Abstract
This study aimed to analyze the clinical characteristics, responsible pathogens, and antibiotic sensitivity of aerobic vaginitis (AV) infection in women in late pregnancy in western China.
We enrolled 246 pregnancy with AV (≥35 weeks gestation) and 204 reproductive non-pregnancy with AV from West China between January 2019 and December 2019. Then, bacterial culture, identification and antibiotic sensitivity testing were performed. Subsequently, we retrospectively analyzed the vaginal microbiota of 250 healthy pregnant women with no AV and compared the maternal features and pregnancy outcomes.
Regarding bacterial diversity, Streptococcus and Lactobacillus were highly abundant in women with AV in late pregnancy, whereas Staphylococcus spp. and other bacteria were significantly more abundant in reproductive non-pregnant women with AV. In addition, 82.5% (343/416) of the single isolate comprised Escherichia coli, group B Streptococcus, Enterococcus faecalis, and Staphylococcus aureus. Among the top 4 isolates, 13.4% (46/343) were multidrug-resistant, but all isolates were highly susceptible to nitrofurantoin. Escherichia coli was 100% susceptible to amikacin, meropenem, ertapenem, and imipenem (100%, 157/157), and gram-positive cocci were 100% (186/186) susceptible to vancomycin and linezolid. Finally, we found that pregnant women with AV had high rates of histories of vaginitis, premature rupture of membranes and neonatal infection.
Our study reveals new insights into AV infection during pregnancy and highlights the different vaginal bacterial microbiome compositions between pregnant and reproductive non pregnant women with AV, these results may translate to treatments that are more cost-effective than current standard treatments.
Keywords: aerobic vaginitis, antibiotic sensitivity, multidrug-resistant, pathogens, pregnancy
1. Introduction
The vaginal microbiome is a complex system that is affected by microorganisms, ethnicity and genes.[1] When aerobic bacteria (or facultative anaerobes) predominate in vaginal flora and the flora lacks lactobacilli, aerobic vaginitis (AV), which is a common vaginal microbiome disorder in females of reproductive age, occurs.[2] AV was first named by Donders et al in 2002 and is characterized by aerobic bacterial infection, vaginal inflammation and parabasal epitheliocyte presence.[3] Among bacterial vaginitis, desquamative inflammatory vaginitis (severe AV) was first proposed in 1965 by Gray and Barnes.[4] The most frequent pathogens responsible for AV are Escherichia coli, Staphylococcus aureus, and coagulase-negative staphylococci (CoNS), such as Staphylococcus epidermidis, group B Streptococcus (GBS), and Enterococcus faecalis.[5–7]
However, little is known about AV during pregnancy. Few studies have been conducted on the microbial diversity differences between pregnant and nonpregnant women with AV.[5] Moreover, the antibiotic sensitivity of pathogens responsible for AV infection is not well known. AV in pregnant women is associated with complications of pregnancy, particularly an increased risk of preterm labor and premature rupture of membranes (PROM).[8,9] The diagnosis and treatment of AV during pregnancy may reduce the risk of negative pregnancy outcomes. For this reason, we aimed to analyze the clinical characteristics, pathogen composition and antibiotic sensitivity of AV infections in women in late pregnancy in western China to identify candidates for prophylaxis and to prevent severe maternal and neonatal outcomes.
2. Methods
2.1. Study design and patients
The case-control study was conducted from January 2019 to December 2019 in the Obstetric and Gynecological Department of West China Second University Hospital, Sichuan University, China. The study was reviewed and approved by the ethics committee of West China Second University Hospital of Sichuan University (Sichuan China).
Written informed consent forms were obtained from all the participants. We collected maternal vaginal discharge from women after 35 weeks of gestation because Schoenmakers et al suggested that this was the optimal time to influence pregnant outcomes.[10] The inclusion criteria were as follows:
-
a)
a singleton pregnancy,
-
b)
≥35 weeks gestation and
-
c)
a vaginal smear AV score ≥ 3 according to Donders’ microscopic diagnostic criteria.[3]
This AV scoring system combines microscopic assessment about lactobacillary grades, number of leukocytes, proportion of toxic leukocytes, background flora, and proportion of parabasal epitheliocytes. The control group comprised reproductive women with AV (20–50 years) from the same gynecological department. The exclusion criteria were as follows:
-
a.
other specific pathogens, including fungal, viral, mycoplasma, chlamydia, or other microbial infections, such as bacterial vaginosis in the female reproductive system;
-
b.
severe medical diseases, such as hypertension, diabetes mellitus, malignant tumor, abnormal immune function, etc,
-
c.
the use of antibiotics within 7 days or
-
d.
unavailable follow-up, such as psychiatric illness. A second control group comprised healthy pregnant women with no AV at ≥35 weeks gestation who underwent regular prenatal check-ups in the same period. They were age-matched to the age ranges of the pregnant women with AV.
2.2. Vaginal discharge collection
We collected vaginal discharge under direct visualization during a speculum examination by using three sterile nylon-flocked swabs (MRC Science and Technology Ltd., Shenzhen, China), the discharge was taken from the posterior fornix or vaginal wall. All sterile nylon-flocked swabs were put into sterile tubes (DIRUI Industrial Company, Changchun, China) immediately and sent to the Department of Laboratory Medicine, West China Second University Hospital, Sichuan University.
2.3. Microscopic observation
One smear was mixed with 1 droplet of saline and then examined by 2 laboratory microbiologists under a 400-field CKX41 inverted microscope (Olympus, Japan). The results of their observations were consistent. Observations and documentation followed the guidelines of both the National System for External Quality Assessment (NSEQA) and the College of American Pathologists (CAP). We used Donders’ AV saline wet mount microscopic diagnostic criteria.[3] If the AV score was ≥3, the other swabs containing vaginal discharge were used for culture and identification. Finally, we identified 246 pregnant and 204 reproductive non pregnant eligible women with AV.
2.4. Microbiological tests
The second swabs were inoculated onto chromID Strepto B agar (Zhuhai Dier Science and Technology Development Limited Company, Zhuhai, China). After incubation for 8 to 48 hours at 35°C to 37 °C, characteristic orange isolates were subcultured on blood agar plates. According to the manufacturer's instructions, 3 quality control stains were performed, Streptococcus agalactiae (the positive control), E. faecalis (gram-positive bacteria as the negative control) and E. coli (gram-negative bacteria as the negative control) to verify the availability of chromID Strepto B agar. The third swabs were analyzed using Columbia agar and 5% sheep blood (Mérieux Shanghai Science and Technology Development Limited Company, Shanghai, China), and microorganisms were identified by VITEK matrix-assisted laser desorption/ionization time of flight mass spectrometry. For the drug susceptibility tests, a VITEK 2 Compact AST-GP system was used for Staphylococcus spp. and Enterococcus spp.; the quality-control strains were S. aureus ATCC 29213 and.E. faecalis ATCC 29212. ATBS TREP5 expression was detected for Streptococcus spp., and the quality-control strain was Streptococcus pneumoniae ATCC 49619. In addition, the quality-control strains for chromID Strepto B agar were Streptococcus agalactiae ATCC 12386, E. faecalis ATCC 29212 and E. coli ATCC 25922. The VITEK2-COMPACT GN13 and K-B methods were applied for Enterobacteriaceae, and the quality-control strains were E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853.
2.5. Retrospective reconsideration information
The pregnant women with AV were asked about their history of vaginitis and followed to evaluate pregnancy outcomes including delivery mode; delivery condition, for example, PROM, referring to membrane rupture before labor;[11] birth weight; Apgar score (low Apgar score[12]); stillbirth (delivery ≥ 28 weeks with no signs of life at birth),[13] and neonatal infections were recorded for 28 days after delivery. Neonatal infection was defined by laboratory-confirmed bacterial infection (positive culture of blood, cerebral spinal fluid, or urine),[14] clinical signs of infection (pneumonia, fever, hypothermia, respiratory distress, etc) or administration of antibiotics for ≥10 days.[15] Then, we collected the data of healthy pregnant women with no AV (the control group) from electronic medical records and/or telephone interviews; this data included vaginal microbiota test results, patient demographics, medical history, delivery mode, delivery condition, birth weight, Apgar score, and neonatal infection status.
2.6. Statistical analysis
The data analysis was performed with SPSS Statistics ver. 20.0 (SPSS Inc., Chicago, IL). Student t test was used to analyze continuous variables, while the comparisons of different species and different positive rates were analyzed by Chi-squared or Fisher exact probability tests. All graphics were accomplished using GraphPad Prism 5.0 (GraphPad Software Inc., CA).
3. Results
3.1. Different vaginal microbial community compositions in AV in pregnant and reproductive non pregnant women
A total of 4.2% (246/5857) of pregnant women (≥35 weeks gestation) were positive for simple AV infection compared to 4.8% (204/4234) of reproductive non pregnant women with AV (χ2 = 2.542, P = .061).They also have no severe medical diseases. The mean age of the pregnant women with AV was 31.1 ± 3.8 years, which was similar to that of the reproductive non pregnant women with AV (32.1 ± 7.7 years, t = 1.79, P = .075). A total of 295 isolates were identified from 224 pregnant women with AV; no bacteria grew on 22 plates and multiple species grew (E. faecalis / CoNS / E coli/ lactobacilli) on 71 plates. We cultured 284 isolates, including 92 with mixed flora (E. faecalis / CoNS / E coli / lactobacilli). A total of 8 women in 204 reproductive non pregnant women with AV had no growth on plates. Flow diagram of the subjects analyzed was shown in Figure 1. The majority (94.3%, 546/579) of isolates belonged to E. coli (32.4%, 177/546), Staphylococcus spp. (21.8%, 119/546), Enterococcus spp. (19.4%, 106/546), Streptococcus spp. (18.7%, 102/546) and Lactobacillus (7.7%, 42/546). In particular, there were no differences between the 2 groups in bacterial diversity, but the constituent ratio significantly differed (χ2 = 32.326, P < .001). We observed a relatively high abundance of Streptococcus spp. (χ2 = 13.812, P < .001) and Lactobacillus (χ2 = 16.034, P < .001) in pregnant women with AV. In contrast, Staphylococcus spp. (χ2 = 37.159, P < .001) and other bacteria (χ2 = 1.59, P = .14) were found at significantly higher abundances in reproductive non pregnant women with AV than in pregnant women with AV (Figure 2). Other bacteria isolated from pregnant women with AV were Gardnerella vaginalis (1.7%,5/295), Candida albicans (0.7%,2/295), Enterobacter cloacae (0.3%,1/295), Enterobacter aerogenes (0.3%,1/295), and Klebsiella pneumoniae (0.3%,1/295). Other bacteria isolated from reproductive non pregnant women with AV were Pseudomonas aeruginosa (3.2%, 9/284), K. pneumoniae (1%,3/284), G. vaginalis (1%,3/284), C. albicans (1%,3/284), E. aerogenes (0.7%,2/284), E. cloacae (0.4%,1/284), Candida parapsilosis (0.4%,1/284), and Proteus vulgaris (0.4%,1/284).
Figure 1.
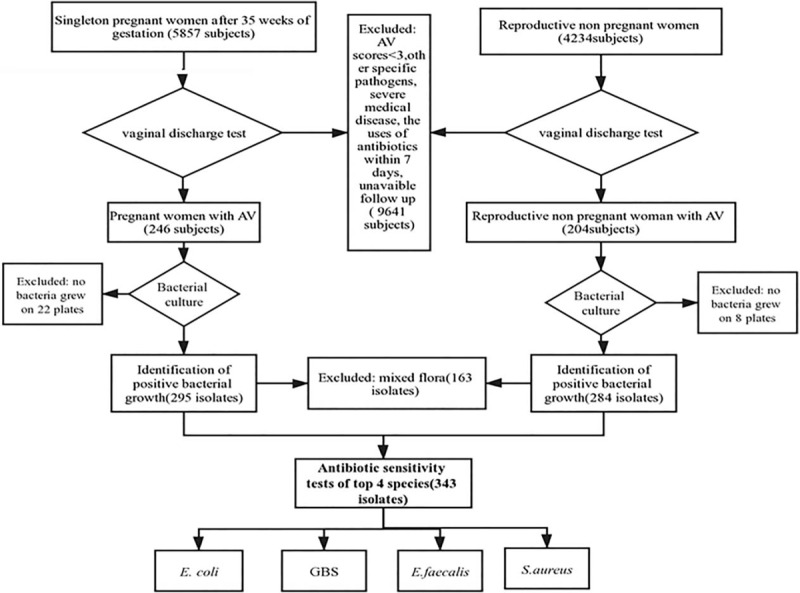
Flow diagram of the subjects analyzed. AV = aerobic vaginitis, GBS = group B Streptococcus.
Figure 2.
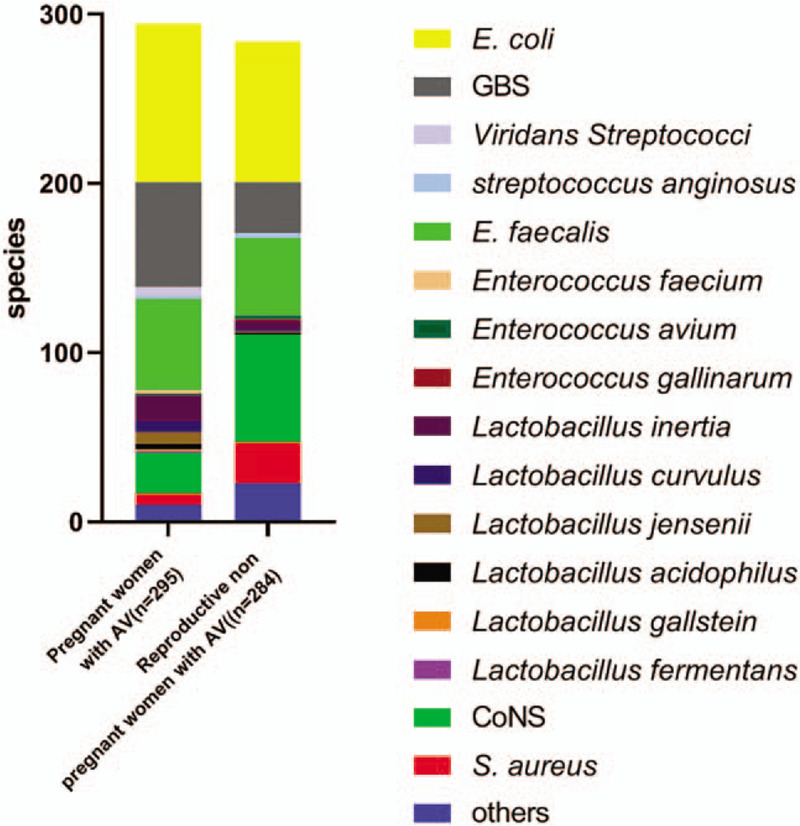
Distribution of bacterial isolates in pregnant and reproductive non pregnant women with aerobic vaginitis. AV = aerobic vaginitis, GBS = Group B Streptococcus, CoNS = Coagulase negative Staphylococci.
3.2. Antibiotic sensitivity tests in maternal AV infection
Antibiotic susceptibility tests were performed on 82.5% (343/416) of the isolates, excluding 163 isolates which belonged to mixed flora (64CoNS, 37Enterococcus feces, 20E coli which considered of opportunistic pathogens and 42 Lactobacillus ), and other species (some species have no tests standard)did not perform antibiotic susceptibility tests. We found that the majority pathogen species were E. coli, GBS, E. faecalis and S. aureus. Out of 343 isolates, 13.4% were antimicrobial-resistant; 11.5% (18/157) of E. coli were extended spectrum beta-lactamase producers, 4.3% (4/92) of GBS were multidrug-resistant, 19% (12/63) of E. faecalis were high-level gentamicin resistance, 20.6% (13/63) of E. faecalis were high-level streptomycin resistance, and 9.7% (3/31) of S. aureus were methicillin-resistant Staphylococcus aureus (MRSA). All isolates were highly susceptible to nitrofurantoin. E. coli were 100% (157/157) susceptible to amikacin, meropenem, ertapenem, and imipenem, and resistance to ampicillin, ampicillin/sulbactam, and cefazolin was less than 40%. Gram-positive cocci were 100% (186/186) susceptible to vancomycin and linezolid and above 50% isolates resistant to erythromycin and clindamycin. Among them, GBS and E. faecalis were highly susceptible to tigecycline and penicillin but resistant to tetracycline. GBS and S. aureus were 100% (94/94) susceptible to quinupristin and dalfopristin. GBS were 100% (92/92) susceptible to ampicillin and more than 50% resistant to levofloxacin and moxifloxacin (Fig. 3).
Figure 3.
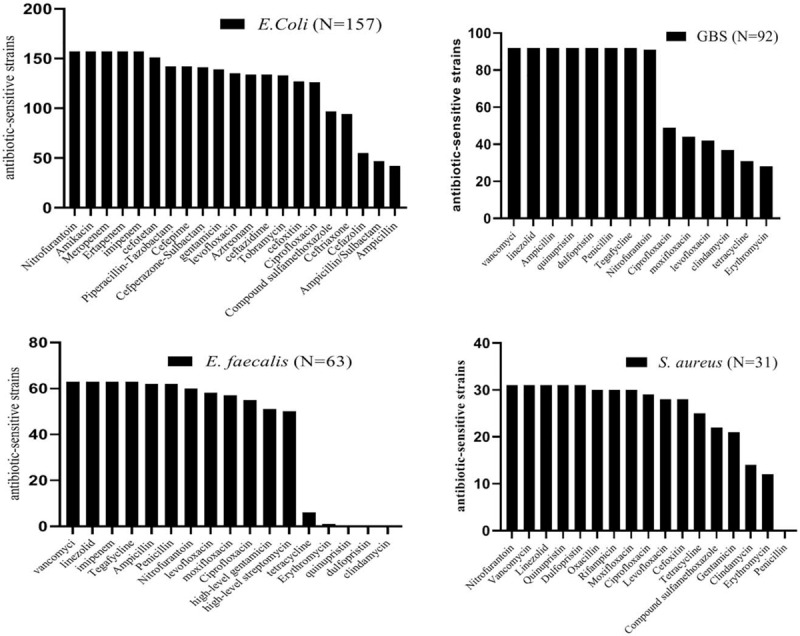
Antibiotic sensitivity tests of the top 4 species in aerobic vaginitis-positive women. GBS = group B Streptococcus.
3.3. Clinical characteristics of AV-positive and healthy pregnant women
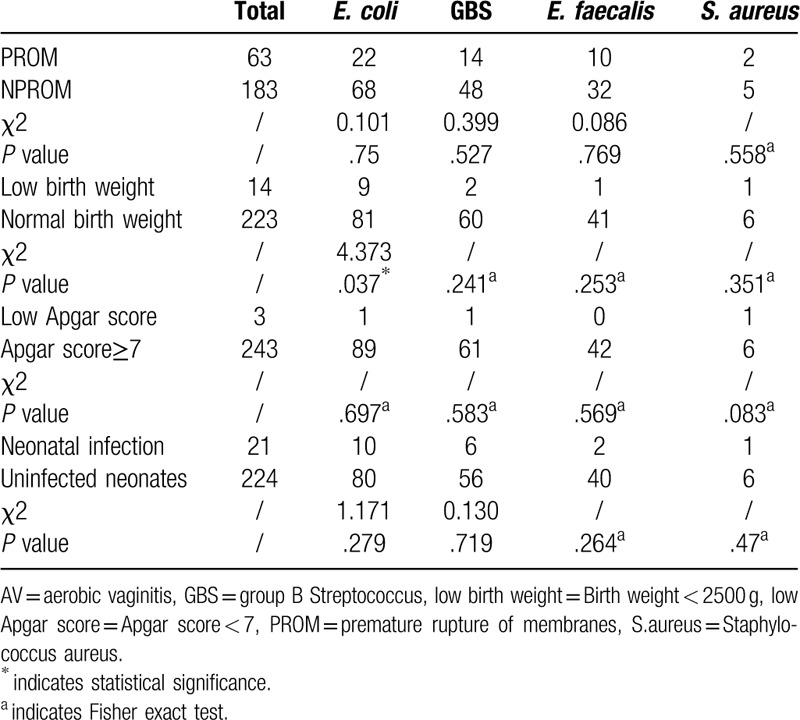
Clinical characteristics, including age, history of vaginitis, PROM, delivery mode, birth weight, Apgar score, stillbirth, and neonatal infection, were recorded after delivery (Table 1). The groups were matched by age and time of delivery. Interestingly, the pregnant women with AV had higher rates of history of vaginitis, PROM and neonatal infection than healthy pregnant women. We found no significant differences between AV-positive and healthy pregnant women with no AV in age, delivery mode, birth weight, or Apgar score (P > .05). No significant differences in PROM, Apgar score, neonatal infection were observed among different bacteria of AV. The maternal infection with E. coli exhibited their infants birth weights lower than average (Table 2).
Table 1.
Clinical factors and pregnancy outcomes of women with aerobic vaginitis and health vaginal microbiota.
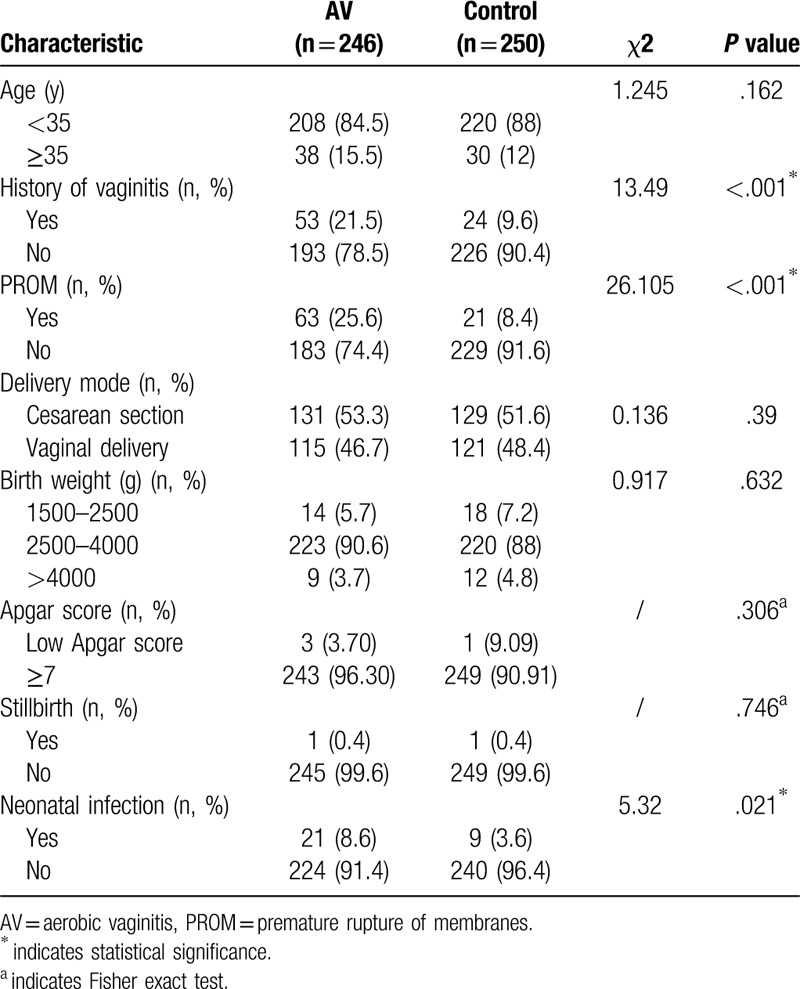
Table 2.
Effect of different bacterial isolates types on pregnancy outcome of pregnant women with aerobic vaginitis (n).
4. Discussion
This was one of the largest observational studies investigating the characterization of AV in late pregnancy, and we found that the isolated pathogens differed between AV-positive pregnant women and reproductive non pregnant women. Furthermore, to our knowledge, this was the first study to evaluate the association between antibiotics and different pathogens in vaginal discharge. Finally, we further strengthened the evidence linking AV with negative neonatal outcomes.
The findings in the current study were consistent with Donders et al's review of the prevalence of AV during pregnancy, which ranged from 4% to 8% but was slightly lower than the 7% to 13% found in non-pregnant women,[16] their prevalence rates were higher than ours possibly because we excluded other types of vaginitis by including only AV-positive women or because the incidence of AV in non pregnant women in western China is lower than that in other areas.
The vaginal bacterial community in women with AV is characterized by high loads of aerobes and low concentrations of Lactobacilli.[16] We also observed a variable mixed flora of E. faecalis, CoNS, E. coli, Lactobacilli in AV-positive women. Several studies concluded that the most common AV-associated organism, or at least the most commonly identified gram-negative pathogen, was E. coli.[16,17] Our study confirmed these findings. E. coli was the most common pathogen among all women, including pregnant women, however Staphylococcus spp. were the most commonly identified gram-positive pathogen in non-pregnant wowen and their compositions varied in different reports. The prevalence of Streptococcus spp., S. aureus, CoNS, and Enterococcus spp. varied from 0.7% to 58.7%, 6% to 37.4%, 0.2% to 41.7%, and 0.3% to 78.3%, respectively.[16,18,19] In particular, GBS,[20]S. aureus,[21] and E. faecalis[19] were found to be the most frequently isolated pathogenic bacteria in different reports. GBS (S. agalactiae) and S. aureus were the predominant gram-positive organisms in nonpregnant women in Nepal.[22] Our study demonstrated that Staphylococcus spp., Enterococcus spp., and Streptococcus spp. dominated in the majority of the AV-positive groups. Among these dominant genera, Staphylococcus and Streptococcus were substantially dissimilar between the pregnant and nonpregnant AV women.
Although not concerning vaginal microbiota, Khan et al found that the gut bacterial community composition differed between pregnant and non pregnant Saudi women, and an increased proportion of antibiotic resistance was observed during pregnancy.[23] To date, there has been little agreement on this. Dammeyer et al[24] found that 3.5% (23/651) of healthy pregnant women harbored antimicrobial-resistant bacteria and 14.3% (93/651) harbored methicillin-susceptible S. aureus (MSSA); the MRSA:MSSA ratio was 3.1%. However, Hetsa[25] reported a high prevalence (76%–100%) of vaginal colonization with antibiotic-resistant isolates among pregnant women.
In our study, 13.4% (46/343) of women harbored antimicrobial-resistant bacteria; 11.5% had extended spectrum beta-lactamase-producing bacteria, 4.3% had multidrug-resistant bacteria, 19% had high-level gentamicin resistance bacteria, 20.6% had high-level streptomycin resistance bacteria, and 9.7% MRSA. The MRSA:MSSA ratio equaled 10.7%. Due to ethnicity and genes, there are possible explanations for the differences. Clindamycin is often used in clinical practice, but considering the emerging resistance to antibiotics, the administration of ampicillin, ampicillin/sulbactam, cefazolin, erythromycin, and clindamycin is insufficient to control relevant bacteria. In theory, we could empirically use nitrofurantoin[26] because there is a low rate of resistance in all AV-associated bacteria; amikacin,[27] meropenem, ertapenem, and imipenem[28] for gram-negative bacilli; and vancomycin and linezolid[29] for gram-positive cocci. We do not recommend levofloxacin or moxifloxacin therapy for GBS which is in accordance with Ji W et al's report.[30] Importantly, consequences for the unborn fetus should be considered. Finally, pregnancy outcomes relates to various factors such as abnormal vaginal flora,[31,32] gestational diabetes,[33] etc our study supports previous research, but we demonstrate that pregnant women without complications (such as gestational diabetes) who suffered from simple AV also had worse pregnancy outcomes. AV maybe as a independent risk factor is associated with adverse pregnancy outcomes. It is feasible to assess the effect of the four main pathogens E. coli, GBS, E. faecalis and S. aureus on pregnancy and neonatal outcome. However, the present study contained too few samples to allow us to draw definite conclusions about the effects of different bacteria on pregnancy and neonatal outcome. Moreover, animal experiment, which is the direct evidence, should be performed in the future, and further research should be conducted to investigate the association between additional risk factors and pregnancy outcomes in women with AV to prevent and/or treat AV early.
In conclusion, our study reveals new insights into AV infection during pregnancy. This finding has implications for future studies designed to explore AV infection and treatment and, in particular, highlights that the isolates of AV infection were different between pregnant and nonpregnant women, which may translate to treatment that is a more cost-effective than current standard treatments.
Acknowledgments
The authors sincerely thank the editors and the anonymous reviewers for their meaningful suggestions on a previous draft.
Author contributions
Data curation: Yuanting Tang, Fan Yu, Luyun Peng.
Supervision: Zhengqiang Hu, Yongmei Jiang.
Writing – original draft: Yuanting Tang.
Writing – review & editing: Fan Yu, Luyun Peng, Zhengqiang Hu, Yongmei Jiang.
Footnotes
Abbreviations: AV = aerobic vaginitis, CoNS = coagulase-negative staphylococci, GBS = group B Streptococcus, MRSA = methicillin-resistant Staphylococcus aureus, PROM = premature rupture of membranes.
How to cite this article: Tang Y, Yu F, Hu Z, Peng L, Jiang Y. Characterization of aerobic vaginitis in late pregnancy in a Chinese population: a STROBE-compliant study. Medicine. 2020;99:25(e20732).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Greenbaum S, Greenbaum G, Moran-Gilad J, et al. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol 2019;220:324–35. [DOI] [PubMed] [Google Scholar]
- [2].Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med 2018;379:2246–54. [DOI] [PubMed] [Google Scholar]
- [3].Donders GG, Vereecken A, Bosmans E, et al. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 2002;109:34–43. [DOI] [PubMed] [Google Scholar]
- [4].Gray LA, Barnes ML. Vaginitis in women, diagnosis and treatment. Am J Obstet Gynecol 1965;92:125–36. [DOI] [PubMed] [Google Scholar]
- [5].Wang C, Fan A, Li H, et al. Vaginal bacterial profiles of aerobic vaginitis: a case-control study. Diagn Microbiol Infect Dis 2020;114981:1–42. [DOI] [PubMed] [Google Scholar]
- [6].Tao Z, Zhang L, Zhang Q, et al. The pathogenesis of streptococcus anginosus in aerobic vaginitis. Infect Drug Resist 2019;12:3745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Seta F, Campisciano G, Zanotta N, et al. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Front Microbiol 2019;10: 2451/1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han C, Li H, Han L, et al. Aerobic vaginitis in late pregnancy and outcomes of pregnancy. Eur J Clin Microbiol Infect Dis 2019;38:233–9. [DOI] [PubMed] [Google Scholar]
- [9].Kaambo E, Africa C. The threat of aerobic vaginitis to pregnancy and neonatal morbidity. Afr J Reprod Health 2017;21:108–18. [PubMed] [Google Scholar]
- [10].Schoenmakers S, Steegers-Theunissen R, Faas M. The matter of the reproductive microbiome. Obstet Med 2019;12:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kuba K, Bernstein PS. ACOG practice bulletin no. 188: prelabor rupture of membranes. Obstet Gynecol 2018;131:1163–4. [DOI] [PubMed] [Google Scholar]
- [12].Chen M, McNiff C, Madan J, et al. Maternal obesity and neonatal Apgar scores. J Matern Fetal Neonatal Med 2010;23:89–95. [DOI] [PubMed] [Google Scholar]
- [13].Blencowe H, Cousens S, Jassir FB, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;4:e98–108. [DOI] [PubMed] [Google Scholar]
- [14].Chan GJ, Lee AC, Baqui AH, et al. Prevalence of early-onset neonatal infection among newborns of mothers with bacterial infection or colonization: a systematic review and meta-analysis. BMC Infect Dis 2015;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smilga AS, Garfinkle J, Ng P, et al. Neonatal infection in children with cerebral palsy: a registry-based cohort study. Pediatr Neurol 2018;80:77–83. [DOI] [PubMed] [Google Scholar]
- [16].Donders G, Bellen G, Grinceviciene S, et al. Aerobic vaginitis: no longer a stranger. Res Microbiol 2017;168:845–58. [DOI] [PubMed] [Google Scholar]
- [17].Dermendjiev T, Pehlivanov B, Hadjieva K, et al. Epidemiological, clinical and microbiological findings in women with aerobic vaginitis. Akush Ginekol (Sofiia) 2015;54:4–8. [PubMed] [Google Scholar]
- [18].Oh KY, Jin CH, Sohn YH, et al. The prevalence of abnormal vaginal flora and predictive factors for intrauterine infection in pregnant Korean women with preterm labor. Clin Exp Obstet Gynecol 2017;44:429–33. [PubMed] [Google Scholar]
- [19].Rumyantseva TA, Bellen G, Savochkina YA, et al. Diagnosis of aerobic vaginitis by quantitative real-time PCR. Arch Gynecol Obstet 2016;294:109–14. [DOI] [PubMed] [Google Scholar]
- [20].Mendling W. Vaginal microbiota. Adv Exp Med Biol 2016;902:83–93. [DOI] [PubMed] [Google Scholar]
- [21].Wang ZL, Fu LY, Xiong ZA, et al. Diagnosis and microecological characteristics of aerobic vaginitis in outpatients based on preformed enzymes. Taiwan J Obstet Gynecol 2016;55:40–4. [DOI] [PubMed] [Google Scholar]
- [22].Tansarli GS, Kostaras EK, Athanasiou S, et al. Prevalence and treatment of aerobic vaginitis among non-pregnant women: evaluation of the evidence for an underestimated clinical entity. Eur J Clin Microbiol Infect Dis 2013;32:977–84. [DOI] [PubMed] [Google Scholar]
- [23].Khan I, Yasir M, Farman M, et al. Evaluation of gut bacterial community composition and antimicrobial resistome in pregnant and non-pregnant women from Saudi population. Infect Drug Resist 2019;12:1749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dammeyer AH, Heinze S, Adler AC, et al. Clinical relevance of colonization with antimicrobial-resistant bacteria (AMRB) and methicillin susceptible Staphylococcus aureus (MSSA) for mothers during pregnancy. Arch Gynecol Obstet 2019;300:1303–16. [DOI] [PubMed] [Google Scholar]
- [25].Hetsa BA, Kumar A, Ateba CN. Characterization of multiple antibiotic resistant clinical strains of Staphylococcus isolated from pregnant women vagina. Folia Microbiol (Praha) 2018;63:607–17. [DOI] [PubMed] [Google Scholar]
- [26].Chu CM, Lowder JL. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol 2018;219:40–51. [DOI] [PubMed] [Google Scholar]
- [27].Vanukuru J, Bagga R, Muthyala T, et al. A clinical and microbiological study of puerperal sepsis in a tertiary care hospital in India. J Matern Fetal Neonatal Med 2019;32:1931–7. [DOI] [PubMed] [Google Scholar]
- [28].Kiponza R, Balandya B, Majigo MV, et al. Laboratory confirmed puerperal sepsis in a national referral hospital in Tanzania: etiological agents and their susceptibility to commonly prescribed antibiotics. BMC Infect Dis 2019;19: 690/1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shrestha LB, Baral R, Poudel P, et al. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr 2019;19: 36/1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ji W, Zhang L, Guo Z, et al. Colonization prevalence and antibiotic susceptibility of Group B Streptococcus in pregnant women over a 6-year period in Dongguan, China. PLoS One 2017;12:e0183083/1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Emmanuel F, Karine F, Martin F, et al. Vaginal mucosal homeostatic response may determine pregnancy outcome in women with bacterial vaginosis: a pilot study. Medicine 2016;95: e2668/1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tibaldi C, Cappello N, Latino MA, et al. Maternal risk factors for abnormal vaginal flora during pregnancy. Int J Gynaecol Obstet 2016;133:89–93. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Xinhong, Liao Qinping, Wang Fengying, et al. Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine 2018;97: e11891/1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


