Abstract
Objective
Endocrine-disrupting chemicals (EDCs) are viewed as a major potential link between the environment and obesity development. We did a systematic review and meta-analysis to examine the association between exposure to EDCs and obesity.
Data sources, design and eligibility criteria
PubMed, Scopus and Web of Science were searched from inception to 6 June 2018 for studies primarily addressing the association between exposure to EDCs after the age of 2 years and anthropometric measures of obesity or body fat. The Newcastle-Ottawa scale was used to assess the risk of bias.
Data extraction and synthesis
Two independent reviewers screened and conducted data extraction and synthesis. A third reviewer resolved disagreements.
Results
A total of 73 studies investigating bisphenol A (32 286 individuals), organochlorine compounds (34 567 individuals), phthalates (21 401 individuals), polybrominated biphenyls (2937 individuals), polycyclic aromatic hydrocarbons (5174 individuals), parabens (4097 individuals), benzoic acid (3671 individuals) and polyfluoroalkyl substances (349 individuals) met our inclusion criteria. Most had a cross-sectional design and low or medium risk of bias. In qualitative analysis, bisphenol A and phthalates were consistently associated with general and abdominal obesity, in children and adults, and some studies suggested this association was age-dependent and gender-dependent. Meta-analysis indicated a significant association between exposure to bisphenol A and overweight (OR 1.254, 95% CI 1.005 to 1.564), obesity (OR 1.503, 95% CI 1.273 to 1.774) and increased waist circumference (OR 1.503, 95% CI 1.267 to 1.783) in adults, and between exposure to 2,5-dichlorophenol and obesity in children (OR 1.8, 95% CI 1.1018 to 3.184).
Conclusion
Most observational studies supported a positive association between obesity and exposure to EDCs. Although causality cannot be determined from these data, they underscore the need to limit human exposure to EDCs in light of the evidence from animal and cell-based studies indicating the effects of these chemicals on adiposity.
PROSPERO registration number
CRD42018074548.
Keywords: endocrinology disrupting chemicals, obesity, abdominal obesity, pediatric obesity
Strengths and limitations of this study.
This systematic review and meta-analysis were conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and used a validated tool for quality assessment of included studies.
Only human studies primarily addressing the association between exposure to endocrine-disrupting chemicals (EDCs) and obesity were included.
This systematic review and meta-analysis analysed the association of a broad range of EDCs and measures of generalised and abdominal obesity.
The meta-analyses were based on a limited number of studies due to the variability in how the measures of association between exposure to EDCs and anthropometric measures of obesity were reported by individual studies.
Introduction
Obesity is a major worldwide health challenge in multiple perspectives. The physiopathology and clinical impacts of excess body fat (BF) are incompletely understood, and there are many difficulties in developing safe and effective long-term therapeutic strategies.1 In addition, obesity-related health costs increase at an alarming rate.2
Development of excess weight is the result of a chronic positive energy balance stemming from the complex interaction between genetic, lifestyle, behavioural and environmental factors.3 Data from experimental studies indicate that endocrine-disrupting chemicals (EDCs) influence the development and progression of obesity.4 These chemicals, so-called environmental obesogens, are functionally defined by their properties to alter lipid metabolism and inappropriately promote adipogenesis and fat accumulation.5 The potential mechanisms underlying their effects are a major focus of research, and a number of them have been proposed.5 6 Obesogens can increase commitment or differentiation of adipocytes from stem cells by activating nuclear receptor signalling pathways that are critical for adipogenesis, such as retinoid X receptor-alpha/peroxisome-proliferator activated receptor gamma7 8 and glucocorticoid receptor.9 Moreover, obesogens lead to the development of unhealthy adipocytes, with reduced insulin sensitivity and decreased thermogenic capacity.10 11 Obesogens may also dysregulate central integration of energy balance and the programming of metabolic setpoints, particularly at critical periods of development, increasing the susceptibility for developing obesity later in life when metabolic homeostasis is challenged by factors such as diet composition and caloric intake.12 13 Moreover, exposure to obesogens may lead to a transgenerational thrifty phenotype, possibly caused by changes in chromatin accessibility and organisation.12
Several human studies addressed whether exposure to EDCs was associated with obesity. However, their findings were varied. To provide a broad picture of the association between human exposure to different EDCs and obesity, we systematically reviewed human studies addressing the association between exposure to these chemicals outside the prenatal and lactation period and measures of excess body weight or adiposity.
Methods
Search strategy and selection criteria
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.14
Inclusion criteria were based on the population, exposure, comparison, outcome and study design (PECOS) approach,15 as follows: (1) population: humans aged over 2 years; (2) exposure: exposure to EDCs assessed by analysis of a biological sample from participants; (3) comparison: participants with higher degrees of exposure versus participants with lower degrees of exposure; (4) outcome: excess weight or adiposity determined by body mass index (BMI), waist circumference (WC) or BF content; and (5) study design: cross-sectional, case–control and cohort studies. We therefore included observational studies addressing the association between exposure to EDCs outside the developmental period and BMI, WC or BF in humans. Studies were excluded if exposure to EDCs was determined by means other than analysis of a biological sample from participants, if exposure was assessed during the prenatal period or lactation, and if a measure of excess weight/adiposity was not considered a primary outcome. Reviews, abstracts, case reports and case series were excluded, in addition to studies addressing the effects of heavy metals, phytoestrogens or the synthetic oestrogen diethylstilbestrol.
PubMed, Scopus and Web of Science were searched from inception to May 3, 2017, and updated on 6 June 6, 2018, with no language restriction, using search terms that were based on a combination of indexed and free-text terms reflecting the exposure and outcomes of interest to the review, and included the following keywords, which were used in combination to execute the search: “endocrine disrupting, endocrine disruptor, endocrine disrupting chemicals, obesity, overweight, obese, body weight, waist circumference, body mass index, adipogenesis, adipose tissue, adipocyte and obesogenic” (online supplementary appendix A). The reference lists of included articles were also manually searched.
bmjopen-2019-033509supp001.pdf (56KB, pdf)
Study selection and data extraction
Study selection was conducted in two phases. In the first phase, three reviewers (BTSB, CMR and NGS) independently screened the titles and abstracts to identify eligible studies according to the PECOS approach. In the second phase, the same two reviewers independently assessed the full-text articles of the eligible studies selected in the first phase. In both phases, disagreements were resolved through discussion, and when there was no consensus, the disagreements were resolved with the participation of a third reviewer (AAA). Data extraction was conducted independently by the same reviewers (BTSB, CMR and NGS) using a predesigned data extraction sheet, with information about sample characteristics, exposure assessment, outcome assessment and risk estimates for relevant comparisons. When necessary to clarify any information, the authors of the included study were contacted by email.
Risk of bias within studies
Risk of bias within studies was assessed using the Newcastle-Ottawa Scale. According to prespecified criteria for risk of bias in sample selection, comparability of subjects in different outcome groups and assessment of outcomes, studies were considered to have a low, medium or high risk of bias (online supplementary appendix B).
bmjopen-2019-033509supp002.pdf (64.7KB, pdf)
Two reviewers independently conducted risk of bias assessment (BTSB and CMR); disagreements were resolved after discussion with a third reviewer (CLL).
Summary measures
The main outcomes assessed in this review were the measures of association between exposure to EDCs and BMI, WC or fat mass.
Meta-analysis
We aggregated the studies into five general groups, according to the type of EDC studied: bisphenol A (BPA), organochlorine (OC) compounds, phthalates (PHTs), brominated compounds (BCs) and other EDCs. Studies assessing OC compounds were further subdivided into those investigating polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), chlorophenol pesticides and triclosan.
The methodological quality of each study was appraised, and sources of heterogeneity, including differences in exposure measurement (eg, categorical vs continuous, any adjustment) and clinical outcome (eg, type of anthropometric measure, categorical vs continuous) were identified. For studies with a similar data source, we included only the study with the largest sample size. Meta-analysis was performed when more than a single study per outcome had a similar design, exposure assessment and outcome measures so that we could have a meaningful pooled effect.
As heterogeneity was high among studies reporting continuous outcome data, only three different categorical outcomes were assessed: prevalent overweight, prevalent obesity and prevalent elevated WC. For each exposure (EDC) and outcome, adjusted OR with 95% CIs were extracted and pooled with random-effect model, as we expected some heterogeneity across the studies. Except for BC studies, we considered OR estimates from the highest versus lower EDCs levels. Because the association between exposure to some brominated metabolites and body mass measures in many studies showed an inverted U-shaped relationship, we collected OR estimates from intermediary categories of metabolite levels. Heterogeneity between study results was evaluated with χ2 test and quantified by I2 statistic (I²>75% considered as high heterogeneity).16 Possible causes of heterogeneity were explored with additional sensitivity analyses clustering the results by age (children vs adults) or by EDC metabolite/compound. Publication bias was assessed with a funnel plot and by using Egger’s regression test (with p<0.05 as an indication of the existence of publication bias). The metan package of STATA V.13.0 software was used for all meta-analysis.
Risk of bias across studies
Clinical heterogeneity of studies was considered by comparing the variability among the participant's characteristics, the assessment of exposure and outcomes. Methodological heterogeneity was assessed by comparing the variability in study design and risk of bias.
Patient and public involvement
No members of the public and patients were directly involved in this study.
Results
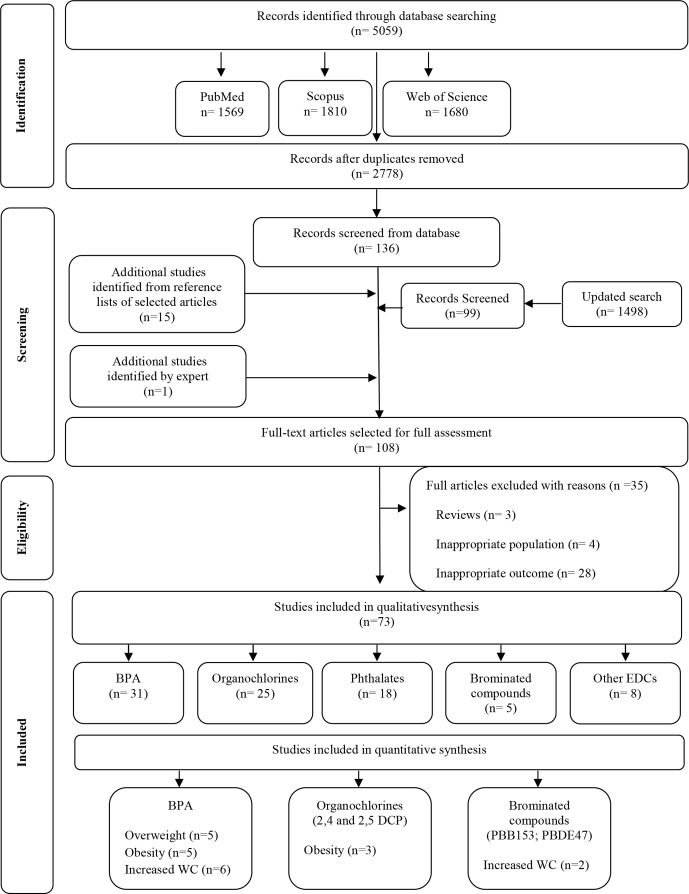
A total of 5059 articles were identified; 108 abstracts were selected for full assessment; and 73 studies met our inclusion criteria (figure 1). Thirty studies17–46 were conducted in the USA, 17 in Europe,47–63 22 in Asia,64–85 2 in Latin America,86 87 1 in Africa88 and 1 in Canada.89 In 72 studies, the anthropometric measures of obesity were assessed by trained health professionals, and in one study, weight and height were self-reported.77 The qualitative association between exposure to the different EDCs examined and obesity found in these studies is summarised in online supplementary figure 1.
Figure 1.
Flow diagram of literature search and selection criteria adapted from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (adapted from Moher et al14). BPA, bisphenol A; DCP, dichlorophenol; EDC, endocrine-disrupting chemical; PBB, polybrominated biphenyl; PBDE, polybrominated diphenyl ether; WC: waist circumference.
bmjopen-2019-033509supp003.pdf (323.9KB, pdf)
Bisphenol compounds
Thirty-one studies17–22 36–40 46–52 63–73 82 86 assessed the association between BPA exposure and obesity (table 1). Three studies37 39 71 additionally assessed other bisphenol compounds. Sixteen studies18–20 22 36 37 40 46 48 63 65–67 70 71 86 were conducted in children or adolescents, and all but 436 40 46 63 were exclusively cross-sectional. Ten studies18–20 22 46 48 63 65 66 86 reported a positive association between exposure to BPA and obesity. In a subgroup analysis based on gender and age, 3 studies65 66 86 indicated the association was significant for girls, and 2 of them for girls aged 8–11 years65 or 9–12 years.66 Moreover, one study22 assessed BF by dual-energy X-ray absorptiometry and found that urinary BPA levels were positively associated with elevated fat mass index in girls but were positively associated with lean body mass in boys. Six studies36 37 40 67 70 71 found no association between exposure to BPA and obesity.
Table 1.
Human studies addressing exposure to BPA and obesity (n=31)
| Authors, year | Country | Study design, quality | Study objective | Source population | Sex and age | Sample size | Sample, compounds (DR) and method | Outcomes | Adjustment for confounding factors | Main findings |
| Carwile et al, 201138 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA] and general and central obesity | General adult population, NHANES 2003–2006 | Female and male, 18–74 years | 2747 | Urine BPA (92%) HPLC-MS |
BMI, WC | Age, sex, race, education, smoking, urinary creatinine | Higher [urinary BPA] associated with higher BMI and WC |
| Shankar et al, 201217 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA] and obesity by gender and race/ethnicity | General adult population, NHANES 2003–2008 | Female and male, >20 years (mean 44.9±0.4 years) | 3967 | Urine BPA (NS) HPLC-GC-MS |
BMI, WC | Age, gender, race/ethnicity, education, smoking, alcohol intake, physical inactivity, diabetes, hypertension, TC | Positive association between [urinary BPA] and higher BMI and WC, independent of gender and race/ethnicity |
| Trasande et al, 201218 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA] and body mass outcomes | Children, NHANES 2003–2008 | Female and male, 6–19 years | 2338 | Urine BPA (96.5%) HPLC-MS |
BMI (sex-standardised and age-standardised z-score) | Age, sex, race/ethnicity, caregiver education, poverty to income ratio, serum cotinine levels, caloric intake, television watching, urinary creatinine | Significant association between [urinary BPA] and obesity |
| Wang et al, 201264 | China | Cross-sectional, 7 | To investigate the association between [urinary BPA] and obesity and insulin resistance | General adult population | >40 years | 3390 | Urine BPA (NS) HPLC-MS |
BMI, WC | Age, sex, education, smoking, urinary creatinine, alcohol drinking, systolic blood pressure, HDL-C, LDL-C, TC, TG, hs-CRP, fasting blood glucose and insulin, ALT, GGT | Higher [urinary BPA] associated with higher BMI and WC |
| Wang et al, 201265 | China | Cross-sectional, 6 | To investigate the association between [urinary BPA] and obesity | Primary and middle school children | Female and male, 8–15 years | 259 | Urine BPA (84.9%) HPLC-MS |
BMI (categories identified according to the Working Group on Obesity in China) | Age, sex, urine specific gravity | Higher [urinary BPA] associated with higher BMI, significant only for girls aged 8–11 years after stratification for age and sex |
| Bhandari et al, 201319 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA] and obesity | General paediatric population, NHANES 2003–2008 | Female and male, 6–18 years | 2664 | Urine BPA (NS) HPLC-MS/MS |
BMI, OB (BMI > p95) | Age, sex, race/ethnicity, parent/guardian education, urinary creatinine, serum cotinine, moderate physical activity | Higher [urinary BPA] associated with obesity |
| Eng et al, 201320 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA] and measures of adiposity and chronic disease risk factors | General paediatric population, NHANES 2003–2010 | Female and male, 6–18 years | 3370 (BMI), 2231 (WC), 3321 (WC-to-height), 775 (%BF) | Urine BPA (95.5%) HPLC-MS |
BMI categories (OW > p85, OB > p95), WC (> p75 or > p90), WC-to-height (> 0.5), %BF, DXA (> p85, age-adjusted and gender-adjusted) | Age, gender, race/ethnicity, urine creatinine, poverty-to-income ratio, serum cotinine, soda consumption | Higher [urinary BPA] associated with higher odds of obesity (BMI > p95) and abnormal WC-to-height ratio |
| Li et al, 201366 | China | Cross-sectional, 6 | To investigate the association between [urinary BPA] and overweight/obesity in school-age children | General population of children and adolescents (from a larger national study of pubertal development and health of adolescents) | Female and male, >9 years | 1326 | Urine BPA (NS) HPLC-fluorescence detection |
Weight (OW > p90), BMI, HC, WC, WC-to-height ratio, skinfold thickness | Age, gender, school grade, residence, paternal and maternal education and OW, playing video games, unbalanced diet, junk food consumption, vegetables or fruit consumption, depression scores, sports/activities | Higher [urinary BPA] associated with higher risk of overweight among girls aged 9–12 years, in a dose-dependent fashion |
| Harley et al, 201346 | USA | Cross-sectional, 7, and prospective, 8 | To investigate the association between [urinary BPA] anthropometric parameters and prevalent OW/OB in children | Subjects from the CHAMACOS cohort | Male and female, 9 years | 311 | Urine BPA (NS) HPLC-MS/MS |
BMI z-score, BMI categories (OW > p85, OB > p95), WC, BF% (bioimpedance) | Urine-specific gravity, maternal prepregnancy BMI, household income, maternal education level, maternal years of residence in the USA, child’s environmental tobacco smoke exposure, soda intake, fast food intake, and sweet consumption at ages 5 and 9 years | [Urinary BPA] at 9 years associated with increased BMI z-score, WC, BF% and prevalent OB/OW at 9 years; [urinary BPA] at 5 years not associated with anthropometric parameters or prevalent OB/OW at 5 or 9 years |
| Choi et al, 201467 | South Korea | Cross-sectional, 4 | To investigate the association between obesity and POPs | Subjects from a medical college in Seoul | Female, 6–14 years | 127 (58 controls, 69 obese) | Urine and serum BPA (NS) GC-MS |
BMI (OB > p85) | None | [Serum and urinary BPA] not associated with obesity |
| Ko et al, 201468 | South Korea | Cross-sectional, 7 | To investigate the association between [urine BPA] and WC | General adult population, from a previous study on integrated exposure to hazardous materials for safety control | Female and male, 44.3±14.6 years | 1030 | Urine BPA (NS) HPLC-MS |
BMI, WC (abdominal OB: > 90 cm for men and > 85 cm for women), %BF* | Age, sex, urinary creatinine (for all outcomes) Age, sex, urinary creatinine, education, income, alcohol consumption, smoking status (for abdominal obesity) | Higher [urinary BPA] associated with higher BMI, WC and BF |
| Ronn et al, 201447 | Sweden | Cross-sectional, 6 | To investigate the association between [serum BPA] and different indices of obesity | General elderly population | Female and male, 70 years | 890 (DXA) and 287 (MRI) | Serum total BPA (98%) Isotope liquid chromatography-MS |
Fat mass by DXA and MRI | Sex, height, lean mass, smoking, exercise habits, educational level, total daily energy intake, alcohol consumption | [Serum BPA] not associated with fat mass or fat distribution |
| Song et al, 201421 | USA | Cross-sectional, 6, and prospective, 8 | To investigate the association between [urinary BPA] and prospective weight change during 10-year follow-up | Adult female non-diabetic (control) population from NHS and NHSII | Female, 53–79 years | 977 | Urine BPA (NS) HPLC-MS |
BMI, weight change (kg) | Age, urinary creatinine, cohort origin, menopausal status, smoking, physical activity, alcohol consumption, AHEI and total energy intake | [Urinary BPA] not associated with baseline BMI Higher [urinary BPA] associated with modestly greater weight gain in a dose-dependent fashion |
| D’Aniello et al, 201548 | Italy | Cross-sectional, 4 | To investigate the association between sleep deprivation/fragmentation, fructose-rich diets and [urinary BPA] and obesity | Children from the teaching hospital and at the local health service outpatient obesity clinics and well-child visits in Salerno | Female and male, 5–16 years | 54 | Urine total (94.4%) and free BPA (90.7%) GC-MS |
BMI (normal p5-p85, OW p85-p95, OB > p95), WC, WC-to-height ratio, WC-to-hip ratio | Urinary creatinine | Higher total and free [urinary BPA] associated with increase in BMI, WC and WC-to-height ratio |
| Geens et al, 201549 | Belgium | Cross-sectional, 6 | To investigate the association between [urinary BPA] and anthropometric data | OW and obese adults from the Endorup trial (Antwerp University Hospital), lean controls from hospital staff and volunteers | Female and male, >18 years | 194 | Urine BPA (>99%) GC-MS |
BMI, WC | Age, gender, weight loss, urinary creatinine | Higher [ urinary BPA] in obese subjects |
| Lee et al, 201569 | South Korea | Cross-sectional, 7 | To investigate the association between [urinary BPA] and obesity | Participants of the Korean Elderly Environmental Panel study | Female and male, >60 years | 558 | Urine BPA (NS), average concentration from five samples collected at intervals from 6 to 12 months HPLC-MS |
BMI, OW (BMI > 25 kg/m2) | Age, sex, LDL-C, alcohol consumption, regular exercise, total calorie intake, fatty acid intake, urinary cotinine, diabetes | Higher [urinary BPA] significantly associated with OW in elderly women |
| Milic et al, 201550 | Serbia | Cross-sectional, 3 | To investigate the occurrence of BPA in morning spot urine and the association between [urinary BPA] and obesity | Residents in Novi Sad, Serbia | Female, 19–59 years | 145 | Urine BPA (29.3%–54.5%) GC-MS |
BMI | Urinary creatinine | [Urinary BPA] not associated with OW and OB |
| Sopon et al, 201570 | Thailand | Cross-sectional, 5 | To investigate exposure of children and adolescents to BPA and the association between [urinary BPA] and obesity | Children and adolescents from two schools in the Patumwan District of Bangkok | Female and male, 3.58–17.17 years | 376 | Urine BPA (75.3%) HPLC-MS |
BMI (OW: z-score > 1.036 or > p85 for age and sex; OB: z-score > 1.64 or > p95 for age and sex) | Urinary creatinine | BPA detection rate significantly higher in obese children, but there was no difference in BPA levels according to BMI category |
| Savastano et al, 201551 | Italy | Cross-sectional, 5 | To investigate the association between [plasma BPA] and visceral obesity | Adult non-diabetic and Caucasian male, enrolled by routine health survey at the ‘Frederico II’ University of Naples outpatient facility | Male, 53.5±5.7 years | 76 | Plasma BPA (NS) ELISA |
BMI and WC | Not stated | Increased [plasma BPA] correlated with increased WC |
| Xue et al, 201571 | India | Cross-sectional, 6 | To investigate the association between [urinary POPs] and obesity | Patients from the Endocrinology Outpatient Department of the Amrita Institute of Medical Sciences, Kochi, India | Male and female, 2–14 years | 103 (49 OW or obese and 27 normal-weight healthy controls) | Urine BADGE, BADGE.2H2O, TBAFs, BPA, BPS, total BPS (70%–99%) LC-MS |
BMI (OW: BMI > p85; OB: BMI > p95) | Age, sex, family income, parent education, physical activity, urinary creatinine | [Urinary bisphenol group compounds] not associated with obesity |
| Hoepner et al, 201640 | USA | Cross-sectional, 7, and prospective, 7 | To investigate the association between [urinary BPA] at 3 and 5 years, and BMI z-score, FMI, %BF, and WC at 5 and 7 years | Participants from the Columbia Center for Children’s Environmental Health New York City birth cohort | Male and female, 3 and 5 years | 408 | Urine BPA (98%) HPLC-MS/MS |
BMI z-score, %BF, FMI, WC | Maternal variables: prepregnancy maternal BMI, race/ethnicity, child variables: sex, birth weight, gestational age at birth, urinary SG, height, (urinary PHT levels) | [Urinary BPA] were not associated with BMI and WC cross-sectionally or prospectively |
| Vafeiadi et al, 201663 | Greece | Cross-sectional, 7, and prospective, 7 | To investigate the association between [urinary BPA] at 2.5 and 4.0 years and BMI, WC, skinfold thickness and prevalent obesity at 2.5 and 4.0 years | Subjects from the Rhea Mother-Child Study | Male and female, at 2.5 and 4.0 years | 500 | Urine BPA (98.8-99.6%) HPLC-EI-MS/MS |
BMI, WC, BMI z-score, WC; abdominal obesity (WC > p90), skinfold thickness | Maternal educational level, maternal age, prepregnancy BMI, working status during pregnancy, child sex, z score of birth weight for gestational age and breastfeeding status | [Urinary BPA] at 4 years positively associated with BMI z-score, WC, skinfold thickness and prevalence of obesity [Urinary BPA] at 2.5 years not associated with anthropometric measures at 2.5 years or prevalence of obesity at 4 years |
| Hong et al, 201772 | South Korea | Cross-sectional, 6 | To investigate the association between [urinary EDCs] and insulin resistance and obesity in healthy, reproductive-aged women | Subjects recruited using local advertisement at a community health and service centre and Ewha Womans University Mokdong Hospital outpatient clinic | Female, 30–49 years | 296 | Urine BPA (NS) HPLC-MS |
BMI, WC | Age, smoking, alcohol consumption, TG, TC, HDL-C, urinary creatinine | [Urinary BPA] positively associated with BMI and WC |
| Li et al, 201722 | USA | Cross-sectional, 6 | To investigate the association between [urinary BPA] and body composition | General adult population, NHANES 2003–2006 | Male and female, 8–19 years | 1860 | Urine BPA (NS) HPLC-MS |
BF% (DXA) | Age, ethnicity/race, height, caregiver's education, family income to poverty ratio, serum cotinine level, daily calorie intake, television/video watching, computer use, survey year, urinary creatinine | [Urinary BPA] positively associated with lean BMI in boys, and positively associated with elevated FMI in girls. Lower [urinary BPA] associated with lower percentage of trunk fat in girls |
| Milosevic et al, 201752 | Serbia | Cross-sectional, 3 | To investigate the association between [urinary BPA] and obesity and abdominal obesity among non-occupationally BPA-exposed women | Residents in the Autonomous Region of Vojvodina, Serbia | Female, 19–50 years | 103 | Urine BPA (35.9%) GC-MS |
BMI, WC, OW/obesity (BMI > 25), WHR, visceral adiposity index | Urinary creatinine | Detectable [urinary BPA] significantly associated with higher WC and WHR. Linear correlation between [urinary BPA] and BMI, WC and WHR among obese women |
| Hao et al, 201773 | China | Cohort, 8 | To investigate the association between [urinary BPA] and incident abdominal obesity | Residents in the Songnan Community, Baoshan District, Shangai, China, free from abdominal obesity at baseline | Male and female, >40 years | 888 | Urine BPA (NS) HPLC-MS |
WC (> 90 cm for men and > 80 cm for women, IDF criteria for Chinese adults) after 4 years | Age, sex, urinary creatinine, BMI, diabetes, smoking, alcohol consumption, education | [Urinary BPA] associated with increased risk of incident abdominal obesity after 4 years |
| Deierlein et al, 201736 | USA | Cohort, 9 | To investigate the association between [urinary EDCs] and changes in adiposity measurements after 8 years, in elementary school-aged girls | Subjects from the puberty cohort studies of the Breast Cancer and Environment Research Program | Female, 6–8 years | 1017 | Urine BPA (>80%) HPLC-MS | BMI, WC, BF% (bioelectrical impedance analysis) | Age, urinary creatinine, race/ethnicity, site of study, caregiver education, early puberty, baseline weight | [Urinary BPA] not associated with changes in adiposity measurements after 8 years |
| Kataria et al, 201737 | USA | Cross-sectional, 5 | To investigate the association between [urinary bisphenols and PHT] and body mass in children | Children from the General Pediatric Clinic at Bellevue Medical Center | Female and male, 10–13 years | 41 | Urine BPA, BPS, BPF (NS) HPLC-MS/MS |
BMI | Urinary creatinine, gender, age, caloric intake, physical activity | [Urinary bisphenols] not associated with BMI |
| Yang et al, 201786 | Mexico | Cross-sectional, 8 | To investigate the association between exposure to BPA and PHTs and obesity | Participants from the 22-year Early Life Exposure in Mexico to Environmental Toxicants cohort | Female and male, 8–14 years | 249 | Urine BPA (85%) LC-MS/MS |
WC, BF (skinfold thickness), BMI z-score | Urine-specific gravity, mother’s age, BMI, years of schooling and smoking status, child’s age and gender | [Urinary BPA] positively associated with skinfold thickness among girls but not boys |
| Liu et al, 201739 | USA | Cross-sectional, 7 | To investigate the association between [urinary BPA, BPF and BPS] and obesity | General adult population, NHANES 2013–2014 | Male and female, >20 years | 1521 | Urine BPA (94.94%), BPF (65.42%), BPS (90.6%) HPLC-MS/MS |
OB and OW defined by BMI, abdominal obesity defined by WC | Age, sex, urinary creatinine, race/ethnicity, education, family income, cigarette smoking, physical activity, total energy intake, BPA, BPF and BPS | [Urinary BPA] associated with general and abdominal obesity |
| Mouneimne et al, 201782 | Lebanon | Cross-sectional, 5 | To investigate the association between [urinary BPA] and metabolic disorders | Residents from the District of the Greater Beirut area, random selection | Male and female, >18 years | 501 | Urine BPA (89%) HPLC-MS | OB defined by BMI | Gender, education, age, smoking status, physical activity | [Urinary BPA] not associated with obesity |
*No description of %BF assessment.
AHEI, Alternative Healthy Eating Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BADGE, bisphenol A diglycidyl ether; BF, body fat; BMI, body mass index; BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; DR, detection rate; DXA, dual-energy X-ray absorptiometry; EDC, endocrine-disrupting chemical; ELISA, enzyme-linked immunosorbent assay; FMI, fat mass index; GC-MS, gas chromatography–mass spectrometry; GGT, gamma-glutamyl transferase; HA, hypothalamic amenorrhea; HC, hip circumference; HDL-C, high-density lipoprotein cholesterol; HPLC-EI-MS/MS, high performance liquid chromatography combined with electrospray ionisation and tandem mass spectrometry; HPLC-GC-MS, high-performance liquid chromatography–gas chromatography/mass spectrometry; HPLC-MS, high performance liquid chromatography-mass spectrometry; HPLC-MS/MS, high performance liquid chromatography-tandem mass spectrometry; hs-CRP, high-sensitivity C reactive protein; IDF, International Diabetes Federation; LC-MS, liquid chromatography-mass spectrometry; LC-MS/MS, isotope dilution-liquid chromatography-tandem mass spectrometry; LDL-C, low-density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey; NHS, Nurses’ Health Study; NHSII, Nurses' Health Study II; NS, not stated; OB, obesity; OW, overweight; PCOS, polycystic ovary syndrome; PHT, phthalate; POP, persistent organic pollutant; SG, specific gravity; TBAFs, tetrabutylamonium fluorides; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WHR, waist-to-hip ratio.
Synthesis of data from 3 cross-sectional studies including 5541 children20 22 46 indicated that BPA exposure was not significantly associated with prevalent overweight, and synthesis of data from 2 cross-sectional studies including 5230 children20 22 indicated that BPA exposure was also not significantly associated with increased WC (figure 2A and table 2).
Figure 2.
Association between exposure to bisphenol A, 2,4-dichlorophenol, 2,5-dichlorophenol and brominated compounds and anthropometric measures of obesity.
Table 2.
Pooled estimates for the association between BPA, DCP or BC exposure and prevalent OW, OB and abdominal OB defined by WC; random-effect models
| EDC | Outcome | Studies (n) | Pooled OR (95% CI) | Heterogeneity | Significance tests of ES=1 | ||||
| Tau2 | χ2 | Df | I2 | P value | |||||
| BPA | |||||||||
| Prevalent OW (subgrouped by age) | |||||||||
| Overall | 5 | 1.321 (1.012 to 1.724) | 0.0382 | 7.36 | 4 | 45.7% | 0.118 | z=2.05 (p=0.041) | |
| Children | 3 | 1.666 (0.821 to 3.382) | 0.2774 | 7.32 | 2 | 72.7% | 0.026 | z=1.41 (p=0.157) | |
| Adults | 2 | 1.254 (1.005 to 1.564) | 0.0000 | 0.04 | 1 | 0.0% | 0.845 | z=2.01 (p=0.045) | |
| Prevalent OB (adults only) | |||||||||
| Overall | 4 | 1.503 (1.273 to 1.774) | 0.0000 | 0.87 | 3 | 0.0% | 0.833 | z=4.81 (p=0.000) | |
| Prevalent increased WC (subgrouped by age) | |||||||||
| Overall | 6 | 1.494 (1.298 to 1.720) | 0.0011 | 5.17 | 5 | 3.3% | 0.395 | z=5.59 (p=0.000) | |
| Children | 2 | 1.623 (0.968 to 2.723) | 0.0434 | 1.29 | 1 | 22.7% | 0.256 | z=1.83 (p=0.067) | |
| Adults | 4 | 1.503 (1.267 to 1.783) | 0.0068 | 3.84 | 3 | 21.8% | 0.280 | z=4.68 (p=0.000) | |
| 2,4-DCP | |||||||||
| Prevalent OB | |||||||||
| Overall | 3 | 1.299 (0.860 to 1.961) | 0.0966 | 8.04 | 2 | 75.1% | 0.018 | z=1.24 (p=0.213) | |
| Children | 2 | 1.558 (0.702 to 3.458) | 0.2828 | 6.63 | 1 | 84.9% | 0.010 | z=1.09 (p=0.276) | |
| Adults | 1 | 1.030 (0.780 to 1.360) | 0.0000 | 0.00 | 0 | – | – | z=0.21 (p=0.835) | |
| 2,5-DCP | |||||||||
| Prevalent OB | |||||||||
| Overall | 3 | 1.629 (1.283 to 2.066) | 0.0102 | 2.56 | 2 | 21.8% | 0.278 | z=4.01 (p=0.000) | |
| Children | 2 | 1.800 (1.018 to 3.184) | 0.1103 | 2.55 | 1 | 60.8% | 0.110 | z=2.02 (p=0.043) | |
| Adults | 1 | 1.620 (1.210 to 2.169) | 0.0000 | 0.00 | 0 | – | – | z=3.24 (p=0.001) | |
| BC | |||||||||
| Prevalent elevated WC (subgrouped by BC compound) | |||||||||
| Overall | 4 | 1.576 (0.846 to 2.938) | 0.0778 | 3.71 | 3 | 19.1% | 0.295 | z=1.43 (p=0.152) | |
| PBB-153 | 1 | 3.500 (1.073 to 11.415) | 0.0000 | 0.00 | 0 | – | – | z=2.08 (p=0.038) | |
| PBDE-153 | 1 | 2.200 (0.581 to 8.329) | 0.0000 | 0.00 | 0 | – | – | z=1.16 (p=0.246) | |
| PBDE-47 | 2 (Wm; M) | 1.041 (0.508 to 2.132) | 0.0000 | 0.44 | 1 | 0.0% | 0.508 | z=0.11 (p=0.912) | |
BC, brominated compound; BPA, bisphenol A; DCP, dichlorophenol; df, degree of freedom; EDC, endocrine-disrupting chemical; ES, estimate effect; M, men; OB, obesity; OW, overweight; PBB, polybrominated biphenyl; PBDE, polybrominated diphenyl ether; WC, waist circumference; Wm, women.
Among 15 studies involving adult participants, 12 studies17 21 38 39 49 51 52 64 68 69 72 73 found a positive association between exposure to BPA and obesity. Two of these studies were prospective; one of them21 reported that higher urinary levels of BPA were modestly associated with greater weight gain in women, whereas the other73 indicated that BPA exposure was positively associated with incident abdominal obesity in men and women.
Synthesis of data from 2 cross-sectional studies including 3006 adults38 64 indicated that BPA exposure was significantly associated with prevalent overweight, with a summary OR of 1.25 (figure 2A and table 2). Synthesis of data from 4 cross-sectional studies including 6248 adults17 39 64 82 indicated that BPA exposure was significantly associated with prevalent obesity, with a summary OR of 1.50 (figure 2A and table 2). Moreover, synthesis of data from 4 cross-sectional studies including 6777 adults17 39 64 68 indicated a significant association between BPA exposure and increased WC, with a summary OR of 1.50 (figure 2A and table 2).
OC compounds
Twenty-five studies23–27 36 42–45 49 53–56 60–62 71 74 80 81 87–89 investigated the association between OC compounds and obesity (table 3). Most obtained data from population-based surveys or other epidemiological studies. Among 12 studies involving children and adolescents,23 25 26 36 42 44 53 56 62 71 74 81 6 reported positive association23 25 26 36 53 81; 4 reported no association42 56 71 74; and 4 reported negative association23 44 53 62 between exposure to specific OC compounds and obesity. Sixteen studies included adults; 11 reported positive association23 24 27 43 45 53–55 60 61 80; 4 reported no association49 87–89; and 7 reported negative association23 44 45 53–55 60 between OC compounds and measures of increased weight or adiposity. Three studies additionally indicated that the association was age26 45 53 or gender26 53 55 dependent. Of note, 5 studies24 36 55 56 74 had a prospective design. Two of them reported positive association between exposure to OCPs and prospective increases in BMI24 and WC55 in adults. One study involving children reported a positive association between exposure to OCPs and prospective changes in adiposity measures in girls aged 6–8 years,36 whereas 2 studies56 74 involving children found no association between exposure to OCPs or PCBs and prospective changes in BMI56 74 or WC.56
Table 3.
Human studies addressing exposure to OC compounds and obesity (n = 25)
| Authors, year | Country | Study design, quality | Study objective | Source population | Sex and age | Sample size | Sample, compounds (DR) and method | Outcomes | Adjustment for confounding factors | Main findings |
| Hue et al, 200789 | Canada | Cross-sectional, 5 | To investigate the association between [plasma OC compounds] and obesity | NS | Male and female, steady body weight, control 38.8±9.4 years (n=16), obese 38.6±7.6 years (n=19), morbidly obese 44.3±9.2 years (n=18) | 53 | Plasma 14 PCBs (28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187) (NS) 11 chlorinated pesticides (β-HCH, HCB, p, p’-DDE, trans-nonachlor, oxychlordane, cis-nonachlor, aldrin, α-chlordane, γ-chlordane) (NS) p, p’-DDT (7.5%) mirex (22.4%) GC-MS |
BMI | Age | [Total plasma OC compounds] not correlated with BMI |
| Dhooge et al, 201053 | Belgium | Cross-sectional, 6 | To investigate the association between exposure to pollutants and body size | Selection from a stratified clustered multistage design, as a random sample of adolescents and adults residing in the study area | Male and female, 14–15 years and 50–65 years | 1679 adolescents, 1583 adults | Serum PCB 118, 138, 153, 180, HCB, p, p-DDE, CALUX analysis of the dioxin fraction of dioxin-like activity in plasma (NS) GC-ECD |
BMI | Adolescents: blood lipids, age, height of father and mother, smoking, sexual maturation (Tanner), food intake. Adults: blood lipids, age, smoking, food intake |
[Serum HCB, sum PCB 118, 153, 180] negatively associated with BMI, and [PCB118] positively associated with BMI in adolescents [Serum sum PCB 138, 153, 180] negatively associated with BMI, and [serum HCB, p, p’-DDE and PCB118, dioxin fraction] positively associated with BMI in adult men [Sum PCB138, 153, 180] negatively associated with BMI, and [HCB, p, p’-DDE and PCB118] positively associated with BMI in adult women |
| Elobeid et al, 201023 | USA | Cross-sectional, 5 | To investigate the association between [serum OC compounds] and BMI/WC | General population, NHANES 1999–2002 | Male and female, 6 years to > 40 years | 2464 | Serum HpCDD, OcDD, oxychlordane, trans-nonachlor, p, p’-DDT (NS) GC-MS |
BMI, WC | Serum TC and TG | [Serum p, p’-DDT] positively associated with WC in all subjects [Serum oxychlordane and HpCDD] positively associated with WC in subjects with detectable levels of these compounds [Serum OcDD] increased with higher WC and BMI [Serum trans-nonachlor] decreased with higher BMI |
| Dirinck et al, 201154 | Belgium | Cross-sectional, 4 | To investigate the association between [serum OC compounds] and BMI, WC, fat mass and HOMA-IR | Outpatients from the weight management clinic of the Department of Endocrinology, Diabetology and Metabolism of the Antwerp University Hospital (obese); hospital staff and volunteers (normal-weight controls) | Male and female, 21–60 years (median 40 years) | 144 | Serum PCB (153, 138, 180, 170, sum PCB), pp-DDE, b-HCH (NS) GC-MS |
BMI, WC, FM (total abdominal, visceral abdominal, subcutaneous abdominal), FM% | None | [Serum PCB 153, 180, 180, sum PCB] negatively correlated with BMI, WC, FM%, total and subcutaneous abdominal adipose tissue [Serum b-HCH] positively correlated with BMI, WC, FM%, and total and subcutaneous abdominal adipose tissue |
| Lee et al, 201124 | USA | Cohort, 7 | To investigate the association between [serum OC compounds] and adiposity, dyslipidaemia, and insulin resistance over 18 years | Non-diabetic controls from the Coronary Artery Risk Development in Young Adults study | Male and female, 18–30 years at baseline (27.2±3.3 years) | 90 | Serum 9°C pesticides (44%–100%), 35 PCB congeners (7%–100%) GC/ID-HRMS |
BMI | Age, sex, race, TG, TC, HDL-C, HOMA-IR, baseline BMI | [Serum p, p’-DDE] and PCBs with > 7 chlorines predicted higher BMI after 18 years (inverted U-shaped curve across quartiles) |
| Twum et al, 201125 | USA | Cross-sectional, 6 | To investigate the association between [urinary OC compounds] and obesity | General population, NHANES 2003–2004, NHANES 2005–2006 | Male and female, 6–19 years | 6770 | Urine 2,4-DCP (92%), 2,5-DCP (99%), ortho-phenylphenol (<40%) HPLC-MS |
BMI, obesity (BMI > p95 for sex and age) | Age, gender, race, income, total fat intake | [Urinary 2,5-DCP] associated with childhood obesity |
| Lee et al, 201255 | Sweden | Cross-sectional, 6, and prospective, 7 | To investigate the association between [plasma POPs] and prevalent abdominal obesity, cross-sectionally and prospectively | Participants from the Prospective Investigation of the Vasculature in Uppsala Seniors | Male and female, 70 years (at baseline) | 970 (cross-sectional) 511 (prospective) |
Plasma 17 PCB (98.7%–100%) 5°C pesticides (p, p’-DDE, trans-nonachlor, HCB, chlordane, cis-chlordane(3.4–100%)) HRGC-HRMS |
WC, abdominal obesity (WC > 102 cm for men and > 88 cm for women) | Total calorie intake, exercise, smoking, alcohol consumption, TG, TC | [Plasma less chlorinated PCBs, p, p’-DDE and dioxin] associated with abdominal obesity (inverted U-shape relation, particularly in women) [Plasma highly chlorinated PCB] inversely associated with abdominal obesity Similar but weaker associations between [plasma POPs] and development of abdominal obesity after 5 years |
| Arrebola et al, 201287 | Bolivia | Cross-sectional, 3 | To describe [serum and adipose tissue OC compounds] in an urban adult population from Bolivia and its association with demographic characteristics | Subjects undergoing non-cancer-related surgery at a general hospital in Santa Cruz de la Sierra | Male and female, >16 years (31.4±12.6 years) | 112 | Serum and adipose tissue p, p’-DDT (50%), p, p’-DDE (93%), HCB (21%), PCB congeners 138,153,180 (56%–80%) GC-ECD |
BMI | None | [Serum and adipose tissue OC compounds] not correlated with BMI |
| Ben et al, 201388 | Tunisia | Cross-sectional, 4 | To describe [serum OC compounds] in the general population of Bizerte, Tunisia, and investigate its association with age, gender and BMI | Subjects visiting the Regional Hospital of Bizerte, in Tunisia | Male and female, >18 years, not pregnant and without critical or heart disease | 113 | Serum HCB, p, p’-DDE, PCB 153, PCB 180 (100%) Dieldrin, heptachlor, PCB 18, 28, 31, 52, 44 (0%) b-HCH, lindane, p, p’-DDD, p, p’-DDT, PCB congeners 101, 149, 118, 138, 194 (1.7%–95.6%) GC-MS |
BMI | Serum lipids | [Serum OCPs and PCB congeners 153, 138, 180 and sum PCB] not associated with BMI |
| Lankester et al., 201343 | USA | Cross-sectional, 7 | To investigate the association between [urinary TCS] and BMI | General population, NHANES 2003–2003 | Male and female, >20 years | 4037 | Urine TCS (75%) HPLC-MS/MS |
BMI | Survey year, sex, age, race, poverty index ratio, urinary BPA, urinary cotinine | [Urinary TCS] positively associated with increased BMI |
| Roos et al, 201360 | Sweden | Cross-sectional, 6 | To investigate the association between [plasma OC compounds] and abdominal obesity | Subjects aged 70 years randomly chosen from the register of community living from Uppsala, Sweden | Male and female, 70 years | 1016 | Plasma 16 PCBs, p, p’-DDE, HCB, TNC (>95.5%); OcDD (80.6%); cis-chlordane, trans-chlordane (<10%) HRGC-HRMS |
BMI, VAT/SAT ratio (determined by MRI) | Gender, education, exercise habits, smoking | [Plasma less chlorinated PCBs, p, p’-DDE, HCB, TNC] positively associated with both VAT and SAT [Plasma highly chlorinated PCBs] inversely related to both VAT and SAT [Plasma PCB189] correlated with VAT/SAT ratio in an inverted U-shaped manner |
| Buser et al, 201426 | USA | Cross-sectional, 7 | To investigate the association between [urinary POP] and BMI z-score, WC and obesity | General adult population, NHANES 2007–2008 and 2009–2010 | Male and female, 6–19 years (mean 12.56±0.1 years) | 1298 | Urine 2,5-DCP (98.5%), 2,4-DCP (90%), TCS (79%) HRGC-HRMS |
BMI z-score, WC, overweight (BMI p85–p95), obesity (BMI > p95) | Age, sex, race/ethnicity, calorie intake, television and video game and computer usage (6–11 years), physical activity (12–19 years), serum cotinine, poverty income ratio, urinary creatinine | [Urinary 2,4-DCP, 2,5-DCP] positively associated with BMI z-score, WC and obesity. After stratification for age, the associations remained significant only in adolescents. |
| Wei et al, 201427 | USA | Cross-sectional, 7 | To investigate the association between [urinary POP] and obesity | General adult population, NHANES 2005–2006, NHANES 2007–2008 | Male and female, 20–85 years | 2931 | Urine 2,4-DCP (92.6%), 2,5-DCP (99%) HPLC-MS |
BMI, obesity (BMI > 30 kg/m2), non-obese (< 30 kg/m2) | Age, gender, race, income, education, total fat intake, physical activity, urinary creatinine | [Urinary 2,5-DCP] positively associated with obesity |
| Li et al, 201544 | USA | Cross-sectional, 6 | To investigate the association between [urinary TCS] and obesity traits | General adult population, NHANES 2003–2010 | Female and male, children (6–19 years) and adults (>20 years) | 2898 children 2066 adults |
Urine TCS (77%–79%) ID-HPLC-MS/MS |
BMI and WC | Race/ethnicity, socioeconomic status, serum cotinine, (urinary BPA) | [Urinary TCS] inversely associated with BMI and WC in children and adults |
| Zong et al, 201545 | USA | Cross-sectional, 7 | To investigate the association between [serum OC compounds] and body fat | General adult population, NHAES 1999–2004 | Female and male, >20 years | 2358 | Serum p, p’-DDE, p, p’-DDT, b-HCH, HxCDD, OcDD, HpCDF, PCB (126, 138, 153, 169, 170, 180, 187, 194, 196,199) (30%–69%) HRGC-HRMS |
FM% (DXA) | Serum lipids, gender, age, ethnicity, education, physical activity, smoking status, alcohol consumption, history of parity and lactation | [Serum b-HCH, HpCDF, OcDD, PCB126] positively associated with trunk FM% (correlations stronger in subjects >40 years); [serum PCB 138, 153, 169, 170, 180, 187, 194, 196] inversely correlated with FM% |
| Tang-Péronard et al, 201556 | Denmark | Cohort, 7 | To investigate the association between [serum POP] at 8–10 years of age and changes in measures of obesity at 14–16 years and 20–22 years | Children form the European Youth Heart Study, Danish component | Male and female, 8-10 years at baseline | 392 | Serum PCB sum (PCB 138, 153, 180), p, p’-DDE, HCB (NS) GC |
BMI z-score, WC, %BF | Baseline obesity, breast feeding, maternal educational level, maternal smoking, maternal BMI, pubertal status, physical fitness (maximal work test), dietary intake | [Serum POP] not associated with subsequent changes in measures of obesity |
| Geens et al, 201549 | Belgium | Cross-sectional, 6 | To investigate the association between [urinary TCS] and anthropometric data and serum thyroid hormones, to evaluate the dynamics of [urinary TCS] during 1 year of weight loss, to estimate daily TCS intake and investigate daily intake differences during weight loss and to evaluate variations in exposure sources according to treatment method for weight loss (bariatric surgery/diet) | OW and obese adults from the Endorup trial (Antwerp University Hospital); lean controls from hospital staff and volunteers | Female and male, >18 years | 194 | Urine TCS (>90%) HPLC-MS |
BMI, WC | Age, gender, weight loss, urinary creatinine | No difference between [urinary TCS] in obese and lean subjects at baseline No significant change of [urinary TCS] during weight loss |
| Xue et al, 201571 | India | Cross-sectional, 6 | To investigate the association between [urinary POPs] and obesity | Endocrinology Outpatient Department of the Amrita Institute of Medical Sciences, Kochi, India | Male and female, 2–14 years | 103 (49 OW or obese and 27 normal-weight healthy controls) | Urine TCS (100%) LC-MS |
BMI (OW defined by BMI > p85 and obesity defined by BMI > p95) | Age, sex, family income, parent education, physical activity, urinary creatinine | (Urinary TCS) not associated with obesity |
| Lee et al, 201674 | South Korea | Cohort, 8 | To investigate the association between [serum OC compounds] and prospective change of metabolic components of metabolic syndrome | Subjects from the Ewha Birth & Growth Cohort study | Female and male, 7–9 years | 214 (158 completed follow-up) | Serum PCB (52, 101, 118, 138, 153, 156, 180), marker PCB (sum 28, 52, 101, 138, 153, 180), dioxin-like PCB (sum 77, 81, 114, 105, 126, 123, 156, 157, 169, 167, 189), nonachlor, HCB, b-HCH, p, p’-DDT, p, p’-DDE) (61.68%–99.53%) PCB (1, 3, 4, 15, 19, 28, 37, 77, 81, 104, 105, 114, 123, 126, 155, 157, 167, 169, 188, 189, 202, 205, 206, 208), oxychlordane, chlordane, heptachlor, heptachlor epoxide, a-HCH, g-HCH, d-HCH, o, p’-DDT, p, p’-DDD, o, p’-DDD, o, p’-DDE (NS) GC-MS |
BMI, BMI z-score | Gender, age, monthly household income, baseline BMI, serum lipids | No association between (serum OC compounds) and change in BMI after 1 year |
| Deierlein et al, 201736 | USA | Cohort, 9 | To investigate the association between [urinary EDCs] and changes in adiposity measurements after 8 years, in elementary-school-aged girls | Subjects from the puberty cohort studies of the Breast Cancer and Environment Research Programme | Female, 6–8 years | 1017 | Urine 2,5-DCP (>80%) TCS (>80%) HPLC-MS |
BMI, WC, BF% (bioelectrical impedance analysis) | Age, urinary creatinine, race/ethnicity, site of study, caregiver education, early puberty, baseline weight | (Urinary 2,5-DCP and TCS) associated with increase in adiposity measurements after 8 years |
| Harmouche et al, 201780 | Lebanon | Cross-sectional, 6 | To investigate serum levels of six indicator PCBs and differences in PCBs levels by gender, age and BMI | Students and employees of Saint Joseph University | Female and male, 17–65 years | 316 | Serum PCB 28, 52, 101, 138, 153, 180 (50%–60%) GC-ECD |
BMI, BF% (bioelectrical impedance analyser) | Total serum lipids, age, gender, smoking status, dairy product, fish and shellfish consumption | (Serum sum PCB) associated with OW and OB in and inverted-U shaped manner |
| Henriquez-Hernandez et al, 201761 | Spain | Cross-sectional, 4 | To investigate the association between exposure to POPs and OB and type two diabetes | Subjects from the Canary Islands Nutrition Survey | Female and male, >18 years | 429 | Serum p, p′-DDT (<50%), DDE (<50%), DDD (<50%), p, p′-DDE (85.8%), p, p′-DDD (<50%), aldrin (64.1%), dieldrin (<50%), endrin (68.3%), HCHα (88.1%), HCHβ (<50%), HCHδ (<50%), HCHγ (55.7%) PCBs 153 (77.2%), 180 (85.1%), 28, 52, 77, 81, 101, 105, 114, 118, 123, 126, 138, 153, 156, 157, 167, 169, 180, 189 (<50%) GC-ECD (OCPs), GC-MS (PCBs) |
BMI, waist-to-hip ratio | None | (Serum p, p’-DDE) higher among OW and OB subjects |
| Karlsen et al, 201762 | Denmark | Cross-sectional, 6 | To investigate the association between [POPs] and obesity | Subjects from the National Hospital of the Farol Islands | Female and male, 5 years | 349 | Serum sum PCB 138, 153, 180 (100%), HCB (100%), p, p’DDE (100%) GC-ECD |
BMI z-score, OW (> p85) | Serum lipids, maternal nationality, age at delivery, prepregnancy BMI, smoking during pregnancy, child’s gender, exclusive breastfeeding duration, child’s fish intake at age 5 years | (Serum OC compounds) inversely associated with BMI z-score |
| Parastar et al, 201781 | Iran | Cross-sectional, 2 | To investigate the association between [urinary pesticides] and obesity in children and adolescents | Selection from households in different areas of Isfahan, Iran | Male and female, 6–18 years | 242 | Urine 2,4-DCP (94.6%), 2,5-DCP (95%), 2,4,5-TCP (85.1%), 2,4,6-TCP (38%) GC-MS |
BMI, BMI z-score, WC | Urinary creatinine, physical activity, fasting blood sugar, blood pressure, TC, HDL-C, LDL-C | [Urinary 2,5-DCP] positively associated with BMI z-score and WC; [urinary 2,4,5-TCP] positively associated with WC; [urinary 2,5-DCP] associated with obesity |
| Kalloo et al, 201842 | USA | Cross-sectional, 8, and prospective, 8 | To investigate the association between [urinary TCS] and adiposity in children | Participants from the Health Outcomes and Measures of the Environment Study, Cincinnati | Male and female, <8 years | 218 | Urine TCS (NS) HPLC-MS/MS |
BMI, WC, %BF | Maternal variables: race, age, education, marital status, household income, age at delivery, BMI, prenatal vitamin use, delivery method, breast feeding, parity, gestational diabetes, hypertensive disorders, urinary cotinine Child variables: age, screen time, diet, physical activity |
No association between [urinary TCS] at the ages of 1–5 and 8 and measures of adiposity at the age of 8 years |
AT, adipose tissue; BF, body fat; BMI, body mass index; BPA, bisphenol A; CALUX, chemical activated luciferase gene expression; DCP, dichlorophenol; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DR, detection rate; DXA, dual-energy X-ray absorptiometry; EDC, endocrine disrupting chemical; FM, fat mass; GC-ECD, gas chromatography–electron capture detector; GC-ID/HMRS, gas chromatography–isotope dilution/high-resolution mass spectrometer; GC-MS, gas chromatography–mass spectrometry; HCB, hexachlorobenzene; HCH, hexachlorohexane; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment-insulin resistance; HpCDD, heptachlorodibenzo-p-dioxin; HpCDF, heptachlorodibenzofuran; HPLC-MS, high-performance liquid chromatography–mass spectrometry; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; HRGC-HRMS, high-resolution gas chromatography–high-resolution mass spectrometry; HxCDD, hexachlorodibenzo-p-dioxin; HxCDF, hexachlorodibenzofuran; ID-HPLC-MS/MS, isotope dilution-high-performance liquid chromatography-tandem mass spectrometry; LC-MS, liquid chromatography-mass spectrometry; LDL-C, low density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey; NS, not stated; OB, obesity; OC, organochlorine; OcDD, octachlorodibenzo-p-dioxin; OCP, organochlorine pesticide; OW, overweight; PCB, polychlorinated biphenyl; PeCDF, pentachlorodibenzofuran; POPs, persistent organic pollutant; SAT, subcutaneous adipose tissue; TC, total cholesterol; TCP, trichlorophenol; TCS, triclosan; TG, triglyceride; TNC, transnonachlordane; VAT, visceral adipose tissue; W, weight; WC, waist circumference.
The individual OC compounds that were examined varied among the studies, and most assessed more than one compound. However, the association between specific OC compounds and obesity in children and adults was overall inconclusive. Pooled data from 2 studies assessing exposure to 2,4-dichlorophenol (DCP) in childhood25 26 and one in adults27 indicated no association with obesity (table 2). Data from 2 studies assessing exposure to 2,5-DCP in childhood25 26 indicated a significant association with obesity (figure 2B and table 2).
Phthalates
Eighteen studies21 28–31 37 41 57–59 67 72 75 76 83–86 examined the association between exposure to PHTs and obesity (table 4). Seven studies30 37 41 67 75 76 86 were conducted in children, 10 in adults21 28 31 57–59 72 83–85 and one in both children and adults.29 An overall positive association between exposure to PHTs and measures of excess weight or adiposity was found; only 4 studies reported inverse associations,29 31 59 86 and 2 reported no association.
Table 4.
Human studies addressing exposure to PHTs and obesity (N=18)72 83
| Authors, year | Country | Study design, quality | Study objective | Source population | Sex and age | Sample size | Sample, compounds (DR) and method | Outcomes | Adjustment for confounding factors | Main findings |
| Stahlhut et al, 200728 | USA | Cross-sectional, 4 | To investigate the association between [urinary PHTs] and abdominal obesity and insulin resistance | General population, NHANES 1999–2002 | Male, >18 years | 1443 | Urine PHT metabolites MBP, MEP, MBzP, MEHHP, MEOHP, MEHP (>80%), MiNP (25%) HPLC-MS |
WC | Age, race/ethnicity, family history of diabetes, dietary fat and caloric intake, physical activity, income, renal function, hepatic function, exposure to tobacco | MBzP, MEHHP, MEOHP and MEP positively associated with increased WC |
| Hatch et al, 200829 | USA | Cross-sectional, 5 | To investigate the association between [urinary PHT metabolites] and BMI and WC | General population, NHANES 1999–2002 | Male and female, 6–80 years | 4836 | Urine MEP, MEHP, MBzP, MBP, MEHHP, MEOHP (>80%), MCP, MNP, MOP (≤80%) HPLC-MS |
BMI, WC | Age, gender, urinary creatinine, height, diet variables, physical activity, race/ethnicity, education, family income, education level, smoking, alcohol consumption, menopausal status, parity, TV/video/computer use | Urinary MBzP] positively associated with BMI and WC in men aged 20–59 years [Urinary MEP] positively associated with BMI and WC in adolescent girls [Urinary MEHP] inversely associated with BMI in adolescent girls and women aged 20–59 years |
| Lind et al, 201257 | Sweden | Cohort, 7 | To investigate the association between [serum PHT monoester] and measures of adiposity after 2 years | Subjects from the Prospective Investigation of the Vasculature in Uppsala Seniors | Male and female, 70 years | 1016 | Serum MEHP, MEP, MiBP, MMP (>96%) LC-MS/MS |
Fat mass (DXA and MRI) | Serum TC and TG, education, exercise, smoking | [Serum MiBP] positively associated WC, total FM, trunk FM, SAT after 2 years in women [MMP] positively associated with trunk fat mass and trunk:leg ratio after 2 years in women |
| Teitelbaum et al, 201230 | USA | Cohort, 7 | To investigate the association between [urinary PHT metabolites] and BMI and WC in children | Children from the Growing Up Healthy prospective cohort study | Male and female, 6–8 years | 387 | Urine MEP, MBP, MCPP, MBzP, MiBP, MEOHP, MECPP, MEHHP (>97%), MEHP (81%–90%) HPLC-MS |
BMI, BMI z-score, WC after 1 year of PHT exposure measurement | Age at baseline, sex, hours of sedentary activity, day of week for reported sedentary activity, MET hours, total caloric intake, race, ethnicity, family income, parental education | [Urinary MEP] and [urinary sum of low molecular-weight PHTs] positively associated with BMI and WC in overweight girls after 1 year |
| Wang et al, 201375 | China | Cross-sectional, 6 | To investigate the association between [urinary PHT metabolites] and BMI and WC in school children | Obese, OW and normal weight (20:10:30) children selected from primary and middle schools in Shangai, China | Male and female, 8–15 years | 259 | Urine MEHP, MEOHP, MECPP, MEHHP, MBP, MiBP, MEP, MCMHP, MHBP, MMP, MCHP (≥94.6%) MBzP (38.6%) MiNP, MOP (0%) Sum MBP, MHBP, MiBP, MMP, MEP Sum MEHP, MECPP, MEHHP, MEOHP, MCMHP, MCHP, MBzP, RPUPLC-ESI-MS/MS |
BMI, WC, normal weight, OW and OB defined according to age and sex-specific criterion (Working Group on Obesity in China) | Age, sex, urine PHT metabolites, urine specific gravity | MEHP and MEP positively associated with BMI and WC |
| Choi et al, 201467 | South Korea | Cross-sectional, 4 | To investigate the association between exposure to POPs and obesity | Participants recruited among subjects from a medical college in Seoul | Female, 6–14 years | 127 (58 controls, 69 obese) | Urine and serum MEP, DBP, DEHP, MEHP, PA, MBzP (NS) GC-MS |
BMI (OB defined by BMI > p85) | None | MEP, DBP and PA positively associated with obesity |
| Song et al, 201421 | USA | Cross-sectional, 6, and cohort, 8 | To investigate the association between [urinary PHT metabolites] and weight change after 10 years | Adult female non-diabetic (control) population from NHS and NHSII | Female, 53–79 years | 977 | Urine PA, MEP, MBzP, Sum of butyl PHTs, DEHP metabolites, total PHTs (NS) HPLC-MS |
BMI, weight change (kg) | Urinary creatinine, cohort origin, age, menopausal status, smoking, physical activity, alcohol consumption, AHEI and total energy intake | Higher [PHT metabolites] associated with modestly greater weight gain in a dose-dependent fashion |
| Hou et al, 201576 | Taiwan | Cross-sectional, 8 | To investigate the association between [urinary PHTs] and obesity and pubertal maturity among adolescents | Children and adolescents selected from primary schools in Tapei, Taiwan | Male and female, 6.5–15 years | 270 | Urine DEHP metabolites (MEHP (78.1%), MEOHP, MEHHP, MECPP (≥99.6%)) LMW PHT metabolites (MMP, MEP, MiBP, MnBP (≥94.8%)) MBzP (94.4%) HMW PHT (DEHP metabolites, MBzP (NS)) UPLC-MS/MS |
BMI, WC, WHR, skin fold thickness, OB defined by BMI (criteria from Taiwan’s Health Promotion Administration and by the Ministry of Health and Welfare) | Age, gender, urinary creatinine | [Urinary PHT metabolites] positively associated with abdominal obesity (assessed by skinfold thickness, WC and WHR), in a dose–response manner |
| Medic et al, 201558 | Serbia | Cross-sectional, 5 | To investigate the association between [urinary DHEP and DEP] and BMI, WC, plasma lipids and lipoproteins | Volunteers randomly recruited during physical examinations at the Institute of Occupational Medicine of Novi Sad, Serbia | Female, 18–55 years | 103 | Urine MEP (24.3%) and MEHP (16.5%) GC-MS |
BMI, WC | None | [Urinary MEHP] positively associated with WC |
| Petrovicova et al, 201659 | Slovakia | Cross-sectional, 6 | To investigate the association between [urinary PHTs], occupation, consumer practices and body composition | Occupationally exposed subjects and non-occupationally exposed from the general population of the Nitra Region in Slovakia | Female and male, >18 years | 129 (45 occupationally exposed subjects, 35 workers from plastic industry, 49 from the general population) | Urine MEHHP, MEOHP, MEHP, MiBP, MnBP (≥82.2%) Sum DEHP HPLC-MS |
BMI, WC, FMI, FFMI, HC, WHR, WHtR, WC > 102 cm (male) or > 88 cm (female) | Gender, occupational exposure | [Urinary MEHP] inversely related to WHtR, BMI, WHR, HC and WC, and positively related to FFMI among women but not men |
| Yaghjyan et al, 201631 | USA | Cross-sectional, 6 | To investigate the association between [urinary PHTs] and individual characteristics, including BMI | General population, NHANES 2001–2012 | Female and male, > 18 years (non-obese, non-pregnant, and non-diabetic) | 6653 | Urine DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP), %MEHP (ratio of MEHP to sum of secondary metabolites) (NS) HPLC-MS |
BMI, OW (BMI 25.0–29.9) | Age, gender, race, smoking, alcohol use, cancer history, daily caffeine consumption, prescription medication, menopausal status, postmenopausal hormone use | [Urinary MEHP:MEHHP] and [urinary %MEHP] inversely associated with the presence of overweight |
| Hong et al, 201772 | Korea | Cross-sectional, 6 | To investigate the association between exposure to EDCs and insulin resistance and obesity in healthy, reproductive-aged women | Women recruited from the community health and service centre and Ewha Womans University Mokdong Outpatient Clinic | Female, 30–49 years | 296 | Urine MEHHP, MEOHP, MnBP (NS) HPLC-MS |
BMI, WC | Age, urinary creatinine, smoking and alcohol status, TG, TC, HDL-C | [Urinary PHTs] not associated with BMI and WC |
| Kataria et al, 201737 | USA | Cross-sectional, 5 | To investigate the association between [urinary bisphenols and PHTs] and body mass in children | Children from the General Paediatric Clinic at Bellevue Medical Centre | Female and male, 10–13 years | 41 | Urine MMP, MEP, MBP, MiBP, MBzP, MCHP, MOP, MCPP, MIDP, MCNP, MNP, MIDP, MCOP, MEHP, MECPP, MEHHP, MEOHP, MCMHP, MHxP, MHpP (NS) HPLC-MS/MS |
BMI | Urinary creatinine, gender, age, caloric intake, physical activity | [Urinary high molecular weight PHT metabolites] positively associated with BMI |
| Yang et al, 201786 | Mexico | Cross-sectional, 8 | To investigate the association between exposure to BPA and PHTs and obesity | Participants from the 22 year Early Life Exposure in Mexico to Environmental Toxicants cohort | Female and male, 8–14 years | 249 | Urine MEP, MBP, MCPP, MiBP, MBzP, MEHP, MEHHP, MEOHP, MECPP (94%–100%) LC-MS/MS |
WC, BF (skinfold thickness), BMI z-score | Urine-specific gravity, mother’s age, BMI, years of schooling and smoking status, child’s age and gender | [Urinary MEHP] positively associated with WC and skinfold thickness, [urinary MEH] inversely associated with skinfold thickness among boys |
| Oktar et al, 201784 | Turkey | Cross-sectional, 1 | To investigate the association between [serum and urinary PHTs] and obesity | Patients from the research hospital of Mustafa Kemal University | Male and female, 17–62 years | 196 | Serum and urine DMP, DEP, DBP, DPP, BBP, DEHP, DOP, GC |
BMI, WC | None | [Urinary and serum PHTs] positively associated with BMI and WC |
| Dong et al, 201785 | China | Cross-sectional, 5 | To investigate the association between [urinary PHT metabolites] and obesity | Participants from the Shangai Food Consumption Survey 2012 | Male and female, >18 years | 2330 | Urine MMP, MEP, MnBP, MiBP, MBzP, MEHP, MEOHP, MEHHP, MECPP, MCMHP LC-MS/MS |
OB and OW defined by BMI, abdominal obesity (> 85 cm for men and > 80 cm for women) | OB: age, gender, education, marriage, smoking, total caloric intake calories, and total fat intake Abdominal obesity: age, marriage, education, smoking status, BMI, total caloric intake, and total fat intake. |
[Urinary MMP, MEHHP, MECPP] associated with abdominal obesity; the association was stronger among young females |
| Lee et al, 201783 | South Korea | Cross-sectional, 6 | To investigate the association between [urinary PHTs] and demographic characteristics | Subjects randomly recruited from the population of the Korean National Human Biomonitoring Survey | Male and female, 18–69 years | 1870 | Urine MnBP, MiBP, MBzP, MCHP, MnOP, MEHP, MEOHP, MEHHP, MiNP, MiDP HPLC-MS |
OB and OW defined by BMI | Urinary creatinine | [Urinary PHT metabolites] not associated with OB or OW |
| Shoaff et al, 201741 | USA | Cohort, 8 | To investigate the association between [urinary PHTs] and measures of adiposity in children | Participants from the Health Outcomes and Measures of the Environment | Male and female, 1 years | 219 | Urine MEP, MnBP, MiBP, MCPP, MBzP, MEHP, MEHHP, MEOHP, MECPP SumDEHP HPLC-MS/MS Measurements conducted six times, from 1 to 8 years |
BMI, WC, %BF at the age of 8 years | Urinary creatinine, maternal age at delivery, race, marital status, insurance, income, education, parity, cotinine, depressive symptoms, midpregnancy BMI, food security, prenatal fruit/vegetable and fish consumption, prenatal vitamin use, child sex, and child age at the 8-year visit | [Urinary MBzP] inversely associated with adiposity; [urinary sum DEHP] at 1 and 5 years associated with decrease and increase in adiposity at 8 years, respectively; [urinary MEP] at 5 and 8 years associated with higher adiposity at 8 years |
AHEI, Alternative Healthy Eating Index; BBP, benzyl butyl phthalate; BF, body fat; BMI, body mass index; BPA, bisphenol A; DBP, dibutyl phthalate; DEHP, diethylhexyl-phthalate; DEP, diethyl phthalate; DMP, dimethyl phthalate; DOP, dioctyl phthalate; DPP, dipentyl phthalate; DR, detection rate; DXA, dual-energy X-ray absorptiometry; EDC, endocrine disrupting chemical; FFMI, fat-free mass index; FM, fat mass; FMI, fat mass index; GC-MS, gas chromatography–mass spectrometry; HC, hip circumference; HDL-C, high-density lipoprotein cholesterol; HMW, high-molecular-weight; HPLC-MS, high-performance liquid chromatography–mass spectrometry; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LMW, low-molecular-weight; MBP, monobuthylphthalate; MBzP, monobenzyl phthalate; MCHP, mono-cyclohexyl phthalate; MCMHP, mono(2-carboxymethylhexyl) phthalate; MCNP, monocarboxylisononyl phthalate; MCOP, mnocarboxyisooctyl phthalate; MCP, mono-cyclohexyl phthalate; MCPP, mono (3-carboxypropyl) phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono-2-ethyl-5 -hydroxyhexyl phthalate; MEHP, monoethylhexyl phthalic acid; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; MET, metabolic equivalent; MHBP, Mono-3-hydroxybutyl phthalate; MHpP, mono-2-heptyl phthalate; MHxP, mono-hexylphthalate; MiBP, mono-isobutyl phthalate; MIDP, mono-8-methyl-1-nonyl-phthalate; MiNP, mono-isononyl phthalate; MMP, mono-methyl phthalate; MnBP, Mono-n-butyl phthalate; MnOP, mono-n-octyl phthalate; MNP, mono-isononyl phthalate; MOP, mono-n-octyl phthalate; NHANES, National Health and Nutrition Examination Survey; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NS, not stated; OB, obesity; OW, overweight; PA, phthalic acid; PHT, phthalate; POPs, persistent organic pollutant; RPUPLC-ESI-MS/MS, reversed-phase ultraperformance liquid chromatography–electrospray ionisation–tandem MS; SAT, subcutaneous adipose tissue; TC, total cholesterol; TG, triglycerides; UPLC-MS/MS, ultraperformance liquid chromatography–tandem mass spectrometry; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio.
Exposure to PHTs was assessed by determining urinary21 28–31 37 41 58 59 67 72 75 76 83–86 or serum57 67 84 levels of PHT metabolites in all studies. The exact set of metabolites varied among studies. Likewise, the specific PHT metabolites associated with measures of obesity also varied. Of note, 6 studies involving both male and female children and/or adults reported age-dependent and gender-dependent associations between urinary concentrations of PHT metabolites and measures of excess body weight.29 30 57 59 85 86
Other EDCs
Five studies24 32 33 55 60 investigated the association between polybrominated biphenyl (PBB) and obesity (online supplementary table 1). Four studies24 32 55 60 were conducted in adults and found no association between exposure to PBB and obesity. Only one study33 was conducted in children and found an inverse relation between exposure to PBB and BMI z-score. Pooled data from 2 studies32 55 indicated that exposure to PBBs was not significantly associated with abdominal obesity (figure 2C and table 2).
bmjopen-2019-033509supp005.pdf (59.2KB, pdf)
Two studies34 35 examining the association between ploycyclic aromatic hydrocarbons and obesity (online supplementary table 2) were conducted in children and found that exposure to these EDCs was positively associated to obesity, defined on the basis of anthropometric measures.
bmjopen-2019-033509supp006.pdf (65.2KB, pdf)
The association between exposure to parabens and obesity was investigated in 3 studies36 71 77 (online supplementary table 2). Xue et al71 reported a positive association between urinary paraben levels and obesity in children, whereas Kang et al77 studied children and adults and described that urinary parabens levels were positively associated with BMI in adults but not in children. Deierlein et al36 found no association between exposure to parabens and prospective changes in adiposity measures among girls. The only study investigating benzoic acid78 (online supplementary table 2) described that in adults low urinary 3-PBA levels were positively associated with obesity, whereas high levels were negatively associated.78 One study investigated exposure to perfluorinated alkylated substances and reported no association with obesity measures in children62 (online supplementary table 2).
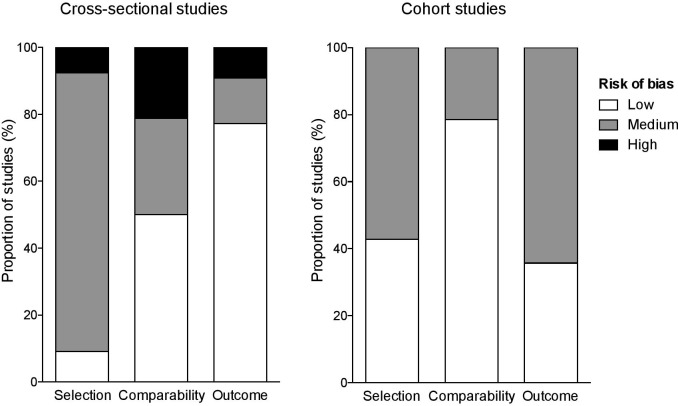
Quality Assessment
Quality assessment using the Newcastle-Ottawa Scale indicated that 65% of cross-sectional studies and all prospective studies had low or medium risk of bias (figure 3 and online supplemenatry table 3). For the studies included in the meta-analysis, no significant publication bias was detected using Egger’s regression test or by visual inspection of the funnel plots (online supplementary figure 2), although the small number of studies limited the reliability of the tests. Online supplementary table 4 presents the reasons for excluding studies from the meta-analysis.
Figure 3.
Quality assessment using the Newcastle-Ottawa scale for risk of bias of studies included in the systematic review.
bmjopen-2019-033509supp007.pdf (52.1KB, pdf)
bmjopen-2019-033509supp004.pdf (44.8KB, pdf)
bmjopen-2019-033509supp008.pdf (144.6KB, pdf)
Discussion
This systematic review of observational studies supports a positive association between exposure to BPA and PHTs and obesity in adults and children outside the early developmental period (aged 2 years or more). Although these data do not establish causation, in light of the evidence from animal and cell-based studies indicating the obesogenic effects of EDCs,4 they reinforce the need for continuing discussion on regulation of human exposure to these compounds.
Six previous systematic reviews addressed the association between exposure to EDCs, either during or outside the developmental period, and increased body weight or other measures of adiposity. Three reviews examined specifically BPA; two were inconclusive (including 2090 and 1891 studies), and one indicated a positive association in both children and adults (including 16 studies).92 One review summarised preclinical and clinical data on exposure to BPA or PHTs and reported positive associations (including 25 studies),93 whereas two assessed a broad range of EDCs and also reported positive associations (including 2494 and 3595 studies).
In contrast to the previous reviews, we used a detailed search strategy with no language restriction, and only studies that defined either generalised or regional obesity as a primary outcome were included. Since adiposity, determined by either anthropometric measures or BF quantification, is a multifactor trait, we viewed this would strengthen our findings. Accordingly, most studies were considered to have a low or medium risk of bias with respect to ascertainment of outcome. In addition, we comprehensively summarised data from a total of 73 studies involving bisphenol compounds, OC compounds, PHTs, PBB, polycyclic aromatic hydrocarbons (PAH), parabens, polyfluoroalkyl substances and benzoic acid.
The studies varied in the number of participants, although there did not appear to be a relationship between the number of participants and whether or not an association between exposure to EDCs and obesity was found. They also varied with respect to the precise method to determine serum or urinary levels of EDCs, the confounders for which the results were adjusted and data analysis. We could therefore not accomplish meta-analysis of all data to present overall estimates of the magnitude of the association between EDCs and obesity. However, data from few studies assessing the association between exposure to BPA, dichlorophenols or brominated compounds and measures of adiposity were pooled. Quantitative synthesis of these data revealed a significant positive association between exposure to BPA and overweight, general and central obesity, and between exposure to 2,5-DCP and obesity.
Most studies assessed exposure to BPA by using robust analytical methods to determine its urinary levels, although only few studies provided detailed information to rule out contamination during sample handling. Urinary BPA levels are considered a more appropriate indicator of exposure when compared with serum/plasma levels.96 Circulating BPA is rapidly metabolised into hydrophilic compounds that are conjugated and excreted in urine. This results in several-fold higher urinary BPA metabolites levels than circulating BPA levels.96 In addition, conjugated BPA (representing most of urinary total BPA) is not found in extraneous sources, minimising the risk of misleading results due to sample contamination.97
A potential concern is that assessment of BPA exposure on the basis of a single urinary and/or serum measurement, as was the case of almost all the included studies, may not be an adequate approach to investigate health outcomes. This is because there may be temporal variability of exposure to BPA, and adverse health effects most likely reflect long-term exposure. Pollack et al98 reported significant variation of urinary BPA levels over a 2-month period in women of reproductive age. On the other hand, data from other studies suggested that measurement in a single sample was predictive of exposure over 3 months.49 99 Moreover, due to its rapid metabolism and excretion, urinary levels of total BPA100 may not be representative of biologically active BPA, with the potential to affect health. Despite these limitations, it is noteworthy that investigations included in this review were conducted on different populations, and most of them pointed to a positive association between exposure to BPA and body size. Moreover, our meta-analysis of cross-sectional data indicated that exposure to BPA was significantly associated with overweight, general and abdominal obesity in adults.
Studies examining exposure to OC compounds in children and adults indicated an overall positive association with obesity; only data from studies assessing 2,4-DCP and 2,5-DCP exposure were pooled in the meta-analysis and indicated a significant association between exposure to 2,5-DCP and obesity. Many studies investigated more than one compound, but the number of studies examining each specific compound was small, leading to inconclusive findings with respect to the association between specific OC compounds and measures of body weight or fat. In adults, the most frequently studied OC compounds were PCBs and OCPs. The number of participants varied considerably between studies, ranging from 53 to 2931, and larger studies (involving more than 1000 participants) more consistently reported negative associations between highly chlorinated PCBs and obesity53–55 60 and positive associations between less chlorinated PCBs24 53 55 60 101 and the pesticide p, p’-dichlorodiphenyldichloroethylene with obesity.24 53 55 60
It is noteworthy that some studies reported no association between exposure to specific less chlorinated PCBs and obesity,24 88 89 101 whereas a similar number of studies indicated positive associations, mostly with a non-linear dose–response association.24 55 60 101 Exposure to specific highly chlorinated PCBs was negatively associated with obesity in four studies,53–55 60 not associated in three studies60 88 89 and positively associated in one studyfi.53 This apparent inconsistency in the direction of the associations may be related to the different concentration ranges for these EDCs found in each study, as has been previously discussed.55 Accordingly, PCB levels were lower in participants from studies that found no association between exposure to these EDCs and obesity.88 89 Therefore, the direction of the associations and also specific features of dose–response association may at least in part reflect the level of exposure of a specific population to these compounds.
Findings from studies investigating exposure to PHTs suggested an overall positive association with obesity, defined by BMI and/or WC, in children/adolescents29 30 67 75 76 and adults.21 28 29 57 58 Exposure to specific PHT chemicals appeared to be associated with obesity in an age-dependent manner and, although less consistently, in a gender-dependent manner. This was the case of diethyl phthalate (assessed by the urinary levels of its metabolite, monoethyl phthalate), which was positively associated with obesity in all studies involving children/adolescents,29 30 67 75 76 but not in adults,29 57 58 and which in some studies was associated with obesity only among girls.29 30 The possibility of an age-dependent and gender-dependent effect of PHTs is essentially speculative, but has been discussed in the light of its well-established estrogenic102 and antiandrogenic effects,103 which may differently affect male and female subjects at different stages of life. This may also reflect other effects of PHTs that possibly vary in different physiological settings, such as inhibition of thyroid hormone action.104
Similarly to BPA, PHTs are rapidly metabolised and excreted, and exposure to PHT sources may vary considerably over time.105 Therefore, a single measurement of PHT metabolites may not reflect long-term exposure to these compounds. However, it was shown that a single measure moderately predicts exposure over some months,105 106 with moderate to high sensitivity to allocate individuals into higher ranges of exposures.106 Another point that deserves discussion is that PHT urinary levels were corrected for variation in urinary dilution differently among the studies, and the best approach for this is still a matter of discussion.106
There were only five studies24 32 33 55 60 addressing the association between exposure to PBBs and obesity, and most reported no association. Too few studies examined PAHs,34 35 parabens71 77 and pyrethroids.78
The association between exposure to some EDCs and obesity raises the question about the potential action of these chemicals as risk factors for obesity-related complications, such as type 2 diabetes and cardiovascular diseases. Because EDCs are lipophilic, they are stored in adipose tissue.107 Adipose tissue, in turn, is affected in complex ways by EDCs and can also be a source of these chemicals to other key sites of metabolic homeostasis regulation in the setting of uncontrolled lipolysis or intentional weight loss.108 The direct actions of EDCs in adipose tissue, in particular, make their relationship to obesity-related complications a complex one. This is because EDCs stored in adipose tissue may act to increase or decrease the risk of these complications. These chemicals may increase the risk of these complications by inducing adipose tissue inflammation independently of obesity or by being released to other tissues and affecting them unfavourably. However, in the scenario where there is no uncontrolled lipolysis, the adipose tissue represents a safe storage site for EDCs, protecting other tissues from their potentially harmful effects.108
The cross-sectional design of most studies precluded determining causality between exposure to EDCs and obesity. Only a few studies had a prospective design, and notably most supported an association between exposure to EDCs and weight and/or WC increase among adults21 24 55 57 73 and children.30 It is also not possible to rule out reverse causality. Since most EDCs are highly lipophilic and stored in adipose tissue, higher levels of these compounds may reflect that obesity is associated with their accumulation. Moreover, it has been argued that obesity or its associated complications could lead to delayed metabolism of EDCs, extending their half-lives and leading to higher levels in serum or urine.109 It is also possible that obese individuals may be more exposed to EDCs by consuming more food or medications, since exposure to EDCs such as BPA, OC compounds and PHTs may occur by oral ingestion.110 111
Additionally, there is the limitation of testing the association between exposure to specific EDCs and obesity in human studies due to the potential confounders beyond the ones that were controlled for in data analysis. Despite adjusting results for various confounding factors, most studies did not consider potential exposure to multiple EDCs itself. Although this could limit establishing an association between a specific EDC and obesity, in a practical view, this may not be important, since humans are exposed to various EDCs simultaneously in the environment. Moreover, although speculative, it has been argued on the basis of data from cell-based studies112 that the effect of exposure to individual EDCs may be low, but combination exposure may have significant effects.28 On the other hand, it has also been discussed that simultaneous exposure to different EDCs may not simply result in additive effects of single exposure, since these compounds may act differently or even oppositely.29 113
It is also not possible to rule out that the associations between exposure to EDCs and measures of obesity outside the early developmental period examined in this review reflect in fact early life exposure, which may permanently alter gene expression patterns that affect metabolic processes.4 Although the circulating half-lives of EDCs are short, current measures of exposure may reflect ongoing exposure since early life, at least for some compounds with still widespread environmental occurrence.
Another methodological limitation was related to meta-analysis conduction. Despite the large number of studies included in this systematic review, only data from a limited number of them were suitable for quantitative synthesis. Different types of summary effects were first designed, considering both BMI/WC as categorical or continous variables. However, with multiple exposure metrics and several outcome measures available, heterogeneity among the studies was considerable and precluded their inclusion in the quantitative synthesis.
Finally, the findings from this meta-analysis must be interpreted carefully considering the risk of publication bias. Although we performed funnel plots and Egger’s weighted regression to explore the presence of publication bias across studies, these methods are limited when fewer than 10 studies are included in meta-analysis.114 Without reliable graphical evidence or statistical testing, we may suspect of publication bias by using qualitative parameters, such as an inadequate search strategy, and the inclusion of only small studies, mainly with funding from the pharmaceutical industry. However, we used a sensitive and specific search strategy and conducted a comprehensive literature review that enabled the retrieval of relevant published articles, which could decrease the chance of publication bias. However, it cannot be completely ruled out.
Conclusion
The findings from the current review indicate a significant association between exposure to BPA and overweight, general and abdominal obesity in adults, and between exposure to 2,5-DCP and obesity in children but are insufficient to support that that these EDCs cause obesity in humans due to the cross-sectional design of most included studies. However, given (1) the qualitative similarity of most data from human studies included in this review; (2) the evidence that exposure to BPA,115 OC compounds116 and PHTs117 induces obesity in animals; and (3) the findings from cell-based and in vitro studies indicating that EDCs affect various physiological pathways that may lead to weight gain,5 the data from human studies summarised herein should be viewed as evidence of the potential hazards of exposure to EDCs. This is particularly important in the current worldwide scenario of ongoing exposure of children and adults to EDCs, not only to chemicals still used for a wide range of purposes but also to compounds that were banned in many countries but have persistent and ubiquitous occurrence in the environment.
Supplementary Material
Footnotes
Contributors: AAA and MSC contributed to the conception of the systematic review and meta-analysis. AAA, MSC, PRSR and FdARN contributed to the design of the study. CMR, BTSB, NGS, AAA and CLL assisted with the selection of papers, data extraction and analysis. CLL conducted the meta-analysis. AAA drafted the manuscript. All authors contributed to the revisions of the manuscript and approved the final manuscript.
Funding: This study was supported by the National Council for Scientific and Technological Development or CNPq (grant number 420562/2016–8).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Heymsfield SB, Wadden TA, Mechanisms WTA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med Overseas Ed 2017;376:254–66. 10.1056/NEJMra1514009 [DOI] [PubMed] [Google Scholar]
- 2.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011;12:131–41. 10.1111/j.1467-789X.2009.00712.x [DOI] [PubMed] [Google Scholar]
- 3.Bouret S, Levin BE, Ozanne SE. Gene-Environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev 2015;95:47–82. 10.1152/physrev.00007.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:E1–150. 10.1210/er.2015-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol 2009;304:19–29. 10.1016/j.mce.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadal A, Quesada I, Tudurí E, et al. Endocrine-Disrupting chemicals and the regulation of energy balance. Nat Rev Endocrinol 2017;13:536–46. 10.1038/nrendo.2017.51 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Pham HT, Janesick AS, et al. Triflumizole is an obesogen in mice that acts through peroxisome proliferator activated receptor gamma (PPARγ). Environ Health Perspect 2012;120:1720–6. 10.1289/ehp.1205383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanayama T, Kobayashi N, Mamiya S, et al. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 2005;67:766–74. 10.1124/mol.104.008409 [DOI] [PubMed] [Google Scholar]
- 9.Regnier SM, Kirkley AG, Ye H, et al. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology 2015;156:896–910. 10.1210/en.2014-1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnier SM, El-Hashani E, Kamau W, et al. Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity 2015;23:1864–71. 10.1002/oby.21174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoucri BM, Hung VT, Chamorro-García R, et al. Retinoid X receptor activation during adipogenesis of female mesenchymal stem cells programs a dysfunctional adipocyte. Endocrinology 2018;159:2863–83. 10.1210/en.2018-00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamorro-Garcia R, Diaz-Castillo C, Shoucri BM, et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Dhana K, Furtado JD, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Med 2018;15:e1002502. 10.1371/journal.pmed.1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Morgan RL, Whaley P, Thayer KA, et al. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121:1027–31. 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol A levels and measures of obesity: results from the National health and nutrition examination survey 2003-2008. ISRN Endocrinol 2012;2012:1–6. 10.5402/2012/965243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol a concentration and obesity prevalence in children and adolescents. JAMA 2012;308:1113–21. 10.1001/2012.jama.11461 [DOI] [PubMed] [Google Scholar]
- 19.Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol 2013;177:1263–70. 10.1093/aje/kws391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eng DS, Lee JM, Gebremariam A, et al. Bisphenol A and chronic disease risk factors in US children. Pediatrics 2013;132:e637–45. 10.1542/peds.2013-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Hauser R, Hu FB, et al. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes 2014;38:1532–7. 10.1038/ijo.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Lai H, Chen S, et al. Gender differences in the associations between urinary bisphenol A and body composition among American children: The National Health and Nutrition Examination Survey, 2003-2006. J Epidemiol 2017;27:228–34. 10.1016/j.je.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elobeid MA, Padilla MA, Brock DW, et al. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999-2002 data. Int J Environ Res Public Health 2010;7:2988–3005. 10.3390/ijerph7072988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee D-H, Steffes MW, Sjödin A, et al. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One 2011;6:e15977. 10.1371/journal.pone.0015977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twum C, Wei Y. The association between urinary concentrations of dichlorophenol pesticides and obesity in children. Rev Environ Health 2011;26:215–9. 10.1515/reveh.2011.029 [DOI] [PubMed] [Google Scholar]
- 26.Buser MC, Murray HE, Scinicariello F. Association of urinary phenols with increased body weight measures and obesity in children and adolescents. J Pediatr 2014;165:744–9. 10.1016/j.jpeds.2014.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y, Zhu J, Nguyen A. Urinary concentrations of dichlorophenol pesticides and obesity among adult participants in the U.S. National health and nutrition examination survey (NHANES) 2005-2008. Int J Hyg Environ Health 2014;217:294–9. 10.1016/j.ijheh.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Stahlhut RW, van Wijngaarden E, Dye TD, et al. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115:876–82. 10.1289/ehp.9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatch EE, Nelson JW, Qureshi MM, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health 2008;7:27. 10.1186/1476-069X-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teitelbaum SL, Mervish N, Moshier EL, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res 2012;112:186–93. 10.1016/j.envres.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaghjyan L, Carlsson NP, Ghita GL, et al. Associations of individual characteristics and lifestyle factors with metabolism of di-2-ethylhexyl phthalate in NHANES 2001-2012. Environ Res 2016;149:23–31. 10.1016/j.envres.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim J-S, Lee D-H, Jacobs DR. Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003-2004. Diabetes Care 2008;31:1802–7. 10.2337/dc08-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erkin-Cakmak A, Harley KG, Chevrier J, et al. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ Health Perspect 2015;123:636–42. 10.1289/ehp.1408417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H-W, Kam S, Lee D-H. Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: the U.S. National health and nutrition examination survey 2003-2008. Environ Res 2014;135:354–60. 10.1016/j.envres.2014.08.032 [DOI] [PubMed] [Google Scholar]
- 35.Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001-2006). Environ Health Perspect 2014;122:299–303. 10.1289/ehp.1307234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deierlein AL, Wolff MS, Pajak A, et al. Phenol concentrations during childhood and subsequent measures of adiposity among young girls. Am J Epidemiol 2017;186:581–92. 10.1093/aje/kwx136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kataria A, Levine D, Wertenteil S, et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res 2017;81:857–64. 10.1038/pr.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 2011;111:825–30. 10.1016/j.envres.2011.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Lehmler H-J, Sun Y, et al. Bisphenol a substitutes and obesity in US adults: analysis of a population-based, cross-sectional study. Lancet Planet Health 2017;1:e114–22. 10.1016/S2542-5196(17)30049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoepner LA, Whyatt RM, Widen EM, et al. Bisphenol A and adiposity in an inner-city birth cohort. Environ Health Perspect 2016;124:1644–50. 10.1289/EHP205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoaff J, Papandonatos GD, Calafat AM, et al. Early-Life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect 2017;125:097008. 10.1289/EHP1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalloo G, Calafat AM, Chen A, et al. Early life Triclosan exposure and child adiposity at 8 Years of age: a prospective cohort study. Environ Health 2018;17:24. 10.1186/s12940-018-0366-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lankester J, Patel C, Cullen MR, et al. Urinary triclosan is associated with elevated body mass index in NHANES. PLoS One 2013;8:e80057. 10.1371/journal.pone.0080057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Zhao J, Wang G, et al. Urinary triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: experience in NHANES 2003-2010. Int J Hyg Environ Health 2015;218:401–6. 10.1016/j.ijheh.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zong G, Grandjean P, Wu H, et al. Circulating persistent organic pollutants and body fat distribution: evidence from NHANES 1999-2004. Obesity 2015;23:1903–10. 10.1002/oby.21161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harley KG, Aguilar Schall R, Chevrier J, et al. Prenatal and postnatal bisphenol a exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect 2013;121:514–20. 10.1289/ehp.1205548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rönn M, Lind L, Örberg J, et al. Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere 2014;112:42–8. 10.1016/j.chemosphere.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 48.D'Aniello R, Troisi J, D'Amico O, D’Amico O, et al. Emerging pathomechanisms involved in obesity. J Pediatr Gastroenterol Nutr 2015;60:113–9. 10.1097/MPG.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 49.Geens T, Dirtu AC, Dirinck E, et al. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ Int 2015;76:98–105. 10.1016/j.envint.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 50.Milić N, Četojević-Simin D, Milanović M, et al. Estimation of in vivo and in vitro exposure to bisphenol A as food contaminant. Food Chem Toxicol 2015;83:268–74. 10.1016/j.fct.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 51.Savastano S, Tarantino G, D'Esposito V, et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med 2015;13:169. 10.1186/s12967-015-0532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milošević N, Jakšić V, Sudji J, et al. Possible influence of the environmental pollutant bisphenol A on the cardiometabolic risk factors. Int J Environ Health Res 2017;27:11–26. 10.1080/09603123.2016.1246654 [DOI] [PubMed] [Google Scholar]
- 53.Dhooge W, Den Hond E, Koppen G, et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int 2010;36:330–7. 10.1016/j.envint.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 54.Dirinck E, Jorens PG, Covaci A, et al. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity 2011;19:709–14. 10.1038/oby.2010.133 [DOI] [PubMed] [Google Scholar]
- 55.Lee D-H, Lind L, Jacobs DR, et al. Associations of persistent organic pollutants with abdominal obesity in the elderly: the prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. Environ Int 2012;40:170–8. 10.1016/j.envint.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 56.Tang-Péronard JL, Jensen TK, Andersen HR, et al. Associations between exposure to persistent organic pollutants in childhood and overweight up to 12 years later in a low exposed Danish population. Obes Facts 2015;8:282–92. 10.1159/000438834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lind PM, Roos V, Rönn M, et al. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ Health 2012;11:21. 10.1186/1476-069X-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medic Stojanoska M, Milankov A, Vukovic B, et al. Do diethyl phthalate (dep) and di-2-ethylhexyl phthalate (DEHP) influence the metabolic syndrome parameters? pilot study. Environ Monit Assess 2015;187:526. 10.1007/s10661-015-4754-5 [DOI] [PubMed] [Google Scholar]
- 59.Petrovičová I, Kolena B, Šidlovská M, et al. Occupational exposure to phthalates in relation to gender, consumer practices and body composition. Environ Sci Pollut Res Int 2016;23:24125–34. 10.1007/s11356-016-7394-6 [DOI] [PubMed] [Google Scholar]
- 60.Roos V, Rönn M, Salihovic S, et al. Circulating levels of persistent organic pollutants in relation to visceral and subcutaneous adipose tissue by abdominal MRI. Obesity 2012;9 10.1038/oby.2012.123 [DOI] [PubMed] [Google Scholar]
- 61.Henríquez-Hernández LA, Luzardo OP, Valerón PF, et al. Persistent organic pollutants and risk of diabetes and obesity on healthy adults: results from a cross-sectional study in Spain. Sci Total Environ 2017;607-608:1096–102. 10.1016/j.scitotenv.2017.07.075 [DOI] [PubMed] [Google Scholar]
- 62.Karlsen M, Grandjean P, Weihe P, et al. Early-Life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol 2017;68:145–53. 10.1016/j.reprotox.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res 2016;146:379–87. 10.1016/j.envres.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 64.Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012;97:E223–7. 10.1210/jc.2011-1989 [DOI] [PubMed] [Google Scholar]
- 65.Wang H-xing, Zhou Y, Tang C-xi, et al. Association between bisphenol a exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health 2012;11:79. 10.1186/1476-069X-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li D-K, Miao M, Zhou Z, et al. Urine Bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One 2013;8:e65399. 10.1371/journal.pone.0065399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J, Eom J, Kim J, et al. Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: a cross-sectional study. Environ Toxicol Pharmacol 2014;38:51–7. 10.1016/j.etap.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 68.Ko A, Hwang M-S, Park J-H, et al. Association between urinary bisphenol A and waist circumference in Korean adults. Toxicol Res 2014;30:39–44. 10.5487/TR.2014.30.1.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee M-R, Kim JH, Choi Y-H, et al. Association of bisphenol a exposure with overweight in the elderly: a panel study. Environ Sci Pollut Res Int 2015;22:9370–7. 10.1007/s11356-015-4087-5 [DOI] [PubMed] [Google Scholar]
- 70.Sopon Pornkunwilaia WN, Jantaratc C, Wachrasindhua S, et al. Urinary bisphenol a detection is significantly associated with young and obese Thai children. Asian Biomedicine 2015;9:363–72. [Google Scholar]
- 71.Xue J, Wu Q, Sakthivel S, et al. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol a diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res 2015;137:120–8. 10.1016/j.envres.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 72.Hong S-H, Sung Y-A, Hong YS, et al. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin Endocrinol 2017;86:506–12. 10.1111/cen.13270 [DOI] [PubMed] [Google Scholar]
- 73.Hao M, Ding L, Xuan L, et al. Urinary bisphenol a concentration and the risk of central obesity in Chinese adults: a prospective study. J Diabetes 2018;10:442-448. 10.1111/1753-0407.12531 [DOI] [PubMed] [Google Scholar]
- 74.Lee HA, Park SH, Hong YS, et al. The effect of exposure to persistent organic pollutants on metabolic health among Korean children during a 1-year follow-up. Int J Environ Res Public Health 2016;13:270. 10.3390/ijerph13030270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Zhou Y, Tang C, et al. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS One 2013;8:e56800. 10.1371/journal.pone.0056800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou J-W, Lin C-L, Tsai Y-A, et al. The effects of phthalate and nonylphenol exposure on body size and secondary sexual characteristics during puberty. Int J Hyg Environ Health 2015;218:603–15. 10.1016/j.ijheh.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 77.Kang H-S, Kyung M-S, Ko A, et al. Urinary concentrations of parabens and their association with demographic factors: a population-based cross-sectional study. Environ Res 2016;146:245–51. 10.1016/j.envres.2015.12.032 [DOI] [PubMed] [Google Scholar]
- 78.Yoo M, Lim Y-H, Kim T, et al. Association between urinary 3-phenoxybenzoic acid and body mass index in Korean adults: 1st Korean national environmental health survey. Ann Occup Environ Med 2016;28:2 10.1186/s40557-015-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo J, Wu C, Lu D, et al. Urinary paraben concentrations and their associations with anthropometric measures of children aged 3 years. Environ Pollut 2017;222:307–14. 10.1016/j.envpol.2016.12.040 [DOI] [PubMed] [Google Scholar]
- 80.Harmouche-Karaki M, Matta J, Helou K, et al. Serum concentrations of polychlorinated biphenyls (PCBs) in a Lebanese population: ENASB study. Environ Sci Pollut Res 2017;24:3705–16. 10.1007/s11356-016-8139-2 [DOI] [PubMed] [Google Scholar]
- 81.Parastar S, Ebrahimpour K, Hashemi M, et al. Association of urinary concentrations of four chlorophenol pesticides with cardiometabolic risk factors and obesity in children and adolescents. Environ Sci Pollut Res Int 2018;25:4516–23. 10.1007/s11356-017-0771-y [DOI] [PubMed] [Google Scholar]
- 82.Mouneimne Y, Nasrallah M, Khoueiry-Zgheib N, et al. Bisphenol a urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environ Monit Assess 2017;189:517. 10.1007/s10661-017-6216-8 [DOI] [PubMed] [Google Scholar]
- 83.Lee K-M, Kho Y, Kim P-G, et al. Urinary levels of phthalate metabolites and associations with demographic characteristics in Korean adults. Environ Sci Pollut Res Int 2017;24:14669–81. 10.1007/s11356-017-9068-4 [DOI] [PubMed] [Google Scholar]
- 84.Oktar S, Sungur S, Okur R, et al. The relationship between phthalates and obesity: serum and urine concentrations of phthalates. Minerva Endocrinol 2017;42:46–52. 10.23736/S0391-1977.16.02295-1 [DOI] [PubMed] [Google Scholar]
- 85.Dong R, Zhou T, Chen J, et al. Gender- and age-specific relationships between phthalate exposures and obesity in Shanghai adults. Arch Environ Contam Toxicol 2017;73:431–41. 10.1007/s00244-017-0441-6 [DOI] [PubMed] [Google Scholar]
- 86.Yang TC, Peterson KE, Meeker JD, et al. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ Res 2017;156:326–33. 10.1016/j.envres.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arrebola JP, Cuellar M, Claure E, et al. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environ Res 2012;112:40–7. 10.1016/j.envres.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 88.Ben Hassine S, Hammami B, Ben Ameur W, et al. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and their relation with age, gender, and BMI for the general population of Bizerte, Tunisia. Environ Sci Pollut Res Int 2014;21:6303–13. 10.1007/s11356-013-1480-9 [DOI] [PubMed] [Google Scholar]
- 89.Hue O, Marcotte J, Berrigan F, et al. Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere 2007;67:1463–7. 10.1016/j.chemosphere.2006.10.033 [DOI] [PubMed] [Google Scholar]
- 90.Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol 2014;44:121–50. 10.3109/10408444.2013.860075 [DOI] [PubMed] [Google Scholar]
- 91.Oppeneer SJ, Robien K. Bisphenol a exposure and associations with obesity among adults: a critical review. Public Health Nutr 2015;18:1847–63. 10.1017/S1368980014002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rancière F, Lyons JG, Loh VHY, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 2015;14:46. 10.1186/s12940-015-0036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stojanoska MM, Milosevic N, Milic N, et al. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017;55:666–81. 10.1007/s12020-016-1158-4 [DOI] [PubMed] [Google Scholar]
- 94.Tang-Péronard JL, Andersen HR, Jensen TK, et al. Endocrine-Disrupting chemicals and obesity development in humans: a review. Obes Rev 2011;12:622–36. 10.1111/j.1467-789X.2011.00871.x [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Hollis-Hansen K, Ren X, et al. Do environmental pollutants increase obesity risk in humans? Obes Rev 2016;17:1179–97. 10.1111/obr.12463 [DOI] [PubMed] [Google Scholar]
- 96.Calafat AM, Koch HM, Swan SH, et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res 2013;15:403. 10.1186/bcr3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koch HM, Kolossa-Gehring M, Schröter-Kermani C, et al. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. J Expo Sci Environ Epidemiol 2012;22:610–6. 10.1038/jes.2012.39 [DOI] [PubMed] [Google Scholar]
- 98.Pollack AZ, Perkins NJ, Sjaarda L, et al. Variability and exposure classification of urinary phenol and paraben metabolite concentrations in reproductive-aged women. Environ Res 2016;151:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahalingaiah S, Meeker JD, Pearson KR, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 2008;116:173–8. 10.1289/ehp.10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thayer KA, Doerge DR, Hunt D, et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int 2015;83:107–15. 10.1016/j.envint.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee D-H, Lee I-K, Porta M, et al. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National health and nutrition examination survey 1999-2002. Diabetologia 2007;50:1841–51. 10.1007/s00125-007-0755-4 [DOI] [PubMed] [Google Scholar]
- 102.Chen F-P, Chien M-H, Chern IY-Y. Impact of low concentrations of phthalates on the effects of 17β-estradiol in MCF-7 breast cancer cells. Taiwan J Obstet Gynecol 2016;55:826–34. 10.1016/j.tjog.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 103.Christen V, Crettaz P, Oberli-Schrämmli A, et al. Antiandrogenic activity of phthalate mixtures: validity of concentration addition. Toxicol Appl Pharmacol 2012;259:169–76. 10.1016/j.taap.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 104.Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 2009;189:67–77. 10.1016/j.toxlet.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 105.Hoppin JA, Brock JW, Davis BJ, et al. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect 2002;110:515–8. 10.1289/ehp.02110515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hauser R, Meeker JD, Park S, et al. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 2004;112:1734–40. 10.1289/ehp.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Darbre PD. Endocrine disruptors and obesity. Curr Obes Rep 2017;6:18–27. 10.1007/s13679-017-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee Y-M, Jacobs DR, Lee D-H, Lee D-H. Persistent organic pollutants and type 2 diabetes: a critical review of review articles. Front Endocrinol 2018;9:712. 10.3389/fendo.2018.00712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emond C, Birnbaum LS, DeVito MJ. Use of a physiologically based pharmacokinetic model for rats to study the influence of body fat mass and induction of CYP1A2 on the pharmacokinetics of TCDD. Environ Health Perspect 2006;114:1394–400. 10.1289/ehp.8805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vandenberg LN, Hauser R, Marcus M, et al. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–77. 10.1016/j.reprotox.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 111.Hauser R, Duty S, Godfrey-Bailey L, et al. Medications as a source of human exposure to phthalates. Environ Health Perspect 2004;112:751–3. 10.1289/ehp.6804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect 2002;110:917–21. 10.1289/ehp.02110917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 2007;115 Suppl 1:98–105. 10.1289/ehp.9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 115.Yang M, Chen M, Wang J, et al. Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology 2016;157:2333–45. 10.1210/en.2015-1926 [DOI] [PubMed] [Google Scholar]
- 116.Arsenescu V, Arsenescu RI, King V, et al. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect 2008;116:761–8. 10.1289/ehp.10554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hao C, Cheng X, Xia H, et al. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 2012;32:619–29. 10.1042/BSR20120042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033509supp001.pdf (56KB, pdf)
bmjopen-2019-033509supp002.pdf (64.7KB, pdf)
bmjopen-2019-033509supp003.pdf (323.9KB, pdf)
bmjopen-2019-033509supp005.pdf (59.2KB, pdf)
bmjopen-2019-033509supp006.pdf (65.2KB, pdf)
bmjopen-2019-033509supp007.pdf (52.1KB, pdf)
bmjopen-2019-033509supp004.pdf (44.8KB, pdf)
bmjopen-2019-033509supp008.pdf (144.6KB, pdf)