Supplemental Digital Content is available in the text
Keywords: diabetic retinopathy, macular edema, teleophthalmology
Abstract
Objective:
To determine cost-effectiveness and the diagnostic accuracy of teleophthalmology (TO) in the detection of macular edema (ME) and various grades of diabetic retinopathy (DR).
Methods:
MEDLINE, EMBASE, and Cochrane databases were searched for TO, ME, and DR on May 25, 2016. The search was updated on April 2, 2019. Pooled sensitivity and specificity for ME and various grades of DR were determined using Meta-Disc software. A systematic review of the articles discussing the cost-effectiveness of TO screening was also performed.
Results:
Thirty-three articles on the diagnostic accuracy and 28 articles on the cost-effectiveness were selected.
Conclusions:
Telescreening is moderately sensitive but very specific for the diagnosis of diabetic retinopathy. Non-mydriatic Teleretinal screening services are cost-effective, decrease clinics workload, and increase patient compliance if provided free of cost in remote low socioeconomic regions.
1. Introduction
Recent surveys indicate that approximately 382 million of the world population and 29.1 million of the US population has diabetes mellitus.[1,2] If no action has been taken, this number will double by 2035.[1] With such a significant portion of the population affected by diabetes, managing complications of this disease are paramount. The most common complication of diabetes is diabetic retinopathy (DR), affecting about 28% of the diabetic population.[3] According to WHO, DR accounts for almost 17% of all cases of blindness in the USA and Europe, and the number of Americans with DR will nearly double from 7.7 million to 14.6 million by the end of 2050.[4] Good glycemic control, early diagnosis, and prompt management of DR can delay the progression of DR into blindness.[5] Despite this knowledge, only about 55% of the diabetic patients in the United States receive retinopathy screening.[6] Similarly, the systematic implementation of DR testing is not common in many low and middle-income countries.[5,7] The main reasons responsible for the poor compliance to the DR screening are high testing expenses, inadequate health care facilities, and limited access to conventional screening strategies.[8] Teleophthalmology recently has gained particular importance as an alternative screening method to overcome the barriers in the face of DR screening.[9]
Teleretinal technology is believed to improve access and reduce the cost of the DR and ME screening. However, the diagnostic accuracy and cost of this screening modality depend on many variables including the use of pupillary mydriasis, an instrument used, the qualifications of the photographer, number of photographic fields, and the image interpreter. Many studies have been done to discuss the utility of this technology, but the literature on the cost-effectiveness and diagnostic accuracy of Teleretinal screening are yet limited. We present the results of a systematic review and meta-analysis of studies evaluating the cost-effectiveness and pooled sensitivity and specificity of TO in DR and ME screening.
2. Methods
2.1. Search strategy and selection criteria
The initial literature search for relevant articles was performed on May 25, 2016, using MEDLINE (PubMed, Ovid), Embase, and Cochrane databases. The search was updated on April 2, 2019. There was no language or time restriction placed on the search. The search strategies included various combinations of text-words and medical subject headings (MeSH) to generate 2 subsets of citations: one for ME or DR, using the MeSH and terms like “macular edema,” “diabetic retinopathy,” “diabetic maculopathy,” “diabetic macular edema,” “diabetic ophthalmopathy,” “diabetic eye disease,” and “diabetic ocular disease” and the other for teleophthalmology using terms and MeSH like “telemedicine,” “telehealth,” “mhealth,” “ehealth,” “medical informatics,” “clinical decision support system,” “computer-assisted decision making,” “information system,” “teleophthalmology diabetes,” “tele maculopathy diabetes,” “tele healthcare,” “mobile health,” “health information technology,” “software-assisted analysis.” The terms from the 2 subsets were combined in 1:1 combination and finally results from all the possible combinations were downloaded into an EndNote library. Based on our research question, we also manually searched the references in all known articles to identify studies that were missed by the initial search. An ethics approval was obtained from the institutional review board (IRB) for this study.
The selection criteria for the included studies were: recruited subjects with macular edema or diabetes mellitus either type 1 or type 2, discussed the cost-effectiveness or cost-utility of teleophthalmology screening, provided data on the sensitivity or specificity of Teleretinal screening. Studies with insufficient data, discussing only the prevalence of diabetes, case reports, and conference papers were excluded, as studies with no enough description of its subjects.
2.2. Study selection
The titles and abstracts of the selected articles were reviewed independently by 3 authors and the articles which met the inclusion criteria were reviewed by the fourth author. Full-text articles that were potentially relevant to the study were also reviewed by all the 4 authors to confirm the eligibility. Disagreements were resolved by mutual consensus and after a detailed group discussion.
2.3. Data abstraction and analysis
Two reviewers extracted data on the study characteristics, cost-effectiveness, and sensitivity and specificity of Teleretinal screening. Disagreements were resolved by consensus and by a discussion with a third reviewer. After carefully assessing the extracted data, pooled sensitivity and specificity were calculated using the Meta-Disc software, RevMan Version 5.3, London, United Kingdom.
2.4. Quality assessment
The quality assessment of all the included articles studies was performed using the RevMan 5.3. Selection, detection, attrition, and reporting bias for all studies was assessed.
3. Results
The combined systematic search strategy identified a total of 3814 articles. After excluding 1201 duplicate items, the remaining 2613 pieces of literature were screened for relevance based on their titles and abstracts, and 2183 articles were further excluded. A total of 430 were deemed potentially eligible and retrieved for a full review. After a detailed review, a total of 369 articles were further excluded for the following reasons: screening strategies other than teleophthalmology (n = 145), telemedicine services of diabetic care (n = 27), cost of diabetic care (n = 26), telemedicine for treatment of DR (n = 48), diabetes prevalence and management (n = 62), telerehabilitation of diabetics (n = 18), telemedicine on visual acuity and retinitis pigmentosa (n = 9), and articles on Teleretinal diabetic prevalence (31). Thirty-three articles on the diagnostic accuracy and 28 articles on the cost-effectiveness were included.
Figure 1 presents a Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA) flowchart of the study selection process along with reasons for study exclusion.
Figure 1.
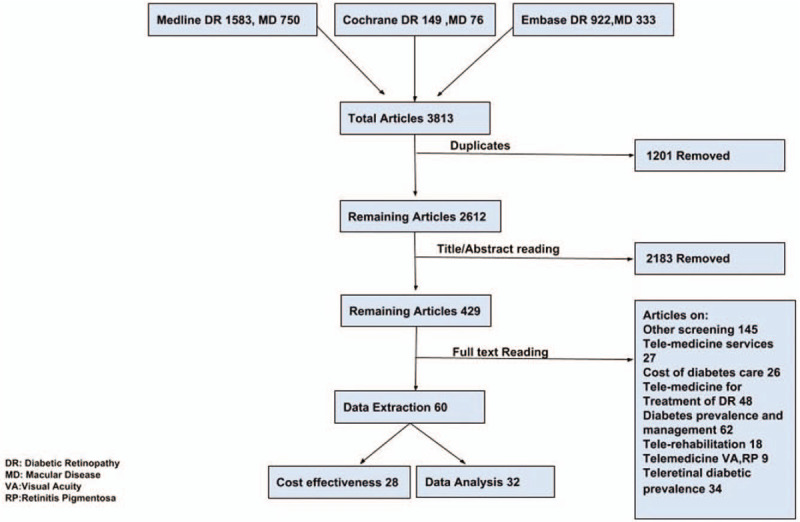
PRISMA flow sheet of the selected studies.
3.1. Summary characteristics of the included studies for diagnostic accuracy
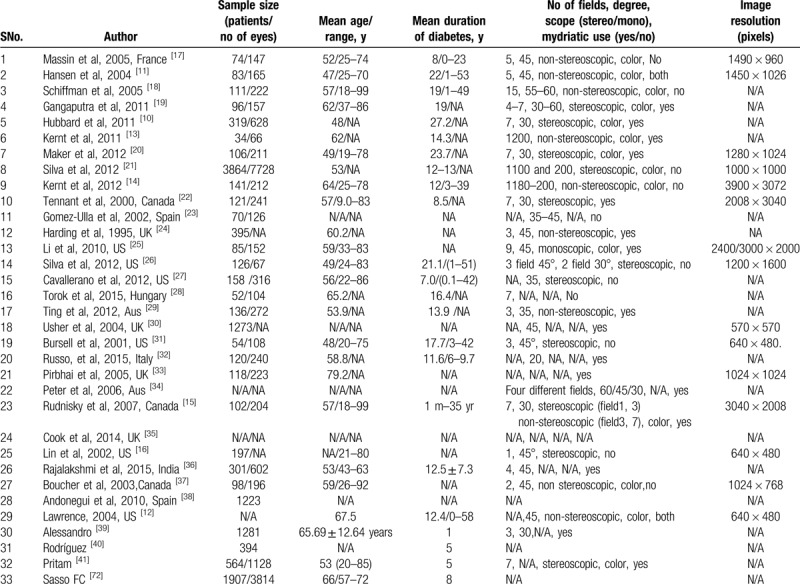
A total of 33 studies were selected for the analysis. All these studies used Teleretinal screening either alone or in comparison to other screening modalities like ophthalmoscopy, slit-lamp examination, or 7-field Early Treatment Diabetic Retinopathy Screening (ETDRS). Both men and women with either type 1 or type 2 studies were included in the recruited studies, except Hubbard et al[10] study which included patients with type 1 DM only. More than 10,000 people from 8 different countries were screened in all the studies. Almost 80% of the total studies were conducted in the UK, Canada, and at various states of the United States. In >50% of the included studies, digital imaging was carried out with mydriasis except for Hansen et al[11] and Lawrence[12] study in which both mydriatic and non-mydriatic images were obtained. Images were transferred through a secure web browser, telemetrically, or via an online network.[13–16] A description of the included studies for diagnostic accuracy is presented in Table 1.
Table 1.
Characteristics of the included studies for the diagnostic accuracy.
4. Discussion
4.1. Diagnostic accuracy
The cumulative diagnostic accuracy of teleophthalmology for diabetic macular edema (DME) showed a mean sensitivity of 59% with a range as <30% and a maximum value of 88%. While specificity ranged from 82% to 98% with a mean of 93% and a standard deviation of about 6%. While for clinically significant macular edema (CSME) these values were 38% and 100%, respectively. The mean for CSME was 66%, the standard deviations for both were 26% and 22%, respectively. The specificity ranged from 75% to 100% with a mean of 94% and a standard deviation of about 8%.
Teleophthalmology was found to be 87% sensitive and 91% specific for the absence of retinopathy. For mild non-diabetic proliferative diabetic retinopathy (NPDR), the total number of articles was 16, with a minimum value of sensitivity 35% and a maximum value of 93% with a mean of 74% and the standard deviation was 0.16481. The mean specificity ranged from none to 98% with a mean of 50% and the standard deviation was 44%. The sensitivity of moderate NPDR was reported in 15 articles having a minimum to maximum range 32% to 100% with a mean and standard deviation of 71% and 19% respectively. These values for specificity ranged from none to 98% with a mean and standard deviation of only 48% and 47% respectively. For severe NPDR, the mean sensitivity was 42% with a standard deviation of 27% and a range from none to 79%. Surprisingly the specificity of telemedicine was very high ranging from 94% to 100% and a mean of 94% having only 1% of standard deviation. As far as individuals with low risk proliferative diabetic retinopathy (PDR) were concerned, the teleophthalmology was found to 75% sensitive with respect to the gold standard ophthalmoscopy with a range from 0% to 100% among different studies. These values were ironically high for mean specificity that was 98% ranging from 94% to 100% among individual studies having a negligible standard deviation of only 2%. Lastly, for high-risk PDR, the minimum sensitivity value of 0.00 and a maximum value of 100% were found among studies while the mean sensitivity was 76% and the standard deviation was 31%. The specificity ranged from 69% to 100% and the mean and the standard deviation was 94% and 9% respectively. The individual sensitivities and specificities of the included studies are tabulated in supplementary table S1.
4.2. Quality assessment of included studies
The detailed quality assessment and risk of bias assessment of the included studies are summarized in supplementary table S2 and shown in Fig. 2 below. The detailed summary of bias assessment is shown in supplementary figure S1. Overall the quality of the studies included in our meta-analysis was high. The allocation concealment might have introduced a high risk of selection bias in the Hansen study.[11] Silva performed 2 similar studies in 2012 and the risk of bias assessment could be done only on one study while the second study had insufficient information. Selection criteria were well defined in almost all studies. Chances for detection bias and attrition bias were low as there not enough unblinded studies or studies reporting incomplete data respectively. Due to complete reporting of outcomes in all studies, the reporting bias was minimal in all studies.
Figure 2.
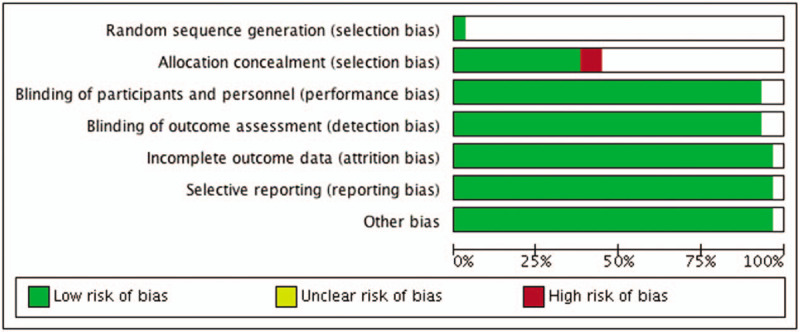
Quality assessment of the included studies shows inclusion of well conducted studies.
4.3. Cost-effectiveness
Cost-effective Teleretinal screening programs have been reported in many clinical settings, including Canada, India, United States, Norway, and the United Kingdom.[42–60] A total of 28 studies were identified assessing the cost-effectiveness of teleophthalmology screening of DR and ME in these clinical settings. The cost analysis was done based on delivery modalities (e.g., telemedicine, clinic camera), screening models like systematic screening versus opportunistic and screening outcomes such as Quality-Adjusted Life-Year (QALY), cost per true positive case detection, DR severity, screening intervals, population, and referral duration (Table 2 ).
Table 2.
Cost effectiveness of teleretinal screening and characteristics of the included studies.
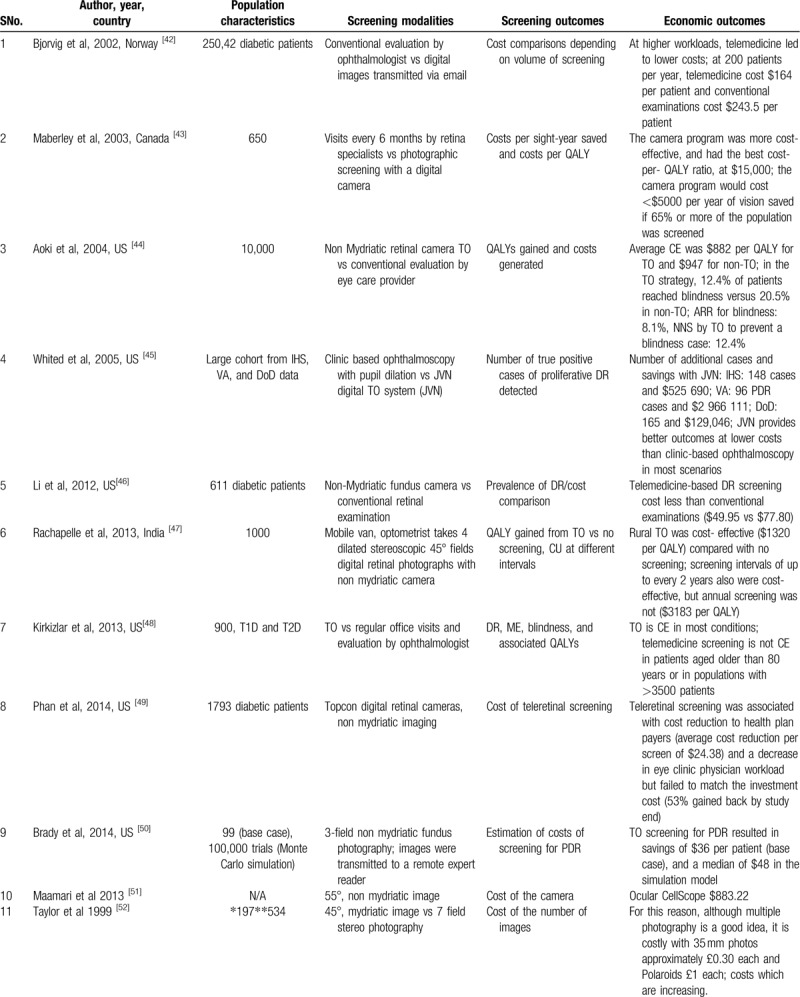
Table 2 (Continued).
Cost effectiveness of teleretinal screening and characteristics of the included studies.
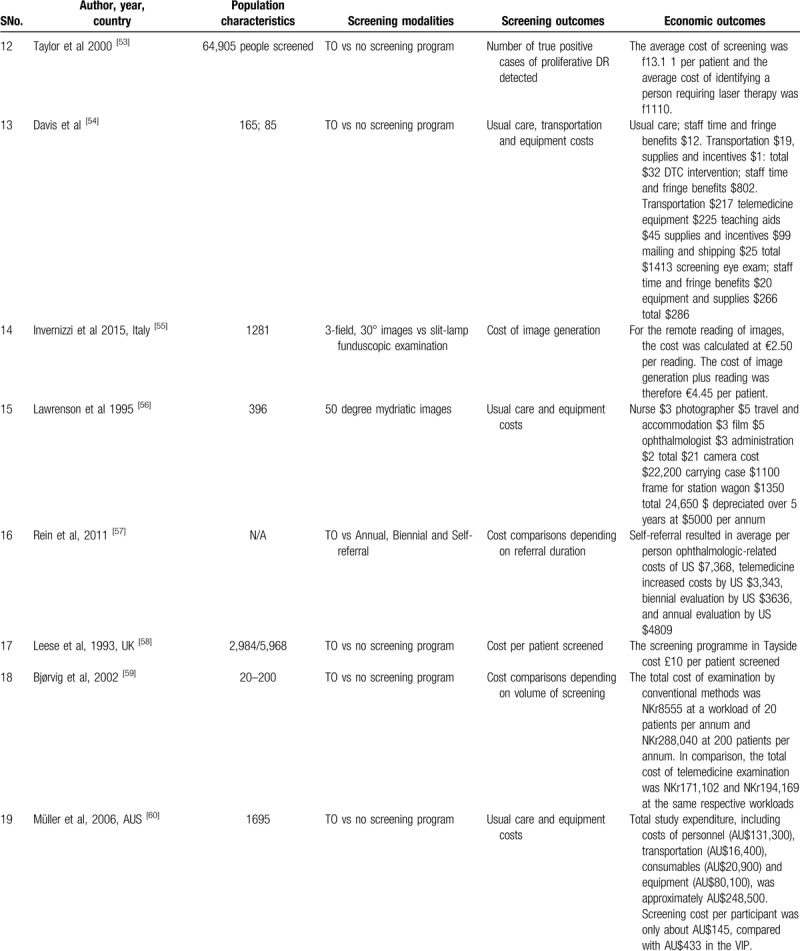
Table 2 (Continued).
Cost effectiveness of teleretinal screening and characteristics of the included studies.
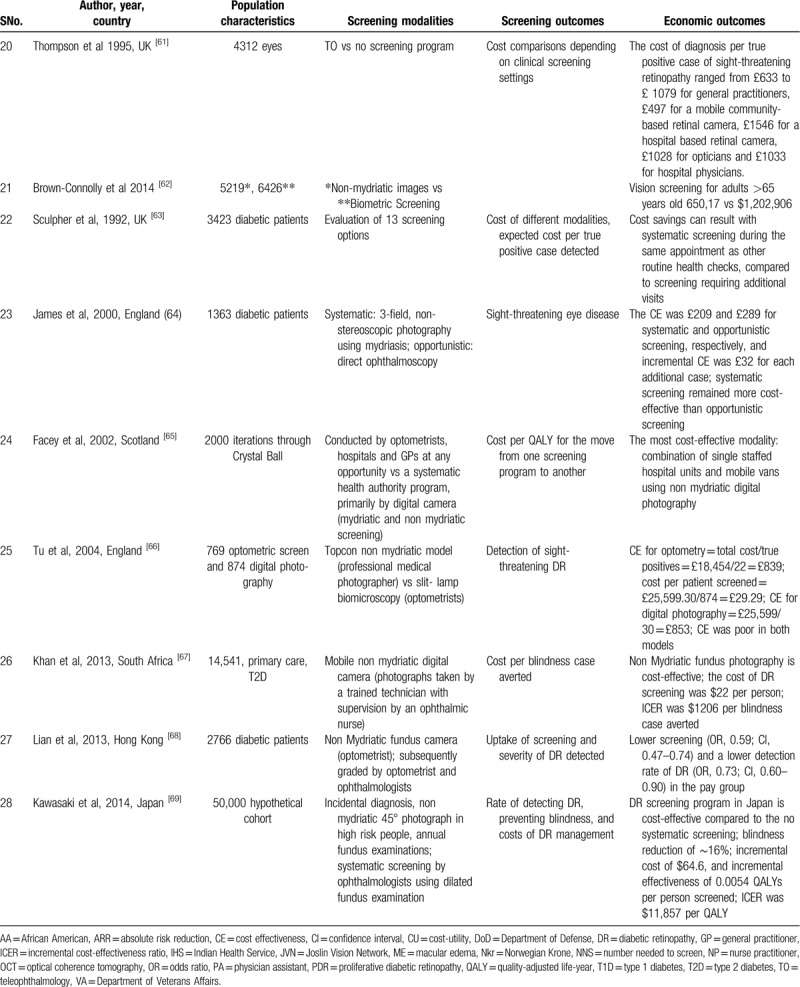
Populations screened at a younger age, higher HbA1c, using insulin, or with high transportation costs derive most of the benefit from Teleretinal screening.[44,48] Besides, disease burden and population size determine the cost-effectiveness of a Teleretinal screening program. Compared with conventional screening, Teleretinal screening was cost-effective at a high workload. A saving of 74$ and NKr 28,7186 per patient was noticed in a population of 200 patients per year.[42,59] At lower workload of 20 patients, the cost-saving for Teleretinal screening was only about NKr 8384 with respect to the conventional screening.[59] However, teleophthalmology (TO) was not economic in patients >80 years of age and in population >3500 patients, similarly multiple photographies with 35 mm photos cost approximately £0.30 extra for each image.[48,52] Furthermore, in many studies, non-mydriatic screening approaches perform well and were cost-effective compared with mydriatic use.[43,65,45,46,67]
Subjects with a high socioeconomic condition or those living in better areas were screened more often but were less likely to have DR detected, suggesting the importance of access to screening.[68] Free screening was associated with a higher compliance rate, higher chances of DR detection, and a decreased workload on clinic physicians. Multiple studies discussed the approximate cost of the usual care like supplies and incentives, transportation charges, staff time, and fringe benefits (19$–32$).[54,56,60] The per-patient cost of screening was estimated to be 24$ to 36$.[49,50] If telemedicine equipment and teaching aids were added the approximate screening cost per participant was about AUS $145 per visit and about 5000 US $ per annum.[54,56,60] The systematic screening model was more cost-effective than the opportunistic screening model in many studies. James et al[64] showed that the cost was £209 and £289 for systematic and opportunistic screening, respectively.
Screening interval is also an important factor in determining the cost-effectiveness of Teleretinal screening in asymptomatic patients screening every 2 years can save 900 to 1863 US $ than annual screening.[47,57] Whited et al[45] compared clinic-based ophthalmoscopy to teleretinal screening at Joslin Vision Network (JVN) to determine the true positive case detection cost. In Indian Health Service (IHS) 148 cases saved $525,690, in the Department of Veterans Affairs (VA) 96 PDR cases saved $2,966,111 while in the Department of Defense (DoD) 165 cases saved $129,046, concluding that JVN provides better outcomes at lower costs than clinic-based ophthalmoscopy in most scenarios. Tu et al[66] in his study showed that true positive detection cost of DR on digital photography by TO was significantly lower (£839) than slit-lamp detection cost of DR (£853) and cost per patient screened was as little as £29.29 per patient.
5. Limitations
Our study did not focus on the prevalence of telemedicine and telescreening in developing countries.
Teleretinal screening would offer more benefits in developing and underdeveloped countries. A population-based survey in Nakuru, Kenya revealed that the prevalence of any type of DR was 35.9% and of severe non-proliferative DR was 13.9%.[70] Similarly, within Asia-Pacific India has a 10% prevalence of DR while Indonesia has a prevalence of 43% for DR. We believe that such developing countries would benefit more from a teleretinal screening program.[71,72]
6. Conclusion
Teleophthalmology is very sensitive and specific for the absence of retinopathy but for the diseased retina, these values have widespread variations. It is highly specific for DME, CSME, PDR, and severe NPDR but non-specific for mild or moderate NPDR. The sensitivity of all kind of retinopathies was not impressive. However, the relatively small cost of Teleretinal screening makes for an attractive platform for both image acquisition, storage, interpretation, and transmission. In contrast to clinical examination and conventional screening, Teleretinal screening reduces the burden in the eye clinic and improves access in remote environments. Moreover, screening a small number of individuals is not economically sound, and a larger population screening with the omission of pupillary mydriasis can be more cost-effective. Telescreening also increases screening compliance and prevent blindness in a high number of a population if the services are offered free of cost.
Author contributions
Data conceptualization: Waqas Ullah.
Data curation: Sana Pathan Khan, Ejaz Chaudary.
Editing & Final proof reading: Yasar Sattar, Ankur Panchal.
Formal analysis: Swapna Anandan, Kaiser Saleem.
Supervision: Haq Nawaz.
Writing – original draft: Kaiser Saleem.
Writing – review: Ejaz Chaudary, Maryam Mukhtar, Waqas Ullah.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CSME = clinically significant macular edema, DME = diabetic macular edema, DoD = Department of Defense, DR = diabetic retinopathy, ETDRS = Early Treatment Diabetic Retinopathy Screening, IHS = Indian Health Service, JVN = Joslin Vision Network, ME = macular edema, NPDR = non-diabetic proliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy, QALY = quality-adjusted life-year, TO = teleophthalmology, VA = Department of Veterans Affairs.
How to cite this article: Ullah W, Pathan SK, Panchal A, Anandan S, Saleem K, Sattar Y, Ahmad E, Mukhtar M, Nawaz H. Cost-effectiveness and diagnostic accuracy of telemedicine in macular disease and diabetic retinopathy: A systematic review and meta-analysis. Medicine. 2020;99:25(e20306).
Financial Disclosure: None.
Ethics: IRB was approved by the institution.
Funding: None.
Competing Interests: None.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].International Diabetes Federation. Diabetes Atlas. 6th ed.Brussels: International Diabetes Federation; 2013. [Google Scholar]
- [2].Centers for Disease Control and Prevention. National Diabetes Statistics Report; 2014. [Google Scholar]
- [3].Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1989;107:237–43. [DOI] [PubMed] [Google Scholar]
- [4].Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull WHO 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- [5].Standards of medical care in diabetes—2014. Diabetes Care 2014;37: suppl: S14–8. [DOI] [PubMed] [Google Scholar]
- [6].Winkler R, Forman A, Morsell A. Maintenance review of NQF-endorsed measures for diabetes, mental health, and musculoskeletal conditions. National Quality Forum; 2010. [Google Scholar]
- [7].Hall M, Felton AM. The St Vincent Declaration 20 years on—defeating diabetes in the 21st century. Diabetes Voice 2009;54:42–4. [Google Scholar]
- [8].Hartnett ME, Key IJ, Loyacano NM, et al. perceived barriers to diabetic eye care. Qualitative study of patients and physicians. Arch Ophthalmol 2005;123:387–91. [DOI] [PubMed] [Google Scholar]
- [9].Cuadros J, Bresnick G. EyePACS: an adaptable telemedicine system for diabetic retinopathy screening. J Diabetes Sci Technol 2009;3:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hubbard LD, Sun W, Cleary PA, et al. Comparison of digital and film grading of diabetic retinopathy severity in the diabetes control and complications trial/29 epidemiology of diabetes interventions and complications study. Arch Ophthalmol 2011;129:718–26. [DOI] [PubMed] [Google Scholar]
- [11].Hansen AB, Sander B, Larsen M, et al. Screening for diabetic retinopathy using a 34 digital non-mydriatic camera compared with standard 35-mm stereo colour transparencies. Acta Ophthalmol Scand 2004;82:656–65. [DOI] [PubMed] [Google Scholar]
- [12].Lawrence MG. The accuracy of digital-video retinal imaging to screen for diabetic retinopathy: an analysis of two digital-video retinal imaging systems using standard stereoscopic seven-field photography and dilated clinical examination as reference standards. Trans Am Ophthalmol Soc 2004;102:321–40. [PMC free article] [PubMed] [Google Scholar]
- [13].Kernt M, Pinter F, Hadi I, et al. Diabetic retinopathy: comparison of the diagnostic features of ultra-widefield scanning laser ophthalmoscopy Optomap with ETDRS 32 7-field fundus photography. Ophthalmologe 2011;108:117–23. [DOI] [PubMed] [Google Scholar]
- [14].Kernt M, Hadi I, Pinter F, et al. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care 2012;35:2459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rudnisky CJ, Tennant MT, Weis E, et al. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology 2007;114:1748–54. [DOI] [PubMed] [Google Scholar]
- [16].Lin DY, Blumenkranz MS, Brothers RJ, et al. Group TD. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol 2002;134:204–13. [DOI] [PubMed] [Google Scholar]
- [17].Massin P, Aubert JP, Eschwege E, et al. Evaluation of a screening program for diabetic retinopathy in a primary care setting Dodia (Dépistage ophtalmologique du diabète) study. Diabetes Metab 2005;31:153–62. [DOI] [PubMed] [Google Scholar]
- [18].Schiffman RM, Jacobsen G, Nussbaum JJ, et al. Comparison of a digital retinal imaging system and seven-field stereo color fundus photography to detect diabetic retinopathy in the primary care environment. Ophthalmic Surg Lasers Imaging 2005;36:46–56. [PubMed] [Google Scholar]
- [19].Gangaputra S, Almukhtar T, Glassman AR, et al. Comparison of film and digital fundus photographs in eyes of individuals with diabetes mellitus. Invest Ophthalmol Vis Sci 2011;52:6168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maker MP, Noble J, Silva PS, et al. Automated Retinal Imaging System (ARIS) compared with ETDRS protocol color stereoscopic retinal photography to assess 41 level of diabetic retinopathy. Diabetes Technol Ther 2012;14:515–22. [DOI] [PubMed] [Google Scholar]
- [21].Silva PS, Cavallerano JD, Sun JK, et al. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol 2012;154:549.e2–59.e2. [DOI] [PubMed] [Google Scholar]
- [22].Tennant MT, Rudnisky CJ, Hinz BJ, et al. Tele-ophthalmology via stereoscopic digital imaging: a pilot project. Diabetes Technol Ther 2000;2:583–7. [DOI] [PubMed] [Google Scholar]
- [23].Gómez-Ulla F, Fernandez MI, Gonzalez F, et al. Digital retinal images and teleophthalmology for detecting and grading diabetic retinopathy. Diabetes Care 2002;25:1384–9. [DOI] [PubMed] [Google Scholar]
- [24].Harding SP, Broadbent DM, Neoh C, et al. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ 1995;311:1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li HK, Hubbard LD, Danis RP, et al. Comparison of multiple stereoscopic and monoscopic digital image formats to film for diabetic macular edema evaluation. Invest Ophthalmol Vis Sci 2010;51:6753–61. [DOI] [PubMed] [Google Scholar]
- [26].Silva PS, Walia S, Cavallerano JD, et al. Comparison of low-light nonmydriatic digital imaging with 35-mm ETDRS seven-standard field stereo color fundus photographs and clinical examination. Telemed J E Health 2012;18:492–9. [DOI] [PubMed] [Google Scholar]
- [27].Cavallerano JD, Silva PS, Tolson AM, et al. Imager evaluation of diabetic retinopathy at the time of imaging in a telemedicine program. Diabetes Care 2012;35:482–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Torok Z, Peto T, Csosz E, et al. Combined methods for diabetic retinopathy screening, using retina photographs and tear fluid proteomics biomarkers. J Diabetes Res 2015;2015:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ting DS, Tay-Kearney ML, Kanagasingam Y. Light and portable novel device for diabetic retinopathy screening. Clin Exp Ophthalmol 2012;40:e40–6. [DOI] [PubMed] [Google Scholar]
- [30].Usher D, Dumskyj M, Himaga M, et al. Automated detection of diabetic retinopathy in digital retinal images: a tool for diabetic retinopathy screening. Diabet Med 2004;21:84–90. [DOI] [PubMed] [Google Scholar]
- [31].Bursell SE, Cavallerano JD, Cavallerano AA, et al. Joslin Vision Network Research Team. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology 2001;108:572–85. [DOI] [PubMed] [Google Scholar]
- [32].Russo A, Morescalchi F, Costagliola C, et al. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J Ophthalmol 2015;159:360.e1–4.e1. [DOI] [PubMed] [Google Scholar]
- [33].Pirbhai A, Sheidow T, Hooper P. Prospective evaluation of digital non-stereo color fundus photography as a screening tool in age-related macular degeneration. Am J Ophthalmol 2005;139:455–61. [DOI] [PubMed] [Google Scholar]
- [34].Peter J, Piantadosi J, Piantadosi C, et al. Use of real-time telemedicine in the detection of diabetic macular oedema: a pilot study. Clin Exp Ophthalmol 2006;34:312–6. [DOI] [PubMed] [Google Scholar]
- [35].Cook S, Staff RT, Goatman KA, et al. Quality assurance in diabetic retinal screening in South Africa. S Afr Med J 2014;104:700–4. [DOI] [PubMed] [Google Scholar]
- [36].Rajalakshmi R, Arulmalar S, Usha M, et al. Validation of smartphone based retinal photography for diabetic retinopathy screening. PLoS One 2015;10:e0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boucher MC, Gresset JA, Angioi K, et al. Effectiveness and safety of screening for diabetic retinopathy with two nonmydriatic digital images compared with the seven standard stereoscopic photographic fields. Can J Ophthalmol 2003;38:557–68. [DOI] [PubMed] [Google Scholar]
- [38].Andonegui J, Serrano L, Eguzkiza A, et al. Diabetic retinopathy screening using tele-ophthalmology in a primary care setting. J Telemed Telecare 2010;16:429–32. [DOI] [PubMed] [Google Scholar]
- [39].Invernizzi A, Bevilacqua MT, Cozzi M, et al. Diabetic retinopathy screening: the first telemedical approach in an Italian hospital. Eur J Ophthalmol 2016;26:369–74. [DOI] [PubMed] [Google Scholar]
- [40].Rodriguez Villa S, Alonso Alvarez C, de Dios Del Valle R, et al. Five-year experience of tele-ophthalmology for diabetic retinopathy screening in a rural population. Arch Soc Esp Oftalmol 2016;91:426–30. [DOI] [PubMed] [Google Scholar]
- [41].Bawankar P, Shanbhag N, Smitha KS, et al. Sensitivity and specificity of automated analysis of single-field non-mydriatic fundus photographs by Bosch DR Algorithm-Comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS One 2017;12:e0189854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bjorvig S, Johansen MA, Fossen K. An economic analysis of screening for diabetic retinopathy. J Telemed Telecare 2002;8:32–5. [DOI] [PubMed] [Google Scholar]
- [43].Maberley D, Walker H, Koushik A, et al. Screening for diabetic retinopathy in James Bay, Ontario: a cost-effectiveness analysis. CMAJ 2003;168:160–4. [PMC free article] [PubMed] [Google Scholar]
- [44].Aoki N, Dunn K, Fukui T, et al. Cost-effectiveness analysis of telemedicine to evaluate diabetic retinopathy in a prison population. Diabetes Care 2004;27:1095–101. [DOI] [PubMed] [Google Scholar]
- [45].Whited JD, Datta SK, Aiello LM, et al. A modeled economic analysis of a digital tele-ophthalmology system as used by three federal health care agencies for detecting proliferative diabetic retinopathy. Telemed J E Health 2005;11:641–51. [DOI] [PubMed] [Google Scholar]
- [46].Li Z, Wu C, Olayiwola JN, et al. Telemedicine based digital retinal imaging vs standard ophthalmologic evaluation for the assessment of diabetic retinopathy. Conn Med 2012;76:85–90. [PubMed] [Google Scholar]
- [47].Rachapelle S, Legood R, Alavi Y, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology 2013;120:566–73. [DOI] [PubMed] [Google Scholar]
- [48].Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic retinopathy in the veterans health administration. Ophthalmology 2013;120:2604–10. [DOI] [PubMed] [Google Scholar]
- [49].Phan A-DT, Koczman JJ, Yung C-W, et al. Cost analysis of teleretinal screening for diabetic retinopathy in a county hospital population. Diabetes Care 2014;37:e252–3. [DOI] [PubMed] [Google Scholar]
- [50].Brady CJ, Villanti AC, Gupta OP, et al. Tele-ophthalmology screening for proliferative diabetic retinopathy in urban primary care offices: an economic analysis. Ophthalmic Surg Lasers Imaging Retina 2014;45:556–61. [DOI] [PubMed] [Google Scholar]
- [51].Maamari RN, Keenan JD, Fletcher DA, et al. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol 2013;98:438–41. [DOI] [PubMed] [Google Scholar]
- [52].Taylor DJ, Fisher J, Jacob J, et al. The use of digital cameras in a mobile retinal screening environment. Diabet Med 1999;16:680–6. [DOI] [PubMed] [Google Scholar]
- [53].Taylor DJ, Jacob JS, Tooke JE. The integration of digital camera derived images with a computer based diabetes register for use in retinal screening. Comput Methods Programs Biomed 2000;62:157–63. [DOI] [PubMed] [Google Scholar]
- [54].Davis R, Mayer-Davis EJ. Cost Effectiveness of a TeleHealth-Based Diabetes Self-Management (DSME) Intervention in a Rural Community. Health Care Delivery—Economic; p. 785. [Google Scholar]
- [55].Invernizzi A, Bevilacqua MT, Cozzi M, et al. Diabetic retinopathy screening: the first telemedical approach in an Italian hospital. Eur J Ophthalmol 2015;26:369–74. [DOI] [PubMed] [Google Scholar]
- [56].Lawrenson RA, Dunn PJ, Worsley D, et al. Discover diabetes: a community based screening programme for diabetic eye disease. N Z Med J 1995;107:172–4. [PubMed] [Google Scholar]
- [57].Rein DB, Wittenborn JS, Zhang X, et al. The cost-effectiveness of three screening alternatives for people with diabetes with no or early diabetic retinopathy. Health Serv Res 2011;46:1534–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Leese GP, Ahmed S, Newton RW, et al. Use of mobile screening unit for diabetic retinopathy in rural and urban areas. BMJ 1993;306:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bjørvig S, Johansen MA, Fossen K. An economic analysis of screening for diabetic retinopathy. J Telemed Telecare 2002;8:32–5. [DOI] [PubMed] [Google Scholar]
- [60].Müller A, Vu HT, Ferraro JG, et al. Rapid and cost-effective method to assess vision disorders in a population. Clin Exp Ophthalmol 2006;34:521–5. [DOI] [PubMed] [Google Scholar]
- [61].Thompson CJ, Leese GP. The evaluation of mobile screening for diabetic retinopathy. Scott Med J 1995;40:5–7. [DOI] [PubMed] [Google Scholar]
- [62].Brown-Connolly NE, Concha JB, English J. Mobile health is worth it! Economic benefit and impact on health of a population-based mobile screening program in New Mexico. Telemed J E Health 2014;20:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sculpher MJ, Buxton MJ, Ferguson BA, et al. Screening for diabetic retinopathy: a relative cost effectiveness analysis of alternative modalities and strategies. Health Econ 1992;1:39–51. [DOI] [PubMed] [Google Scholar]
- [64].James M, Turner DA, Broadbent DM, et al. Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ 2000;320:1627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Facey K, Cummins E, Macpherson K, et al. Health Technology Assessment Report 1: Organisations of Services for Diabetic Retinopathy Screening. Glasgow: Health Technology Board for Scotland; 2002. [Google Scholar]
- [66].Tu KL, Palimar P, Sen S, et al. Comparison of optometry vs digital photography screening for diabetic retinopathy in a single district. Eye (Lond) 2004;18:3–8. [DOI] [PubMed] [Google Scholar]
- [67].Khan T, Bertram MY, Jina R, et al. Preventing diabetes blindness: cost effectiveness of a screening programme using digital non-mydriatic fundus photography for diabetic retinopathy in a primary health care setting in South Africa. Diabetes Res Clin Pract 2013;101:170–6. [DOI] [PubMed] [Google Scholar]
- [68].Lian JX, McGhee SM, Gangwani RA, et al. Screening for diabetic retinopathy with or without a copayment in a randomized controlled trial: influence of the inverse care law. Ophthalmology 2013;120:1247–53. [DOI] [PubMed] [Google Scholar]
- [69].Kawasaki R, Akune Y, Hiratsuka Y, et al. Cost-utility analysis of screening for diabetic retinopathy in Japan: a probabilistic Markov modeling study. Ophthalmic Epidemiol 2014;22:4–12. [DOI] [PubMed] [Google Scholar]
- [70].Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-Saharan Africa: meeting the challenges of an emerging epidemic. BMC Med 2013;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chua J, Lim CX, Wong TY, et al. Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila) 2018;7:3–16. [DOI] [PubMed] [Google Scholar]
- [72].Sasso FC, Pafundi PC, Gelso A, et al. Telemedicine for screening diabetic retinopathy: The NO BLIND Italian multicenter study. Diabetes Metab Res Rev 2019;35:e3113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


