Abstract
Introduction
The learning healthcare system (LHS) underpinned by data analysis and feedback to clinical care providers is thought to improve quality of care. The work aimed to implement an LHS for antibiotic prescribing in primary care in England.
Method
Deidentified patient-level data from general practices were processed and analysed at regular intervals (fortnightly increments). A dashboard application was developed and implemented displaying analytical graphics to give periodic feedback to clinicians, tailored to each clinical site. Benchmarking parameters were established by the analysis of two large national primary care datasets allowing peer-to-peer comparisons. To date, the dashboard is available to 70 English practices.
Conclusions
Successful implementation and uptake of the secure technical LHS infrastructure for the analysis and feedback to clinicians of their antibiotic prescribing demonstrate a great appetite for this type of frequent prescribing review in primary care, combining advanced data analytics with tailored feedback.
Keywords: medical informatics, patient care, primary health care, public health, information systems
Introduction
Antimicrobial resistance (AMR) has become a priority area for the WHO as it is responsible for over 700 000 deaths annually.1 Extensive use of antibiotics leads to rapid development of AMR, which, along with the slow development of new compounds to treat bacterial infections, poses a catastrophic threat for human health.
Approximately 80% of all antibiotics in the UK are prescribed in primary care, equating to 3 million antibiotics each month.2 Clinical Commissioning Groups (CCGs) within England are clinically led statutory National Health Service (NHS) bodies responsible for the planning and commissioning of healthcare services for their local areas, made up of an elected governing body of general practitioners (GPs), clinical care providers, care consultants and lay members.3 NHS England makes payments to each CCG to reflect the quality of services that they commission. This budget is adjusted for factors such as the average practice list size within the CCG, the average spend per patient for the CCG and the historic spend of practices within the CCG.4 The ‘top down’ approach allows the CCGs medicines management team to review each practice’s performance and contribution to whether the CCG meets its targets. One target includes the Quality Outcomes Framework, a points system for a set of known indicators, where practices are financially rewarded based on how they are performing. These measurements are commonly used by CCGs to review the performance of all practices in their region.5 Additionally, quality premiums (QPs) are awarded to the CCG for improvements in the quality of services they commission,6 for example, the Commissioning for Quality and Innovation (CQUIN) payment scheme assess how each CCG has commissioned to improve health.7
To help optimise antimicrobial use in primary care, the 2016/17 CQUIN aimed to reduce antibiotic consumption and encouraged a prescribing review within 72 hours of commencing antibiotic treatment.8 QP measures for 2018/2019 also aimed to reduce AMR by targeting: (1) a reduction in gram-negative blood stream infections, (2) a reduction of inappropriate antibiotic prescribing for urinary tract infections (UTI) in primary care and (3) a sustained reduction of inappropriate prescribing in primary care, based on the UK government targets of halving inappropriate prescribing by 2020/2021.9 Practices were also encouraged to establish antimicrobial stewardship programmes within CCGs to educate GPs about their contribution to overprescribing of antibiotics and the emergence of AMR.10
These measures have led to a reduction in antibiotic prescribing rates in recent years; however, it is estimated that 20% of all antibiotic prescriptions in primary care are still inappropriate, equating to 20 000 antibiotics unnecessarily issued every day.11 Antibiotic prescribing also varies substantially between regions,12 practices and within a practice by common infection,13 suggesting that penalising individual practices for prescribing more than that of the national average may not consider more complex patient populations.
The current approaches to monitor and feedback prescribing insights to practices have a varied and short-lived effect. Indicator methods to improve prescribing change each year meaning that there is little way to compare a practice’s performance over time.5 In addition, monitoring is often infrequent (quarterly or annually); meaning the effect of individual practice-specific interventions may become diluted when averaging practice-level prescribing at large intervals. A continuous effort from CCGs, general practices and various stewardship programmes across the UK has helped to reduce overall prescribing, but substantial inequality still exists. It is vital that practices with a complex patient population are not penalised for continuing to treat patients who truly need medicines. For example, those patients with a poor quality of life and/or living conditions, but fall within a low-risk age-sex category, known as the Specific Therapeutic group Age-sex Related Prescribing Unit (STAR PU) which is used to standardise and quantify prescribing rates by age and sex, per 1000 registered patients, will still need treatment but prescribing an antibiotic will be classified as inappropirate because they fall within a low risk STAR PU category.
A learning health system is a system that is aligned for continuous improvement through the assembly of data from various sources, the analysis of the data and regular feedback of findings to instigate a change in practice.14 The UK government stated as part of their 5-year national plan to reduce AMR a need to ‘Use electronic prescribing data to give healthcare providers feedback on guidance compliance and prescribing rates’.15 The current project aimed to build an interoperable infrastructure that can provide feedback to general practices on their antibiotic prescribing, which is tailored to their characteristics and is independent of the software system as used by the practices (most general practices in the UK use one of three). This infrastructure will analyse antibiotic prescribing and patient characteristics at fortnightly intervals and compare the results of the participating practices to comparable data from large national datasets (the Clinical Practice Research Datalink16 17 and the Secure Anonymised Information Linkage (SAIL) Databank18) in order to provide peer comparisons and deliver tailored results back to each practice.
Methods
User requirements
Working alongside stakeholders, the user requirements of a system to analyse and feedback information on how to better optimise antibiotic prescribing were developed through a series of face-to-face workshops and an online questionnaire. Stakeholders included general practitioners, prescribing advisers, microbiologists, as well as members from the National Institute for Health and Care Excellence (NICE) and Public Health England (PHE) involved in antimicrobial guideline development.
Data source
Deidentified patient-level data from primary care electronic health records (EHRs) were made available to the project following a privacy impact assessment and the approval and signing of a data-sharing agreement by the data custodians. Data were acquired through a trusted third party from multiple general practices and sent through a secure connection (the private NHS Health and Social Care Network (HSCN)) to a data safe haven for analysis. The data included information about infection-related consultations, for example, the Read code entered during the consultation, the type and dose of antibiotic prescription (when prescribed) and calendar time, as well as patient characteristics, such as, age at consultation, sex, ethnicity, body mass index, smoking status and comorbidity information. At point of study enrolment, a bulk data extraction from each practice containing historic data for the past 2 years was obtained to allow analysis of a practices historic baseline prescribing before the implementation of the infrastructure. Subsequent incremental data were sent every fortnight to the data safe haven for repeated analyses.
Infrastructure
The data safe haven, with a connection to the NHS private broadband network, HSCN, allows secure analysis of data. EHR data were translated to a common data model meaning all data received from different IT systems were ready for combined analyses. The infrastructure comprises one server for the import of the data and the use of a Virtual Desktop Infrastructure for the analyses, as well as a server to host a web-based dashboard for general practices to log on to (via a self-hosted authentication provider19) and view their practice’s results. Following analyses, summary results were stored in a document-oriented database on the HSCN web application server for exclusive use of the data visualisations. The data visualisations were developed using the R Studio 2020 package Shiny, and the ‘Shiny Modules’ design pattern was used to scale from single visualisation pages to multivisualisation pages.20 An iterative design process was employed to codevelop useful analyses and visualisations with stakeholders, always updating and incorporating end-user feedback.
Data analysis and feedback of results
Four main themes of analysis emerged:
Longitudinal fortnightly prescribing rates, displaying the variability in the rate of antibiotic prescribing for each practice, compared with national averages.
The type of antibiotic prescribing by infection.
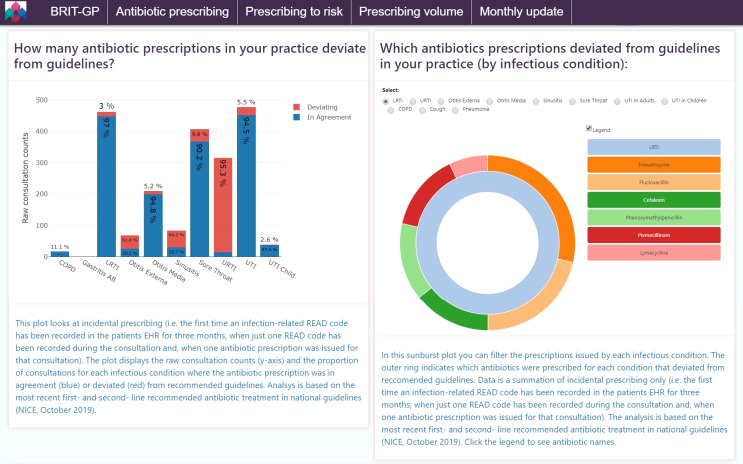
Antibiotics that deviate from recommended guidelines (NICE) for each infection (figure 1).
How a practice prescribes antibiotics in relation to a patient’s predicted risk of an infection-related poor outcome.
Figure 1.
An example of the general practitioner (GP) antibiotic prescribing dashboard where prescriptions may deviate from recommended guidelines. Incidence (acute) antibiotic prescriptions for each infection are compared with the first-line and second-line recommended antibiotic for common infections by the National Institute for Health and Care Excellence. GPs can see the frequency and rate of deviating antibiotic prescription (left) as well as they type of antibiotic prescription (right) by indication. COPD, chronic obstructive pulmonary disease; EHR, electronic health record; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection; UTI, urinary tract infection.
Results for each individual practice are fed back to practice staff, compared with those from very large national datasets to deliver tangible information tailored to each practice.
Discussion
This work focused on developing the infrastructure to support optimisation of antibiotic prescribing in primary care in England using a learning health system.
To date, this project has successfully developed and implemented a trustworthy system within the HSCN capable of extracting and processing patient-level deidentified data and fed back actionable results to individual practices. In contrast to traditional feedback that compromises summary statistics, aggregated practice-level analyses, CCG and regional level assessments, this web-based platform is able to deliver information on prescribing at both the practice and patient level for each practice. Demonstrating a mechanism that allows practitioners to: query prescribing patterns by infection; observe prescriptions that deviate from recommended guidelines; improve prescribing based on the risk of hospitalisation in addition to symptom severity; as well as obtaining a holistic overview of their prescribing volume compared with peers. These mechanisms in turn will help optimise antibiotic prescribing and improve patient care. The analysis has observed significant variability in the prescribing of antibiotics, for example, some practices prescribed antibiotics to patients with an upper respiratory tract infection just 10% of the time, while other practices prescribe antibiotics 80% of the time, a problem well recognised nationally.12 13
Involving stakeholders throughout the process gave the infrastructure validity, codesigning a tool with end-users ensured each element of the tool was necessary and enhanced the utility of the product for each clinical need. Regular communication with stakeholders throughout the design process and during the pilot phases allowed for continuous modification to the platform in use, making it more fit for purpose with each redeployment. Once more, involvement of stakeholders at each stage has encouraged uptake and repeated regular use of the tool. Stakeholders recognise that the product was developed to assist them in their day-to-day work and contains features they personally recommended. This codesign and feedback process will continue to focus the analysis and the development of additional features within the platform that fit the evolving needs of each practice, providing continuous support to optimise antibiotic prescribing.
To validate the data displayed in the dashboards, first, all consultations resulting in antibiotic prescriptions were extracted from a large national database (~5.2 million patients) and the associated medical codes were grouped by infection type and reviewed by two clinicians. In order to evaluate any missing codes, the codes that occurred more frequently (OR >3) on the date of an antibiotic prescription compared with control were also reviewed and added to the code lists where clinically relevant. The second stage of validation involved visiting partner practices and reviewing their dashboard data. Here, we were able to further improve the grouping of the code lists as well as the data processing steps taken in the analysis. However, the limitation remains that until coding improves there will inevitably be some incidences displaying an unrealistic representation of a consultation (e.g., a prescription deviated from recommended guidelines but in reality, this may have been appropriate but inadequately entered into the system). Our partner practices agree that as part of this study, focus on improved coding will improve their practices dashboards for review.
One major challenge to overcome was the translation of coding across the three different systems as practices also used different versions of the same systems producing discrepancies in medical coding with no mapping catalogue available to translate different versions to one common data model. In the NHS in England, there is a challenge to get very different and disconnected systems to communicate, but this work demonstrates that this can be done on a system-wide level within primary care.
The infrastructure is currently active in 70 primary care centres across England. Future work includes a formal evaluation of the impact the platform has on antibiotic prescribing post implementation, as well as supporting a full scale roll out across the UK, of which discussions are ongoing with PHE. However, the project has observed an association between frequent review of practice-specific dashboards and behavioural changes within our partner practices. Recent feedback includes the desire to identify individual prescribers within a practice for prescribers to be accountable for their actions. Furthermore, practices are enthusiastic to investigate new interventions within their own practice and assess the effect each intervention has, for example, there is interest in reviewing how rescue packs for patients with chronic obstructive pulmonary disease are issued, as well as piloting a point-of-care test to distinguish between bacterial and viral infections.
Ultimately, the platform gives back control to practices, ensuring they are equipped to monitor, but also take responsibility for, their antibiotic prescribing. Additionally, practices that are targeted for overprescribing now have the evidence to defend (when necessary) their prescribing decisions using the more in-depth and detailed analysis compared with traditional aggregated prescribing assessment at the practice and CCG levels. Furthermore, a practice can pilot different interventions and review the impact each intervention has on their prescribing overtime, allowing for rapid uptake of interventions that work and rapid removal of those that fail; optimising antibiotic utility further and improving the quality of care their patients receive. This in turn can instigate a variety of changes within different practices, ultimately optimising prescribing across the UK as a whole. In the future, this infrastructure can be made available for other priority areas in health, adopting a data-driven approach to improve patient care, delivering research that is relevant, effective and can have a real impact on public health.
Footnotes
Twitter: @v1kki_p
Contributors: VP and CM performed data analysis and design and development of shiny visualisations. ET designed and implemented the infrastructure and web-based dashboards. TPvS processed data into a common data model and performed data analysis. VP drafted the first manuscript. TPvS and ET provided input and edits to later versions.
Funding: This study was cofunded by Connected Health Cities and the NIHR Manchester Biomedical Research Centre. Connected Health Cities is a Northern Health Science Alliance led programme funded by the Department of Health and delivered by a consortium of academic and NHS organizations across the north of England.
Disclaimer: The interpretation and conclusions contained in this study are those of the authors alone, and not necessarily those of the MHRA, NHSA, NHS or the Department of Health.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.O'Neill J. Tackling drug-resistant infections globally: final report and recommendations, 2016. Available: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf [Accessed Nov 2019].
- 2.Public Health England English surveillance programme for antimicrobial utilisation and resistance (ESPAUR), 2018. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843129/English_Surveillance_Programme_for_Antimicrobial_Utilisation_and_Resistance_2019.pdf [Accessed Oct 2019].
- 3.NHS Clinical Commissioners About CCGs. Available: https://www.nhscc.org/ccgs/ [Accessed Nov 2019].
- 4.British Medical Association The GP practice prescribing budget, 2018. Available: https://www.bma.org.uk/advice/employment/gp-practices/service-provision/prescribing/advice-for-dispensing-gps/the-gp-practice/the-gp-practice-prescribing-budget [Accessed Nov 2019].
- 5.NHS Digital Quality outcomes frameworks (QOF), 2019. Available: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/quality-outcomes-framework-qof
- 6.NHS England Information on quality premium, 2018. Available: https://www.england.nhs.uk/wp-content/uploads/2018/04/annx-b-quality-premium-april-18.pdf [Accessed Nov 2019].
- 7.NHS England Commissioning for quality and innovation, 2019. Available: https://www.england.nhs.uk/nhs-standard-contract/cquin/ [Accessed Nov 2019].
- 8.NHS England Commissioning for quality and innovation (CQUIN), 2016. Available: https://www.england.nhs.uk/wp-content/uploads/2016/03/cquin-guidance-16-17-v3.pdf [Accessed Oct 2019].
- 9.NHS England Information on quality premium, 2016. Available: https://www.england.nhs.uk/publication/technical-guidance-annex-b-information-on-quality-premium/ [Accessed Nov 2019].
- 10.The National Institute for Health and Care Excellence (NICE) Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use, 2015. Available: https://www.nice.org.uk/guidance/NG15/chapter/recommendations#antimicrobial-stewardship [Accessed Oct 2019].
- 11.Bostock N. Gps write 20,000 unnecessary antibiotic prescriptions per day, officials suggest, 2018. Available: https://www.gponline.com/gps-write-20000-unnecessary-antibiotic-prescriptions-per-day-officials-suggest/article/1458080 [Accessed Oct 2019].
- 12.Mölter A, Belmonte M, Palin V, et al. . Antibiotic prescribing patterns in general medical practices in England: does area matter? Health Place 2018;53:10–16. 10.1016/j.healthplace.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Palin V, Mölter A, Belmonte M, et al. . Antibiotic prescribing for common infections in UK general practice: variability and drivers. J Antimicrob Chemother 2019;74:2440–50. 10.1093/jac/dkz163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The learning healthcare project. background: learning healthcare system. Available http://www.learninghealthcareproject.org/section/background/learning-healthcare-system (Accessed March 2020). [Google Scholar]
- 15.HM Government Tackling antimicrobial resistance 2019–2024: The UK’s five-year national action plan, 2019. Available: https://www.gov.uk/government/publications/uk-5-year-action-plan-for-antimicrobial-resistance-2019-to-2024 [Accessed Nov 2019]. [DOI] [PubMed]
- 16.Clinical practice research Datalink. CPRD: UK data driving real-world evidence. Available: https://cprd.com/home [Accessed Nov 2019].
- 17.Herrett E, Gallagher AM, Bhaskaran K, et al. . Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford DV, Jones KH, Verplancke J-P, et al. . The Sail Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res 2009;9:157. 10.1186/1472-6963-9-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keycloak Keycloak - Open Source Identity and Access Management, 2014. Available: https://www.keycloak.org/ [Accessed Oct 2019].
- 20.Cheng J. Modularizing shiny APP code, 2017, 2019. Available: https://shiny.rstudio.com/articles/modules.html [Accessed Nov 2019].