Abstract
Background
Pruritus is the most common symptom in patients with skin disease. Psoriasis and atopic dermatitis are clinically distinct inflammatory diseases. Interleukins are cytokines which play key roles in inflammatory signaling pathways.
Materials and Methods
Cross-sectional study was conducted among patients with psoriasis and atopic dermatitis: 59 psoriatic patients, 56 AD patients, and 49 matched healthy controls. Interleukins 4, 13, 31, 33 serum levels were assayed by ELISA and results were compared using SPSS. Itch severity and disease severity were measured and correlation with interleukin levels was determined using SPSS.
Results
The serum levels of IL-4, -13, -31, -33 were elevated in atopic dermatitis patients compared to controls. Itch and disease severity were not correlated with elevated serum levels of these interleukins. In psoriasis, the levels of IL-4 and -31 were elevated compared to controls, whereas the levels of IL-13 and -33 were lower than controls. The levels of measured interleukins in psoriasis did not correlate with itch and disease severity.
Conclusion
IL-31 is the key mediator for pruritus in both AD and Ps patients. IL-4/31 axis and IL-33/13 axis play distinct roles in the pathogenesis of Atopic dermatitis and Psoriasis. Interleukin serum levels were not correlated with itch and disease severity in both conditions.
Keywords: psoriasis, atopic dermatitis, pruritus, interleukins
Introduction
Pruritus (Itch) is the most common symptom experienced by patients with skin disease. Additionally, many systemic diseases can be associated with itch. The pathogenesis of itch is complex and multifactorial as discussed by several reviews.1–3
Psoriasis (Ps) and atopic dermatitis (AD) are clinically distinct inflammatory diseases. The two diseases differ in their age of onset such as AD appears in early childhood affecting 15–20% of all children and only 1–10% of adults worldwide whereas psoriasis is rare in young children and appears in early adulthood affecting 2–3% of the population.4 Both conditions are associated with pruritus that can affect several aspects of life in these patients.5,6 Our knowledge about the pathophysiology of pruritus in these conditions is still incomplete especially in relation to Ps.
Itch in AD has been studied extensively clinically and biochemically.7,8 The mechanisms that may be involved in Ps and AD itch are complex and multifactorial,9 however, the role of inflammation is likely to be significant since several inflammatory mediators elevated in both conditions are known to cause itch and anti-inflammatory treatments can reduce inflammatory signs as well as itch.8,10
Interleukins are cytokines which play key roles in pro-inflammatory and anti-inflammatory signaling pathways. Interleukins 4, 13, 31 and 33 have been studied in multiple skin diseases including psoriasis and atopic dermatitis and targeted therapy for some of these interleukins are used for treatment of these conditions.11 IL-4 and IL-13 signaling actions are mediated through binding to four different types of receptors, namely, IL-4R, Type II IL-4R, Type II IL-13R, and IL-13Rα2, explaining the multiple physiological effects common between these two interleukins.12,13 IL-31 is a pro-inflammatory cytokine produced by CD4+ T helper cells.14 The actions of IL-31 are mediated via its binding to IL-31R; a heterodimeric receptor of 2 subunits, IL-31 receptor alpha (IL-31RA) and oncostatin-M receptor beta (OSMR). 14. IL-33 is a new member of the IL-1 cytokine family which plays a key role in the induction of Th2 cytokines production.15 IL-33 is considered to be an “alarmin” cytokine; its secretion from damaged tissues and inflammatory sites activates multiple signaling pathways. 15. IL-33 actions are mediated by the receptor T1/ST2.15
In the current study, interleukins IL-4, -13, -31 and -33 were examined in serum of patients with Ps and AD in order to look for possible links between itch and severity scores as measured by clinical scores.
Materials and Methods
Study Subjects
A comparative cross-sectional study was conducted in 2019 and 2020 among patients with Ps and AD. The study consisted of 59 psoriatic patients, 56 AD patients, and 49 healthy controls of matched age and sex. Ps and AD patients with chronic pruritus (more than 6 weeks) were recruited from the dermatology clinic in the King Abdullah University Hospital (KAUH). An ethical approval was obtained from the research and ethics committee of the Jordan University of science and technology (IRB # 64/2019). This study was conducted in accordance with the Declaration of Helsinki. After written informed consent was obtained, clinical assessments were recorded.
Disease and Itch Severity Assessment
For patients with Ps, the disease severity was assessed by Psoriasis area severity Index and Body surface area (PASI/BSA) and Dermatology Life Quality Index (DLQI). For patients with AD, severity was assessed by Scoring Atopic Dermatitis index, Eczema area severity Index (SCORAD/EASI). Itch severity in Ps was measured by Pruritus grading system (PGS)16 and visual analogue scale (VAS), while itch in AD was measured by PGS and Investigator Global Assessment scale (IGA).
ELISA
Blood samples (5 mL) were collected from the peripheral vein of the subjects (Ps, AD and healthy controls) and were left to clot in a plain tube for about 2 hours. Serum was separated soon after being centrifuged using Centrifuge 5810 R at 2000 rpm (rotation per minute) for 5 minutes. Serum samples were subdivided into small aliquots and stored at −80 °C until tested for Interleukin levels. Human IL-33, IL-31, IL-13, and IL-4 ELISA kits were purchased from Abbexa, UK. All interleukins have a test range of 15.6–1000 pg/mL and a sensitivity of 9.38 pg/mL for IL-33, 5.5 pg/mL for IL-31, 6.7 pg/mL for IL-13 and 5.7 pg/mL for IL-4. Interleukin concentrations in serum samples were determined using the ELISA kits of each interleukin according to the manufacturer’s instructions. Absorbance at 450 nm was determined on an ELx800 Microplate Reader (BioTek Instruments, Winooski, VT, USA). For values over the range of detection, serial dilutions were done and the final values were presented multiplied by the appropriate dilution factor. The values represent the concentrations in pg/mL.
Statistical Analysis
The statistical analysis was performed with the use of SPSS software version 22.0. The data were presented as a mean value according to the normal distribution and the number of patients in the group. Normal distribution was tested with Kolmogorov–Smirnov test, accepting p < 0.05 as the value different from normal distribution. Correlations between serum interleukin levels in the patients and control groups and the correlations between disease severity in both Ps and AD patients were assessed using U Mann–Whitney test. The Spearman rank test was used to evaluate the relationship between serum levels of interleukins and itch severity. Differences with P value less than 0.05 were considered to be statistically significant.
Results
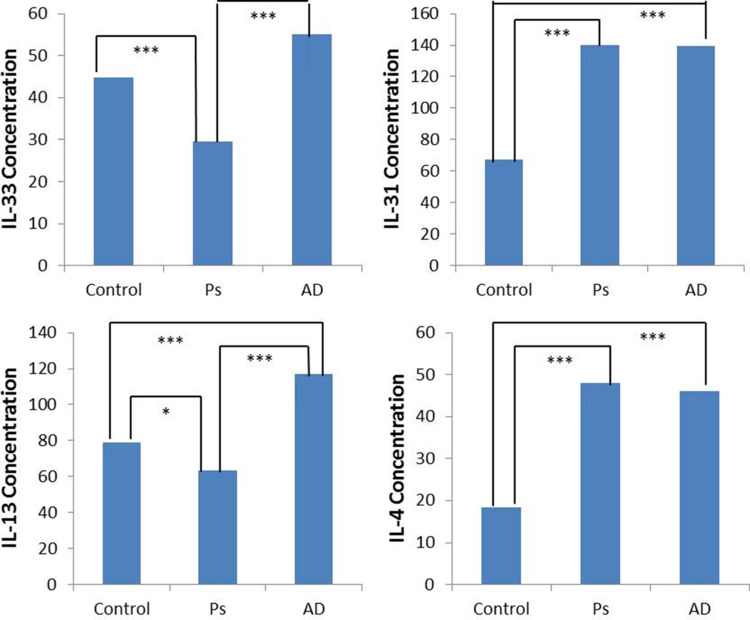
A total of 59 Ps patients and 56 AD patients were enrolled in this study. Additionally, 49 healthy controls were recruited with matching age and sex for comparisons with no itch. The average age of Ps patients was 35 years old (range 5–71 years old) and for AD patients it was 26 years old (range 1–88 years old). The male to female ratio was approximately 1:1 in all three studied groups. Serum levels of IL-33, IL-31, IL-13 and IL-4 were determined using standard ELISA procedures. The mean serum levels of IL-33 in Ps patients were 29.55 pg/mL compared with 44.88 pg/mL in controls (Figure 1). In comparison, IL-33 mean level in AD patients was 55.02 pg/mL as shown in Figure 1. As shown in Table 1, IL-33 mean serum levels were significantly lower in the Ps group compared to AD and control groups (P < 0.0001). However, there was no significant difference in IL-33 mean serum levels between the AD and control groups (P = 0.765). For IL-31, the mean serum levels were higher in Ps patients (139.75 pg/mL) compared with controls (67.07 pg/mL) (Figure 1). Similarly, IL-31 serum levels were higher in AD patients (139.35 pg/mL) in comparison to the control group (Figure 1). The difference in IL-31 levels in both Ps and AD in comparison to controls was found to be statistically significant (P < 0.0001) as shown in Table 1. IL-13 mean serum levels in the Ps group were found to be higher (6356.35 pg/mL) than the control (79.13 pg/mL) and AD (116.8 pg/mL) group levels (Figure 1). The difference in IL-13 levels in both Ps and AD in comparison to the control group is statistically significant (Ps vs control, P= 0.02; AD vs control, P= 0.0001) as shown in Table 1. A significantly higher levels of IL-13 in the serum of AD patients were seen when compared to the levels in the Ps group (P = 0.0001). In the case of IL-4, a similar trend to that of IL-13 was seen such as IL-4 mean serum levels were higher in both Ps (47.94 pg/mL) and AD patients (45.98 pg/mL) when compared to the control group (18.34 pg/mL) (Figure 1). The difference is statistically significant (P= 0.0001) for both groups as shown in Table 1.
Figure 1.
Comparison of IL-33, IL-31, IL-13 and IL-4 mean serum levels in the Psoriasis group (Ps), Atopic Dermatitis group (AD) and healthy controls (Control) using ELISA assays. The values represent the concentrations in pg/mL. The stars (*) are used to represent P values of significance such as *Represents P ≤ 0.05 and ***Represents P ≤ 0.001.
Table 1.
Pairwise Comparisons Between IL-33/31/13/4 Serum Levels in PS Patients, AD Patients and Healthy Controls Using Mann Whitney U-test. The Stars (*) are Used to Represent Levels of Significance; P ≤ 0.05
| Group | IL-33 | IL-31 | IL-13 | IL-4 | ||||
|---|---|---|---|---|---|---|---|---|
| Z | P-value | Z | P-value | Z | P-value | Z | P-value | |
| PS - Control | −5.81 | 0.0001* | −4.38 | 0.0001* | −2.33 | 0.020* | −7.67 | 0.0001* |
| AD - Control | −.298 | 0.765 | −4.12 | 0.0001* | −4.96 | 0.0001* | −8.01 | 0.0001* |
| PS - AD | −6.38 | 0.0001* | −.200 | 0.841 | −6.44 | 0.0001* | −2.40 | 0.016* |
Next, we investigated the correlation between interleukins-4, -13, -31, -33 serum levels and both disease and itch severity in Ps and AD patients. For Ps patients, we examined disease severity using PASI/BSA, DLQI, and for itch severity, we used PGS and VAS. Each patient was given a score according to these scales. Using the Spearman correlation test, the results show that there are no statistically significant correlations between IL-33, IL-31, IL-13 and IL-4 and the aforementioned scoring systems as shown in Tables 2 and 3. We also examined disease and itch severity in AD patients using SCORAD, EASI, DLQI, PGS and IGA and each patient was given a score. Using the Spearman correlation test, the results show, similar to Ps patients, that there are no statistically significant correlations between IL-33, IL-31, IL-13 and IL-4 and the aforementioned soring systems as shown in Table 4.
Table 2.
Relationship Between IL-33/31/13/4 Serum Levels in AD Patients and Itch Score Systems (PGS/SCORAD/DLQI/EASI) Using Spearman Correlation Test
| Variable | Statistics | IL-33 | IL-31 | IL-13 | IL-4 |
|---|---|---|---|---|---|
| SCORAD | r | 0.047 | 0.244 | −.145 | 0.078 |
| P | 0.774 | 0.125 | 0.347 | 0.589 | |
| EASI | r | 0.065 | 0.300 | −.180 | 0.270 |
| P | 0.689 | 0.057 | 0.243 | 0.055 | |
| DLQI | r | 0.016 | 0.019 | −.019 | 0.079 |
| P | 0.922 | 0.908 | 0.902 | 0.581 | |
| PGS | r | −.081 | 0.085 | −.063 | 0.244 |
| P | 0.619 | 0.598 | 0.684 | 0.085 |
Table 3.
Relationship Between IL-33/31/13/4 Serum Levels in AD Patients and IGA Itch Score System Using the Mann–Whitney U-test
| Variable | IGA | N | Mean Rank | Sum of Ranks | Z | P-value |
|---|---|---|---|---|---|---|
| IL-33 | Mild vs. | 15 | 20.80 | 312.00 | −.126 | 0.900 |
| Moderate/severe | 25 | 20.32 | 508.00 | |||
| IL-31 | Mild vs. | 15 | 21.40 | 321.00 | −.162 | 0.871 |
| Moderate/severe | 26 | 20.77 | 540.00 | |||
| IL-13 | Mild vs. | 16 | 27.72 | 443.50 | −2.04 | 0.041 |
| Moderate/severe | 28 | 19.52 | 546.50 | |||
| IL-4 | (Mild) vs. | 19 | 23.97 | 455.50 | −.755 | 0.450 |
| (Moderate/severe) | 32 | 27.20 | 870.50 |
Table 4.
Relationship Between IL-33/31/13/4 Serum Levels in Ps Patients and Itch Score Systems (PASI/DLQI/PGS/VAS) Using Spearman Correlation Test
| Variable | Statistics | IL-33 | IL-31 | IL-13 | IL-4 |
|---|---|---|---|---|---|
| PASI | r | −.044 | 0.134 | −.006 | −.240 |
| P | 0.780 | 0.399 | 0.969 | 0.086 | |
| DLQI | r | 0.059 | 0.096 | −.154 | −.221 |
| P | 0.711 | 0.544 | 0.324 | 0.115 | |
| PGS | r | 0.013 | 0.100 | 0.061 | −.189 |
| P | 0.935 | 0.528 | 0.698 | 0.179 | |
| VAS | r | −.041 | 0.095 | −.041 | −.224 |
| P | 0.797 | 0.550 | 0.793 | 0.111 |
Discussion
Psoriasis (Ps) and atopic dermatitis (AD) result in chronic inflammation which can be treated by anti-cytokine targeted therapies and systemic immunosuppressive drugs. The distinction between the two diseases is manifested by the type of T helper cells involved in which AD is linked to Th2 cells and increased production of IL-4, IL-13, IL-31 among others, whereas psoriasis is linked to Th1 and Th17 cells and increased production of IL-17, IL-22 and IL-23 among others. The role of IL-4 and IL-13 as culprits in the pathogenesis of AD led to the use of Dupilumab for the treatment of AD.17 Dupilumab is designed to bind IL-4Ra, the receptor subunit common to type I and type II receptors for IL-4 and IL-13. A role for IL-17/23 axis in psoriasis pathogenesis is further manifested by the efficacy of several agents targeting these pathways.
In the current study, we investigated the correlation between serum levels of several inflammatory cytokines (IL-4, -13, -31, -33), pruritus and disease severity in both Ps and AD patients. In patients with AD, significant correlation was found between elevated serum levels of IL-4, -13, -31 compared to healthy controls (Table 1 and Figure 1). As for patients with Ps, the levels of interleukins-31 and −4 were significantly elevated compared to healthy controls (Table 1 and Figure 1). However, the levels of interleukins-13 and −33 were significantly lower than both controls and AD patients. This indicates distinct inflammatory signaling pathways are activated in Ps and AD.
In a study by Neis et al, the mRNA levels of IL-31 were shown to be increased in AD lesions but not psoriatic plaques and the levels of IL-31 in AD lesions correlated with the expression of IL-13 and IL-4.18 In another study, it was found that the expression of IL-31 in primary bronchial epithelial cells is dependent on IL-4 as treatment of these cells with anti-IL-4 antibodies resulted in a marked reduction in the expression of IL-31.19 The association between IL-31 and IL-4 is further confirmed by a recent study which shows that IL-31 receptor alpha (IL-31-RA) expression and its association with IL-31 in dendritic cells were enhanced in the presence of IL-4.20 Nattkemper and co-authors investigated the transcriptome of pruritic skin from AD and Ps patients in addition to controls. Their results identified IL-31 transcript, among others, as being overexpressed in both itchy atopic and psoriatic skin.21
IL-33 is a member of the IL-1 family with an increased expression in barrier tissues including the skin, stomach and lungs. IL-33 functions as an alarm signal “alarmin” secreted subsequent to tissue damage and trauma to activate and recruit different cells of the immune system.15 Duffen et al identified a link between IL-33 and IL-13 in which high levels of IL-33 increased serum levels of IL-13. The authors point to IL-33/IL-13 axis as key player in promoting anti-inflammatory macrophage differentiation in adipose tissue.22 IL-33 activation of IL-13 has been also implicated in cutaneous fibrosis and up-regulation of immune system in relation to candidiasis infection.23,24 Additionally, a role for IL-33 in the activation of type 2 innate immune cells has been recently discovered.25 Studies confirm that keratinocytes release IL-33 to activate dendritic cells in AD lesions which subsequently, activates Th2 cells to produce IL-31, IL-13 and IL-4 resulting in the induction of a plethora of genes in keratinocytes secondary to breaching of barrier function and pruritic symptoms. In a study by Li et al, the authors show increased serum levels of IL-33 in Ps patients compared to controls, however, IL-33 serum levels did not correlate with PASI scores.26 Other studies showed conflicting results for serum levels of IL-33 in Ps patients.27,28 Low levels of IL-33 in Ps patient compared to AD patients could be explained by the fact that AD lesions are associated with more significant barrier disruption compared to Ps plaques which could explain why AD patients are more susceptible to recurrent infections.
The disease severity score for AD was measured using 2 scoring systems, SCORAD and EASI, however, no correlation between serum levels of investigated interleukins and disease severity was found (Table 2). Also, the levels of these interleukins did not correlate with intensity of itch as measured by 2 pruritus scales (PGS and IGA) (Tables 2 and 3). This could be possibly explained by the fact that pruritus in AD is multifactorial involving several mechanisms and other inflammatory cytokines. This lack of significant association was also demonstrated by other studies.29,30 However, a systematic review on IL-31 found serum IL-31 levels to correlate with pruritus intensity indicating that IL-31 is a key mediator in inducing pruritus.31 The levels of interleukins in Ps did not correlate with disease or itch severity (measured by PASI/BSA, VAS, PGS) which indicates that other mediators in Ps pathogenesis are also implicated. Results of previous studies on correlation between pruritus and Ps severity have been conflicting with some showing no correlation and others showing significant correlation.28,32–34
Conclusions
Interleukin-31 seems to be a main itch mediator in both Ps and AD via induction by IL-4. The lack of significant barrier disruption in Ps explains lack of induction of IL-33/13 when compared to AD. No significant correlation was found between serum levels of interleukins-4, -13, -31, -33 and both disease and itch severity in AD and Ps patients.
Acknowledgment
This study has been approved and supported by Deanship of Research at Jordan University of Science and Technology grant number 64/2019.
Disclosure
The authors declare no conflict of interest.
References
- 1.Millington GWM, Collins A, Lovell CR, et al. British Association of Dermatologists’ guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. Br J Dermatol. 2018;178(1):34–60. doi: 10.1111/bjd.16117 [DOI] [PubMed] [Google Scholar]
- 2.Weisshaar E, Szepietowski JC, Dalgard FJ, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99(5):469–506. doi: 10.2340/00015555-3164 [DOI] [PubMed] [Google Scholar]
- 3.Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142(5):1375–1390. doi: 10.1016/j.jaci.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CE, van de Kerkhof P, Czarnecka-Operacz M. Psoriasis and atopic dermatitis. Dermatol Ther (Heidelb). 2017;7(Suppl S1):31–41. doi: 10.1007/s13555-016-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaaz K, Szepietowski JC, Matusiak Ł. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol. 2019;99(2):175–180. doi: 10.2340/00015555-3065 [DOI] [PubMed] [Google Scholar]
- 6.Szepietowski JC, Reich A. Itch in psoriasis management. Curr Probl Dermatol. 2016;50:102–110. [DOI] [PubMed] [Google Scholar]
- 7.Zeidler C, Pereira MP, Huet F, Misery L, Steinbrink K, Ständer S. Pruritus in autoimmune and inflammatory dermatoses. Front Immunol. 2019;10:1303. doi: 10.3389/fimmu.2019.01303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol. 2016;51(3):263–292. [DOI] [PubMed] [Google Scholar]
- 9.Szepietowski JC, Reich A. Pruritus in psoriasis: an update. Eur J Pain. 2016;20(1):41–46. doi: 10.1002/ejp.768 [DOI] [PubMed] [Google Scholar]
- 10.Ayasse MT, Buddenkotte J, Alam M, Steinhoff M. Role of neuroimmune circuits and pruritus in psoriasis [published online ahead of print, 2020 Jan 18]. Exp Dermatol. 2020. doi: 10.1111/exd.14071 [DOI] [PubMed] [Google Scholar]
- 11.Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73. doi: 10.1016/j.coi.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 12.Zamorano J, Rivas M, Perez G. Interleukin-4: a multifunctional cytokine. Immunologia. 2003;22(2):215–224. [Google Scholar]
- 13.Seyfizadeh N, Seyfizadeh N, Gharibi T, Babaloo Z. Interleukin-13 as an important cytokine: a review on its roles in some human diseases. Acta Microbiol Immunol Hung. 2015;62(4):341–378. doi: 10.1556/030.62.2015.4.2 [DOI] [PubMed] [Google Scholar]
- 14.Gangemi S, Quartuccio S, Casciaro M, Trapani G, Minciullo PL, Imbalzano E. Interleukin 31 and skin diseases: a systematic review. Allergy Asthma Proc. 2017;38(6):401–408. doi: 10.2500/aap.2017.38.4080 [DOI] [PubMed] [Google Scholar]
- 15.Cayrol C, Girard JP. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281(1):154–168. doi: 10.1111/imr.12619 [DOI] [PubMed] [Google Scholar]
- 16.Al-Qarqas F, Al-Aboosi M, Al-Shiyab D, Bataineh A. Using pruritus grading system for measurement of pruritus in patients with disease associated with itch. J Med. 2012;46(1):39–44. [Google Scholar]
- 17.Gooderham MJ, Hong HC, Eshtiaghi P, Papp KA. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(Suppl 3):S28–S36. doi: 10.1016/j.jaad.2017.12.022 [DOI] [PubMed] [Google Scholar]
- 18.Neis MM, Peters B, Dreuw A, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118(4):930–937. doi: 10.1016/j.jaci.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 19.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132(2):446–54.e5. doi: 10.1016/j.jaci.2013.03.050 [DOI] [PubMed] [Google Scholar]
- 20.Miake S, Tsuji G, Takemura M, et al. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced Ccl 17 and Ccl 22 production in dendritic cells: implications for atopic dermatitis. Int J Mol Sci. 2019;20(16):4053. doi: 10.3390/ijms20164053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nattkemper LA, Tey HL, Valdes-Rodriguez R, et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Invest Dermatol. 2018;138(6):1311–1317. doi: 10.1016/j.jid.2017.12.029 [DOI] [PubMed] [Google Scholar]
- 22.Duffen J, Zhang M, Masek-Hammerman K, et al. Modulation of the IL-33/IL-13 axis in obesity by IL-13Rα2. J Immunol. 2018;200(4):1347–1359. doi: 10.4049/jimmunol.1701256 [DOI] [PubMed] [Google Scholar]
- 23.Rankin AL, Mumm JB, Murphy E, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010;184(3):1526–1535. doi: 10.4049/jimmunol.0903306 [DOI] [PubMed] [Google Scholar]
- 24.Tran VG, Kim HJ, Kim J, et al. IL-33 enhances host tolerance to Candida albicans kidney infections through induction of IL-13 production by CD4+ T cells. J Immunol. 2015;194(10):4871–4879. doi: 10.4049/jimmunol.1402986 [DOI] [PubMed] [Google Scholar]
- 25.Herbert DR, Douglas B, Group ZK. 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. Int J Mol Sci. 2019;20(9):2276. doi: 10.3390/ijms20092276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Liu L, Rui W, et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology. 2017;233(1):37–46. doi: 10.1159/000471798 [DOI] [PubMed] [Google Scholar]
- 27.Balato A, Lembo S, Mattii M, et al. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol. 2012;21(11):892–894. doi: 10.1111/exd.12027 [DOI] [PubMed] [Google Scholar]
- 28.Mitsui A, Tada Y, Takahashi T, et al. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol. 2016;41(2):183–189. doi: 10.1111/ced.12670 [DOI] [PubMed] [Google Scholar]
- 29.Ozceker D, Bulut M, Ozbay AC, et al. Assessment of IL-31 levels and disease severity in children with atopic dermatitis. Allergol Immunopathol (Madr). 2018;46(4):322–325. doi: 10.1016/j.aller.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Siniewicz-Luzeńczyk K, Stańczyk-Przyłuska A, Zeman K. Correlation between serum interleukin-31 level and the severity of disease in children with atopic dermatitis. Postepy Dermatol Alergol. 2013;5(5):282–285. doi: 10.5114/pdia.2013.38356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Wu K, Zeng Q, Xiang Y, Gao L, Huang J. Serum interleukin-31 level and pruritus in atopic dermatitis: a meta-analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(2):124–130. doi: 10.11817/j.issn.1672-7347.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Abdallah F, Pichon C. Evidence on the direct correlation between miR-31 and IL-22 axis in IMQ-induced psoriasis. Exp Dermatol. 2019;28(11):1336–1340. doi: 10.1111/exd.14001 [DOI] [PubMed] [Google Scholar]
- 33.Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383. doi: 10.3389/fimmu.2019.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SM, Kim GW, Kim HS, Ko HC, Kim MB, Kim BS. Characteristics of pruritus according to morphological phenotype of psoriasis and association with neuropeptides and interleukin-31. Ann Dermatol. 2020;32(1):1–7. doi: 10.5021/ad.2020.32.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]