Abstract
Surgical management of cancer may induce stress and increase the likelihood of cancer metastasis and recurrence. Appropriate surgical and anesthetic techniques may affect the patient's outcome. Although numerous studies have been performed, conflicting results have been obtained regarding the effect of anesthetic techniques on the outcome of patients with cancer. We conducted this study to evaluate the association of anesthetic techniques with overall and recurrence-free survival in patients who had undergone gastric cancer surgery.
This retrospective study reviewed the electronic medical records of patients, who had visited our hospital and had been diagnosed with gastric cancer between July 1st, 2006 to June 30th, 2016. Univariate analysis of the potential prognostic factors was performed using the log-rank test for categorical factors, and parameters with a P-value < .05 at the univariate step were included in the multivariate regression analysis. Propensity Score Matching was performed to account for differences in baseline characteristics: propofol or desflurane, in a 1:1 ratio.
A total of 408 patients anesthetized with desflurane (218) and propofol (190) were eligible for analysis. After propensity matching, 167 patients remained in each group. The overall mortality rate was significantly higher in the desflurane group (56%) than in the propofol group (34%) during follow-up (P < .001). In addition, a greater percentage of patients in the desflurane group (41%) exhibited postoperative metastasis than those in the propofol group (19%, P < .001).
The authors found some association between types of anesthesia used and the long-term prognosis of gastric cancer. Propofol-based total intravenous anesthesia improved survival and reduced the risk of recurrence and metastasis during the 5-year follow-up period after gastric cancer surgery.
Keywords: desflurane, propofol, stomach cancer
1. Introduction
Gastric cancer is the fourth most common cause of cancer-related death, primarily because most patients present with the advanced stage of the disease.[1] Surgical resection is a crucial intervention and remains the mainstay in the treatment of gastric cancer.[2] However, the surgical insult itself can induce an acute systemic inflammatory response.[3] Moreover, several studies have reported that surgery increases shedding of malignant cells into the blood and lymphatic circulations,[4] local and systemic levels of growth factors,[5] and the presence of circulating tumor cells, which is associated with shorter overall survival.[6–8]
Numerous effects of various anesthetic drugs on perioperative immune status have been documented.[9] Moreover, accumulating evidence emphasizes the importance of the perioperative period in determining the risk of postoperative cancer recurrence and metastasis.[10] Therefore, the choice of the anesthetic drug and analgesic approach used during surgery has long been proposed to influence oncological outcomes.[11] Volatile anesthetics (VA) and propofol are widely used for general anesthesia. More recent cellular in vitro and in vivo studies exploring pathways by which choice of the anesthetic drug may directly or indirectly promote or prevent cancer. In vitro studies showed that VA, such as isoflurane and sevoflurane, suppress natural killer (NK) cell cytotoxicity, and increase insulin-like growth factors and hypoxia-inducible factor-1α, which can increase the malignancy potential of cancer cells via proliferation, migration, angiogenesis, and chemoresistance.[12–14] On the other hand, a number of studies have demonstrated that propofol may exert antitumor effects via various mechanisms, including promotion of NK cell cytotoxicity, and reduction of colon cancer cell motility and invasion.[15] The neuroepithelial cell transforming 1 gene has a role in promoting migration in breast adenocarcinoma; propofol anesthesia is associated with reduced neuroepithelial cell transforming 1 expression in vitro.[16] Ren et al[17] reported that propofol not only potentiated the expression of cluster of differentiation 28 co-stimulator on peripheral T-helper cells, but also increased the ratio of interferon-c/interleukin-4, indicating that it might initiate the activation and differentiation of T-helper lymphocytes, a key step in antitumor immune responses. These results lead to the hypothesis that administering patient propofol anesthesia may provide survival advantages. To the best of our knowledge, only 1 study has reported the effect of VA and propofol on the survival of patients with gastric cancer.[18]
In this retrospective study, we investigated the association of propofol and desflurane anesthesia with long-term survival, local recurrence, and postoperative distant metastasis of patients undergoing gastric cancer surgery.
2. Material and methods
2.1. Ethical approval
The ethics committee of the Tri-Service General Hospital (TSGH), Taipei, Taiwan, Republic of China approved the study design on May 9th, 2017 (TSGH IRB No: 1–106- 05–094) and waived the requirement for obtaining informed consent and patient records.
2.2. Study population
This retrospective study included patients who visited our hospital and were diagnosed with gastric cancer between July 1st, 2006 to June 30th, 2016. We obtained the following information: demographic data; cancer stage; American Society of Anesthesiology (ASA) score; duration of surgery and anesthesia; type of anesthetic used; degree of differentiation of tumor; preoperative or postoperative adjuvant chemotherapy, and/or radiation therapy; and length of hospital stay. The status of patients until July 1st, 2017 were retrieved from the medical records and electronic database of the TSGH.
2.3. Inclusion and exclusion criteria
No form of premedication was used before induction of anesthesia. On arrival at the operating room, regular monitoring of parameters such as noninvasive blood pressure, electrocardiography (lead II), pulse oximetry, end-tidal carbon dioxide and securing a radial artery line and central venous catheter were initiated for each patient throughout the surgery. The type of anesthesia was selected according to the anesthetist's preference. A total of 408 cases with ASA scores II–III, who had undergone elective gastric cancer surgery for tumor-node-metastasis (TNM) stage I– IV gastric cancer under propofol (propofol group, n = 190) or desflurane (desflurane group, n = 218) anesthesia were considered for inclusion in the study. Fifty-two cases were excluded from the analysis. The exclusion criteria were combined propofol with VA or epidural anesthesia, incomplete data, or age < 20 years (Fig. 1).
Figure 1.
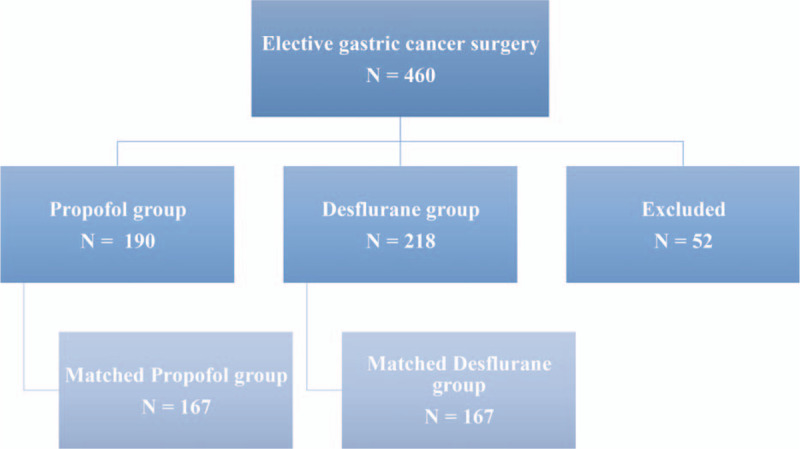
Flow diagram detailing the selection of patients included in the retrospective analysis. Fifty-two patients were excluded due to combined propofol anesthesia with inhalation anesthesia, incomplete data, or age less than 20 yr.
2.4. Anesthesia induction
In the propofol group (n = 190), anesthesia was induced with intravenous fentanyl (2 μg/kg) and 2% lidocaine (1.5 mg/kg). Continuous infusion of propofol (Fresofol 1%) was initiated subsequently using Schneider's kinetic model of target-controlled infusion (TCI; Fresenius Orchestra Primea, Fresenius Kabi AG, Bad Homburg, Germany) with an effect-site concentration (Ce) of 4.0 μg/mL. Rocuronium (0.6 mg/kg) was administered when patients lost consciousness, followed by tracheal intubation. Anesthesia was maintained using TCI with propofol Ce 3 μg/mL to 4 μg/mL and an oxygen flow of 0.3 L/min with 100% fractional inspired oxygen concentration. Repetitive bolus injections of cisatracurium and fentanyl were administered as required throughout the procedure. In the desflurane group (n = 218), anesthesia was induced with intravenous fentanyl (2 μg/kg), 2% lidocaine (1.5 mg/kg), and propofol (1.5–2 mg/kg). When patients lost consciousness, 0.6 mg/kg rocuronium or 1.5 mg/kg to 2.0 mg/kg succinylcholine was administered, followed by endotracheal intubation. Anesthesia was maintained by 8% to 12% desflurane in a 100% oxygen flow of 0.3 L/min under a closed system. Repetitive bolus injections of cisatracurium and fentanyl were administered as required throughout the procedure.
2.5. Main outcome
The main end-point was overall survival, which was compared between the groups that had received propofol or desflurane as the main anesthetic agent. Survival time was defined as the interval between the date of surgery and the date of outcome, emigration, or end of follow-up in May 2017.
2.6. Data collection
The retrospectively collected data included anesthetic technique; time since the earliest included patient (which served as a surrogate of the calendar year); sex; age at the time of surgery; TNM stage of the primary tumor; preoperative functional status, such as metabolic equivalents (patients were grouped according to whether their metabolic equivalents were greater than or equal to 4 or less than 4 because the perioperative cardiac and long-term risks increase in patients with less than 4 metabolic equivalents during most normal daily activities[19]); use of adjuvant chemotherapy; use of postoperative non-steroidal anti-inflammatory drugs (NSAIDs); grade of surgical complications determined using the Clavien-Dindo classification; postoperative recurrence; and postoperative metastasis. Preoperative morbidity was assessed using the ASA physical status score of I (least morbidity) to V (highest), as determined by the anesthesiologist preoperatively. Ten-year survival in patients with multiple comorbidities was predicted using the Charlson Comorbidity Index (CCI) of 0 (least comorbidity) to 37 (highest). The grade of surgical complication was scaled from 0 (none) to V (maximum) according to the Clavien-Dindo classification. These variables were chosen as potential confounders as they have either been shown, or posited, to affect the outcome.
2.7. Statistical analysis
Patient characteristics and death rates were compared between different anesthetics using the t-test or the chi-squared test. Survival and type of anesthesia were depicted visually by a Kaplan-Meier survival curve. The relationship between the choice of anesthetic (propofol or desflurane) and survival was analyzed using the Cox proportional-hazards model with and without adjustment for sex, age, ASA score, TNM stage, chemotherapy, and local recurrence. Subgroup analysis was performed for postoperative distant metastasis because a significant interaction of metastasis was found with the relationship between the type of anesthesia and survival. Results were presented as mean ± standard deviation or number (percentage) or as median with interquartile range, as appropriate. The SPSS software (version 16.0.1; IBM Corp, Armonk, NY) was used for the statistical analyses; P-values < .05 were considered statistically significant.
Propensity Score Matching using preoperative variables was performed to select the most similar propensity scores (with calipers set at 0.2 standard deviation of the logit of the propensity score) for the anesthetic drugs used: propofol or desflurane in a 1:1 ratio. Since ‘time since the earliest included patient’ and ‘ASA stage’ were still significantly different between two groups, further exact matching of these 2 variables was carried out to enhance the comparability. Data were presented as mean ± standard deviation or number (percentage), as appropriate; P-values < .05 were considered statistically significant.
3. Results
Our study included 408 patients with gastric cancer, out of which 190 received propofol and 218 received desflurane. The patient characteristics and treatment details are shown in Table 1. Time since the earliest included patient was shorter in the desflurane group (5.0 ± 2.4 years) than in the propofol group (5.5 ± 2.1 years, P = .012). There were no age differences between the desflurane (66.0 ± 15.0 years) and propofol (65.0 ± 14.0 years, P = .456) groups. The CCI score was higher in the desflurane group (5.9 ± 2.4) than in the propofol group (5.5 ± 2.5, P = .090). The desflurane group had significantly more patients with an ASA score of III (P < .001) and preoperative functional status of less than 4 metabolic equivalents (P = .013). The TNM stage differed significantly between the desflurane and propofol groups (P = .006). Patients in the propofol group were more prone to have TNM stage I cancer, and patients in the desflurane group were more prone to have TNM stage III cancer. Moreover, the tumor size was significantly larger in the desflurane group (> 3 cm, 58%) than in the propofol group (> 3 cm, 56%) (P < .001).
Table 1.
Patients’ and treatment characteristics for overall group and matched group after propensity scoring.
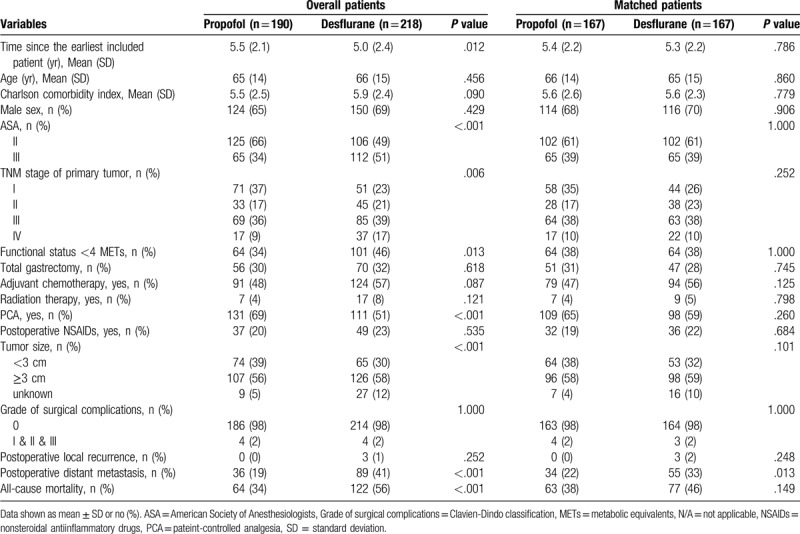
The overall mortality rate was significantly higher in the desflurane group (56%) than in the propofol group (34%) during follow-up (P < .001). In addition, a greater percentage of patients in the desflurane group (41%) exhibited postoperative metastasis than in the propofol group (19%, P < .001). A greater percentage of patients in the desflurane group (1%) exhibited postoperative recurrence than in the propofol group (0%, P = .252). The use of patient-controlled analgesia (fentanyl) was significantly higher in the propofol group (131, 69%) than in the desflurane group (111, 51%) during follow-up (P < .001). No significant differences were found between the groups in terms of sex, adjuvant chemotherapy, use of postoperative NSAIDs, or grade of surgical complications.
Due to the significant differences in baseline characteristics between the 2 groups, we used a series of algorithms to obtain a propensity score. After matching, 167 pairs were formed. (Table 1).
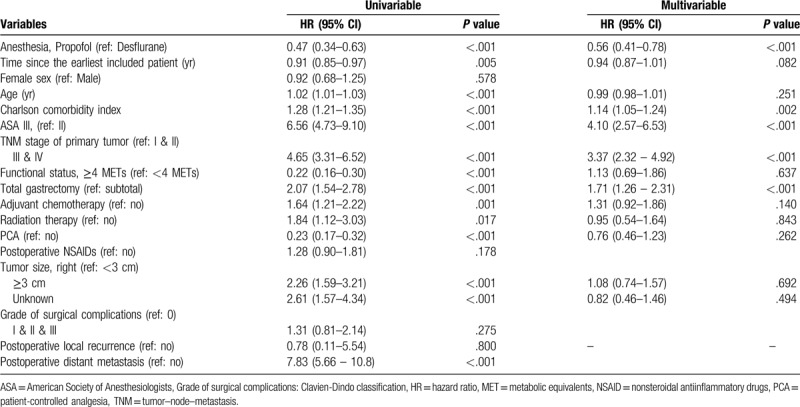
The overall mortality risk associated with the use of propofol and desflurane during gastric cancer surgery is shown in Table 2. Overall survival from the date of surgery, grouped according to the anesthesia type and other variables, was compared separately using a univariable Cox model, and subsequently, a multivariable Cox regression. Other variables that significantly increased the risk of death after multivariable analysis were higher ASA scores, higher TNM stage and total gastrectomy (Table 2).
Table 2.
Cox proportional hazards regression for mortality: univariable and multivariable models for overall patients.
The subgroup analyses for TNM Stage, presence of postoperative metastasis, gastrectomy, and disease progression are shown in Table 3. Due to the interaction effect between the type of anesthesia and TNM stage (P = .754) and postoperative metastasis (P = .002) on survival, all analyses were stratified by these 2 variables. Patients who received propofol also exhibited better survival than those who received desflurane, regardless of the presence or absence of postoperative metastasis. For patients with postoperative distant metastasis, the crude hazard ratio (HR) was 1.16 (95% confidence interval [CI], 0.77–1.74; P = .477), the propensity score-adjusted HR was 1.06 (95% CI, 0.70–1.59; P = .788), and the propensity score-matched HR was 1.36 (95% CI, 0.86–2.15; P = .186). For patients without postoperative metastasis, the crude HR was 0.45 (95% CI, 0.28–0.71; P < .001), the propensity score-adjusted HR was 0.66 (95% CI, 0.41–1.06; P = .086), and the propensity score-matched HR was 0.66 (95% CI, 0.41–1.08; P = .101; Table 3).
Table 3.
Subgroup analyses for TNM stage, presence of postoperative metastasis, gastrectomy, and disease progression (propofol vs desflurane).
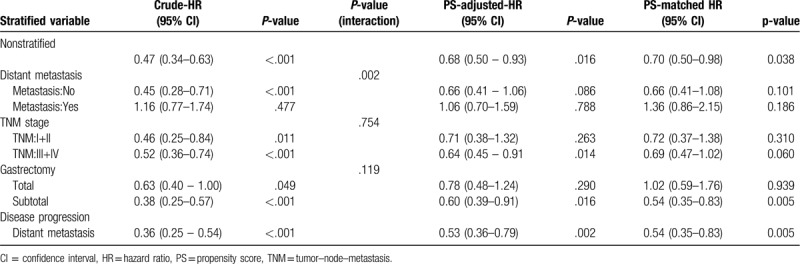
Patients who received propofol exhibited better survival than those who received desflurane, regardless of their TNM stage. For lower TNM stages (I and II), the crude HR was 0.46 (95% CI, 0.25–0.84; P = .11), the propensity score–adjusted HR was 0.71 (95% CI, 0.38–1.32; P = .263), and the propensity score–matched HR was 0.72 (95% CI, 0.37–1.38; P = .310). For higher TNM stages (III and IV), the crude HR was 0.52 (95% CI, 0.36–0.74; P < .001), the propensity score-adjusted HR was 0.64 (95% CI, 0.45–0.91; P = .014), and the propensity score-matched HR was 0.69 (95% CI, 0.47–1.02; P = .060; Table 3).
Patients who received propofol exhibited better survival than those who received desflurane, regardless of whether they underwent total or subtotal gastrectomy. For subtotal gastrectomy, the crude HR was 0.38 (95% CI, 0.25–0.57; P < .001), the propensity score-adjusted HR was 0.60 (95% CI, 0.39–0.91; P = .016), and the propensity score-matched HR was 0.54 (95% CI, 0.35–0.83; P = .005). For total gastrectomy, the crude HR was 0.63 (95% CI, 0.40–1.00; P = .049), the propensity score-adjusted HR was 0.78 (95% CI, 0.48–1.24; P = .290), and the propensity score- matched HR was 1.02 (95% CI, 0.59–1.76; P = .939). Distant metastasis showed at the crude HR was 0.36 (95% CI, 0.25–0.54; P < .001), the propensity score-adjusted HR was 0.53 (95% CI, 0.36–0.79; P = .002), and the propensity score- matched HR was 0.54 (95% CI, 0.35–0.83; P = .005; Table 3).
In summary, the present study demonstrated overall better outcomes for propofol anesthesia, as shown in Fig. 2A, B and C.
Figure 2.
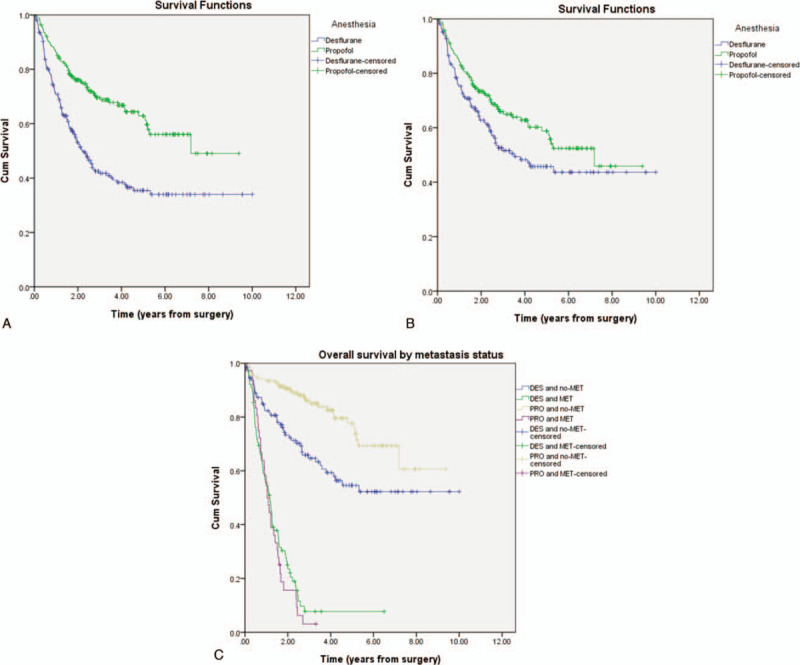
(A) Overall survival curves from the date of surgery by anesthesia type. (B) Overall survival curves from the date of surgery by anesthesia type after Propensity Score Matching. (C) Overall survival curves from the date of surgery by metastasis.
4. Discussion
This retrospective study included 408 patients who underwent elective gastric cancer surgery and evaluated the long-term survival of patients receiving propofol-based total intravenous anesthesia compared with desflurane-based anesthesia. Our results showed that propofol-based anesthesia improved survival and relatively reduced the risk of distant metastasis compared to desflurane-based anesthesia. Our study has considerable implications in relation to prognostic factors, indicating that the propofol-based anesthetic technique may have an early beneficial effect on long-term outcome after gastric cancer surgery. Our results are consistent with those of Zheng et al[18], who reported that total intravenous anesthesia is associated with improved survival in gastric cancer.
Many studies have investigated the association between propofol anesthesia and gastric cancer surgery outcome. Yang et al[20] reported that propofol exerts an inhibitory effect on the growth and survival of gastric cancer cells by interfering with the degradation of inhibitor of growth 3. In addition, Peng et al[21] showed that propofol effectively inhibits proliferation and induces apoptosis of gastric cancer cells, partly due to the downregulation of matrix metalloproteinase (MMP)-2 expression by miR- 451. Moreover, Zhang et al[22] found that propofol inhibits proliferation, migration, and invasion of gastric cancer MKN45 cells by up-regulating miR-195 and then inactivating the Janus kinase/signal transducers and activators of activation and nuclear factor kappa-light-chain-enhancer of activated B-cell pathways. The authors suggested that propofol could serve as an effective therapeutic medicine for gastric cancer treatment. Taken together, various in vitro studies have shown that propofol exhibits antitumor properties. In a clinical study with a large sample size (n = 897 per group), Zheng et al[18] revealed that total intravenous anesthesia was associated with an HR of 0.65 after a multivariate analysis of known confounders in the matched group. In a multivariable analysis according to surgical specialty, the mortality of patients undergoing gastrointestinal surgery with VAs (isoflurane or sevoflurane) (44.2% [223/504]) was significantly worse than with total intravenous anesthesia (32.8% [137/418]).[11] Previously, we have also reported that propofol anesthesia is associated with better survival in colon cancer surgery, irrespective of the TNM stage.[23,24]
Only a few studies have been conducted to study the association between cancer cells and desflurane, especially gastric cancer cells. Müller-Edenborn et al[25] showed that desflurane reduces subsequent migration of colon cancer cells in vitro through down- regulation of MMP-9. Woo et al[26] also showed that leukocyte count is higher in the desflurane group than in the propofol group, 1 hour after induction and 24 hours postoperatively. The NK cell count is significantly reduced, 1 hour after induction in the propofol group, but not in the desflurane group. The authors suggested that desflurane anesthesia is associated with less adverse immune responses than propofol anesthesia during surgery for breast cancer.[26] However, Iwasaki et al[27] reported that desflurane increases the gene and protein expression of vascular endothelial growth factor-A, MMP-11, chemokine (C-X-C motif) receptor 2, and transforming growth factor beta-1 in ovarian cancer cells, and suggested that desflurane could enhance ovarian cancer metastatic potential. Therefore, the role of desflurane as an antitumor agent is still controversial, especially in gastric cancer and further investigations are needed to determine the role of desflurane as an antitumor agent.
The impact of anesthetic management on the recurrence and metastasis of cancer has been investigated, but previous studies have reported conflicting findings due to variations in protocols, experimental environments, and subject species. These include variations in the type of surgery, blood transfusion, hypothermia, and evaluation of postoperative complications. Surgical stimulation may affect cancer immunity during the perioperative period by releasing tumor cells into circulation, suppressing the cellular immune system, and augmenting angiogenesis.[28] The number of circulating cancer cells before and during surgery has been shown to be a strong predictor of recurrence.[29] These discrepancies can be explained by various factors influencing the immune system during the perioperative period. In the present study, we considered a wide range of aspects of perioperative care, including the use of opioid analgesia and regional anesthesia, since these may also alter the immune response and influence cancer recurrence, and thus, survival. Opioids are currently a major component in the management of both perioperative and acute postoperative pain. Opioids play an important role in physiological and pathophysiological processes, including inflammation, tumor growth, and metastasis. Many studies have evaluated the correlation between opioid receptors and tumor progression. Nguyen et al[30] showed that morphine promotes the development of established tumors by increased μ-opioid receptor expression, and impairs survival in transgenic mice with breast cancer. In a review article, Liang et al[31] concluded that the role of opioids in immune function involves various complicated mechanisms, such as their effect on tumor progression.
Chester et al[32] reported that NK cells directly exert cellular cytotoxicity against local tumor growth and metastasis, but this may be related to the dose of opioids. The κ- opioid receptor ligands act as anti-angiogenic factors to directly regulate the growth of normal and neoplastic cells by modulating the pro-oncogenic vascular endothelial growth factors and epidermal growth factor receptors, resulting in inhibition of tumor growth.[33] In our study, fentanyl was administered to the patients intraoperatively and postoperatively; compared with that used in other studies, we used relatively small doses and did not observe any deleterious effect of perioperative opioids on the recurrence and metastasis of gastric cancer. This suggests that the result of opioid- induced immunomodulation is affected by the type of surgery and cancer, and dose of opioids,[34,35] and further investigations are needed.
NSAIDs inhibit prostaglandin synthesis via inhibition of cyclooxygenases, and have been shown to be safe in the perioperative setting, reducing post-operative prostate cancer metastases and mortality.[36] Forget et al[37] showed that intraoperative use of ketorolac or diclofenac in patients with breast cancer was associated with prolonged disease-free and overall survival, particularly if administered shortly before the surgery. In our study, postoperative administration of NSAIDs had no effect on the outcome, recurrence, or metastasis, and further investigations are needed.
Our study has certain limitations. A retrospective study may have uncontrolled and unrecognized biases. Moreover, the choice of anesthetic drugs depended on the anesthetists’ preference. A previous study has shown that perioperative blood transfusions might promote cancer cell growth.[38] Information regarding blood transfusion was incomplete in our medical records. However, in our clinical practice, the frequency of perioperative blood transfusion is very low (less than 1%). There were more patients with an ASA score of III or IV, metastatic cancer, and larger tumor size in the desflurane group; however, statistical methods can be used to control such variations, and we conducted propensity-score matching to address this issue. Finally, although the results for factors such as metastasis, TNM, and surgical type differed non-significantly between the groups (P > .05), the trend of better survival in the propofol group should be noted.
In conclusion, the results of our study support the theory that propofol-based total intravenous anesthesia reduces the risk of recurrence and metastasis during the 5-year follow-up period after gastric cancer surgery.
Acknowledgments
The authors thank the Cancer Registry Group of Tri- Service General Hospital for the clinical data support.
Author contributions
Conceptualization: Nian-Cih Huang, Zhi-Fu Wu.
Data curation: Hou-Chuan Lai, Chueng-He Lu, Yi-Hsuan Huang.
Formal analysis: Meei-Shyuan Lee, Han-Ting Lin, Chen-Heng Hsu.
Investigation: Nian-Cih Huang.
Resources: Meei-Shyuan Lee.
Supervision: Zhi-Fu Wu.
Writing – original draft: Nian-Cih Huang.
Writing – review & editing: Zhi-Fu Wu.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, TNM = tumor-node-metastasis, VA = volatile anesthetics.
How to cite this article: Huang NC, Lee MS, Lai HC, Lin HT, Huang YH, Lu CH, Hsu CH, Wu ZF. Propofol-based total intravenous anesthesia improves survival compared to desflurane anesthesia in gastric cancer surgery: a retrospective analysis. Medicine. 2020;99:25(e20714).
NCH this author developed the theory and performed the computations. MSL this author helped verified the analytical methods. HCL this author developed the theory and performed the computations. HTL this author helped verified the analytical methods. YHH this author helped conceived of the presented idea. CHL this author helped conceived of the presented idea. CHH this author helped conceived of the presented idea. ZFW this author helped supervised the project.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Christina F, Daniel D, Amanda P, et al. Global Burden of Disease Cancer Collaboration. Global the global burden of cancer 2013. JAMA Oncol 2015;1:505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dikken JL, van de Velde CJH, Coit DG, et al. Treatment of resectable gastric cancer. Therap Adv Gastroenterol 2012;5:49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 2015;12:213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamaguchi K, Takagi Y, Aoki S, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 2000;232:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fisher B, Gunduz N, Coyle J, et al. Presence of a growth- stimulating factor in serum following primary tumor removal in mice. Cancer Res 1989;49:1996–2001. [PubMed] [Google Scholar]
- [6].Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 2006;33:S9–14. [DOI] [PubMed] [Google Scholar]
- [7].Peach G, Kim C, Zacharakis E, et al. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer 2010;102:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gasparri ML, Savone D, Besharat RA, et al. Circulating tumor cells as trigger to hematogenous spreads and potential biomarkers to predict the prognosis in ovarian cancer. Tumour Biol 2016;37:71–5. [DOI] [PubMed] [Google Scholar]
- [9].Inada T, Yamanouchi Y, Jomura S, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004;59:954–9. [DOI] [PubMed] [Google Scholar]
- [10].Gottschalk A, Sharma S, Ford J, et al. The role of the perioperative period in recurrence after cancer surgery. Anesth Analg 2010;110:1636–43. [DOI] [PubMed] [Google Scholar]
- [11].Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016;124:69–79. [DOI] [PubMed] [Google Scholar]
- [12].Stollings LM, Jia LJ, Tang P, et al. Immune modulation by volatile anesthetics. Anesthesiology 2016;125:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 2003;97:1331–9. [DOI] [PubMed] [Google Scholar]
- [14].Huang H, Benzonana LL, Zhao H, et al. Prostate cancer cell malignancy via modulation of HIF-1(pathway with isoflurane and propofol alone and in combination. Br J Cancer 2014;111:1338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miao Y, Zhang Y, Wan H, et al. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother 2010;64:583–8. [DOI] [PubMed] [Google Scholar]
- [16].Ecimovic P, Murray D, Doran P, et al. Propofol and bupivacaine in breast cancer cell function in vitro - role of the NET1 gene. Anticancer Res 2014;34:1321–31. [PubMed] [Google Scholar]
- [17].Ren XF, Li WZ, Meng FY, et al. Differential effects of propofol and isoflurane on the activation of T-helper cells in lung cancer patients. Anaesthesia 2010;65:478–82. [DOI] [PubMed] [Google Scholar]
- [18].Zheng X, Wang Y, Dong L. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther 2018;11:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery-Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 2002;94:1052–64. [DOI] [PubMed] [Google Scholar]
- [20].Yang C, Gao J, Yan N. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep 2017;37:587–93. [DOI] [PubMed] [Google Scholar]
- [21].Peng Z, Zhang Y. Propofol inhibits proliferation and accelerates apoptosis of human gastric cancer cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol Res 2016;15: 10.4238. [DOI] [PubMed] [Google Scholar]
- [22].Zhao S, Zhang Y, Liu Y. Optimization of preparation conditions for calcium pectinate with response surface methodology and its application for cell encapsulation. Int J Biol Macromol 2018;115:29–34. [DOI] [PubMed] [Google Scholar]
- [23].Wu ZF, Lee MS, Wong CS. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology 2018;129:931–41. [DOI] [PubMed] [Google Scholar]
- [24].Lai HC, Lee MS, Lin KT, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in intrahepatic cholangiocarcinoma surgery. Medicine 2019;98:e18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Muller-Edenborn B, Roth-Z’graggen B, Bartnicka K, et al. Volatile anesthetics reduce invasion of colorectal cancer cells through down-regulation of matrix metalloproteinase-9. Anesthesiology 2012;117:293–301. [DOI] [PubMed] [Google Scholar]
- [26].Woo JH, Baik HJ, Kim CH, et al. Effect of propofol and desflurane on immune cell populations in breast cancer patients: a randomized trial. J Korean Med Sci 2015;30:1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iwasaki M, Zhao H, Jaffer T, et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget 2016;7:26042–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106–15. [DOI] [PubMed] [Google Scholar]
- [29].Koch M, Kienle P, Hinz U, et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg 2005;241:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nguyen J, Luk K, Vang D, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth 2014;113: Suppl1: i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liang X, Liu R, Chen C, et al. Opioid system modulates the immune function: A review. Transl Perioper Pain Med 2016;1:5–13. [PMC free article] [PubMed] [Google Scholar]
- [32].Chester C, Fritsch K, Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol 2015;6:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamamizu K, Hamada Y, Narita M. Opioid receptor ligands regulate angiogenesis in development and in tumours. Br J Pharmacol 2015;172:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cummings KC, Xu F, Cummings LC, et al. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population-based study. Anesthesiology 2012;116:797–806. [DOI] [PubMed] [Google Scholar]
- [35].Yardeni IZ, Beilin B, Mayburd E, et al. Relationship between fentanyl dosage and immune function in the postoperative period. J Opioid Manag 2008;4:27–33. [DOI] [PubMed] [Google Scholar]
- [36].Patel MI, Subbaramaiah K, Du B, et al. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2- independent mechanism. Clin Cancer Res 2005;11:1999–2007. [DOI] [PubMed] [Google Scholar]
- [37].Forget P, Bentin C, Machiels JP, et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease- free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113: Suppl1: i82–7. [DOI] [PubMed] [Google Scholar]
- [38].Wu HL, Tai YH, Lin SP, et al. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4030 patients. Sci Rep 2018;8:13345. [DOI] [PMC free article] [PubMed] [Google Scholar]


