Abstract
Background
Colorectal cancer (CRC) is one of the most common malignant tumors in the digestive system. The lncRNA HCP5 has been reported to affect the progression of tumor in several types of cancer. Here, in this research, we focus on the role and function of lncRNA HCP5 in human colorectal cancer.
Materials and Methods
Tissue samples from colorectal cancer patients were used for detecting the expression of HCP5 by qRT-PCR. Proliferation, migration, invasion and apoptotic cells were assessed by CCK-8, colony formation, transwell assays and flow cytometry in SW480 and HCT-116 cells. The interactions between miR-299-3p and HCP5 or PFN1 were analyzed and confirmed by online database and luciferase reporter assays. The changes in PFN1 and AKT proteins were measured by Western blot. In vivo experiment was used to confirm the role of HCP5 in CRC.
Results
The expression of HCP5 had a higher level in colorectal cancer samples and cells by qRT-PCR, comparing with the normal colorectal tissues and human normal colon epithelial cell. It was revealed that knockdown of HCP5 inhibited viabilities, migration and invasion, while inducing apoptosis in SW480 and HCT-116 cells. Then, HCP5 negatively regulated the expressions of miR-299-3p, which negatively regulated the expressions of PFN1 by targeting PFN1. Furthermore, miR-299-3p inhibitor could alleviate the inhibiting effect by si-HCP5 on cell process of SW480 and HCT-116 cells. In addition, the lncHCP5/miR-299-3p/PFN1 axis could affect the progression of CRC through activating the AKT signaling. Last, we confirmed that knockdown of HCP5 inhibited the progression of CRC with an in vivo experiment.
Conclusion
The experiments and analyses support our hypothesis that knockdown of lncRNA HCP5 suppresses the progression of colorectal cancer by miR-299-3p/PFN1/AKT axis.
Keywords: lncRNA HCP5, miR-299-3p, PFN1, AKT, human colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors in the world, ranking the third among all malignant tumors in incidence and the fourth in mortality, following the lung cancer, liver cancer and gastric cancer.1 In 2018, more than 1.8 million new cases and nearly 900,000 deaths were estimated around the world for CRC.2 Similarly, CRC is the 4th most usual malignancy in China and the incidence continues to increase.3 Nevertheless, the molecular mechanism of CRC development still remains unclear. Therefore, further study on the mechanism of CRC development and the discovery of valuable molecular markers is of great scientific significance and clinical value for the early diagnosis, prognosis evaluation and targeted therapy of CRC.
Studies have shown that lncRNAs could function in affecting the occurrence and development of diseases, especially tumors.4,5 LncRNA does not encode proteins, but can regulate gene expression in multiple layers and through multiple pathways such as sponging the target-miRNAs.6 The correlation between lncRNA and CRC has also attracted more and more attention.7 LncRNAs can participate in the regulation of colorectal tumor cell proliferation, invasion and metastasis through the action of a similar oncogene or tumor suppressor gene.8,9 Due to the high specificity of lncRNA expression in tumors, lncRNA can be used as a diagnostic and prognostic indicator for colorectal cancer, and is expected to become a new target for disease treatment.10,11 Human major histocompatibility complex p5 (lncRNA HCP5) has been regarded as a novel genetic locus in clinical thyroid disease.12 The abnormal expression of HCP5 exists in most cancers and affects the progression of tumors in many ways. Previous study has shown that HCP5 contributes to epithelial-mesenchymal transition (EMT) in colorectal cancer through ZEB1 activation and interacting with miR-139-5p.13 Their results indicated that HCP5 promoted the occurrence of EMT in CRC cells, as an oncogenic factor, through miR-139-5p/ZEB1/Wnt signaling pathway.13 However, this study was to investigate the role and mechanism of lncRNA HCP5 in CRC development and progression.
MicroRNAs (miRNAs, ~20 nt) are a class of non-coding small RNAs that are highly conserved in evolution.14,15 Increasing evidence indicates miRNAs play an important role in cell proliferation and tumor formation by regulating gene expression at the post-transcriptional level.16 For example, miR-299-3p usually acts as a tumor suppressor, restricting the development of varieties of cancers. However, the role and function of miR-299-3p remains to be elusive in CRC. Profilin 1 (PFN1) plays an important role in actin dynamics by regulating actin polymerization in response to extracellular signals.17 PFN1 generally has a higher expression in CRC, and would be an anti-cancer drug target to participate in the progression of CRC.18 Previous studies have revealed that PFN1 could activate the AKT signaling in breast cancer and regulate the integrin/focal adhesion kinase pathway in gastric cancer.19,20 As we know, AKT often acts as a key factor to participate in varieties of cancers.21 The functions of the AKT signaling were induced by the phosphorylation of AKT, and the activated the AKT signaling could affect most cell processes, including the proliferation and apoptosis.22,23 Hence, investigating the AKT signaling could help us found the underlying mechanism.
Here in this research, we focus on studying the role and the underlying mechanism of lncRNA HCP5 in human colorectal cancer, as well as investigating the effect of miR-299-3p/PFN1/AKT axis in progression of CRC.
Materials and Methods
Tissue Samples
The tissue samples and matched adjacent normal tissues were obtained from 20 colorectal cancer patients (8 males and 12 females; age: 55–70 years old) at Xi’an Central Hospital between 2018 and 2019 with written informed consent. This study was reviewed and approved by the Ethics Review Committee of Xi’an Central Hospital and was performed in accordance with Declaration of Helsinki. Every patient provided their informed consent in writing prior to their participation in the study.
Cells Culture
Human normal colon epithelial cell, NCM460, and human colon cancer cell lines, SW480, HCT-116 and HT-29 were purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in the DMEM medium with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco). All cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Cell Transfections
After cells were grown up to 60% confluence, cells were transfected with a small interfering RNA (siRNA) targeting HCP5 (si-HCP5; 100 pmol), si-PFN1 (100 pmol), a miR-299-3p mimic (100 pmol), a miR-299-3p inhibitor (100 pmol) and a negative control (Invitrogen, Shanghai, China) by using Lipofectamine 3000 (Invitrogen, USA).
Cell Viabilities
Three thousand transfected SW480 cells or HCT116 cells were plated in 96-well plates. After inoculation: 0, 24, 48, or 72 h., 10 μL Cell Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology, Shanghai, China) solution was pipetted and let incubation for another 4 hours at 37°C and 5% CO2. Absorbance was measured at 450 nm through a Microplate Reader (Bio-Rad, USA).
Colony Formation Assays
Five hundred cells were seeded into a 6-well plate. After two weeks, the cells were fixed and stained with 0.1% crystal violet solution. The numbers of colonies were counted under an inverted microscope.
Apoptotic Cells
Apoptotic cells were analyzed through Annexin V-FITC/PI (Invitrogen, USA). Cells were washed, re-suspended, and stained by 5 μL of Annexin VFITC and 10 μL of PI. After 15 min in dark, cells were measured through a FACScan (Beckman Coulter, USA).
Migration and Invasion
The 24-well transwell chamber (8µm), with or without 50 μl Matrigel (CorningLife Sciences, Corning, NY, USA) was used to detect cell migratory and invasive cells. Cells were fixed with 100 μl of serum-free medium and seeded into the upper chamber, and 500 μl of DMEM containing 20% serum was added to the lower chamber. After 48 h of incubation, the cells attached to the lower surface of the upper chamber stained with crystal violet, and analyzed under a microscope.
qRT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen). cDNAs were synthesized with a reverse transcription kit (Invitrogen). qRT-PCR analysis was performed with SYBR Premix Ex Taq II (TaKaRa, Dalian, China). For mRNA and miRNA, GAPDH and U6 were used as internal controls, respectively. The primers in this article are in Table 1. The relative expressions were calculated by using 2−ΔΔCt method.
Table 1.
The Primers in This Article are as Follows
| Gene Name | Primer Sequence (5ʹ to 3ʹ) |
|---|---|
| HCP5 | Forward: 5ʹ-GCTGGACGATTCTCCTCACACT-3ʹ Reverse: 5ʹ-CTCCTCTCCAGGCACAGGTAAT-3’ |
| miR-299-3p | Forward: 5ʹ-GGTTTACCGTCCCACAT-3ʹ Reverse: 5ʹ-GAACATGTCTGCGTATCTC-3’ |
| PFN1 | Forward: 5ʹ-CATCGTGGGCTACAAGGACTCG-3ʹ Reverse: 5ʹ-CCAAGTGTCAGCCCATTCACGT-3’ |
| GAPDH | Forward: 5ʹ-ATCCACGGGAGAGCGACAT-3ʹ Reverse: 5ʹ-CAGCTGCTTGTAAAGTGGAC-3’ |
| U6 | Forward: 5ʹ-ACAGATCTGTCGGTGTGGCAC-3ʹ Reverse: 5ʹ-GGCCCCGGATTATCCGACATTC-3’ |
Fluorescent in situ Hybridization (FISH)
FISH assay was performed using Ribo™ Fluorescent in Situ Hybridization Kit (RiboBio, Guangzhou, China). Nucleus was stained via DAPI, which was designed and synthesized by Ribobio. Cy3 fluorescent dye was selected to label HCP5. Fluorescence detection was conducted via the confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany).
Luciferase Reporter Assays
Luciferase report gene vectors (pRL-TK, Promega) containing HCP5 Wild Type (WT) or HCP5 mutant type and PFN1 3ʹUTR WT or PFN1 3ʹUTR Mut were transfected into SW480 cells. MiR-299-3p mimic or miR-299-3p inhibitor or NC was co-transfected with reporter plasmids for 48h. The relative luciferase activity was determined using Dual-Luciferase Reporter Assay System (Promega). All plasmids were made from Genepharma, China.
Western Blotting
Cell and tissue lysates were prepared by RIPA Lysis Buffer (Beyotime, Shanghai, China). Proteins were separated and transferred to PVDF membrane (Bio-Rad, USA). After incubations with anti-PFN1 (1:1000, abcam, UK), anti-p-AKT (1:1000, abcam, UK), anti-t-AKT (1:1000, abcam, UK) and anti-GAPDH (1:1000, abcam, UK) overnight, the membrane had incubation with goat anti-rabbit IgG secondary antibody and detection through chemiluminescence. GAPDH was a reference.
Xenograft Mouse Model
A total of 5 × 106 transfected SW480 cells were subcutaneously injected into six-week-old male nude mice (n = 5 per group). Tumor volume was measured every 3 days according to the following formula: volume = 1/2 × length × width2. Animal protocols, housing, and care were performed with the approval of the Ethics Committee of Xi’an Central Hospital and conducted according to the guidelines set forth in the National Institutes of Health’s (NIH) “Guide for the Care and Use of Laboratory Animals” (8th edition).
Immunohistochemistry (IHC)
After fixation in 4% formalin, the tumor xenografts were embedded in paraffin and cut into 4-μm sections. The sections were incubated with anti-Ki67 antibody (1:200, Abcam, Cambridge, UK) at 4°C overnight. Then, using biotinylated secondary antibodies to incubate for 1h at room temperature, visualized with diaminobenzidine substrate (Sigma-Aldrich, St Louis, MO, USA). Immunohistochemistry (IHC) images were taken using an Olympus microscope.
Statistical Analysis
Experiments were performed in triplicates. Data analysis was carried out through Graphpad Prism 6.0 (GraphPad, USA). The data were shown as mean ± SD. P-values were calculated by ANOVA, with P<0.05 considered as significant.
Results
Dysregulated HCP5 in Colorectal Cancer Samples and Cells
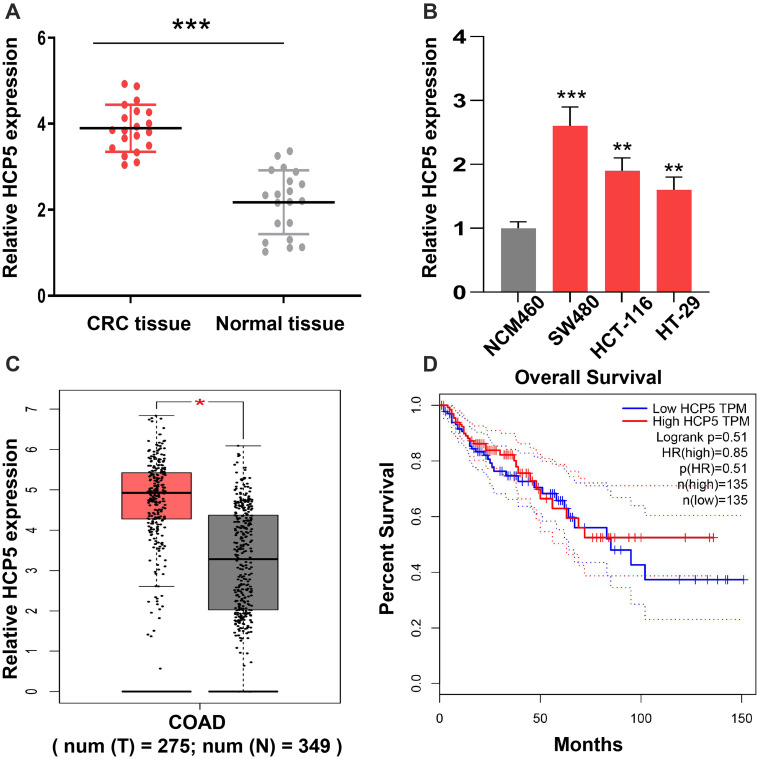
The expression levels of HCP5 in colorectal cancer tissues and para-carcinoma tissues were examined at RNA level. As shown in Figure 1A, the expressions of HCP5 were elevated in colorectal cancer tissues compared to normal tissues. Meanwhile, we detected the expression of HCP5 in CRC cells, SW480, HCT116 and HT-29 and normal colonic epithelial cell line (NCM460). In Figure 1B, the expression of HCP5 in SW480 has the highest level in these four cell lines, and the levels of HCP5 in all three cancer cells exceed the level in NCM460. Subsequently, analyzing the expression in TCGA database (http://gepia.cancer-pku.cn/), we found similar result on expression of HCP5 with ours. The expression of HCP5 has a higher level in COAD (Colon adenocarcinoma) tumors than in normal tissues (Figure 1C). But the prognosis results with distinct groups of high or low expression did not show a statistical difference (Figure 1D). Above these results, we speculated the lncRNA HCP5 might participate in the progression of CRC.
Figure 1.
Dysregulated HCP5 in colorectal cancer patients. (A) The level of HCP5 in CRC tissues and adjacent normal tissues was examined by RT-qPCR. (B) The expression levels of HCP5 in cells were detected by RT-qPCR. (C) The level of HCP5 in CRC cells was analyzed on TCGA database. (D) The prognosis results of HCP5 was analyzed on TCGA database. N = 3, *P < 0.05, **P < 0.01, ***P < 0.001.
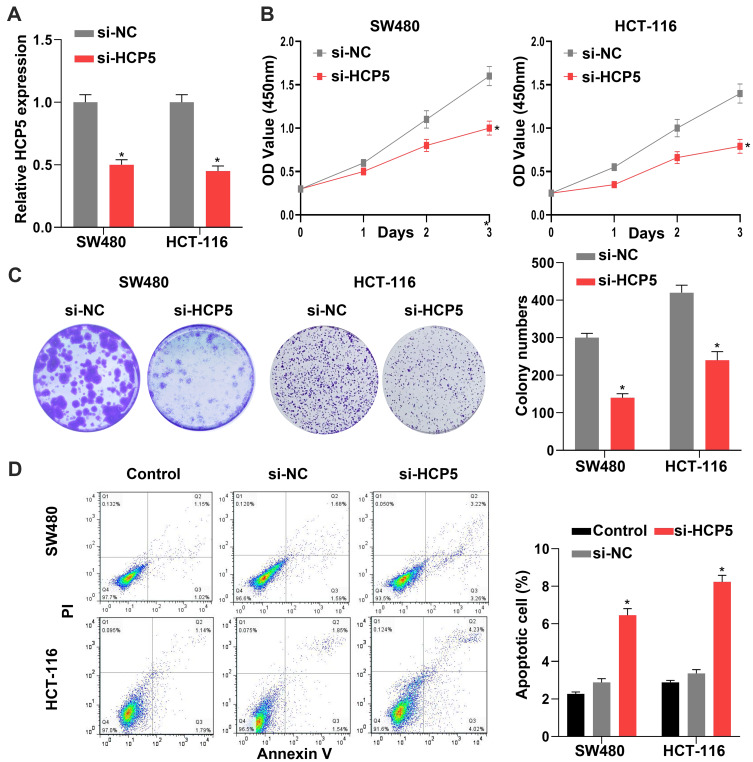
Knockdown of HCP5 Inhibited the SW480 and HCT-116 Cell Proliferation
To investigate the role and function of HCP5 in CRC cells, we chose the siRNA to downregulate the expression of HCP5. Knockdown of HCP5 with si-HCP5 could decrease the expression level of HCP5 in SW480 and HCT-116 cells (Figure 2A). And a further exploration of the effect on proliferation when SW480 and HCT-116 cells transfected with si-HCP5 or si-NC (negative control) was examined by CCK-8 and colony formation assays. We found that both the SW480 and HCT-116 cell viability and colony-forming ability were inhibited by si-HCP5 (Figure 2B and C). In addition, comparing with Control (non-transfection) and si-NC group, si-HCP5 elevated the apoptotic rate of SW480 and HCT-116 cells in Figure 2D. Thus, knockdown of HCP5 could affect the proliferation and apoptosis of SW480 and HCT-116 cells.
Figure 2.
Knockdown of HCP5 inhibited the SW480 cell proliferation. (A) The expression levels of HCP5 in cells were detected by RT-qPCR in SW480 and HCT-116 cells. (B) The relative cell viability was detected by CCK-8 assay in SW480 and HCT-116 cells. (C) The cell proliferation was evaluated with the colony formation assay in SW480 and HCT-116 cells. (D) The cell apoptosis was evaluated by flow cytometry; N = 6, *P < 0.05.
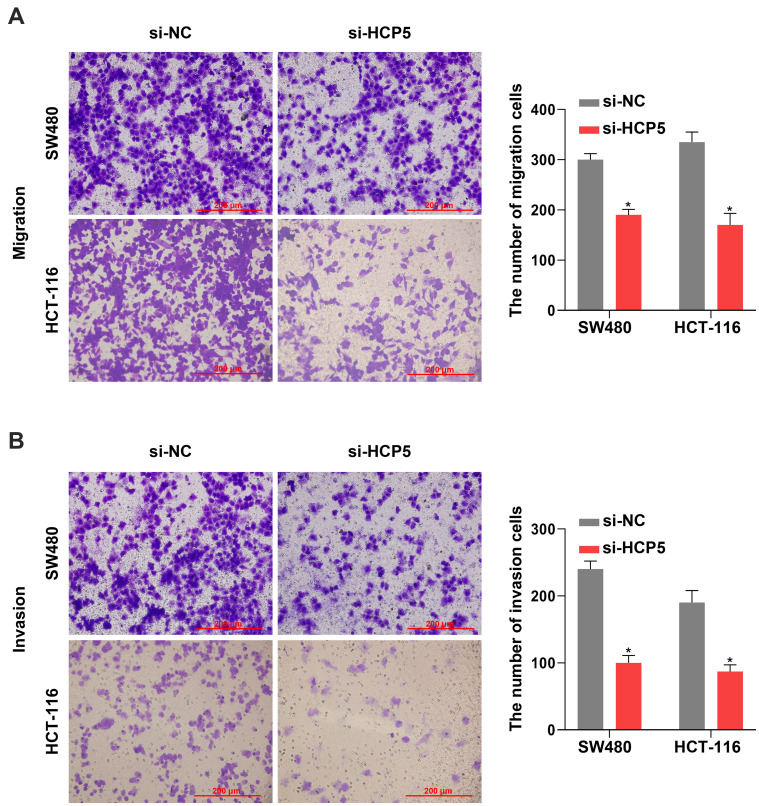
Knockdown of HCP5 Suppressed the SW480 and HCT-116 Cell Migration and Invasion
Besides the proliferation, the cell abilities of migration and invasion also reflected whether the cell process was influenced. It presented that downregulation of HCP5 decreased the number of migration and invasion SW480 and HCT-116 cells in comparison with si-NC (Figure 3A and B). Similarly with proliferation, knockdown of HCP5 suppressed the migration and invasion in SW480 and HCT-116 cells.
Figure 3.
Knockdown of HCP5 suppressed the SW480 and HCT-116 cell migration and invasion. (A) The migration was examined in SW480 and HCT-116 cells by Transwell assay. (B) The invasion was examined in SW480 and HCT-116 cells by Transwell assay. Scale bar: 200 µm. N = 3, *P < 0.05.
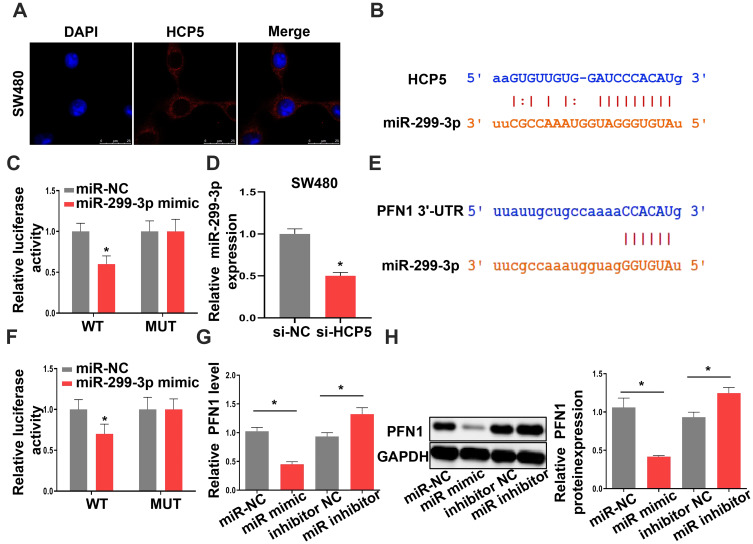
miR-299-3p Is a Target of HCP5 and PFN1 Is a Target of miR-299-3p
lncRNAs could function as a molecular sponge of miRNAs when lncRNAs are located at cytoplasm. We first investigate the subcellular localization of lncRNA HCP5 by Fluorescence in situ hybridization (FISH). The result showed that the lncRNA HCP5 is located at cytoplasm (Figure 4A). Meanwhile, bioinformatic prediction (http://starbase.sysu.edu.cn/) presented that miR-299-3p has a binding site on HCP5 (Figure 4B). The luciferase reporter system result indicated miR-299-3p mimic only weakened the luciferase activity of the WT-HCP5 plasmid in SW480 cells, but not affected the MUT-HCP5 plasmid (Figure 4C). Subsequently, we detected the expression level of miR-299-3p and its expression was increased when SW480 cell transfected with si-HCP5 (Figure 4D). And miRNAs also negatively regulate the target gene in cell process. PFN1 is a target of miR-299-3p by bioinformatic prediction (http://starbase.sysu.edu.cn/) (Figure 4E). In Figure 4F, the luciferase activities in SW480 cells with WT-3ʹ-UTR-PFN1 plasmid were notably decreased, whereas there was no obvious difference in SW480 cells with MUT-type. Next, the mRNA and protein levels of PFN1 were examined. After SW480 cells transfecting with mimic and inhibitor, both the mRNA and protein levels of PFN1 were declined by miR mimic, but miR-299-3p inhibitor increased the expression of PFN1 (Figure 4G and H). These data supported that HCP5 could sponge the miR-299-3p and miR-299-3p reduced the expression of PFN1.
Figure 4.
MiR-299-3p is a target of HCP5 and PFN1 is a target of miR-299-3p. (A) FISH assay was used to confirm the location of HCP5 in SW480 cells. (B) The binding site between HCP5 and miR-299-3p. (C) Luciferase activity was examined in SW480 cells. (D) The expression levels of miR-299-3p in SW480 cells. (E) The binding site between PFN1 and miR-299-3p. (F) Luciferase activity was examined in SW480 cells. (G) The mRNA levels of PFN1 in SW480 cells. (H) The protein levels of PFN1 in SW480 cells. N = 3, *P < 0.05.
HCP5 Might Affect the Functions of SW480 and HCT-116 Cells Through miR-299-3p/PFN1 Axis
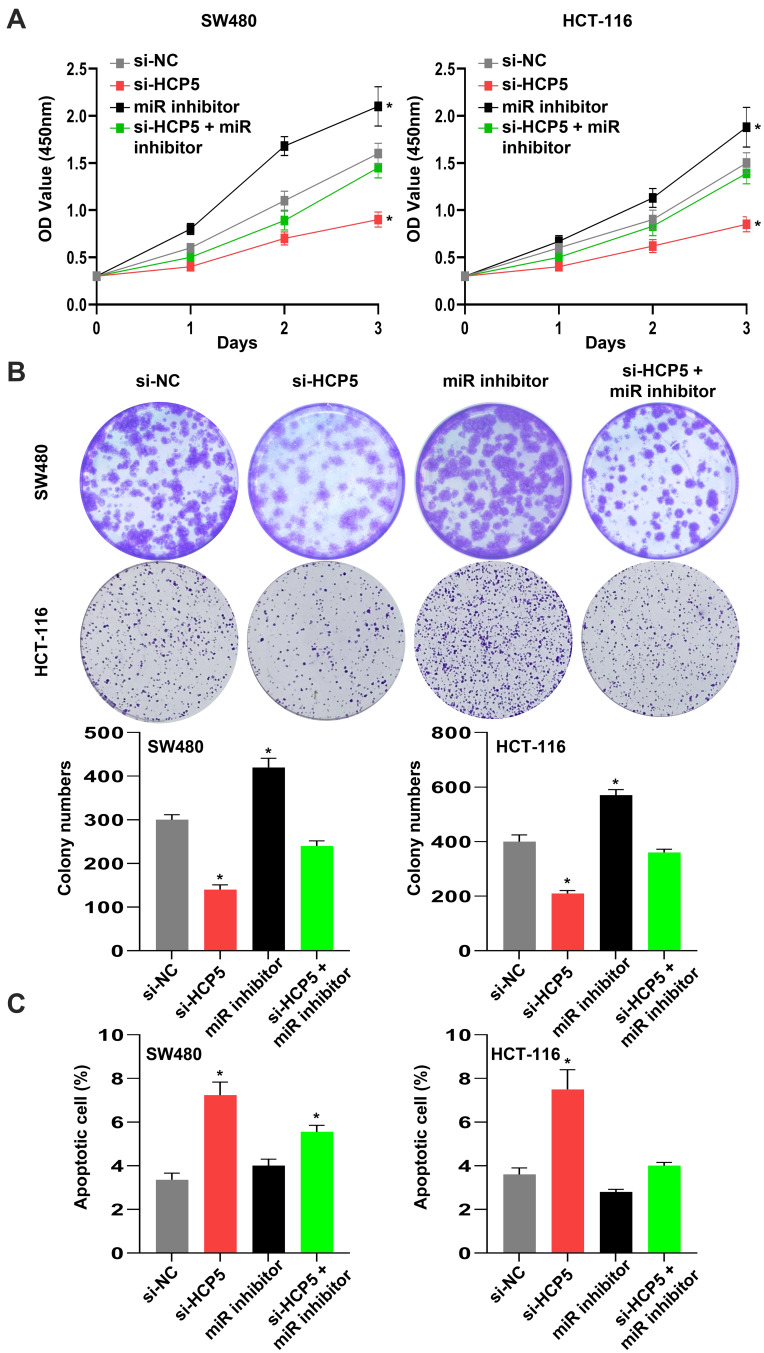
According to Figure 4, we have reason to assume that HCP5 functions via miR-299-3p and PFN1. Therefore, miR-299-3p inhibitor could alleviate the inhibition of si-HCP5 in SW480 and HCT-116 cells. As shown in Figure 5A and B, miR-299-3p inhibitor bated inhibition of si-HCP5 on the cell viability and colony formation ability of SW480 cells and HCT-116 cells. Moreover, the apoptosis in SW480 cells also happened a weaker attenuation when cells were treated with miR-299-3p inhibitor (Figure 5C). Thus, we considered that HCP5 might affect the functions of SW480 cells through miR-299-3p/PFN1 axis.
Figure 5.
HCP5 might affect the functions of SW480 cells through miR-299-3p/PFN1 axis. (A) The relative cell viability was detected by CCK-8 assay. (B) The cell proliferation was evaluated with the colony formation assay. (C) The cell apoptosis was evaluated by flow cytometry. N = 6, *P < 0.05.
HCP5/miR-299-3p/PFN1 Axis Could Activate the AKT Signaling
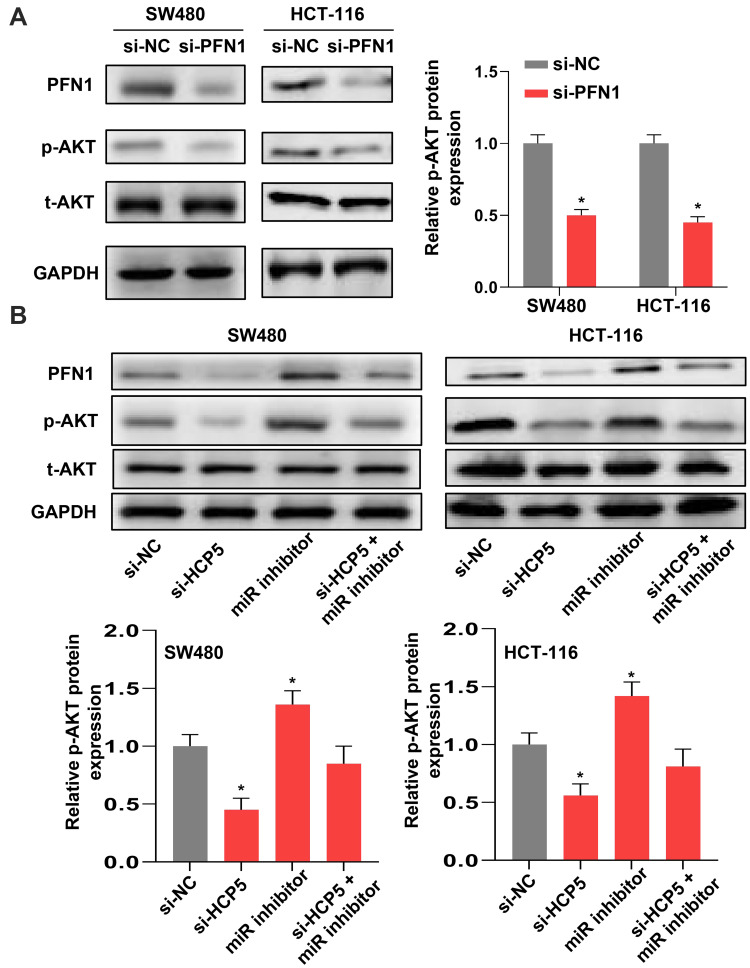
To investigate the underlying mechanism of PFN1 in CRC, we tried to examine the AKT signaling in SW480 cells. Due to the AKT signaling is usually involved in most cancers, especially in progression of colorectal cancer. As shown in Figure 6A, the protein level of p-AKT was decreased when cells transfected with si-PFN1, comparing with si-NC. Moreover, we further verified that the expression level of p-AKT was influenced by the HCP5/miR-299-3p/PFN1 axis (Figure 6B). Knockdown of HCP5 could downregulate the phosphorylation level of AKT in both SW480 and HCT-116 cells. In all, we deem that HCP5/miR-299-3p/PFN1 axis could activate the AKT signaling.
Figure 6.
HCP5/miR-299-3p/PFN1 axis could activate the AKT signaling. (A) and (B) The protein levels of PFN1, p-AKT and t-AKT in SW480 and HCT-116 cells. N = 3, *P < 0.05.
In vivo Experiment Confirmed That Knockdown of HCP5 Inhibited the Progression of CRC
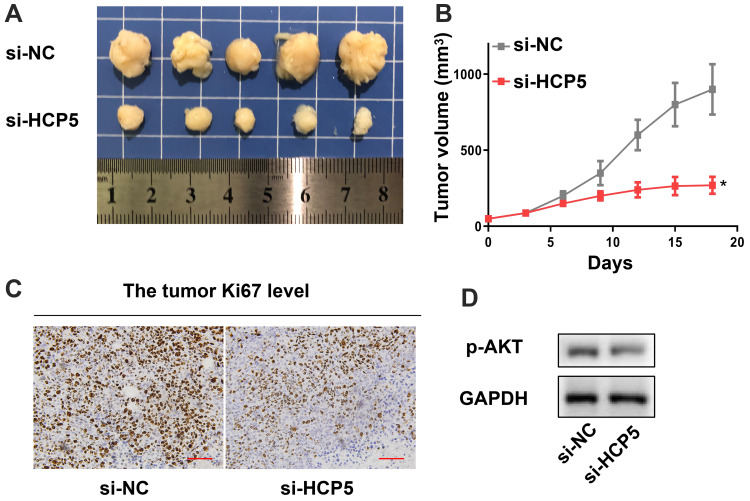
Finally, we confirmed knockdown of HCP5 inhibited the progression of CRC with an in vivo model. We found si-HCP5 retarded the growth of tumors (Figure 7A and B). And both the level of ki67 and p-AKT appeared an obvious decline compared with the si-NC group (Figure 7C and D). These in vivo experiments indicated knockdown of HCP5 could retard the progression of CRC.
Figure 7.
In vivo experiment confirmed that knockdown of HCP5 inhibited the progression of CRC. (A) The image of tumors. (B) The tumor volume. (C) The Ki67 level in tumor. (D) The p-AKT level in tumor. Scale bar: 50 µm; N = 5, *P < 0.05.
Discussions
Based on our results, we elaborated that lncHCP5 affects the progression of CRC, as an oncogenic factor. Relative cell experiments revealed that lncHCP5 elevated the expression of PFN1 by sponging miR-299-3p and then promoted the progression of CRC. Our results that HCP5 could inhibit apoptosis, induce proliferation, migration and invasion process were in accordance with the previous studies, which indicated that HCP5 might be an oncogenic factor. For example, Zhao et al indicated that HCP5 was up-regulated in oral squamous cell carcinoma and enhanced the ability of cell invasion by miR-140-5p/SOX4 axis.24 Meanwhile, HCP5 could expedite the progression of prostate cancer through miR-4656/CEMIP pathway, as reported by Hu et al.25 In addition, we established an in vivo model to confirm knockdown of HCP5 retarded the progression of SW480 cells. In summary, we consider HCP5 to act as an oncogenic factor in CRC.
Moreover, miR-299-3p and PFN1 in CRC were found to affect the development of CRC. Although miRNAs often function by altering the target protein expression, lots of studies suggest that the change in the relative protein is the key factor in the progression of cancers.26,27 PFN1 was identified as a target of the miR-299-3p subsequently. Meanwhile, we verified that miR-299-3p could down-regulate the expression of PFN1 by the Dual-luciferase reporter system. This study is the first example that draws the correlation between the miR-299-3p and the expression of PFN1 in CRC. In addition, studies have been reported that miR-299-3p often played a role of tumor suppressor in most cancers. For instance, Yu et al reported miR-299-3p represses the progression of cervical cancer cell by reducing the expression of TCF4.28 Chen et al revealed that miR-299-3p inhibits the proliferation and migration of thyroid cancer by targeting SHOC2.29 Moreover, previous study has indicated that PFN1 accelerates the development of CRC.18 These studies uncover the roles of miR-299-3p and PFN1 in malignancy and have no difference with our results.
In addition, the AKT signaling is an important pathway to regulate the progression of CRC.30 Our results indicated the HCP5/miR-299-3p/PFN1 axis could activate the AKT signaling in SW480 and HCT-116 cells. The underlying mechanism of HCP5 in CRC might be that HCP5 elevated the expression of PFN1 by sponging the miR-299-3p eventually to induce the phosphorylation of AKT. Meanwhile, this assumption was verified by our in vivo experiment. Inhibition of HCP5 with si-HCP5 obviously decreased the volume of tumors in SW480 xenograft tumors. Both Ki67 and p-AKT level also displayed a weakening trend in xenograft tumors.
However, some piece of deficiencies were still in our study. Besides the roles and functions of the factor in our experiment, both the HCP5 and miR-299-3p could have excess biological functions. They possibly played their roles in CRC through the multilevel regulation leading to the synergistic effects. Our findings only revealed the correlation between the HCP5 and miR-299-3p, some other downstream targets are still to study. How HCP5 affects the other miRNAs or miR-299-3p biomolecules such as kinases or enzymes involved ubiquitination is also beyond the scope of this study, which may also play an important role in the development of CRC. Our next plan is to investigate the HCP5 further to uncover the underlying biological process.
In conclusion, the experiments and analysis support our hypothesis that knockdown of lncRNA HCP5 suppresses the progression of colorectal cancer by miR-299-3p/PFN1/AKT axis.
Abbreviations
HCP5, human major histocompatibility complex p5; CRC, colorectal cancer; PFN1, profilin 1; TCGA, the Cancer Genome Atlas; qRT-PCR, quantitative RT-PCR; COAD, Colon adenocarcinoma; p-AKT, phosphorylated-Protein Kinase B; t-AKT, total-Protein Kinase B.
Ethics and Consent Statement
This study was performed with the approval of the Research Ethics Committee of Xi’an Central Hospital. In addition, written informed consent forms were signed by all the patients who participated in this research. All animal experiments were performed with the approval of the animal ethics committee of Xi’an Central Hospital and conducted according to the guidelines set forth in the National Institutes of Health’s (NIH) “Guide for the Care and Use of Laboratory Animals” (8th edition).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Song MY, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–U1217. doi: 10.1053/j.gastro.2014.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Chen WQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Froberg JE, Lee JT. Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci. 2014;39(1):35–43. doi: 10.1016/j.tibs.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amirkhah R, Naderi-Meshkin H, Shah JS, Dunne PD, Schmitz U. The intricate interplay between epigenetic events, alternative splicing and noncoding RNA deregulation in colorectal cancer. Cells-Basel. 2019;8(8):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang XJ, Wang W, Hann SS. Interactions among lncRNAs, miRNAs and mRNA in colorectal cancer. Biochimie. 2019;163:58–72. doi: 10.1016/j.biochi.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 7.Li H, Ma SQ, Huang J, Chen XP, Zhou HH. Roles of long noncoding RNAs in colorectal cancer metastasis. Oncotarget. 2017;8(24):39859–39876. doi: 10.18632/oncotarget.16339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He RZ, Luo DX, Mo YY. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019;6(1):6–15. doi: 10.1016/j.gendis.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi YJ, Wang D, Wang JP, Yu WD, Yang JC. Long non-coding RNA in the pathogenesis of cancers. Cells-Basel. 2019;8(9):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LY, Cho KB, Li Y, Tao G, Xie ZX, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci. 2019;20(22):5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang LL, Xu JC, Wang M, et al. LncRNA HCP5A promotes follicular thyroid carcinoma progression via miRNAs sponge. Cell Death Dis. 2019;10(9). doi: 10.1038/s41419-019-1868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C, Sun JJ, Liu WF, et al. Long noncoding RNA HCP5 contributes to epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation and interacting with miR-139-5p. Am J Transl Res. 2019;11(2):953–963. [PMC free article] [PubMed] [Google Scholar]
- 14.Kabekkodu SP, Shukla V, Varghese VK, et al. Cluster miRNAs and cancer: diagnostic, prognostic and therapeutic opportunities. Wires Rna. 2019;11(2):e1563. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Urrutia E, Montes LPB, Cervantes DLD, Perez-Plasencia C, Campos-Parra AD. Crosstalk between long non-coding RNAs, micro-RNAs and mRNAs: deciphering molecular mechanisms of master regulators in cancer. Front Oncol. 2019;9. doi: 10.3389/fonc.2019.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandellini P, Rancati T, Valdagni R, Zaffaroni N. miRNAs in tumor radiation response: bystanders or participants? Trends Mol Med. 2014;20(9):529–539. doi: 10.1016/j.molmed.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 17.Skruber K, Warp P, Henty-Ridilla J, Vitriol E. Profilin-1 controls actin network organization and homeostasis through coordination with other assembly factors. Biophys J. 2020;118(3):31a. doi: 10.1016/j.bpj.2019.11.350 [DOI] [Google Scholar]
- 18.Zhao JX, Xu JH, Lv J. Identification of profilin 1 as the primary target for the anti-cancer activities of Furowanin A in colorectal cancer. Pharmacol Rep. 2019;71(5):940–949. doi: 10.1016/j.pharep.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 19.Das T, Bae YH, Wells A, Roy P. Profilin-1 overexpression upregulates PTEN and suppresses AKT activation in breast cancer cells. J Cell Physiol. 2009;218(2):436–443. doi: 10.1002/jcp.21618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng YJ, Zhu ZX, Zhou JS, et al. Silencing profilin-1 inhibits gastric cancer progression via integrin β1/focal adhesion kinase pathway modulation. World J Gastroenterol. 2015;21(8):2323–2335. doi: 10.3748/wjg.v21.i8.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3(9):1266–1273. doi: 10.1001/jamaoncol.2016.4975 [DOI] [PubMed] [Google Scholar]
- 22.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–+. doi: 10.1200/JCO.2014.59.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, Pan SM, Xiao Y, Liu QQ, Xu JC, Jia L. LINC01296/miR-26a/GALNT3 axis contributes to colorectal cancer progression by regulating O-glycosylated MUC1 via PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38(1). doi: 10.1186/s13046-019-1367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao JL, Bai XJ, Feng C, Shang XH, Xi YL. Long non-coding RNA HCP5 facilitates cell invasion and epithelial-mesenchymal transition in oral squamous cell carcinoma by miR-140-5p/SOX4 axis. Cancer Manag Res. 2019;11:10455–10462. doi: 10.2147/CMAR.S230324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu RG, Lu ZJ. Long non-coding RNA HCP5 promotes prostate cancer cell proliferation by acting as the sponge of miR-4656 to modulate CEMIP expression. Oncol Rep. 2020;43(1):328–336. doi: 10.3892/or.2019.7404 [DOI] [PubMed] [Google Scholar]
- 26.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–U38. doi: 10.1038/nature07242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mork S, Pletscher-Frankild S, Caro AP, Gorodkin J, Jensen LJ. Protein-driven inference of miRNA-disease associations. Bioinformatics. 2014;30(3):392–397. doi: 10.1093/bioinformatics/btt677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Zhao JD, Yang H. MiR-299-3p inhibits proliferation and invasion of cervical cancer cell via targeting TCF4. Eur Rev Med Pharmacol Sci. 2019;23(13):5621–5627. doi: 10.26355/eurrev_201907_18296 [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Qi M, Yang Q, Li JY. MiR-299-3p functions as a tumor suppressor in thyroid cancer by regulating SHOC2. Eur Rev Med Pharmacol Sci. 2019;23(1):232–240. [DOI] [PubMed] [Google Scholar]
- 30.Afrin S, Giampieri F, Gasparrini M, et al. Dietary phytochemicals in colorectal cancer prevention and treatment: a focus on the molecular mechanisms involved. Biotechnol Adv. 2020;38:107322. doi: 10.1016/j.biotechadv.2018.11.011 [DOI] [PubMed] [Google Scholar]