < .001) (Fig. 2). The Egger test did not indicate a risk of publication bias for preterm birth rate (P = .823).
Figure 2.
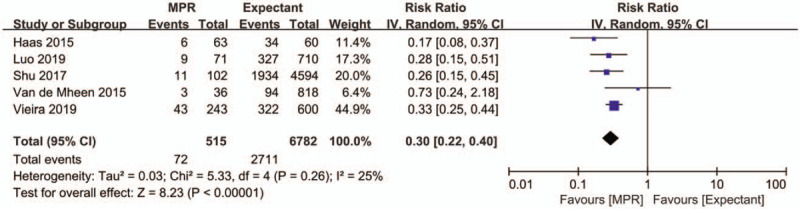
Forest plot depicting the effect of multifetal pregnancy reduction of dichorionic diamniotic twin pregnancy vs expectant management on preterm birth rate. IV = inverse variance MPR = multifetal pregnancy reduction.
3.5. Birth weight
Four studies with 5763 patients reported the effects of MPR of DCDA twin pregnancy on birth weight. Newborns in the MPR group had significantly higher birth weight than those in the expectant management group, with an MD of 548.10 g (95% CI 424.04–672.15, P < .001) (Fig. 3). The Egger test did not indicate a risk of publication bias for birth weight (P = .411).
Figure 3.
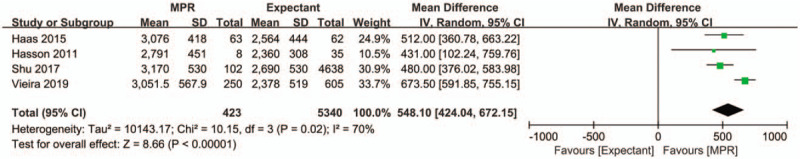
Forest plot depicting the effect of MPR of dichorionic diamniotic twin pregnancy vs expectant management on birth weight. IV = inverse variance MPR = multifetal pregnancy reduction.
3.6. Miscarriage rate
Five studies with 7355 patients provided data on the effect of MPR of DCDA twin pregnancy on the rate of miscarriage. The pooled estimate of miscarriage rate showed no significant increase in the MPR group when compared to the expectant management group (RR: 1.57, 95% CI 0.90–2.75, P = .11) (Fig. 4). The Egger test did not indicate a risk of publication bias for miscarriage rate (P = .206).
Figure 4.
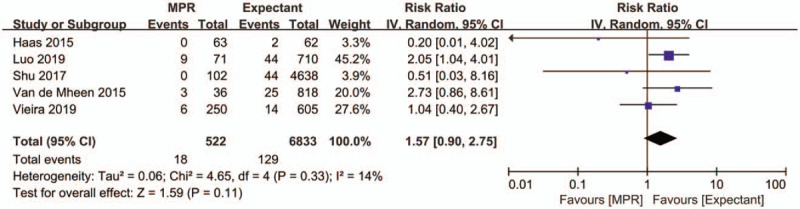
Forest plot depicting the effect of multifetal pregnancy reduction of dichorionic diamniotic twin pregnancy vs expectant management on miscarriage rate of dichorionic diamniotic twins. IV = inverse variance, MPR = multifetal pregnancy reduction.
3.7. Sensitivity analysis
Sensitivity analysis evaluating the rates of preterm birth and miscarriage and birth weight was performed, with the omission of 2 studies with serious risk of bias.[12,17] Consistent with the overall estimates, the re-calculated effect size still favored MPR with low risk of preterm birth (RR: 0.24, 95% CI 0.17–0.35, P < .001) and high birth weight (MD: 486.50 g, 95% CI 403.59–569.41, P < .001), and the risk of miscarriage remained comparable between the groups (RR: 1.10, 95% CI 0.29–4.18, P = .88) (Fig. 5A-C). Notably, the heterogeneity in the analysis of birth weight decreased from I2 = 70% to 0% when the study of Vieira et al[17] was removed. We found that this excluded study did not match the groups for important covariates, which may have caused the heterogeneity.
Figure 5.
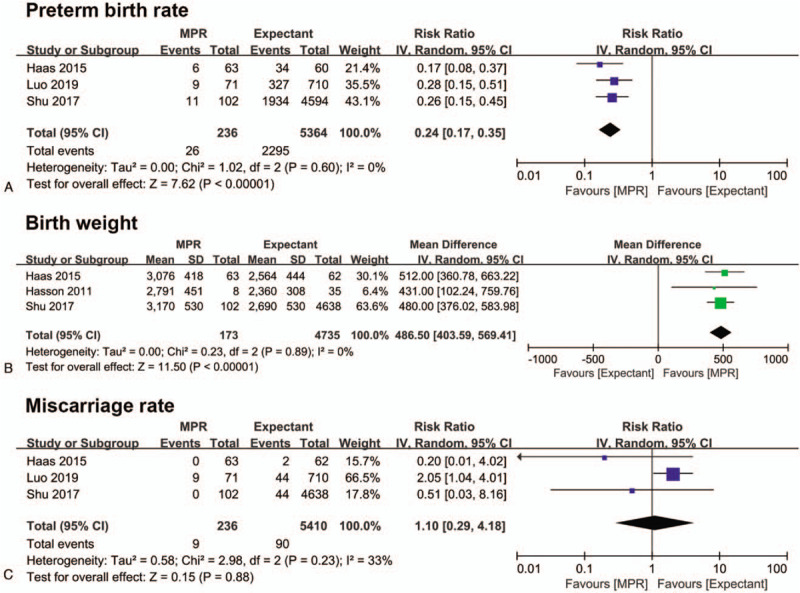
Forest plot depicting the effect of multifetal pregnancy reduction of dichorionic diamniotic twin pregnancy on preterm birth rate (A), birth weight (B), and miscarriage rate (C) as compared to expectant management. The recalculated result was obtained by sensitivity analysis. IV = inverse variance, MPR = multifetal pregnancy reduction.
3.8. Secondary outcomes
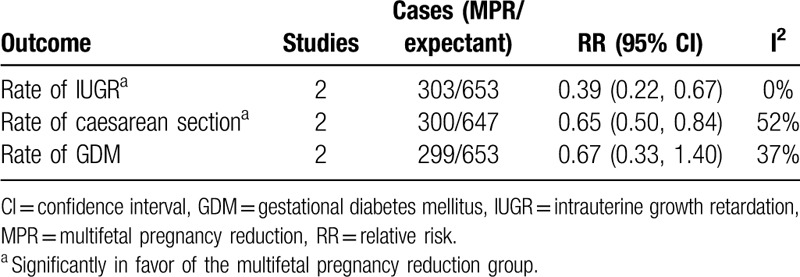
Two studies[11,17] with 956 participants assessed the effect of MPR of DCDA twin pregnancy on the rates of IUGR, cesarean section, and GDM (Table 3). The MPR group showed a significantly lower rate of IUGR (RR: 0.39, 95% CI 0.22–0.67, P < .001) and cesarean section (RR: 0.65, 95% CI 0.50–0.84, P = .001) than the expectant management group, while the rate of GDM was comparable between the 2 groups (RR: 0.67, 95% CI 0.33–1.40, P = .29). The forest plots are depicted in the Supplemental Content (Fig. S1).
Table 3.
Multifetal pregnancy reduction versus expectant management: other pregnancy outcomes.
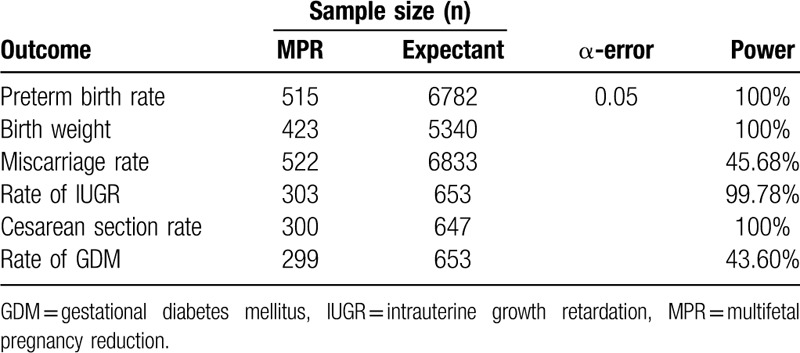
3.9. Power analyses
Since there were only 6 studies in this meta-analysis, we decided to carry out a power analysis to critically evaluate the sample size. As shown in Table 4, the analysis of preterm birth rate, birth weight, and rates of IUGR and Caesarean section achieved adequate power (>80%), which means our sample size was sufficient for these indicators. However, concerning the rates of miscarriage and GDM, the power was found to be lower than 50%. Therefore, we could not rule out the possibility that the nonsignificant effect of MPR on the rates of miscarriage and GDM in DCDA twins group was due to the insufficient sample size.
Table 4.
Power analysis of the perinatal outcomes using Power Analysis and Sample Size software.
4. Discussion
4.1. Main findings
The major finding of the current meta-analysis is that MPR of DCDA twin pregnancy to singleton before 15 weeks of gestation improved perinatal outcomes including preterm birth rate and birth weight without increasing the risk of miscarriage. Moreover, MPR of DCDA twin pregnancy was associated with a lower risk of IUGR and cesarean section than expectant management, while the rate of GDM was comparable between both modalities.
4.2. Comparison with other studies
Preterm birth and low birth weight are the leading causes of perinatal morbidity and mortality in twins. According to a previous systematic review,[29] MPR of DCDA twin pregnancy (n = 17) was associated with a longer median gestation period (38.0 vs 34.9 weeks) and higher birth weight (2922 g vs 2474 g) than expectant management (n = 47), consistent with our study results. The preterm birth rate had meaningfully improved after MPR across all the 5 included studies, except in the study conducted by Van de Mheen et al.[12] This study defined preterm birth as birth before 32 gestational weeks and did not match for important covariates, such as a history of preterm birth, which might explain their null results.
Another question that arises is whether the neonatal outcomes of singletons reduced from twins are comparable to those of initial singletons? According to the national data of the United States, singletons demonstrated a preterm birth rate (<37 weeks) of 8.13%,[3] with a mean birth weight of 3296 g, which is nearly 1 kg higher than that of twins (2336 g).[30] In contrast, our results showed that twins reduced to singletons had a relatively higher preterm birth rate of 14.0% and improved birth weight, with an MD of 548.10 g. Similarly, a previous meta-analysis of MPR in triplets also suggested that reduced twins retained their suboptimal growth potential as triplets,[16] lending support to our study findings. Therefore, prevention is still considered the first-line therapy for multiple pregnancies, and MPR can be reserved as a remediation measure.
The risk of miscarriage secondary to MPR is still a controversial issue. In two previous meta-analyses on MPR of trichorionic triplet pregnancy to twins showed no association with a high risk of miscarriage.[15,16] Stone et al[31] in a large single-center study, reported that the rate of pregnancy loss after MPR in patients initially carrying twins is 2.1%, which was comparable to our results. Of note, all the studies pooled in Figure 4 showed comparable results between groups, except the study by Luo et al[13]; in their study, transvaginal MPR before 8 weeks of gestation was carried out before genetic screening. Previous study has proven that early (6–8 weeks) transvaginal MPR is associated with higher rates of miscarriage than late (11–14 weeks) transabdominal MPR,[32] which might have contributed to the high miscarriage rate in their study.
4.3. Possible reasons behind the findings
MPR can eliminate a few but not all etiologies that supposedly compromise the growth trajectory of twins. First, twins have more constraints than singletons in the uterine environment with limited potential to increase their volume.[33] Second, limited maternal resources and exhausted intrauterine environment cannot assist in the adequate growth of both fetuses as the pregnancy progresses.[33,34] Furthermore, the placental size and growth potential of twins are inferior to those of singletons and are partly predetermined before MPR is carried out.[35] Besides, some epigenetic changes related to energy balance regulation were observed in twins[36] which cannot be rectified by MPR. Considering the above reasons, MPR can improve the perinatal outcomes of twins, but not to the level of naturally conceived singletons.
Regarding safety, MPR can be easily carried out in DCDA twin pregnancies as placental vascular anastomoses do not exist between both fetuses, so each fetus is considered a separate entity. According to Luo et al,[13] all pregnancy losses occur at least 2 weeks after MPR, and this long interval between the procedure and the loss supports the hypothesis that excess loss with MPR is attributed to the resorption of fetal residues and accompanying chronic inflammation rather than the procedure itself.[37]
4.4. Implications for clinical practice
According to the American College of Obstetricians and Gynecologists,[38] when a patient requires information regarding MPR, nondirective counseling should be offered, and all necessary information should be provided. Considering the absence of a randomized trial, the data from our meta-analysis provides the best existing evidence for counseling in early gestation. Specifically, MPR is the first choice as far as perinatal morbidity related to preterm birth is concerned. Although the procedure might cause a non-significant increase in pregnancy loss, the overall miscarriage rate of 3.4% is generally acceptable. If the foremost concern is to save 2 live infants, then expectant management is also considered as a rational choice with advanced neonatology. Finally, the patients’ autonomy based on their unique situation should be fully respected.
4.5. Strengths and limitations
This is the first meta-analysis that compared perinatal outcomes of MPR with those of expectant management in DCDA twin pregnancies. Although only 6 studies fulfilled the inclusion criteria, the sample size of the analysis was large, allowing us to draw some conclusions. Besides, sensitivity analysis confirmed the credibility of our findings.
However, the present meta-analysis has some limitations. First, although the available databases were systematically searched, the eligible studies were all retrospective in nature, and the important baseline characteristics were not properly matched in some studies, which might have increased the risk of confounding bias. Second, on comparing our data with published data, the definition of preterm birth and miscarriage in all the included studies were inconsistent, which may have introduced some heterogeneity between the studies. Finally, the conclusions about the rates of miscarriage and GDM should be taken with caution considering our insufficient sample size to achieve adequate power.
5. Conclusions
In summary, our meta-analysis showed that MPR of DCDA twin pregnancy to singleton prevents preterm birth and low birth weight and is not associated with an increased risk of miscarriage. MPR can be reserved as a remediation measure to improve the perinatal outcomes of DCDA twins. Our findings should be interpreted with caution since only retrospective studies were included, and confounding bias was considered inevitable.
Author contributions
Conceptualization: Bihui Jin, Jing Shu.
Data curation: Bihui Jin, Qiongxiao Huang, Jing Shu.
Formal analysis: Mengxia Ji, Zhizhi Yu.
Funding acquisition: Bihui Jin.
Investigation: Qiongxiao Huang, Mengxia Ji.
Methodology: Qiongxiao Huang, Zhizhi Yu.
Software: Mengxia Ji, Zhizhi Yu.
Supervision: Jing Shu.
Writing – original draft: Bihui Jin.
Writing – review & editing: Jing Shu.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DCDA = dichorionic diamniotic, GDM =gestational diabetes mellitus, IUGR = intrauterine growth retardation, MD = mean difference, MPR = multifetal pregnancy reduction, RR = relative risk.
How to cite this article: Jin B, Huang Q, Ji M, Yu Z, Shu J. Perinatal outcomes in dichorionic diamniotic twins with multifetal pregnancy reduction versus expectant management: a systematic review and meta-analysis. Medicine. 2020;99:25(e20730).
This research was funded by the Natural Science Foundation of Zhejiang Province (grant number LQ19H040005); Medical and Health Science and Technology Research Program of Zhejiang Province (grant number 2018KY220).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Ferraretti AP, Goossens V, Kupka M, et al. Assisted reproductive technology in Europe, 2009: results generated from European Registers by ESHRE. Hum Reprod 2013;28:2318–31. [DOI] [PubMed] [Google Scholar]
- [2].Kulkarni AD, Jamieson DJ, Jones HW, Jr, et al. Fertility treatments and multiple births in the United States. N Engl J Med 2013;369:2218–25. [DOI] [PubMed] [Google Scholar]
- [3].Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2017. Natl Vital Stat Rep 2018;67:1–50. [PubMed] [Google Scholar]
- [4].Practice Committee of the American Society for Reproductive Medicine. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril 2017;107:901–3.28292618 [Google Scholar]
- [5].Jain T, Harlow BL, Hornstein MD. Insurance coverage and outcomes of in vitro fertilization. N Engl J Med 2002;347:661–6. [DOI] [PubMed] [Google Scholar]
- [6].Zork N, Biggio J, Tita A, et al. Decreasing prematurity in twin gestations: predicaments and possibilities. Obstet Gynecol 2013;122:375–9. [DOI] [PubMed] [Google Scholar]
- [7].Chauhan SP, Scardo JA, Hayes E, et al. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol 2010;203:305–15. [DOI] [PubMed] [Google Scholar]
- [8].Grantz KL, Grewal J, Albert PS, et al. Dichorionic twin trajectories: the NICHD fetal growth studies. Am J Obstet Gynecol 2016;215:221.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Santana DS, Cecatti JG, Surita FG, et al. Twin pregnancy and severe maternal outcomes: the World Health Organization multicountry survey on maternal and newborn health. Obstet Gynecol 2016;127:631–41. [DOI] [PubMed] [Google Scholar]
- [10].Glazebrook C, Sheard C, Cox S, et al. Parenting stress in first-time mothers of twins and triplets conceived after in vitro fertilization. Fertil Steril 2004;81:505–11. [DOI] [PubMed] [Google Scholar]
- [11].Haas J, Sasson AM, Barzilay E, et al. Perinatal outcome after fetal reduction from twin to singleton: to reduce or not to reduce? Fertil Steril 2015;103:428–32. [DOI] [PubMed] [Google Scholar]
- [12].van de Mheen L, Everwijn SM, Knapen MF, et al. Pregnancy outcome after fetal reduction in women with a dichorionic twin pregnancy. Hum Reprod 2015;30:1807–12. [DOI] [PubMed] [Google Scholar]
- [13].Luo L, Fan X-Z, Jie H-Y, et al. Is it worth reducing twins to singletons after IVF-ET? A retrospective cohort study using propensity score matching. Acta Obstet Gynecol Scand 2019;98:1274–81. [DOI] [PubMed] [Google Scholar]
- [14].Shu L, Zhang Y, Wang J, et al. A case-control study on pregnancy outcome of fetal reduction to single fetus from dizygotic twins after in vitro fertilization and intracytoplasmic sperm injections [in Chinese]. Chin J Reprod Contracept 2017;37:743–5. [Google Scholar]
- [15].Anthoulakis C, Dagklis T, Mamopoulos A, et al. Risks of miscarriage or preterm delivery in trichorionic and dichorionic triplet pregnancies with embryo reduction versus expectant management: a systematic review and meta-analysis. Hum Reprod 2017;32:1351–9. [DOI] [PubMed] [Google Scholar]
- [16].Zipori Y, Haas J, Berger H, et al. Multifetal pregnancy reduction of triplets to twins compared with non-reduced triplets: a meta-analysis. Reprod Biomed Online 2017;35:296–304. [DOI] [PubMed] [Google Scholar]
- [17].Vieira LA, Warren L, Pan S, et al. Comparing pregnancy outcomes and loss rates in elective twin pregnancy reduction with ongoing twin gestations in a large contemporary cohort. Am J Obstet Gynecol 2019;221:253.e1–8. [DOI] [PubMed] [Google Scholar]
- [18].Hasson J, Shapira A, Many A, et al. Reduction of twin pregnancy to singleton: does it improve pregnancy outcome? J Matern Fetal Neonatal Med 2011;24:1362–6. [DOI] [PubMed] [Google Scholar]
- [19].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [20].Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Everwijn S, van de Mheen L, Ravelli A, et al. Reduction in dichorionic twin pregnancy, a retrospective cohort study. Am J Obstet Gynecol 2012;206:S228–9. [Google Scholar]
- [24].Gupta S, Fox N, Feinberg J, et al. Adverse pregnancy outcomes in twin pregnancies reduced to singleton pregnancies and ongoing twin pregnancies. Ultrasound Med Biol 2015;41:S39. [DOI] [PubMed] [Google Scholar]
- [25].Sasson AM, Haas J, Barzilay E, et al. To reduce or not to reduce? Perinatal outcome of pregnancies after twin-to-singleton reduction. Am J Obstet Gynecol 2015;212:S375–6. [Google Scholar]
- [26].Vieira L, Warren L, Robles B, et al. Comparison of pregnancy outcomes in 2-to-1 multifetal pregnancy reduction and ongoing twins. Am J Obstet Gynecol 2018;218:S317–8. [Google Scholar]
- [27].Luo L, Jie H, Chen M, et al. Is it worth reducing twins to singletons after IVF-et? A retrospective cohort study using propensity score matching. Fertil Steril 2017;108:e378–9. [DOI] [PubMed] [Google Scholar]
- [28].Gupta S, Fox NS, Feinberg J, et al. Outcomes in twin pregnancies reduced to singleton pregnancies compared with ongoing twin pregnancies. Am J Obstet Gynecol 2015;213:580.e1–5. [DOI] [PubMed] [Google Scholar]
- [29].Lust A, De Catte L, Lewi L, et al. Monochorionic and dichorionic twin pregnancies discordant for fetal anencephaly: a systematic review of prenatal management options. Prenat Diagn 2008;28:275–9. [DOI] [PubMed] [Google Scholar]
- [30].Committee on Practice Bulletins-Obstetrics. Society for maternal-fetal medicine. practice bulletin no. 169: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol 2016;128:e131–46. [DOI] [PubMed] [Google Scholar]
- [31].Stone J, Ferrara L, Kamrath J, et al. Contemporary outcomes with the latest 1000 cases of multifetal pregnancy reduction (MPR). Am J Obstet Gynecol 2008;199:406 e1–4. [DOI] [PubMed] [Google Scholar]
- [32].Haas J, Barzilay E, Hourvitz A, et al. Outcome of early versus late multifetal pregnancy reduction. Reprod Biomed Online 2016;33:629–34. [DOI] [PubMed] [Google Scholar]
- [33].Blickstein I. Is it normal for multiples to be smaller than singletons? Best Pract Res Clin Obstet Gynaecol 2004;18:613–23. [DOI] [PubMed] [Google Scholar]
- [34].Blickstein I, Goldman RD, Mazkereth R. Adaptive growth restriction as a pattern of birth weight discordance in twin gestations. Obstet Gynecol 2000;96:986–90. [DOI] [PubMed] [Google Scholar]
- [35].Alexander JM, Hammond KR, Steinkampf MP. Multifetal reduction of high-order multiple pregnancy: comparison of obstetrical outcome with nonreduced twin gestations. Fertil Steril 1995;64:1201–3. [DOI] [PubMed] [Google Scholar]
- [36].Begum G, Stevens A, Smith EB, et al. Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J 2012;26:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chaveeva P, Kosinski P, Puglia D, et al. Trichorionic and dichorionic triplet pregnancies at 10-14 weeks: outcome after embryo reduction compared to expectant management. Fetal Diagn Ther 2013;34:199–205. [DOI] [PubMed] [Google Scholar]
- [38].Committee on Ethics. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol 2017;130:e158–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


