“Pneumonia” is a Greek word meaning “inflammation of the lungs.” This illness is frequently acquired from exposure in the community and is therefore called community-associated pneumonia (CAP); rarely, it is acquired during/or after hospitalization, when it is referred to as nosocomial or hospital-associated pneumonia (HAP). The onset can be acute or chronic. This chapter focuses on acute, community-acquired pneumonia and its complications in infants and children.
ACUTE PNEUMONIA
Acute pneumonia is a common infection affecting infants and children, capable of causing significant morbidity and mortality.1 The clinical presentations and agents of pneumonia in children and infants are different compared with adults. An accurate measurement of the worldwide incidence of childhood pneumonia is difficult because of the many ways of defining pneumonia. In this chapter, acute pneumonia is defined as an acute lower respiratory tract infection (LRTI) with fever (>37.8°C), presence of lower respiratory tract signs, and radiologic evidence of a lung abnormality in one or both lungs.2
The estimated annual incidence of pneumonia in North America and Europe is 36 to 40 cases/1000 population in children younger than 5 years and is 11 to 16 cases/1000 in those 5 to 14 years of age.2 Every year, 1 to 4/1000 children are hospitalized in the United States with LRTI.3 The incidence of pneumonia is almost twofold higher in American Indian and Alaska native infants and children,4 and is 10- to 12-fold higher in the developing countries.5 In the developing world, pneumonia is the third leading cause of death in children, accounting for over 1.9 million deaths annually in children younger than 5 years.5, 6 In developed countries, acute pneumonia is associated with morbidity, with low mortality.
Etiologic Agents and Epidemiology
Multiple microbes cause LRTI in infants and children and establishing a microbial diagnosis is difficult. In two recent studies of immunocompetent hospitalized patients etiologic agents of pneumonia were confirmed in 79% to 85%.2, 7 However, these investigations to detect the etiology involved performing multiple laboratory tests, some only available in research laboratories. Other studies have confirmed etiologic agents of pneumonia in a minority of children.8 For some organisms, particularly viruses, Mycoplasma, and Chlamydiaceae, microbial etiology is inferred by detection of microorganisms in the upper respiratory tract. For others, serologic analysis or nucleic acid assay (polymerase chain reaction (PCR)) is the preferred method. Pyogenic bacteria present the most difficult challenge because some pathogens frequently coexist with or are normal upper respiratory tract flora. Bacteremia confirms the cause but is present in only 1% to 10% of hospitalized children with bacterial pneumonia.2, 7, 9 In most cases of acute pneumonia, extensive or invasive testing is not warranted. Epidemiologic information is frequently useful in guiding differential diagnosis and management (see Chapter 23, Respiratory Tract Symptom Complexes). Certain pathogens, particularly respiratory syncytial virus (RSV), rhinoviruses, influenza viruses, and Mycoplasma, are seasonal. In other instances, the pattern of family illness provides a clue to the causative agent.
For purposes of management, the relative importance of etiologic agents in series of patients who have been extensively evaluated is extrapolated to patients with similar clinical syndromes, physical findings, and laboratory results. Table 36-1 lists the common etiologic agents of acute pneumonia in children.
TABLE 36-1.
Microbial Causes of Community-Acquired Pneumonia in Childhood
| Age | Etiologic Agentsa | Clinical Features |
|---|---|---|
| Birth–3 weeks | Group B streptococcus | Part of early-onset septicemia; usually severe |
| Gram-negative enteric bacilli | Frequently nosocomial; occurs infrequently within 1 week of birth | |
| Cytomegalovirus | Part of systemic cytomegalovirus infection | |
| Listeria monocytogenes | Part of early-onset septicemia | |
| Herpes simplex virus | Part of disseminated infection | |
| Treponema pallidum | Part of congenital syndrome | |
| Genital Mycoplasma or Ureaplasma | From maternal genital infection; afebrile pneumonia | |
| 3 weeks–3 months | Chlamydia trachomatis | From maternal genital infection; afebrile, subacute, interstitial pneumonia |
| Respiratory syncytial virus (RSV) | Peak incidence at 2–7 months of age; usually wheezing illness (bronchiolitis/pneumonia) | |
| Parainfluenza viruses (PIV), especially type 3 | Similar to RSV, but in slightly older infants and not epidemic in the winter | |
| Streptococcus pneumoniae | The most common cause of bacterial pneumonia | |
| Bordetella pertussis | Primarily causes bronchitis; secondary bacterial pneumonia and pulmonary hypertension can complicate severe cases | |
| 3 months–5 years | RSV, PIV, influenza, hMPV, adenovirus, rhinovirus | Most common causes of pneumonia |
| Streptococcus pneumoniae | Most likely cause of lobar pneumonia; incidence may be decreasing after vaccine use | |
| Haemophilus influenzae | Type b uncommon with vaccine use; nontypable stains cause pneumonia in immunocompromised hosts and in developing countries | |
| Staphylococcus aureus | Uncommon, although CA-MRSA is becoming more prevalent | |
| Mycoplasma pneumoniae | Causes pneumonia primarily in children over 4 years of age | |
| Mycobacterium tuberculosis | Major concern in areas of high prevalence and in children with HIV | |
| 5–15 years |
Mycoplasma pneumoniae |
Major cause of pneumonia; radiographic appearance variable |
| Chlamydophila pneumoniae | Controversial, but probably an important cause in older children in this age group | |
CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; HIV, human immunodeficiency virus; hMPV, human metapneumovirus.
Ranked roughly in order of frequency. Uncommon causes with no age preference: enteroviruses (echovirus, coxsackievirus), mumps virus, Epstein–Barr virus, Hantavirus, Neisseria meningitidis (often group Y), anaerobic bacteria, Klebsiella pneumoniae, Francisella tularensis, Coxiella burnetii, Chlamydophila psittaci. Streptococcus pyogenes occurs sporadically or especially associated with varicella-zoster virus infection.
Neonates and Young Infants
Pneumonia in neonates can manifest as early-onset disease (within 5 to 7 days of life), or late-onset disease after 7 days of life. Most infections in the first week of life are caused by organisms acquired from the maternal genital tract through aspiration of either infected amniotic fluid or genital secretions. Group B streptococcus is the most frequent cause of early-onset pneumonia.10 Group B streptococcus, Listeria monocytogenes, Escherichia coli, and other gram-negative bacilli can cause severe respiratory distress resembling hyaline membrane disease, usually as a part of a widespread systemic infection. Prenatal and perinatal risk factors, including preterm delivery, maternal chorioamnionitis, and prolonged rupture of membranes, increase the risk for development of neonatal pneumonia. Hematogenous dissemination can also occur from an infected mother. Pneumonia due to Chlamydia trachomatis, which becomes symptomatic >2 to 3 weeks after birth, occurs in about 10% of infants born to women who carry this organism in their genital tract. Bordetella pertussis infection can lead to pulmonary hypertension (simulating pneumonia) or secondary bacterial pneumonia. Viruses are a less common cause of pneumonia in neonates compared with older infants. Severe pneumonia can be the result of congenital or perinatal infection with cytomegalovirus (CMV), herpes simplex virus (HSV), or Treponema pallidum. Genital Mycoplasma species and Ureaplasma urealyticum can cause LRTI in very-low-birthweight infants.
Infants, Children, and Adolescents
Traditionally, viruses have been considered to be the most common cause of acute LRTI in children between 1 and 36 months of age. However, in a recent study of acute pneumonia in immunocompetent, hospitalized children between 2 months to 17 years of age, bacteria were identified in 60%, viruses in 45%, Mycoplasma species in 14%, Chlamydophila pneumoniae in 9%, and mixed bacterial–viral infections in 23% of the cases.7
Viruses
Overall, viruses account for approximately 14% to 35% of CAP in childhood.11 However, when categorized by age, they accounted for 80% of CAP in children <2 years compared with 49% in those >2 years of age.12 RSV is the predominant respiratory tract viral pathogen. Other viruses include human metapneumovirus (hMPV),13 parainfluenza viruses (types 1, 2, and 3), influenza viruses (A and B), adenoviruses, rhinoviruses, and enteroviruses. Rhinoviruses have been recovered by culture in 2% to 24% cases of childhood pneumonia.2, 14, 15 Varicella-zoster virus (VZV), CMV, and HSV typically cause LRTI in immunocompromised children. Recently, human parechovirus 1 (HPeV-1), a picornavirus, was identified to cause LRTI in young children.16 Coronavirus became a global concern in 2003, causing the severe acute respiratory syndrome (SARS)17; although children were infected, the clinical course was mild with no documented death.18, 19 Infections with RSV, hMPV, and influenza viruses occur during the winter season whereas infections with parainfluenza viruses and rhinoviruses are more common in spring and autumn; adenovirus infections can occur throughout the year.
Mycoplasma pneumoniae and Chlamydophila pneumoniae
In one study, Mycoplasma pneumoniae was detected in 30% of children with CAP.20 Harris et al.21 found that children >5 years of age had a higher rate of Mycoplasma infection (42%) compared with children <5 years of age (15%). Coinfections with either Streptococcus pneumoniae (30%) or Chlamydophila pneumoniae (15%) are common.22 Infections due to M. pneumoniae occur in 2- to 4-year epidemic cycles.23 Unlike respiratory viruses that cause rapid transmission among family members, transmission of M. pneumoniae is slow with a median interval of 3 weeks between cases in family members.24 Between 9% and 20% of cases of CAP in children of all ages are associated with recovery of C. pneumoniae;11 the median age is 35 months.7 Asymptomatic carriage of C. pneumoniae is well documented and confounds assessment of pathogenicity.
Bacterial Pathogens
Bacterial pneumonia is more common in children living in developing countries, presumably due to chronic malnutrition, crowding, and chronic injury to the respiratory tract epithelium from exposure to cooking and heating with biomass fuels without adequate ventilation.25
Various tests that determine bacterial products in blood, respiratory tract secretions, and urine have been used in an attempt to ascribe a causative role of bacteria but are positive in fewer than 10% of cases.2, 7, 9, 26–30 Evidence from multiple sources indicates that S. pneumoniae is the single most common cause of bacterial pneumonia beyond the first few weeks of life.2, 11, 31 The serotypes that cause uncomplicated pneumonia in the United States are generally similar to those that cause bacteremia or acute otitis media (see Chapter 123, Streptococcus pneumoniae). Pneumococcal pneumonia occurs in all age groups.2, 26, 32
In the United States and Europe, the frequency of Haemophilus influenzae type b (Hib) infection, including pneumonia, has been markedly reduced in the past decade because of widespread immunization with the Hib conjugate vaccine.33 Pneumonia due to nontypable H. influenzae is also uncommon in the United States except in children with underlying chronic lung disease, immunodeficiencies, or aspiration. Recently, a virulent strain of community-associated, methicillin-resistant Staphylococcus aureus (CA-MRSA) carrying virulence factors including the Panton–Valentine leukocidin has emerged as an important agent of pneumonia, including, the United States,34 causing life-threatening necrotizing pneumonia. Streptococcus pyogenes (group A streptococcus) is not a frequent cause of acute pneumonia. However, both staphylococcal and streptococcal pneumonia are rapidly progressive and severe, frequently leading to hypoxemia and pleural effusion within hours. Other bacteria, especially Gram-negative organisms, are rare causes of pneumonia in previously healthy children.
Occasional Pathogens
A variety of epidemiologic and host factors prompt consideration of specific organisms (Table 36-2 ). The most important of these is Mycobacterium tuberculosis (MTB), which should always be suspected if there is a history of exposure, in the presence of hilar adenopathy, or when pneumonia does not respond in a typical fashion to therapy or with passage of time. In North America and Europe, primary MTB in children is most common among those born to recent immigrants from countries with a high prevalence of infection, after contact with infected adults, or in HIV-infected individuals.
TABLE 36-2.
Occasional Causes of Pneumonia in Special Circumstances
| Organism | Risk Factors | Diagnostic Methods |
|---|---|---|
| Histoplasma capsulatum | Exposure in certain geographic areas (Ohio and Mississippi river valley, Caribbean) | Culture of respiratory tract secretions; urine antigen; serum immunodiffusion antibody test; and serum histoplasma complement fixation antibody test |
| Coccidioides immitis | Exposure in certain geographic areas (southwestern United States, Mexico, and Central America) | Culture of respiratory tract secretions; serum immunodiffusion antibody test |
| Blastomyces dermatitidis | Exposure in certain geographic areas (Ohio, Mississippi, St. Lawrence river valleys) | Culture of respiratory tract secretions; serum immunodiffusion antibody test |
| Legionella pneumophila | Exposure to contaminated water supply | Culture or direct fluorescent assay of respiratory tract secretions; antigen test on urine |
| Francisella tularensis | Exposure to infected animals, usually rabbits | Acute and convalescent serology |
| Pseudomonas pseudomallei (melioidosis) | Travel to rural areas of Southeast Asia | Culture of respiratory tract secretions; acute and convalescent serology |
| Brucella abortus | Exposure to infected goats, cattle, or their products of conception; ingestion of unpasteurized milk | Acute and convalescent serology |
| Leptospira spp. | Exposure to urine of infected dogs, rats, or swine, or to water contaminated by their urine | Culture of urine; acute and convalescent serology |
| Chlamydophila psittaci | Exposure to certain infected birds (often parakeets) | Acute and convalescent serology |
| Coxiella burnetii | Exposure to infected sheep | Acute and convalescent serology |
| Hantavirus | Exposure to dried mouse dung in a closed structure (opening cabins after winter closure) | Acute and convalescent serology; PCR test on the respiratory tract secretions |
PCR, polymerase chain reaction.
Residence in or travel to certain geographic areas suggests consideration of certain pathogens. Coccidioides immitis is endemic in the southwestern United States, northern Mexico, and parts of Central and South America. Histoplasma capsulatum is endemic in the eastern and central United States and Canada. Some other pathogens such as Chlamydophila psittaci and Coxiella burnetii are transmitted from infected birds, animals, or humans. Pneumocystis jirovecii (P. carinii) causes pneumonia in HIV-infected infants at 3 to 6 months of age, in severely malnourished children, and in other immunocompromised or immunosuppressed hosts. Legionella pneumophila is a rare cause of pneumonia in children but is considered with certain environmental exposures and in immunocompromised individuals.
Pathogenesis and Pathology
Pneumonia occurs in a child who lacks systemic or secretory immunity to a pathogenic organism. Invasion of the lower respiratory tract or lung usually occurs at a time when normal defense mechanisms are impaired, such as after a viral infection, during chronic malnutrition, or with exposure to environmental pollutants. High density of aerosal exposure or hematogenous spread can occasionally cause bacterial pneumonia.
The pulmonary defense mechanisms against LRTI consist of: (1) physical and physiologic barriers; (2) humoral and cell-mediated immunity; and (3) phagocytic activity. Physical barriers of the respiratory tract include the presence of hairs in the anterior nares that can trap particles >10 μm in size, configuration of the nasal turbinates, and acute branching of the respiratory tract. Filtration and humidification capacities of the upper airways, mucus production, and protection of the airway by the epiglottis and cough reflex (eliminating particles between 2 and 10 μm) are protective physiologic functions. Mucociliary transport moves microscopic amounts of normally aspirated oropharyngeal flora and particulate matter up the tracheobronchial tree, minimizing the presence of bacteria below the carina. However, particles less than 1 μm can escape into the lower airways. Immunoglobulin A (IgA), which has good antibacterial and antiviral activities, is the major antibody secreted by the upper airways; IgG and IgM primarily protect the lower airways. In addition, other substances found in alveolar fluid – including surfactant, fibronectin, complement, lysozyme, and iron-binding proteins – have antimicrobial activity. The LRT has four distinct populations of macrophages. Of these, the alveolar macrophages are the pre-eminent phagocytic cells that ingest and kill bacteria. Viral infection (especially due to influenza virus), high oxygen concentration, uremia, and use of alcohol and/or drugs can impair the function of the alveolar macrophages, predisposing to pneumonia. Cell-mediated immunity plays an important role in certain pulmonary infections such as those caused by M. tuberculosis and Legionella species.
Viral Pneumonia
Three pathologic patterns are seen with viral pulmonary infections: bronchiolitis, interstitial pneumonia, and parenchymal infection. The first two patterns often overlap.35, 36 Viral pneumonia is characterized by neutrophilic infiltration of the lumen of the airway with lymphocytic infiltration of the interstitium and parenchyma of the lungs.37 Giant cell formation can be seen in infections due to measles or CMV, or in children with immune deficiency. Viral inclusions within the nucleus of respiratory cells can be seen in adenoviral pneumonia.38, 39 Air trapping with resultant disturbances in ventilation–perfusion ratio can occur from obstructed or obliterated small airways and thickened septa impeding oxygen diffusion. Necrosis of bronchial or bronchiolar epithelium can be seen in severe, sometimes fatal, viral infections (e.g., adenovirus infection).
Bacterial Pneumonia
Five pathologic patterns are seen with bacterial pneumonia: (1) parenchymal infection/inflammation/consolidation of a lobe or a segment of a lobe (lobar pneumonia, the classic pattern of pneumococcal pneumonia); (2) primary infection of the airways and surrounding interstitium (bronchopneumonia, often seen with Streptococcus pyogenes and Staphylococcus aureus); (3) necrotizing parenchymal pneumonia that occurs after aspiration; (4) caseating granulomatous disease, as seen with tuberculous pneumonia; and (5) peribronchial and interstitial disease with secondary parenchymal infiltration, as seen when viral pneumonia (usually due to influenza or measles) is complicated by bacterial infection.39 Bacterial pneumonia is associated with diffuse polymorphonuclear infiltration. The airspaces become filled with transudates or exudates, impairing oxygen diffusion. The proximity of alveoli and a rich pulmonary vascular bed increase the risk for complications, such as bacteremia, septicemia, or shock.
Clinical Manifestations
Use of clinical symptoms and signs to differentiate among sites and causes of LRTI is also discussed in Chapter 23, Respiratory Tract Symptom Complexes. The symptoms of pneumonia are varied and nonspecific. Acute onset of fever, rapid breathing (tachypnea), and cough are the classic symptom complex of pneumonia.40 Fever may be absent in very young infants and in infections due to Chlamydia trachomatis, B. pertussis, and Ureaplasma. Some children have a prodrome of low-grade fever and rhinorrhea prior to developing lower respiratory tract symptoms. There is no single sign that is pathognomonic for pneumonia. Tachypnea, nasal flaring, decreased breath sounds, and auscultatory crackles (crepitations or rales) are specific signs of LRTI. Crackles can be absent in a dehydrated patient. Guidelines developed by the World Health Organization (WHO) for the clinical diagnosis of pneumonia in developing countries highlight tachypnea and retractions as the two best indicators of LRTI.41 Tachypnea is defined as >50 breaths/minute (min) in infants <12 months of age, >40 breaths/min in those between 1 and 5 years of age, and >30 breaths/min in children >5 years of age. Palafox et al. observed that, in children <5 years of age, of all the clinical signs of pneumonia, tachypnea (as defined by WHO) had the highest sensitivity (74%) and specificity (67%) for radiologically confirmed pneumonia but it was less sensitive and specific in early disease.42
Tachypnea can occur in other conditions such as asthma, cardiac disease, and metabolic acidosis. Crackles and bronchial breathing were reported to have sensitivity of 75% but specificity of only 57%43 for pneumonia. Isolated wheezing or prolonged expiration is associated with bronchiolitis and is uncommon in bacterial pneumonia.44 The sensitivity and specificity of clinical findings for predicting the presence of radiographically evident pneumonia have been evaluated in a number of studies.44–47 In one study, the combination of a respiratory rate >50 breaths/min, oxygen saturation < 96%, and the presence of nasal flaring in children <12 months of age was highly associated with radiographically confirmed pneumonia.48 About three-fourths of children with radiographically confirmed pneumonia appear ill. Severity of illness correlates with the likelihood of a bacterial cause. Approximately 6% to 25% of children <5 years of age with fever >39°C and a white blood cell (WBC) count >20,000/mm3 without an alternative source of major infection and with no symptoms or signs of lower respiratory tract disease have radiographically confirmed pneumonia.47, 49
A Medline search from 1982 to 1995 of studies that considered observer agreement of clinical examination suggested that observed clinical signs were better than auscultatory signs50; interobserver agreement was low in recognizing crackles, retractions, and wheezing, but high in determining respiratory rate and cyanosis. However, neither respiratory rate nor cyanosis is a specific or sensitive indicator of hypoxia. Oxygen saturation should be measured in any child with respiratory distress, especially if the child has retractions or decreased level of activity.51
Neonates and Young Infants
The neonate with bacterial infection due to group B streptococcus, Listeria monocytogenes, or gram-negative bacilli usually manifests respiratory distress in the first few hours of life. Septicemia and infection at other sites, including the meninges, can dominate the clinical presentation. Pneumonia in children younger than 2 months of age is usually characterized by tachypnea (respiratory rate >60 breaths/min), intercostal retractions, or both.52 In very young infants, particularly those who are born prematurely, fever may be absent and apneic spells may be the most prominent initial finding of LRTI from any cause.53 Infants with C. trachomatis pneumonia present insidiously between 3 weeks and 3 months of age with staccato cough, tachypnea, crackles on pulmonary auscultation, and absence of fever. Significant laboratory findings include eosinophilia and elevated total serum IgM concentration.54–56
Infants, Children, and Adolescents
Viral Pneumonia
The onset of viral pneumonia is usually gradual and occurs in the context of a preceding upper respiratory tract illness (URI) (rhinorrhea, low-grade fever, and decreased appetite) in the patient or family members. There is then increase in irritability, respiratory congestion, cough, posttussive emesis, and fever. The patient may not appear toxic although hypoxia can be marked, particularly in a young infant, whose initial presentation can be apnea. The auscultatory findings are not anatomically confined but diffuse and bilateral, consisting of wheezing and crackles. Adenovirus usually produces signs and symptoms similar to other viral infections but it can also cause severe pneumonia similar to a bacterial infection, especially in immunocompromised hosts.
Bacterial Pneumonia
The onset of bacterial pneumonia is usually abrupt but may follow several days of mild URI. The patient with pyogenic bacterial pneumonia is usually ill and toxic-appearing with high fever, rigors, and tachypnea. Respiratory distress and hypoxemia, however, can be absent or mild unless there is widespread disease or a large pleural effusion. Cough occurs later in the course of the illness when the debris from the involved lung is swept into the upper airway. Unilateral pleuritic chest pain or abdominal pain in the presence of radiographically demonstrated infiltrate is a specific sign of bacterial pneumonia. Unless there is a parapneumonic effusion, auscultatory findings are usually few (especially in infants) and are focal and limited to an anatomic segment. These include decreased tactile and vocal fremitus on palpation, diminished air entry with rales, and dullness to percussion over the involved area of the lung. Presence of wheezing, in an otherwise healthy child, usually excludes pyogenic bacterial pneumonia.
Other Pathogens
The major symptoms of LRTI due to Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Coxiella burnetii (Q fever) are fever and cough that persist for more than 7 to 10 days. The onset of pneumonia caused by M. pneumoniae is not usually well demarcated but malaise, headache, sore throat, fever, and photophobia occur early, and sometimes subside when gradually worsening, nonproductive cough ensues. Although coryza is an unusual symptom, ear infection with or without bullous myringitis can occur with M. pneumoniae infections. Findings on physical examination and auscultation can be minimal, most commonly dry or musical crackles. The presence of Stevens–Johnson syndrome or hemolytic anemia suggests M. pneumoniae infection. M. pneumoniae can cause severe disease in persons with sickle-cell disease in whom acute chest syndrome is common. Chlamydophila pneumoniae infection usually causes bronchospasm and can cause an acute exacerbation of asthma. Q fever, caused by Coxiella burnetii, has an acute onset with intractable headache, fever, and cough with round parenchymal opacities on chest radiograph.
Differential Diagnosis
Pneumonia is highly probable in children with fever, cough, tachypnea, and shortness of breath (dyspnea) in whom chest radiograph demonstrates pulmonary infiltrates. There are many alternative diagnoses, particularly in the absence of fever or with chronic or relapsing symptoms and signs. These include foreign-body aspiration, asthma, gastroesophageal reflux, cystic fibrosis, congestive cardiac failure, systemic vasculitis, and bronchiolitis obliterans. Children who develop chemical pneumonia after ingestion of volatile hydrocarbons can have severe necrotizing pneumonia with high fever and peripheral neutrophil counts exceeding 15 000/mm3.
Laboratory Findings and Diagnosis
Radiograph
Routine use of chest radiography did not change the clinical outcome in most cases in a study evaluating ambulatory children >2 months of age with acute LRTI.57, 58 Prescription of an antibiotic was more frequent in those who underwent radiography (61% versus 53%). However, chest radiography is necessary to confirm the presence and determine the location of pneumonia in the following patients: those<12 months of age with acute LRTI who are hospitalized or severely ill; those who have recurrent disease or chronic medical conditions; those who develop complications; and those in whom the diagnosis is uncertain. The chest radiograph can appear falsely normal in children examined early in the course of pneumonia or in dehydrated patients.44 Chest radiography is insensitive in differentiating bacterial from nonbacterial pneumonia; however, combined with clinical findings, it is accurate in excluding most cases of bacterial pneumonia.59, 60
Bilateral diffuse infiltrates are seen with pneumonia caused by viruses, Pneumocystis jirovecii, Legionella pneumophila and occasionally M. pneumoniae. Both Chlamydophila pneumoniae and M. pneumoniae (Figure 36-1 ) cause focal radiographic abnormalities, which are out of proportion to clinical findings. Distinctly confined lobar or segmental abnormality or a large pleural effusion suggests bacterial infection (Figure 36-2 ). Rarely, M. pneumoniae or adenovirus can manifest with these findings.61–63 Round appearance of infiltrate is common in children younger than 8 years of age and is most often due to Streptococcus pneumoniae.
Figure 36-1.
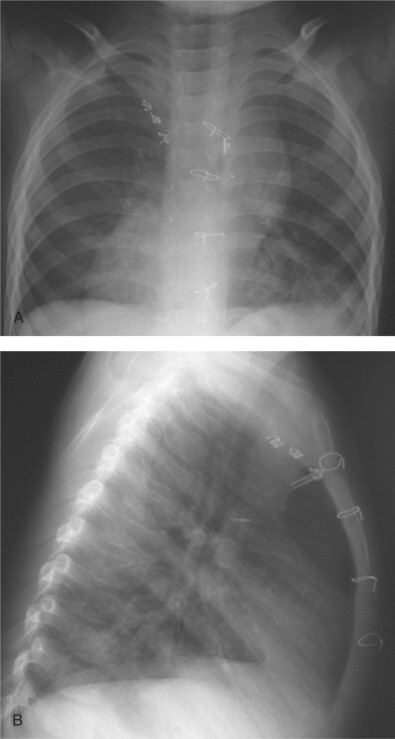
(A) Posteroanterior and (B) lateral plain radiographs of a 2-year-old child with dextrocardia, complex congenital heart disease, and Mycoplasma pneumoniae pneumonia. Note bilateral patchy alveolar infiltrates in both lower lobes.
(Courtesy of E.N. Faerber and S.S. Long, St. Christopher's Hospital for Children, Philadelphia, PA.)
Figure 36-2.
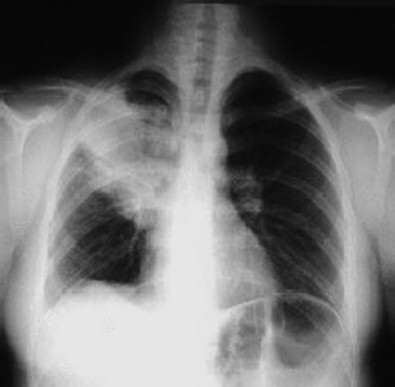
Plain radiograph showing consolidative pneumonia in the right upper lobe, typical of acute bacterial pneumonia.
Enlarged or calcified hilar lymph nodes suggest tuberculosis or a fungal infection such as histoplasmosis, and can also occur in Mycoplasma pneumonia and in patients with cystic fibrosis. Tuberculosis is highly likely in an adolescent with epidemiologic risk factors and apical disease or cavitation. Pneumatoceles (thin-walled air–fluid-filled cavities) resulting from alveolar rupture are usually associated with infection due to Staphylococcus aureus but can be seen in infections due to Streptococcus pneumoniae, S. pyogenes, Hib, other gram-negative bacteria, or anaerobes. Involvement of the lower lobes, particularly with recurrent infections, suggests aspiration pneumonia, or if confined to the same site, pulmonary sequestration. Recurrent bacterial pneumonia involving the same anatomic area suggests congenital anomaly or foreign body whereas recurrences in different areas suggest an abnormality of host defense, cystic fibrosis, or other causes (see Chapter 37, Persistent and Recurrent Pneumonia).
A chest radiograph is rarely useful in following the clinical course of a child with acute pneumonia who is recovering as expected. Radiographic improvement lags clinical changes significantly; complete resolution is expected in children 4 to 6 weeks after onset. Follow-up radiography is indicated for children with lobar collapse, complicated pneumonia, recurrent pneumonia, and round pneumonia (to exclude tumor as the cause).64, 65
Laboratory Tests
Indices of host response (acute-phase reactants), including peripheral WBC, white blood cell differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level, best detect invasive infections, particularly those caused by bacteria. Viral pneumonia is associated with a less brisk rise of acute-phase reactants than bacteria pneumonia. However, some viral agents, especially adenovirus, influenza, and measles virus, can induce a host response similar to that of invasive bacterial infection. In a prospective study examining the utility of routinely obtaining the acute-phase reactants in children with pneumonia, the authors concluded that these tests do not stand alone as indicators of bacterial versus viral pneumonia.66, 67
Diagnosis of Specific Agents
The rigor of an investigation for specific causative agents in pneumonia depends on the severity of illness, the presence of underlying disease, and clinical manifestations. Mildly to moderately ill ambulatory patients can often be managed empirically without specific diagnostic tests. Under circumstances in which the identity of a specific etiologic diagnosis is desired, a number of investigations may be warranted. Some of these circumstances include all patients admitted to the hospital with pneumonia, patients with underlying medical conditions, and when there is a community outbreak caused by an organism that has recently been recognized to be contagious (i.e., an emerging infection).
Viruses
A viral pathogen is best identified by recovering the organism in tissue culture or by detection of viral products (antigens or nucleic acid) in respiratory tract secretions. Combined real-time PCR can rapidly detect common viral and atypical bacterial agents of CAP.68 However, both false-positive and false-negative results can occur when the specimen is improperly obtained or transported and/or the tests are suboptimally performed. A nasopharyngeal wash or aspirate is the most sensitive specimen because it contains infected epithelial cells. The presence of a viral agent in the upper respiratory tract does not exclude the presence of secondary bacterial pneumonia. Clinical correlation is necessary. Obtaining acute and convalescent sera to assess rising antibodies to various viruses is usually confined to research settings.
Other Pathogens
M. pneumoniae can be detected most effectively by PCR methodology but the test may not be available in most hospital or commercial laboratories. Mycoplasma culture is available in some commercial and hospital laboratories but can take 3 weeks to complete. Cold agglutinins (IgM antibodies that agglutinate human red cells) are found in 30% to 75% of individuals with M. pneumoniae pneumonia during the acute phase of the disease.69 The titer correlates with the severity of disease; a titer of 1:64 or greater has a high predictive value for M. pneumoniae infection. The cold agglutinin test result can be positive in lower titers in infections due to adenovirus, influenza virus, Epstein–Barr virus, and CMV, as well as in lymphoma. The test can be negative in young children and in those with mild disease. Testing for serum IgM and IgA antibodies to M. pneumoniae is positive in 80% of cases during the early convalescent period70; specificity and reproducibility may be suboptimal. Examining paired sera is the most definitive test.
Chlamydophila pneumoniae infection is identifiable serologically, by isolation of the organism in tissue culture, or by PCR, although none of these tests may be readily available.71 Serology is also effective in detecting infections with other agents that cause atypical pneumonia, namely C. psittaci and Coxiella burnetii.
When tuberculosis is considered, a Mantoux skin test (a 5 TU intradermal test, purified protein derivative (PPD)) should be placed on the patient as well as on all the immediate family members and other significant contacts. In acutely ill patients, the PPD skin test can be nonreactive because of general or specific anergy to MTB antigen. When the suspicion of tuberculosis is strong, multiple respiratory tract specimens should be obtained; specimens include sputum (spontaneous or induced), gastric aspirate, and/or bronchoalveolar lavage. Gastric aspirates are superior to bronchoscopic specimens in infants with tuberculosis72 and should be obtained in patients with suspected primary pulmonary infection or miliary disease without cough.
Bacterial Pathogens
The diagnosis of most bacterial causes of pneumonia is problematic. Young children do not effectively cough up sputum, resulting in a specimen contaminated with saliva. In older children, a sputum sample is considered appropriate for microbiologic evaluation when Gram stain reveals <10 squamous epithelial cells and >25 neutrophils per low-power field, and a predominant organism. Nasopharyngeal cultures are not usually reliable specimens because many bacterial pathogens are also common commensals. Further, noncommensal organisms residing in the upper airway may not be the causative agent of LRTI. Tracheal aspiration is useful for culture if performed with direct laryngoscopy. However, culture samples obtained via a catheter directly passed through a tracheostomy, endotracheal tube, or deep nasotracheal tube have limitations due to frequent contamination with upper respiratory tract organisms. (They could be evaluated as for a sputum sample.) Quantitative culture performed on a bronchoalveolar lavage specimen is useful, with isolate colony count >104/mL considered significant. Blood culture is specific but insensitive. A recent study demonstrated that transthoracic needle aspiration (lung tap) in hospitalized children with clinical pneumonia had a high microbiologic yield and was relatively safe.31 However, this procedure is not widely performed in the United States. A diagnostic thoracocentesis should be considered in patients with more than a minimal pleural effusion, when the etiology is obscure, or when mechanical removal is indicated. A lung biopsy and/or a broncho-alveolar lavage may be necessary to confirm the diagnosis in patients who are seriously ill, immunocompromised, intubated, or who are not responding to empiric therapy.
Management
Indications for Hospitalization
Hypoxemia with Sao2 <92% is the single most important indication for hospitalization because a hypoxemic child is at greater risk of death than an adequately oxygenated child.43 Other indications include cyanosis, rapid respiratory rate (RR >70 breaths/min in an infant or >50 breaths/min in a child), apnea, dyspnea, expiratory grunting, dehydration, toxic appearance, poor oral intake, recurrent pneumonia, underlying medical condition, or uncertain observation at home.
Supportive Therapy
Oxygen Therapy and Ventilatory Support.
Oxygenation is assessed continuously by measuring oxygen saturation or arterial P o 2. Hypoxic infants and children may not appear cyanotic until they are terminally ill. Mental agitation, clinically evident as increased irritability, may be an indication of hypoxemia. Therefore, supplemental oxygen therapy is indicated in any patient whose oxygen saturation is persistently 92% or less. Sole reliance on pulse oximetry values is hazardous in ill patients because hypercarbia is an important sign of impending respiratory failure, especially in the tiring young infant who may have relatively preserved oxygenation. Blood gas should be evaluated to detect impending respiratory failure and provide ventilatory support when indicated.
Fluid Therapy.
Rapid breathing, fever, and fatigue increase the fluid requirements in a child with acute LRTI. Most patients can be hydrated orally if they are given small volumes of fluids frequently. Intravenous hydration may be necessary for intubated or seriously ill children with very rapid breathing because of increased likelihood for pulmonary aspiration. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) can be seen in approximately one-third of patients hospitalized with pneumonia.73
Nutrition.
Malnutrition has been associated with a worse prognosis of pneumonia. Infants and small children fare better if fed in small quantities and more frequently to prevent pulmonary aspiration.74 Seriously ill or intubated children may require placement of an enteral feeding tube or parenteral nutrition.
Fever and Pain Management.
Persistent and high fever increases the basal metabolic rate and oxygen consumption. Similarly, pain interferes with the depth of breathing and with the ability to cough effectively. Therefore, it is important that the patient is kept comfortable by using age-appropriate antipyretic or analgesic agents or both.
Antimicrobial Therapy
Treatment of pneumonia in infants and children is often empiric because of the difficulty in proving etiology. Because viral LRTI is most frequent in previously healthy children, antibiotics are only administered when findings suggest bacterial infection.
Optimal antibiotic treatment of pneumonia in infants and children has not been determined by randomized controlled clinical trials. Recommendations are based on the most likely etiologic agents at different ages and in various settings. Because pathogens of pneumonia in neonates are similar to those of sepsis, broad-spectrum antibiotics like ampicillin and gentamicin are appropriate in this age group. A macrolide antibiotic is appropriate for Chlamydia trachomatis and Ureaplasma. In infants <1 month of age the preferred macrolide is azithromycin because azithromycin is not known to cause hypertrophic pyloric stenosis.75 For pertussis, the dose is 10 mg/kg per day on each of 5 days. Amoxicillin (80 to 90 mg/kg per day) is effective empiric therapy for febrile children with pneumonia; alternatives include high-dose amoxicillin-clavulanate (14:1 preparation), cefuroxime axetil, or cefdinir.76 In children >5 years of age in whom atypical organisms (Mycoplasma or Chlamydophila pneumoniae) are suspected, initial treatment with a macrolide or doxycycline (recommended only after 7 years of age) may be appropriate if pyogenic bacterial pneumonia is not likely. For a hospitalized child beyond the neonatal period with uncomplicated pneumonia, initial parenteral (intravenous) therapy with ampicillin is appropriate, even in areas with penicillin-nonsusceptible Streptococcus pneumoniae. Some experts recommend cefuroxime, ceftriaxone, cefotaxime, or ampicillin-sulbactam, and use higher doses of beta-lactam agents than in the era prior to penicillin-nonsuspectibility.77–79 While the use of vancomycin, clindamycin, or linezolid is not recommended for initial treatment of uncomplicated CAP, these drugs are considered if infection due to CA-MRSA is suspected, if pneumonia is unresponsive to initial antibiotics, or in those patients allergic to beta-lactam agents.80 Other antimicrobial agents may be chosen if a likely pathogen is identified, the case has clinical or epidemiologic features strongly suggestive of a particular infection, or the evolution of the disease suggests a more specific cause.
Opinions differ about the frequency with which viral pneumonia is complicated by bacterial superinfection.81 There is a good deal of evidence, however, that withholding antibiotics from hospitalized children with pneumonia clinically compatible or proven to be of viral origin is safe and is preferable to empiric antibiotic treatment.82 Use of specific antiviral therapy depends on the pathogen, the severity of the clinical course, and availability of effective nontoxic therapy. Use of ribavirin for the treatment of RSV and acute LRTI is guided by recommendations from the American Academy of Pediatrics,83, 84 although the value of treatment has been questioned.85, 86
Prognosis and Sequelae
Mortality due to CAP is uncommon beyond infancy in Europe and North America because of improved and enhanced immunization rates, early access to medical care and availability of antimicrobial therapy. Most healthy children with acute LRTIs recover without sequelae. However, in some patients, especially premature infants, immunocompromised hosts, or in children with chronic lung, neuromuscular, or cardiovascular diseases, complications can develop from an acute LRTI.
In the late 1990s, there was a significant increase in the relative incidence of complications from bacterial pneumonia in infants and children living in North America,87, 88 the exact reason for which is still obscure.89 Since 2000, universal immunization with pneumococcal conjugate vaccine in children less than 2 years of age has reduced the frequency of complications due to pneumococcal pneumonia,90 and the overall complications due to presumed bacterial pneumonia. The complications of bacterial pneumonia include: necrotizing pneumonia, parapneumonic effusion, empyema, pneumatocele formation, and lung abscess.
Several epidemiologic studies have linked asthma and other respiratory problems occurring later in childhood to viral bronchiolitis or atypical pneumonia in infancy.91–95 Although many etiologic agents, including RSV and Chlamydia in particular, have been implicated, bacterial pneumonia has not been specifically found to have long-term sequelae. One study suggested that chronic cough can follow C. trachomatis pneumonia in infancy96 and another has suggested that asthma can follow Chlamydophila pneumoniae infection.97 A study of 35-year-old adults, whose parents had been interviewed 18 years previously, showed that those reported to have had pneumonia before age 7 years demonstrated a significant reduction of forced expiratory volume and forced vital capacity,98 confirming findings of prior studies.99, 100 Several longitudinal studies of lung function in children with bronchiolitis have suggested that lung function abnormalities may have preceded the acute infectious illness.101–103 Thus, it remains unclear whether childhood pneumonia causes subsequent pulmonary abnormalities.
Prevention
Most respiratory viral infections are transmitted by direct inoculation from hands contaminated with respiratory secretions on to conjunctival and nasal mucosa. Spread by airborne droplets also occurs occasionally. Hand hygiene by caregivers or medical personnel, before and after contact with patients, is the single most important method of preventing hospital-associated infections. Spread of infection by small droplets can be reduced by placing the patient in a negative-pressure room. All caregivers should wear facemasks and goggles.
The development of vaccines for the prevention of pneumonia is complicated by the large number of etiologic agents. Universal use of Hib conjugate vaccine has eliminated invasive Hib disease. Universal immunization with pneumococcal conjugate vaccine has significantly reduced the incidence of pneumococcal pneumonia in children,104, 105 and in elderly contacts through herd immunity.106, 107
RSV bronchiolitis and pneumonia can be reduced in high-risk infants by passive immunoprophylaxis using palivizumab, a monoclonal antibody directed at the fusion protein of RSV, which can be administered intramuscularly every month to infants and young children at high risk for infection (see Chapter 225, Respiratory Syncytial Virus).108, 109 Annual vaccination against influenza is recommended for individuals >6 months of age with underlying medical conditions such as chronic lung diseases (including mild asthma), neuromuscular disorders, congenital cardiac conditions, and diabetes because of high risk of complications or more severe respiratory disease. Annual influenza vaccine is also recommended for all healthy children between 6 and 59 months of age to reduce morbid disease in them and the burden of disease in the community.110 It is anticipated that the incidence of bacterial pneumonia in children, especially that caused by Staphylococcus aureus and Streptococcus pyogenes, will decrease substantially in the future with increased immunization rates for influenza and varicella viruses in young children.
PLEURAL EFFUSION, PARAPNEUMONIC EFFUSION, AND EMPYEMA
Pleural effusion is the presence of demonstrable fluid of any character between the visceral and parietal pleurae. Pleural effusions are classified as being a transudate or an exudate based on the biochemical characteristics of the fluid. The relative concentration of pleural fluid protein to serum protein is >0.5 in an exudate versus <0.5 in a transudate (Table 36-3 ). Exudates can have an infectious or noninfectious cause whereas transudates are less often caused by infections. Noninfectious causes of pleural effusions are listed in Table 36-4 . Several drugs, including hydralazine, nitrofurantoin, dantrolene, amiodarone, methysergide, procarbazine, bromocriptine, methotrexate, and agents associated with a lupus-like reaction, are associated with pleural effusions. Parapneumonic effusions are inflammatory fluid collections adjacent to a pneumonic process, seen in about 40% of cases of bacterial pneumonia.84 They can be classified as complicated or uncomplicated on the basis of various characteristics, particularly pH, glucose, and lactate dehydrogenase (LDH) concentrations (see Table 36-3).111 The term empyema is used when a parapneumonic fluid becomes purulent or seropurulent.
TABLE 36-3.
Biochemical Characteristics of Parapneumonic Pleural Effusions
| Laboratory Value | Uncomplicated Effusion transudate | Complicated Effusion exudate |
|---|---|---|
| pH | > 7.2 | < 7.1 |
| Glucose level | >40 mg/dL | <40 mg/dL |
| Lactate dehydrogenase concentration | <1000 IU/mL | >1000 IU/mL |
| Pleural protein:serum protein | <0.5 | >0.5 |
TABLE 36-4.
Noninfectious Causes of Pleural Effusion in Children
| Transudate | Exudate | |
|---|---|---|
| Hypoalbuminemia | Spontaneous chylothorax | Malignancy |
| Congestive heart failure | Posttrauma or postsurgical | Collagen vascular disease |
| Cirrhosis with ascites | Postoperative chylothorax | Pancreatitis |
| Myxedema | Pulmonary lymphangiectasia | Subphrenic or other intra-abdominal abscess |
| Peritoneal dialysis | Uremic pleuritis | |
| Central venous catheter leak | Sarcoidosis | Drug reaction |
| Fluid mismanagement | Dressler syndrome (postmyocardial infarction) | Meig syndrome (pelvic tumor) |
| Adult respiratory distress syndrome |
Parapneumonic effusion (PPE) usually occurs as a complication of pyogenic bacterial pneumonia but can occasionally occur secondary to other etiologic agents (e.g., Mycoplasma) or as a consequence of an infection from another contiguous site. PPE can be complicated (CPPE) or uncomplicated (UPPE). CPPE and empyema represent a different spectrum of the same disease.112 The estimated incidence of empyema in children is approximately 3.3 per 100,000.84 Both CPPE and empyema are serious illnesses, often associated with significant morbidity113, 114 but with low mortality rates. Seventy percent of complicated pneumonia occurs in children <4 years of age; pneumatoceles (defined as air–fluid-filled alveoli) occur predominantly in children <3 years of age.115
Etiologic Agents
During the latter 1990s Streptococcus pneumoniae, especially serotype 1, emerged as the most common isolate from children with complicated parapneumonic effusion.116 With the introduction of universal conjugated pneumococcal vaccination in the United States, the incidence of CPPE due to vaccine-serotype S. pneumoniae has decreased,87 although serotype 1 and other nonvaccine serotypes appear to be emerging.117 Likewise, universal childhood immunization against Hib has also significantly reduced the incidence of CPPE due to this agent. CA-MRSA is an important cause of pneumonia and CPPE in children.104, 118 In South Asia, Staphylococcus aureus is the most common cause of CPPE or empyema.119 Less frequently, group A streptococcus, Pseudomonas aeruginosa, mixed anaerobic pathogens, Mycobacterium species and, rarely, fungi can be etiologic agents.120 Although effusions have been described in pneumonia due to M. pneumoniae and viruses,121–123 they are rarely large enough to require intervention. In several large studies, PPEs are found to be sterile in 22% to 58% of cases.116, 124–128
Pathogenesis and Pathologic Findings
Under normal circumstances the pleural space contains 0.3 mL of fluid per kilogram body weight. The pleural circulation is maintained by a delicate balance between secretion and absorption of pleural fluid by lymphatic vessels in the pleura. When this balance is disturbed, fluid accumulates. Various infectious agents induce pleural effusion by different mechanisms. Effusion can result from a sympathetic response to a bacterial infection (by elaboration of cytokines), extension of infection, an immune-complex phenomenon (e.g., pneumococcal infections) or as a hypersensitivity reaction (e.g., rupture of tuberculous granulomas). Replication of microorganisms in the subpleural alveoli precipitates an inflammatory response resulting in endothelial injury, capillary permeability, and extravasation of pulmonary interstitial fluid into the pleural space. The pleural fluid is readily infected because it lacks opsonins and complement. Bacteria also interfere with the host defense mechanism by production of endotoxins and other toxic substances. Anaerobic glycolysis results from further accumulation of neutrophils and bacterial debris. This in turn causes pleural fluid to become purulent and acidic (i.e., empyema). The acidic environment of the pleural fluid suppresses bacterial growth and interferes with antibiotic activity. With disease progression, more inflammatory cytokines are released and there is activation of coagulation leading to deposition of fibrin.
The American Thoracic Society has divided empyema into three stages: (1) exudative phase, in which the pleural fluid has low cellular content; (2) fibrinopurulent phase, in which frank pus containing increased neutrophils and fibrin is formed. This fibrinous pus coats the inner surfaces of the pleura, interfering with lung expansion. Fibrin also leads to loculations within the pleural space; and (3) organizational phase (late stage), in which fibroblasts migrate into the exudate from visceral and parietal pleurae, producing a nonelastic membrane called the pleural peel. Before the availability of antibiotics, spontaneous drainage sometimes occurred by rupture through the chest wall (empyema necessitans) or into the bronchus (bronchopleural fistula). At present, such events are rare and are usually seen due to antibiotic-resistant, nosocomially acquired bacteria or insidiously progressive actinomycosis.
The acidic pleural pH is the basis for biochemical tests that differentiate uncomplicated effusions (which usually resolve with antibiotic therapy alone) from complicated effusions (that benefit from more aggressive drainage and other interventions); see Table 36-3.
Clinical and Radiographic Manifestations
Necrotizing pneumonia or effusion should be suspected when the response of a lobar, lobular, or alveolar pneumonia to appropriate antibiotic therapy is slow, or if there is clinical deterioration during treatment. Early in the disease, the symptoms of pleural effusion can be nonspecific and include malaise, lethargy, and fever. This is followed by cough and rapid breathing. Pain in the chest and/or in the abdomen develops on the involved side, associated with high fever, chills, and rigors. The child may guard (splint) or lie on the involved side of the chest in an attempt to minimize pain. As the effusion progresses, so does difficulty in breathing (dyspnea). On physical examination, the patient is usually ill and toxic-appearing, febrile, with significant tachypnea and shallow respirations (to minimize pain). Scoliosis may be noted on the involved side and the affected side may be tender to palpation. Breath sounds are usually grossly diminished on auscultation. Crackles from an associated pneumonia may be audible. Pleural rub is usually audible when there is a small fibrinous exudate within the pleural space. The percussion note on the involved side is dull when the effusion is free-flowing; by contrast, dullness can disappear as the effusion organizes.
Chest radiography is more sensitive than physical examination, especially in detecting small pleural effusions. Blunting of the costophrenic angle, thickening of the normally paper-thin pleural shadow, and a subpulmonic density all suggest pleural effusion (Figure 36-3 ). Movement and layering of fluid on lateral decubitus films differentiate free effusions from loculated collections, pulmonary consolidation, and pleural thickening. In large effusions containing more than 1000 mL of fluid, compression of the lung and shift of the trachea away from the effusion occur.129 Computed tomography (CT) is not routinely necessary to differentiate simple parapneumonic effusions from empyema or for recognizing loculations within an empyema.130 However, CT often gives diagnostic information regarding the parenchymal component of the process not discernible by conventional radiographs.131 Ultrasonography is usually more useful in the management than in the detection of effusions; it helps to localize and estimate the size of an effusion precisely.
Figure 36-3.
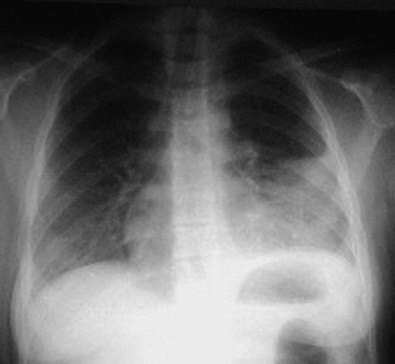
Plain radiograph showing left lower lobe pneumonia and a parapneumonic effusion, typical of acute bacterial pneumonia.
Laboratory Findings and Diagnosis
A sample of pleural fluid is obtained. The fluid should be centrifuged if it is cloudy on gross inspection; persistent cloudiness is suggestive of a chylothorax. Putrid odor is pathognomonic of anaerobic infection but is present in only half of such infections.
Pleural fluid is analyzed by biochemical (pH, glucose, and LDH levels), hematologic (total WBC count and differential), special staining techniques, and culture. If the diagnosis of pneumonia is not clear, other tests may be helpful, such as cellular histology and flow cytometry for malignancy. The usefulness of pH and glucose level in guiding the management of effusions is derived from data in adults.132, 133 The results of one small pediatric series support a similar interpretation in children.124 To enhance the accuracy of tests for pH and glucose, the fluid must be obtained in a heparinized syringe with the exclusion of air, placed on ice, and tested promptly. If immediate performance of such tests is not possible, the fluid should be stored at 0°C for no more than 2 hours. The pH can be lowered if the fluid is stored at room temperature and raised upon exposure to air. Effusions that are likely to require aggressive management (CPPEs) can be differentiated from those that do not require such management by gross appearance of pus, or by the pleural fluid having a pH <7.1, a glucose level <40 mg/dL, and a LDH concentration >1000 IU/mL.133
Neutrophil predominance in pleural fluid suggests an acute process, such as pneumonia, pancreatitis, pulmonary embolism, or intra-abdominal abscess.134 Lymphocytic predominance suggests tuberculosis, fungal, or Mycoplasma infection but can also be due to chylothorax; mixed mononuclear cell types suggest a more chronic process, such as malignancy, uremia, or collagen vascular disease. Pleural fluid eosinophilia is not usually helpful in determining the cause of an effusion in children,135 although eosinophilia is consistently found in effusions accompanying pneumonia due to various forms of paragonimiasis.136
Bacteriologic examination of pleural fluid always includes Gram stain and culture for aerobic and anaerobic bacteria. Acid-fast stain and culture for Mycobacterium tuberculosis, and fungal stain and culture, should be obtained from both the pleural fluid and sputum when clinically indicated. The possibility of isolating a pathogen from either the pleural fluid or blood varies widely, from 8% to 76%.124–126,137–140 Direct antigen testing is not considered useful because of technical problems associated with all the current tests available.
A Mantoux (PPD) skin test should be performed in patients with epidemiologic risk factors or clinical manifestations possibly suggesting tuberculosis. Anergy is unusual in the presence of pleural effusion.141 If organisms are not seen on acid-fast stains of the sputum or pleural fluid, a pleural biopsy (and thoracoscopy when possible) should be performed because granulomas are observed in 50% to 80% of pleural biopsy specimens compared with a 10% rate of isolation of Mycobacterium in cultures of pleural fluid in patients with tuberculous effusions.141, 142 Pleural biopsy is also useful in diagnosing other granulomatous infections (histoplasmosis, other fungal infections, and Francisella tularensis) and malignancies.
Cytokine levels, especially interleukin-8, are significantly higher in empyema. They may be useful to differentiate CPPE from UPPE.143, 144
Management
The optimal management of PPEs in children is controversial. Data underlying many of the decision points come from studies in adults, although effusions in children differ in the following ways from those in adults: the spectrum of causative organisms is not the same; underlying airway and lung diseases are less common and are different in children; and the capacity of the lung and pleural space to recover fully without aggressive intervention is greater in children. There have been no prospective pediatric trials comparing management schemes and, therefore, no optimal evidence-based management has been established.
The traditional approach to the management of PPEs is initially to obtain fluid by needle aspiration, primarily to establish a microbial etiology; this is followed by administration of antibiotic therapy and observation of the child's clinical course. In most cases, symptoms resolve. A more aggressive approach is taken if any of the following is present: persistence of fever or toxicity; rapid reaccumulation of the pleural effusion; respiratory compromise because of the size of the effusion. Under these circumstances, a drainage tube is initially inserted using ultrasonographic guidance. Benefit of fibrinolytic agents is controversial. Surgical intervention is considered in moderately severe cases.
In severely affected individuals, more aggressive approaches may be appropriate. Some experts advocate immediate thoracoscopic examination of the pleural space, lysis of the adhesions, and placement of a large-diameter drainage tube.127 With the availability of less invasive video-assisted thoracoscopic surgery (VATS), there is increasing opinion that VATS is the best treatment for patients with CPPE and empyema. In one study, treatment failure and mortality rates were higher in patients who had nonoperative therapies (chest tube placement, antibiotics, and/or fibrinolytic therapy) compared with primary operative therapy (VATS and thoracotomy).145 In addition, it was noted in this study that fibrinolytic therapy, using streptokinase or urokinase, was associated with more complications as systemic absorption of streptokinase after intrapleural instillation resulted in coagulopathy.146 Another study concluded that VATS was both safe and effective as well as superior to chest tube drainage for large loculated empyemas147 when used early in the course of the illness. Bronchoscopy may be indicated in anaerobic infections to hasten drainage or the removal of a foreign body. Pleural decortication is reserved for patients who have entrapment of lung with persistent and severe restrictive lung disease.
Antimicrobial Therapy
When choosing an antibiotic for the treatment of CPPE or empyema, consideration must be given to the following: probable pathogens predicted from patient's age; clinical circumstances; Gram stain of the pleural fluid; and radiographic appearance. The clinical circumstances include whether the infection was community- or hospital-acquired, the host is immunocompetent or immunocompromised, and/or has an underlying medical condition. In most cases, empiric therapy should adequately treat Streptococcus pneumoniae, CA-MRSA, and group A streptococcus. In addition, therapy should include adequate coverage for anaerobic bacteria in patients at risk for aspiration. In cases where atypical pathogens are suspected, a macrolide (for children <7 years) or doxycycline (for children >7 years) may be added. When the specific causative pathogen is determined, the antibiotic spectrum should be narrowed. Duration of parenteral therapy and total treatment is individualized on the basis of clinical response and adequacy of drainage. A minimal duration of 4 weeks is usual for CPPE; treatment should be prolonged when drainage is delayed and systemic manifestations are protracted.
In circumstances in which the effusion persists and the microbial etiology is unknown, it is important to remember that fever, anorexia, and toxicity can be prolonged, even when the choice of antibiotics is correct – due to inflammatory response within the pleural space. Therefore, additions or changes in appropriately selected antibiotic therapy should be avoided.
Prognosis
The mortality rate for CPPE in previously healthy children is between 0% and 3%.145 Historically, mortality was highest in small infants and with infection due to Staphylococcus aureus. Long-term follow-up data of patients with PPEs are limited. The capacity of the child's lung to recover is great and even patients with prolonged morbidity rarely require decortication. Patients are usually asymptomatic at follow-up but radiographs may show pleural thickening,125, 128 which regresses over months. Mild abnormalities occur with equal frequency in children treated with and without chest tube drainage.148 Re-expansion of the affected segment of the lung is an important consideration regarding need for surgical intervention, even if clinical improvement has ensued.
NECROTIZING PNEUMONIA AND LUNG ABSCESS
Necrotizing pneumonia usually occurs as a consequence of a localized lung infection by particularly virulent, pyogenic bacteria. Necrotizing pneumonia in an otherwise healthy child can resolve without further complications after antimicrobial treatment, or it can be followed by the formation of a lung abscess or a pneumatocele (blebs in the lung parenchyma created by coalescence of alveolar spaces following rupture of septa) or bronchopleural fistula. Lung abscesses can be the outcome of widely variable pathogenic processes, such as: (1) a consequence of necrotizing pneumonia; (2) localized infection after aspiration of heavily infected mouth secretions (sometimes along with a foreign body); (3) focal infection of the lung that occurs during high-grade bacteremia or as a consequence of septic emboli; and (4) complication of a subacute or chronic airway infection seen as a late consequence of cystic fibrosis, after prolonged intubation, or after an infection with hospital-associated (nosocomial) bacteria. In the immunocompromised host, necrotizing pneumonia can be caused by bacteria and/or fungi that invade vessel walls, with subsequent lung infarction (see Chapter 38, Pneumonia in the Immunocompromised Host).
Etiologic Agents
Community-acquired bacterial pneumonia can result in necrosis of the lung parenchyma, which is often discovered on chest radiography or CT in a child with prolonged fever and ill appearance.90, 149 As in most cases of bacterial pneumonia, the causative pathogen may not be identified. When a pathogen is isolated, most often it is Streptococcus pneumoniae, or, less commonly, Staphylococcus aureus, (especially CA-MRSA) or Streptococcus pyogenes. Hemoptysis can be a manifestation of necrotizing pneumonia. Pneumococcal necrotizing pneumonia, if not accompanied by parapneumonic effusion, usually resolves with antimicrobial treatment alone. Infection with either S. pneumoniae or Staphylococcus aureus can cause pneumatoceles. Occasionally, abscesses can form from pneumatoceles or as a complication of staphylococcal pneumonia.150–153 Rarely, severe Mycoplasma pneumoniae pneumonia is accompanied by abscess formation.154 Lung abscess is frequently accompanied by PPE.155
Pneumonia associated with aspiration of bacteria from the oropharynx, or after aspiration of regurgitated stomach contents, is particularly likely to cause necrosis and abscess formation. Anaerobic bacteria are frequently recovered from lung abscesses, accounting for 30% to 70% of isolates.153, 155 Peptostreptococcus spp., Bacteroides spp., Prevotella spp., and Veillonella spp. are most common. Facultative aerobic pathogens include β-hemolytic streptococci (Lancefield groups C and G).
Single or multiple lung abscesses can result from right-sided endocarditis, severe septicemia (usually with S. aureus), or infarction/infection following endovascular infection of the large veins in the neck (Lemierre disease).156 The bacteriology reflects the primary pathogens, which can be S. aureus (common pathogen), members of the Streptococcus anginosus group, or Fusobacterium necrophorum (Lemierre disease).
Abscesses in intubated infants and children are usually due to hospital-associated pathogens.157 Abscesses can develop in the later stages of cystic fibrosis secondary to chronic bronchiectasis. In such cases, Staphylococcus aureus, Pseudomonas aeruginosa, and mycobacteria are considered.153, 158
Table 36-5 shows the common microorganisms isolated in several series of children with lung abscesses.153, 155, 157, 159, 160
TABLE 36-5.
Microbiology of Lung Abscesses in Childrena
| Organisms | Percent Cases | |
|---|---|---|
| Aerobic and facultative bacteria | Staphylococcus aureus | 19 |
| Streptococcus pneumoniae | 10 | |
| Other streptococci | 32 | |
| Haemophilus influenzae | 6 | |
| Pseudomonas aeruginosa | 13 | |
| Escherichia coli | 9 | |
| Other gram-positive organisms | 7 | |
| Other gram-negative organisms | 6 | |
| Anaerobic bacteria | Bacteroides speciesb | 25 |
| Prevotella melaninogenica | 9 | |
| Peptostreptococcus species | 21 | |
| Fusobacterium species | 5 | |
| Veillonella species | 8 | |
| Other gram-positive organisms | 8 | |
| Other gram-negative organisms | 3 | |
| Fungi | 10 | |
| Mycobacteria | 1 | |
Note: more than one organism can be isolated from a lung abscess
Includes some Prevotella melaninogenica (formerly Bacteroides melaninogenica). Data compiled from references 153, 155, 157, 159, and 160.
Pathogenesis
Necrotizing pneumonia appears as a consequence of severe lobar or alveolar pneumonia in which the confined infection results in parenchymal damage. Such necrosis can result in abscess formation in some instances when treatment is adequate, and more frequently when it is delayed or inadequate.
Aspiration and obstruction of the airways predispose to lung abscess. Risk factors for aspiration include: decreased level of consciousness due to neurologic disease, anesthesia, alcohol, or drugs; neuromuscular disorders depressing the gag reflex; esophageal abnormalities; gastroesophageal reflux; and prolonged endotracheal intubation. Periodontal disease predisposes to bacterial hypercontamination of aspirated material.155 The mechanism of abscess formation in otherwise healthy children is most often from an obstruction due to an aspirated foreign body, with growth of bacteria distal to the obstruction. Abnormal drainage, as seen in congenital pulmonary sequestration, lobar emphysema, and pneumatocele formation, predisposes to abscess formation through the same mechanism.
Any high-grade bacteremia or heavy seeding of bacteria into the pulmonary circulation via the systemic venous system can also lead to lung abscess formation. Pulmonary infarction associated with septic embolization increases the likelihood of abscess formation. Chronic airway disease, cystic fibrosis, congenital ciliary dysfunction, or bron chiectasis increases susceptibility to lung abscess. Instrumentation, either during surgery or in intensive care, raises the likelihood of pulmonary infection and abscess formation, probably through a similar pathogenic pathway.157 In addition, impairment of humoral or cellular immune responses results in greater susceptibility to lung abscess.
Clinical Manifestations
Clinical manifestations of necrotizing pneumonia are similar to, but usually more severe than, those of nonnecrotizing pneumonia due to the same bacteria. Prolonged fever and a toxic appearance or persistent hypoxia, despite appropriate antimicrobial therapy, is characteristic. The evolution from necrotizing pneumonia to abscess is frequently insidious.161
Lung abscess typically develops approximately 1 to 2 weeks after aspiration of oropharyngeal or gastric material. The site of involvement is usually the lobe that was dependent at the time of aspiration. Fever is the most common sign in patients with lung abscess.153 Cough, dyspnea, and sputum production are present in approximately half of patients.153, 162 Chest pain and hemoptysis can also occur. Putrid breath or a mass effect is occasionally the sole or predominant manifestation (Figure 36-4 ).163
Figure 36-4.
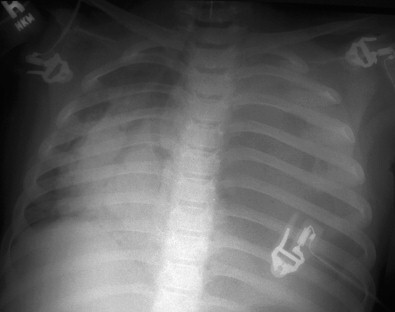
Anaerobic pleural empyema in a 5-year-old girl who came to medical attention because of a 1-month history of abdominal pain, tiredness, and constipation, but no history of an aspiration event, fever, respiratory distress, or cough. This radiograph was obtained after an acute respiratory event during evaluation for constipation. Note complete opacification of the left hemithorax with severe shift of the heart and trachea to the right. Three liters of putrid pus was drained, revealing a left lower lobe abscess. Gram stain and culture revealed polymicrobial anaerobic and facultative oropharyngeal flora.
(Courtesy of E.N. Faerber and S.S. Long, St. Christopher's Hospital for Children, Philadelphia, PA.)
The differential diagnosis of lung abscess includes other necrotizing infections such as tuberculosis, nocardiosis, fungal infections, melioidosis, paragonimiasis, and amebic abscess. Certain noninfectious diseases, such as malignancy, sarcoidosis, and pulmonary infarction, can produce lesions that mimic abscess on chest radiographs.
Diagnosis
Necrotizing pneumonia is suspected in a child when the symptoms do not respond to appropriate antibiotic treatment of a pneumonic consolidation. A chest radiograph can reveal a radiolucent lesion, but CT is more discerning. Decreased parenchymal contrast enhancement on CT correlates with impending necrosis and cavitation.130
The radiographic diagnosis of lung abscess is based on finding an air–fluid level in a cavity at least 2 cm in diameter, with a well-defined wall.157 In about 20% of cases, a chest radiograph may not be diagnostic initially. Lung abscesses are most commonly found in the right upper, right lower, and left lower lobes.164
CT is often useful to define the extent of disease, underlying anomalies, and the presence or absence of a foreign body (Figure 36-5 ). Bronchoscopy is diagnostic, and therapeutic on many occasions to facilitate the removal of a foreign body or to promote the drainage of purulent fluid if this has not occurred spontaneously.155 Ultrasound or CT-guided transthoracic aspiration of lung abscess successfully identifies the etiologic agent in >90% of cases.165 It is only required in complex cases or when the etiology cannot reasonably be ascertained from the clinical circumstances.
Figure 36-5.
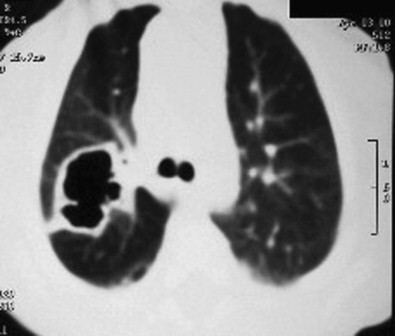
Lung windows of computed tomography study showing right upper lobe abscess.
Specimens for culture, other than those obtained by bronchoscopy or direct aspiration of the lung, are of limited value. Quantitative culture of bronchoalveolar lavage fluid improves the accuracy of identification of aerobic and anaerobic bacteria as causes of lung abscess.155, 166
Management
Prolonged antibiotic therapy is the mainstay of treatment for necrotizing pneumonia and lung abscess. Duration of therapy is based on clinical response and is usually 4 weeks or at least 2 weeks after the patient is afebrile and shows clinical improvement. Parenteral therapy is usually initiated. Two randomized clinical trials that involved 72 adults found clindamycin to be superior to penicillin for the treatment of anaerobic lung abscess.167, 168 A clinical trial in children found no difference between these two drugs.169 Parenteral clindamycin is an appropriate empiric therapy for children with suspected S. Aureus (including MRSA) or anaerobic lung infection. Combination therapy with ticarcillin or piperacillin and a β-lactamase inhibitor, with or without an aminoglycoside, is considered when necrotizing pneumonia follows aspiration in a hospitalized child or in a child for whom an Enterobacteriaceae (e.g., Escherichia coli, Klebsiella, etc.) or Pseudomonas aeruginosa infection is suspected on clinical grounds (as in cystic fibrosis) or has been identified as an isolate from a percutaneous lung aspirate.
Necrotizing pneumonia or abscess is frequently complicated by parapneumonic effusion, which benefits from percutaneous drainage or other invasive procedures (as mentioned earlier). Percutaneous abscess drainage157 carries the hazard of bronchopleural fistula, with prolonged morbidity or the necessity for surgical repair.157, 170 Nevertheless, it should be considered in those patients with continued systemic illness, 5 to 7 days after initiation of antibiotic therapy, especially if lesions are peripheral or if bronchoscopy fails to drain a more central lesion. Drainage may also be necessary if an abscess is >4 cm in diameter, causes mediastinal shift, or results in ventilator dependency.171 Surgical wedge resection or lobectomy is rarely required, and is reserved for cases in which medical management and drainage fail or bronchiectasis has occurred.
Prognosis and Complications
Necrotizing pneumonia in otherwise healthy children usually resolves with antibiotic treatment alone.153 Similarly, 80% to 90% of lung abscesses resolve with antibiotic therapy alone (provided that the bronchial obstruction is removed). Fever has an average duration of 4 to 8 days. The most common complication of lung abscess is intracavitary hemorrhage with hemoptysis or spillage of abscess contents and spread of infection to other parts of the lung.172 Other complications are empyema, bronchopleural fistula, septicemia, cerebral abscess, and inappropriate secretion of antidiuretic hormone.172
ACKNOWLEDGMENT
The authors acknowledge significant contributions of K. McIntosh and M. Harper in the second edition of Principles and Practices of Pediatric Infectious Diseases.
References
- 1.Bulletin of the World Health Organization . Global Estimate of the Incidence of Clinical Pneumonia among Children under Five Years of Age. World Health Organization; Geneva: 2004. p. 12. [PMC free article] [PubMed] [Google Scholar]
- 2.Juven T, Mertsola J, Waris M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Farha T, Thompson AH. The burden of pneumonia in children in the developed world. Pediatr Respir Rev. 2005;6:76–82. doi: 10.1016/j.prrv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Peck AJ, Holman RC, Curns AT. Lower respiratory infections among American Indian and Alaska native children and general population of US children. Pediatr Infect Dis J. 2005;24:342–351. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries and Risk Factors in 1990 and Projected to 2000. World Health Organization; Cambridge MA: 1996. [Google Scholar]
- 6.Zar HJ. Pneumonia in HIV infected and HIV-uninfected children in developing countries: epidemiology, clinical features and management. Curr Opin Pulm Med. 2004;3:176–182. doi: 10.1097/00063198-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Michelow IC, Olsen K, Lozano J. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 8.Ruuskanen O, Mertsola J. Childhood community-acquired pneumonia. Semin Respir Infect. 1999;14:163–172. [PubMed] [Google Scholar]
- 9.Kennedy M, Bates DW, Wright SB. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46:393–400. doi: 10.1016/j.annemergmed.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Barnett ED, Klein JO. Bacterial infections of the respiratory tract. In: Remington and Klein: Infectious Diseases of the Fetus and Newborn Infant, 5th ed. Saunders, pp 999–1013.
- 11.British Thoracic Society Standards of Care Committee BTS guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(Suppl. 1) doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart CA, Beeching NJ, Duerden BI. Respiratory infections: proceedings of the eighth Liverpool Tropical School Bayer symposium of microbial diseases held on 3 February 2001. J Med Microbiol. 2002;51:903–914. doi: 10.1099/0022-1317-49-1-5. [DOI] [PubMed] [Google Scholar]
- 13.Williams JV, Harris PA, Tollefson SJ. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants an children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiskanen-Kosoma T, Korppi M, Jokinen C. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986–991. doi: 10.1097/00006454-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kellner G, Popow-Kraupp T, Kundi M. Contribution of rhinoviruses to respiratory viral infections in childhood: a prospective study in mainly hospitalized infant population. J Med Virol. 1988;25:455–469. doi: 10.1002/jmv.1890250409. [DOI] [PubMed] [Google Scholar]
- 16.Abed Y, Boivin G. Human parechovirus types 1, 2 and 3 infections in Canada. Infect Dis. 2006:12969–12975. doi: 10.3201/eid1206.051675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaki-Korpela P, Paivi Hyypia T. Paraechoviruses, a novel group of human Picornaviruses. Ann Med. 2001;33:466–471. doi: 10.3109/07853890109002095. [DOI] [PubMed] [Google Scholar]
- 17.Cooke FJ, Shapiro DS. Global outbreak of severe acute respiratory syndrome. Int J Infect Dis. 2003;7:80–85. doi: 10.1016/S1201-9712(03)90001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hon KLE, Leung CW, Cheng WTF. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay B. SARS poses challenges for MDs treating pediatric patients. CMAJ. 2003;168:457. [PMC free article] [PubMed] [Google Scholar]
- 20.Korppi M, Heiskanen-Kosma T, Kleemola M. Incidence of community acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology. 2004;9:109–114. doi: 10.1111/j.1440-1843.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris JA, Kolokathis A, Cambell M. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865–871. doi: 10.1097/00006454-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Klig JE, Shah NB. Office pediatrics: current issues in lower respiratory infections in children. Curr Opin Pediatr. 2005;17:111–118. doi: 10.1097/01.mop.0000150599.31091.f0. [DOI] [PubMed] [Google Scholar]
- 23.Clyde WA., Jr Clinical overview of typical Mycoplasma pneumoniae infections. Clin Infect Dis. 1993;17(Suppl 1):S32–S36. [PubMed] [Google Scholar]
- 24.Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different population of patients. Clin Infect Dis. 1993;17:S37–S46. doi: 10.1093/clinids/17.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 25.Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis. 1990;12(suppl 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 26.Korpii M, Heiskanen-Kosoma T, Jalonen E. Aetiology of community- acquired pneumonia in children treated in hospital. Eur J Pediatr. 1993;152:24–30. doi: 10.1007/BF02072512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claesson BA, Trolifors B, Brolin I. Etiology of community-acquired pneumonia in children based on antibody responses to bacterial and viral antigens. Pediatr Infect Dis J. 1989;8:856–862. doi: 10.1097/00006454-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Lankinen KS, Ruutu P, Nohynek H. Pneumococcal pneumonia diagnosis by demonstration of pneumolysin antibodies in precipitated immune complexes: a study in 350 Philippine children with lower respiratory infection. Scand J Infect Dis. 1999;31:155–161. doi: 10.1080/003655499750006209. [DOI] [PubMed] [Google Scholar]
- 29.Nohynek H, Skola J, Laine E. The causes of hospital-treated acute lower respiratory tract infection in children. Am J Dis Child. 1991;145:618–622. doi: 10.1001/archpedi.1991.02160060036016. [DOI] [PubMed] [Google Scholar]
- 30.Toikka P, Nikkari S, Ruuskanen O. Pneumolysin PCR-based diagnosis of invasive pneumococcal infection in children. J Clin Microbiol. 1999;37:33–637. doi: 10.1128/jcm.37.3.633-637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuori-Holopainen E, Salo E, Saxon H. Etiological diagnosis of childhood pneumonia by use of intrathoracic needle aspiration and modern microbiological methods. CID. 2002;4:583–590. doi: 10.1086/338642. [DOI] [PubMed] [Google Scholar]
- 32.Wubbel L, Muniz L, Ahmed A. Etiology and treatment of community- acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1999;18:98–104. doi: 10.1097/00006454-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Russell FM, Buttery J. Vaccine development for capsulate bacteria causing pneumonia. Curr Opin Pulm Med. 2003;9:227–232. doi: 10.1097/00063198-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Banithia S, Meka VG, Pillai SK. A fatal case of necrotizing pneumonia caused by community-associated methicillin-resistant Staphylococcus aureus. Infect Dis Clin Pract. 2005;13:132–138. [Google Scholar]
- 35.Grisom NT, Wohl ME, Kirkpatrick JA., Jr Lower respiratory infections: how infants differ from adults. Radiol Clin North Am. 1978;16:367–387. [PubMed] [Google Scholar]
- 36.Grisom NT. Pneumonia in children and some of its variants. Radiology. 1988;167:297–302. doi: 10.1148/radiology.167.2.3357939. [DOI] [PubMed] [Google Scholar]
- 37.Aherne W, Bird T, Court SD. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carball G, Siminovich M, Murtagh P. Etiological, clinical and pathologic analysis of 31 fatal cases of acute respiratory tract infection in Argentinian children under 5 years of age. Rev Infect Dis. 1990;12:S1074–S1080. doi: 10.1093/clinids/12.supplement_8.s1074. [DOI] [PubMed] [Google Scholar]
- 39.Anderson VM, Turner T. Histopathology of childhood pneumonia in developing countries. Rev Infect Dis. 1991;13(Suppl 6):S470–S476. doi: 10.1093/clinids/13.supplement_6.s470. [DOI] [PubMed] [Google Scholar]
- 40.McCracken GH., Jr Diagnosis and management of pneumonia in children. Pediatr Infect Dis J. 2000;19:924–928. doi: 10.1097/00006454-200009000-00036. [DOI] [PubMed] [Google Scholar]
- 41.The WHO Young Infants Study Group Serious infections in young infants in developing countries: rationale for a multicenter study. Pediatr Infect Dis J. 1999;18(10 Suppl):S4–S7. doi: 10.1097/00006454-199910001-00002. [DOI] [PubMed] [Google Scholar]
- 42.Palafox M, Guiscarfe H, Reyes H. Diagnostic value of tachypnoea in pneumonia defined radiologically. Arch Dis Child. 2000;82:41–45. doi: 10.1136/adc.82.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth A, Carty H, Hart CA. Clinical predictors of hypoxemia in children with pneumonia. Ann Trop Paediatr. 1998;18:31–40. doi: 10.1080/02724936.1998.11747923. [DOI] [PubMed] [Google Scholar]
- 44.Zukin DD, Hoffman JR, Cleveland RH. Correlation of pulmonary signs and symptoms with chest radiographs in the pediatric age group. Ann Emerg Med. 1986;15:792–796. doi: 10.1016/s0196-0644(86)80374-2. [DOI] [PubMed] [Google Scholar]
- 45.Leventhal JM. Clinical predictors of pneumonia as a guide to ordering chest roentgenograms. Clin Pediatr. 1982;21:730–734. doi: 10.1177/000992288202101205. [DOI] [PubMed] [Google Scholar]
- 46.Alario AJ, McCarthy PL, Markowitz R. Usefulness of chest radiographs in children with acute lower respiratory tract disease. J Pediatr. 1987;111:187–193. doi: 10.1016/s0022-3476(87)80065-3. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly LF. Practical issues concerning imaging of pulmonary infection in children. J Thoracic Imaging. 2001;16:238–250. doi: 10.1097/00005382-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Mahabee-Gittens ME, Grupp-Phelan J. Identifying children with pneumonia in the emergency department. Clin Pediatr. 2005;44:427–435. doi: 10.1177/000992280504400508. [DOI] [PubMed] [Google Scholar]
- 49.Bachur R, Perry H, Harper MB. Occult pneumonias: empiric chest radiographs in febrile children with leucocytosis. Ann Emerg Med. 1999;33:166–173. doi: 10.1016/s0196-0644(99)70390-2. [DOI] [PubMed] [Google Scholar]
- 50.Margolis P, Gadomski A. Does this infant have pneumonia? JAMA. 1998;279:308–313. doi: 10.1001/jama.279.4.308. [DOI] [PubMed] [Google Scholar]
- 51.Margolis PA, Ferkol TW, Marsocci S. Accuracy of the clinical examination in detecting hypoxemia in infants with respiratory illness. J Pediatr. 1994;124:552–560. doi: 10.1016/s0022-3476(05)83133-6. [DOI] [PubMed] [Google Scholar]
- 52.Singhi S, Dhawan A, Kataria S. Clinical signs of pneumonia in infants under 2 months. Arch Dis Child. 1994;70:413–417. doi: 10.1136/adc.70.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruhn FW, Mokrohisky ST, McIntosh K. Apnea associated with respiratory syncytial virus infection in young infants. J Pediatr. 1977;40:382–386. doi: 10.1016/s0022-3476(77)80697-5. [DOI] [PubMed] [Google Scholar]
- 54.Beem MO, Saxon EM. Respiratory-tract colonization and distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977;296:306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- 55.Harrison HR, English MG, Lee CK. Chlamydia trachomatis infant pneumonitis: comparison with matched controls and other infant pneumonitis. N Engl J Med. 1978;298:702–708. doi: 10.1056/NEJM197803302981303. [DOI] [PubMed] [Google Scholar]
- 56.Tipple MA, Beem MO, Saxon EM. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics. 1979;63:192–197. [PubMed] [Google Scholar]
- 57.Swingler GH, Hussey GD, Zwarenstein M. Randomized controlled trial of clinical outcome after chest radiograph in ambulatory acute lower-respiratory infection in children. Lancet. 1998;351:404–408. doi: 10.1016/S0140-6736(97)07013-X. [DOI] [PubMed] [Google Scholar]
- 58.Swingler GH, Zwarebstein M. Chest radiograph in acute respiratory infections in children. Cochrane Database Syst Rev. 2000:2. doi: 10.1002/14651858.CD001268. [DOI] [PubMed] [Google Scholar]
- 59.Courtoy I, Lande AE, Turner RB. Accuracy of radiographic differentiation of bacterial from nonbacterial pneumonia. Clin Pediatr (Phila) 1989;28:261–264. doi: 10.1177/000992288902800604. [DOI] [PubMed] [Google Scholar]
- 60.Clements H, Stephenson T, Gabriel V. Rationalised prescribing for community acquired pneumonia: close loop audit. Arch Dis Child. 2000;83:320–324. doi: 10.1136/adc.83.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han BK, Son JA, Yoon HK. Epidemic adenoviral lower respiratory tract infection in pediatric patients: radiographic and clinical characteristics. Am J Roentgenol. 1998;170:1077–1080. doi: 10.2214/ajr.170.4.9530062. [DOI] [PubMed] [Google Scholar]
- 62.Brolin I, Wernstedt L. Radiographic appearance of mycoplasma pneumonia. Scand J Respir Dis. 1978;59:179–189. [PubMed] [Google Scholar]
- 63.Putman CE, Curtis AM, Simeone JF. Mycoplasma pneumonia: clinical and roentgenographic patterns. Am J Roentgenol. 1975;125:417–422. doi: 10.2214/ajr.124.3.417. [DOI] [PubMed] [Google Scholar]
- 64.Gibson NA, Hollman AS, Paton JY. Value of radiological follow-up of childhood pneumonia. Br Med J. 1993;307:1117. doi: 10.1136/bmj.307.6912.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heaton P, Arthur K. The utility of chest radiography in the follow-up of pneumonia. NZMed J. 1998;111:315–317. [PubMed] [Google Scholar]
- 66.Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J. 1997;10:1125–1129. doi: 10.1183/09031936.97.10051125. [DOI] [PubMed] [Google Scholar]
- 67.Nohynek H, Valkeila E, Leinonen M. Erythrocyte sedimentation rate, white blood cell count and serum C-reactive protein in assessing etiologic diagnosis of acute lower respiratory infections in children. Pediatr Infect Dis J. 1995;14:484–490. doi: 10.1097/00006454-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Templeton KE, Scheltinga SA, van den Eeden WC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casell GH, Cole BC. Mycoplasma as agents of human disease. N Engl J Med. 1981;304:80–89. doi: 10.1056/NEJM198101083040204. [DOI] [PubMed] [Google Scholar]
- 70.Waris M, Toikka P, Saarinen T. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998;38:3155–3159. doi: 10.1128/jcm.36.11.3155-3159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammerschlag MR. Atypical pneumonias in children. Adv Pediatr Infect Dis. 1995;10:1–39. [PubMed] [Google Scholar]
- 72.Vallejo J, Ong LT, Starke JR. Clinical features, diagnosis and treatment of tuberculosis in infants. Pediatrics. 1994;94:941–947. [PubMed] [Google Scholar]
- 73.Russell TM. Bacterial pneumonias: management and complication. Paediatr Respir Rev. 2000;1:14–20. doi: 10.1053/prrv.2000.0014. [DOI] [PubMed] [Google Scholar]
- 74.Khoshoo V, Edell D. Previously healthy infants may have increased risk of aspiration during respiratory syncytial viral bronchiolitis. Pediatrics. 1999;104:1389–1390. doi: 10.1542/peds.104.6.1389. [DOI] [PubMed] [Google Scholar]
- 75.Tiwari T, Murphy TV, Moran J. Recommended antimicrobial agents for the treatment and post-exposure prophylaxis of pertussis; CDC guidelines. Morbid Mortal Wkly Rep MMWR. 2005;54(RR-14):1–16. [PubMed] [Google Scholar]
- 76.Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Curr Opin Infect Dis. 2003;16:271–277. doi: 10.1097/00001432-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 77.McIntosh K. Current concepts: community acquired pneumonia in children. N Engl J Med. 2002;346:429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 78.Jadavji T, Law B, Lebel MH. A practical guide for the diagnosis and treatment of pediatric pneumonia. CMAJ. 1997;156:S703–S711. [PMC free article] [PubMed] [Google Scholar]
- 79.Buckingham SC, Brown SP, Joaquin VH. Breakthrough bacteremia and meningitis during treatment with cephalosporins parenterally for pneumococcal pneumonia. J Pediatr. 1998;132:174–176. doi: 10.1016/s0022-3476(98)70510-4. [DOI] [PubMed] [Google Scholar]
- 80.Jantausch BA, Deville J, Adler S. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant Gram-positive bacterial pathogens. Pediatr Infect Dis J. 2003;22(suppl 9):S164–S171. doi: 10.1097/01.inf.0000086956.45566.55. [DOI] [PubMed] [Google Scholar]
- 81.Korppi M, Leinonen M, Koskela M. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr Infect Dis J. 1989;8:687–692. doi: 10.1097/00006454-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 82.Hall CB, Powell KR, Schanbel KC. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial virus infection. J Pediatr. 1988;113:266–271. [PubMed] [Google Scholar]
- 83.American Academy of Pediatrics Committee on Infectious Diseases Use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics. 1993;92:501–504. [PubMed] [Google Scholar]
- 84.American Academy of Pediatrics Committee on Infectious Diseases Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics. 1996;97:137–140. [PubMed] [Google Scholar]
- 85.Law BJ, Wang EE, MacDonald N. Does ribavirin impact on the hospital course of children with respiratory syncytial virus (RSV) infection? An analysis using the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) RSV database. Pediatrics. 1997;99:E7. doi: 10.1542/peds.99.3.e7. [DOI] [PubMed] [Google Scholar]
- 86.Wald ER, Dashefsky B, Green M. In re: ribavirin: a case of premature adjudication? J Pediatr. 1988;112:154–158. doi: 10.1016/s0022-3476(88)80143-4. [DOI] [PubMed] [Google Scholar]
- 87.Hardie WD, Bokulic R, Garcia VF. Pneumococcal pleural empyemas in children. Clin Infect Dis. 1996;22:1057–1063. doi: 10.1093/clinids/22.6.1057. [DOI] [PubMed] [Google Scholar]
- 88.Tan TQ, Mason EO, Jr, Barson WJ. Clinical characteristics and outcome of children with pneumonia attributable to penicillin-susceptible and penicillin- nonsusceptible Streptococcus pneumoniae. Pediatrics. 1998;102:1369–1375. doi: 10.1542/peds.102.6.1369. [DOI] [PubMed] [Google Scholar]
- 89.Tan TQ, Mason EO, Wald ER. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002;110:1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- 90.Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003;22:499–504. doi: 10.1097/01.inf.0000069764.41163.8f. [DOI] [PubMed] [Google Scholar]
- 91.Mok JY, Simpson H. Outcome for acute bronchitis, bronchiolitis and pneumonia in infancy. Arch Dis Child. 1984;59:306–309. doi: 10.1136/adc.59.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pullan CR, Hey EN. Wheezing, asthma and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castro-Rodriguez JA, Holberg CJ, Wright AL. Association of radiologically ascertained pneumonia before age 3 yr with asthma like symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 94.Clark CE, Coote JM, Silver DA. Asthma after childhood pneumonia: six year follow up study. Br Med J. 2000;320:1514–1516. doi: 10.1136/bmj.320.7248.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weber MW, Milligan P, Giadom B. Respiratory illness after severe respiratory syncytial virus disease in infancy in the Gambia. J Pediatr. 1999;135:683–688. doi: 10.1016/s0022-3476(99)70085-5. [DOI] [PubMed] [Google Scholar]
- 96.Harrison HR, Taussig LM, Fulginiti VA. Chlamydia trachomatis and chronic respiratory disease in childhood. Pediatr Infect Dis J. 1982;1:29–33. doi: 10.1097/00006454-198201000-00008. [DOI] [PubMed] [Google Scholar]
- 97.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis and adult onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 98.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood and adult lung function. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 99.Watkins CJ, Burton P, Leeder S. Doctor diagnosis and maternal recall of lower respiratory illness. Int J Epidemiol. 1982;11:62–66. doi: 10.1093/ije/11.1.62. [DOI] [PubMed] [Google Scholar]
- 100.Shaheen SO, Sterne JA, Tucker JS. Birth weight, childhood lower respiratory tract infection and adult lung function. Thorax. 1998;53:549–553. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez FD, Morgan WJ, Wright AL. Diminished lung function as a factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 102.Tager IB, Hanrahan JP, Tosteson TD, et al. Lung function, pre- and post-natal smoke [DOI] [PubMed]
- 103.Young S, O'Keefe PT, Arnott J. Lung function, airway responsiveness and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72:16–24. doi: 10.1136/adc.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaplan SL. Decline of invasive pneumococcal infections in children. Pediatrics Mason E, Wald ER. 2004;113:443–449. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 105.Whitney CG. Impact of conjugate pneumococcal vaccines. Pediatr Infect Dis J. 2005;24:729–730. doi: 10.1097/01.inf.0000174138.25465.ec. [DOI] [PubMed] [Google Scholar]
- 106.Lexau CA, Lynfield R, Danila R. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 107.Shafnoori S, Ginocchio CC, Greenburg AJ. Impact of pneumococcal conjugate vaccine and the severity of winter influenza-like illnesses on invasive pneumococcal infections in children and adults. Pediatr Infect Dis J. 2005;24:10–16. doi: 10.1097/01.inf.0000148930.41928.0d. [DOI] [PubMed] [Google Scholar]
- 108.The IMPACT-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 109.Meissner HC, Long SS, the Committee on Infectious Diseases and the Committee on Fetus and Newborn American Academy of Pediatrics revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1447–1452. doi: 10.1542/peds.112.6.1447. [DOI] [PubMed] [Google Scholar]
- 110.American Academy of Pediatrics . Influenza. In: Pickering LK, editor. Red Book Report of the Committee on Infectious Diseases. 27th ed. American Academy of Pediatrics; Elk Grove Village, IL: 2006. pp. 401–411. [Google Scholar]
- 111.Light RW, Girad WM, Jenkinson SG. Parapneumonic effusions. Am J Med. 1980;68:985–986. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 112.Barnes NP, Hull J, Thomson AH. Medical management of parapneumonic pleural disease. Pediatr Pulmonol. 2005;39:127–134. doi: 10.1002/ppul.20127. [DOI] [PubMed] [Google Scholar]
- 113.Khakoo GA, Goldstraw P, Hansell DM. Surgical treatment of parapneumonic empyema. Pediatr Pulmonol. 1996;22:348–356. doi: 10.1002/(SICI)1099-0496(199612)22:6<348::AID-PPUL3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 114.Campbell PW., III New developments in pediatric pneumonia and empyema. Curr Opin Pediatr. 1995;7:278–282. doi: 10.1097/00008480-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 115.Kunyoshi V, Cataneo DC, Cataneo AJ. Complicated pneumonia with empyema and/or pneumatocele in children. Pediatr Surg Int. 2006;22:186–190. doi: 10.1007/s00383-005-1620-5. [DOI] [PubMed] [Google Scholar]
- 116.Byington CL, Spencer LY, Johnson TA. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–440. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 117.Byington CL, Korgenski K, Daly J. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006;25:250–254. doi: 10.1097/01.inf.0000202137.37642.ab. [DOI] [PubMed] [Google Scholar]
- 118.Alfaro C, Fergie J, Purcell K. Emergence of community-acquired methicillin- resistant Staphylococcus aureus in complicated parapneumonic effusions. Pediatr Infect Dis J. 2005;24:274–276. doi: 10.1097/01.inf.0000154332.66085.de. [DOI] [PubMed] [Google Scholar]
- 119.Baranwal AK, Singh M, Marwaha RK. Empyema thoracis: a 10 year comparative review of hospitalized children from South Asia. Arch Dis Child. 2003;88:1009–1014. doi: 10.1136/adc.88.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaffe A, Balfour-Lynn IM. Management of empyema in children. Pediatr Pulmonol. 2005;40:148–156. doi: 10.1002/ppul.20251. [DOI] [PubMed] [Google Scholar]
- 121.Fine NL, Smith LR, Sheedy PF. Frequency of pleural effusions in mycoplasma and viral pneumonias. N Engl J Med. 1970;283:790–793. doi: 10.1056/NEJM197010082831505. [DOI] [PubMed] [Google Scholar]
- 122.Grix A, Giammona ST. Pneumonitis with pleural effusion in children due to Mycoplasma pneumoniae. Am Rev Respir Dis. 1974;109:665–671. doi: 10.1164/arrd.1974.109.6.665. [DOI] [PubMed] [Google Scholar]
- 123.Han BK, Son JA, Yoon HK. Epidemic adenoviral lower respiratory tract infection in pediatric patients: radiographic and clinical characteristics. AJR Am J Roentgenol. 1998;170:1077–1080. doi: 10.2214/ajr.170.4.9530062. [DOI] [PubMed] [Google Scholar]
- 124.Hoff SJ, Neblett WW, Edwards KM. Parapneumonic empyema in children: decortication hastens recovery in patients with severe pleural infections. Pediatr Infect Dis J. 1991;10:194–199. [PubMed] [Google Scholar]
- 125.Freij BJ, Kusmiesz H, Nelson JD. Parapneumonic effusions and empyema in hospitalized children: a retrospective review of 227 cases. Pediatr Infect Dis. 1984;3:578–591. doi: 10.1097/00006454-198411000-00021. [DOI] [PubMed] [Google Scholar]
- 126.Chonmaitree T, Powell KR. Parapneumonic pleural effusion and empyema in children. Review of a 19-year experience, 1962–1980. Clin Pediatr (Phila) 1983;22:414–419. doi: 10.1177/000992288302200603. [DOI] [PubMed] [Google Scholar]
- 127.Doski JJ, Lou D, Hicks BA. Management of parapneumonic collections in infants and children. J Pediatr Surg. 2000;35:265–270. doi: 10.1016/s0022-3468(00)90022-8. [DOI] [PubMed] [Google Scholar]
- 128.McLaughlin FJ, Goldmann DA, Rosenbaum DM. Empyema in children: clinical course and long-term follow-up. Pediatrics. 1984;73:587–593. [PubMed] [Google Scholar]
- 129.Sahn SA. The differential diagnosis of pleural effusions. West J Med. 1982;137:99–108. [PMC free article] [PubMed] [Google Scholar]
- 130.Donnelly LF, Klosterman LA. CT appearance of parapneumonic effusions in children: findings are not specific for empyema. AJR Am J Roentgenol. 1997;169:179–182. doi: 10.2214/ajr.169.1.9207521. [DOI] [PubMed] [Google Scholar]
- 131.Donnelly LF, Klosterman LA. The yield of CT of children who have complicated pneumonia and noncontributory chest radiography. AJR Am J Roentgenol. 1998;170:1627–1631. doi: 10.2214/ajr.170.6.9609186. [DOI] [PubMed] [Google Scholar]
- 132.Light RW, Girard WM, Jenkinson SG. Parapneumonic effusions. Am J Med. 1980;69:507–512. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 133.Light RW, MacGregor MI, Ball WC., Jr Diagnostic significance of pleural fluid pH and PCO2. Chest. 1973;64:591–596. doi: 10.1378/chest.64.5.591. [DOI] [PubMed] [Google Scholar]
- 134.Light RW, Erozan YS, Ball WC., Jr Cells in pleural fluid: their value in differential diagnosis. Arch Intern Med. 1973;132:854–860. [PubMed] [Google Scholar]
- 135.Adelman M, Albelda SM, Gottlieb J. Diagnostic utility of pleural fluid eosinophilia. Am J Med. 1984;77:915–920. doi: 10.1016/0002-9343(84)90542-4. [DOI] [PubMed] [Google Scholar]


