Abstract
A rising number of non-tuberculous mycobacterial (NTM) isolates are being identified in UK clinical practice. There are many uncertainties around the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD), including its epidemiology, diagnosis, treatment and prevention. Regional variations in how patients with NTM-PD are managed reflects the lack of standardised pathways in the UK. Service optimisation and multidisciplinary working can improve the quality of care for patients with NTM-PD, including (1) better identification of patients at risk of NTM-PD and modification of risk factors where applicable; (2) standardisation of reference laboratory testing to offer clinicians access to accurate and prompt information on NTM species and drug sensitivities; (3) development of recognised specialist NTM nursing care; (4) standardisation of NTM-PD imaging strategies for monitoring of treatment and disease progression; (5) establishment of a hub-and-spoke model of care, including clear referral and management pathways, dedicated NTM-PD multidisciplinary teams, and long-term patient follow-up; (6) formation of clinical networks to link experts who manage diseases associated with NTM; (7) enabling patients to access relevant support groups that can provide information and support for their condition; and (8) development of NTM research groups to allow patient participation in clinical trials and to facilitate professional education.
Keywords: bacterial infection, immunodeficiency, infection control, opportunist lung infections, rare lung diseases, respiratory Infection, bronchiectasis, imaging/CT MRI
Introduction
Between 1995 and 2012, the incidence of non-tuberculous mycobacterial (NTM) culture positive isolates increased eightfold in England, Wales and Northern Ireland from 0.9 per 100 000 population in 1995 to 7.6 per 100 000 in 2012. This incidence is now similar to that of tuberculosis (TB), cases of which are now declining.1–3 Around 200 species of Mycobacteria have been identified over a spectrum of pathogenicity, although most do not cause disease in humans.4–6 While the rise in incidence may be driven by factors such as improved awareness and better diagnostic tools, it is likely to be a true representation of the increased frequency with which these robust environmental organisms are now isolated.
The majority of clinical NTM cases manifest as pulmonary disease (non-tuberculous mycobacterial pulmonary disease (NTM-PD)), with a smaller number affecting extrapulmonary sites such as the skin and soft tissue.7 8 Mycobacterium avium complex (MAC, including M. avium, M. intracellulare and M. chimaera), and M. abscessus are associated with around 90% of the total number of reported cases of NTM-PD.9–11
Uncertainties around the management of NTM-PD include its epidemiology, diagnosis, treatment and prevention. These are addressed to some extent in the 2017 British Thoracic Society (BTS) guidelines12; however, variations in care remain for UK patients with NTM-PD. Paediatric NTM-PD is rare outside of the cystic fibrosis (CF) population, and hence the evidence base on which to guide management decisions is even smaller than for adult NTM-PD.13 An in-depth discussion of paediatric NTM-PD is beyond the scope of this article, although we recommend that such cases are managed in consultation with specialist centres. Using a clinical case of NTM-PD, we will review the current status and challenges of patient management and consider practical ways in which NTM services may be optimised in the future. While the focus of this article is the UK, many issues are relevant to the global management of NTM-PD.
Case: A 67-year-old slim-build woman with a history of smoking-associated chronic obstructive pulmonary disease (COPD) presents to her community COPD clinic with a productive cough and unintentional weight loss. Her COPD is managed with inhaled corticosteroids in combination with long-acting bronchodilators, and she has recently experienced recurrent respiratory infections, despite repeated courses of antibiotics. She also has ongoing gastro-oesophageal reflux disease (GORD). Chest X-rays carried out by the COPD team look generally similar to previous imaging, though occasional nodularity that appeared to resolve on repeat imaging was noted.
Risk factors
Various factors can increase the risk of developing NTM-PD; these are outlined in box 1 and discussed in more detail as follows. Immunocompromise is a major risk factor for NTM-PD, whether it is caused by the use of immunosuppressive drugs, by a systemic illness such as rheumatoid arthritis (RA), HIV or malignancy, or by a primary immunodeficiency.12 14 15 The use of biological agents, such as antitumour necrosis factor drugs, to treat RA and other autoimmune diseases has also been shown to increase the risk of NTM infection.16
Box 1. Factors which increase the risk of developing non-tuberculous mycobacterial pulmonary disease12–21.
Alcohol misuse
Biological agents
Chronic kidney disease
Diabetes
Female gender
Gastro-oesophageal reflux disease
Immunocompromise, primary or secondary to disease or drug therapies
Inhaled corticosteroids
Low body mass index
Pneumoconiosis
Underlying structural lung disease, for example, bronchiectasis, and COPD (chronic obstructive pulmonary disease)
NTM also causes pulmonary infections in apparently immunocompetent hosts, and those with underlying structural lung damage are at greatest risk.17 There is a high prevalence of NTM-PD in patients with CF and bronchiectasis.15 18 19 As in the case described, COPD is also a common predisposing condition for NTM-PD, with the risk increased further when patients are using inhaled corticosteroids, particularly at high doses.20 21
Other risks for NTM-PD in immunocompetent patients include host factors such as low body mass index (BMI), female gender and vitamin D deficiency, and the presence of comorbidities such as GORD, diabetes and chronic kidney disease.14 15 Some of these factors are modifiable and, where possible, clinicians and patients should address them to reduce NTM infection risk.14
We recommend that appropriate assessments are performed, although their extent depends on the patient’s clinical features plus available local resource. An initial screen should be carried out on all adult patients diagnosed with NTM-PD, comprising a thorough review of medication history, assessment for underlying disease leading to immunocompromise and HIV testing.
As bronchiectasis is commonly associated with NTM-PD, we suggest testing immunoglobulins in all patients with bronchiectasis to exclude an immunological basis for the structural lung disease. Other conditions, such as CF and alpha 1 antitrypsin deficiency, should be excluded in line with local policies. The presence of significant bronchiectasis or a characteristic pattern of microbial isolation could suggest underlying CF, which should be excluded.
If these tests do not identify any specific risk factors, we recommend discussing the case with immunologists or clinicians with expertise in immunodeficiency to determine whether further assessment is required.
Presentation
Like our case, patients with an underlying aetiology, such as COPD or bronchiectasis, may present with respiratory symptoms and may be seen in a specialist service. This provides an opportunity for NTM infection to be recognised by respiratory teams and the patient referred for diagnostic tests. Patients with no underlying aetiology usually present with symptoms to primary care. The possibility of NTM infection may not be recognised initially, resulting in a potential diagnostic delay. If patients are referred to secondary care, they may be seen in a general respiratory clinic with little experience of diagnosing and managing NTM-PD.
There is an urgent requirement for clear referral pathways for NTM-PD, both from primary to secondary care and from secondary care to NTM specialists. In addition, education will highlight the diverse presentations of NTM-PD to all relevant healthcare providers.
Case: Our patient has risk factors for the development of NTM-PD, including low BMI due to weight loss and slim build, structural lung disease, GORD and inhaled corticosteroids. She has presented with respiratory symptoms to her COPD clinic so she is referred for diagnostic tests.
Diagnosis of NTM-PD
Microbiological
UK and US guidelines provide microbiological diagnostic criteria for NTM-PD. Unlike TB, a patient must have two or more positive sputum samples, or one positive bronchial wash/lavage or compatible histopathological findings with one positive culture of the same NTM species.12 22
To establish the diagnosis of NTM-PD, the collection of three good-quality sputum specimens on different days is preferred. Potential causes of pulmonary disease must also be excluded, such as other bacteria or fungi.12 22
Accurate mycobacterial speciation enables clinicians and scientists to determine its potential clinical significance, identify available antimicrobial options, record epidemiology and characterise new species. All NTM isolates from respiratory samples should be identified to at least species level using validated molecular or mass spectrometry techniques.12 Suggested microbiological tests are outlined in table 1.12 22
Table 1.
Recommended microbiological tests to support a diagnosis of NTM-PD
| Test | Sensitivity | Specificity | Cost | Availability | Comment |
| Tests to detect NTM in respiratory samples12 22 | |||||
| Microscopy (AFB smear) | Low | Moderate | Low | High | Rapid, but variable sensitivity and cannot distinguish NTM from Mycobacterium tuberculosis |
| Direct molecular detection on primary sample | Moderate | High | Moderate | Low | NTM-specific: costly and less sensitive than conventional AFB culture. Limited uptake: TB PCR is useful as a rule-out test for NTM on smear-positive samples |
| Mycobacterial culture | High | High | Low | High | Most sensitive method to detect NTM from respiratory samples. Optimal results require the use of both solid and liquid media. Slow results, taking days to weeks |
| Tests to speciate and type NTM in respiratory samples12 22 | |||||
|
Moderate | High | Moderate/high | Low/moderate | Enables species identification, including subspecies of M. abscessus. May require phenotypical drug sensitivity testing to determine inducible macrolide resistance |
| WGS | High | High | Moderate/high | Low/moderate | Improved discrimination of strains enables WGS to be used to investigate possible person-to-person transmission events |
AFB, acid-fast bacilli; MALDI-TOF, matrix-assisted laser desorption ionisation-time of flight; NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacterial pulmonary disease; PCR, polymerase chain reaction; TB, tuberculosis; WGS, whole-genome sequencing.
There is regional variation in the microbiological diagnosis of NTM-PD in the UK, and patients may have different tests depending on their route of referral and which laboratory performs the tests. This makes it difficult to compare national data, although the National Reference Laboratories in the UK are currently undertaking whole-genome sequencing on all positive mycobacterial cultures received.
This lack of standardisation of diagnostic testing is a significant barrier to a timely diagnosis of NTM-PD, which in turn delays treatment, if required. In addition, the increased incidence of NTM culture-positive isolates has placed additional pressure on laboratories and will do so for the foreseeable future unless an appropriate solution is found.
Clinical
UK guidelines also provide criteria for a clinical diagnosis of NTM-PD. This requires the presence of respiratory symptoms alongside appropriate radiology, plus the exclusion of other diagnoses.12
It can be difficult to distinguish clinically meaningful NTM-PD from NTM colonisation, which is indicative of a patient with previously damaged lungs and impaired local host defences and no evidence of ongoing NTM-driven disease.
Radiological
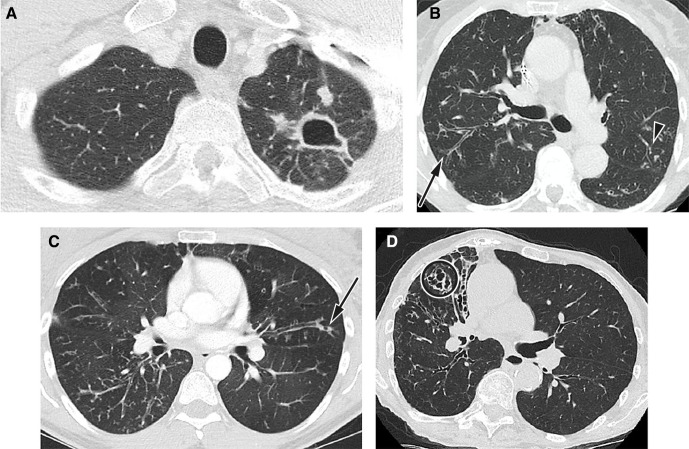
Radiology is a key component in the diagnosis and follow-up of NTM-PD. All patients with suspected NTM-PD should have computerised tomography (CT) imaging of the thorax, as the chest X-ray is often normal or non-specific.12 Examples of imaging seen with NTM infection are shown in figure 1. Systems for scoring NTM-PD radiology have been developed but are not widely used outside of research studies or specialist centres.23–25
Figure 1.
Radiological imaging features typical of NTM lung infection. (A) Fibrocavitatory NTM-PD in a patient who has M. kansasii. Axial CT image shows a cavity in the apex of the left upper lobe with adjacent satellite nodules. (B) Nodular–bronchiectatic NTM-PD. CT image from a 75-year-old female patient with MAC. Axial CT image at the carina shows bronchiectasis and bronchial wall thickening in the posterior segment of the right upper lobe (arrow) with additional scattered areas of peripheral bronchiectasis, mucus plugging and centrilobular nodularity in the left upper (arrowhead) and lower lobes. (C) Nodular–bronchiectatic NTM-PD in a patient with variant cystic fibrosis who has grown MAC and M. abscessus. Axial CT image just below the carina shows widespread bronchiectasis and bronchial wall thickening with centrilobular nodules in the right lower lobe. Peripheral mucus plugging is a more common finding in NTM-PD and air bubbles or lucency is often seen within the mucus (arrow). There is an ancillary finding of air trapping causing lucent areas of lung. (D) Nodular–bronchiectatic NTM-PD in a patient who has MAC. There is more severe bronchiectasis in the middle lobe, which is cystic and varicose in appearance (circle). There are ancillary findings of chest wall deformity with volume loss in the right hemithorax and little chest wall body fat. Reproduced by the kind permission of Dr Joanne Cleverley and Dr Besma Musaddaq of the Department of Radiology, Royal Free Hospital, London, UK. CT, computerised tomography; MAC, Mycobacterium avium complex; NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacterial pulmonary disease.
Case: Our patient underwent a CT scan and sputum samples were collected for analysis. The CT scan showed underlying emphysema with inflammatory changes in the middle lobe, lingular bronchiectasis and non-cavitating nodularity in both lungs. Sputum microbiology was smear-negative for AFB; however, two cultures grew mycobacterial species after 2 weeks. This was subsequently confirmed as M. avium. The COPD team want to know whether or not she should be treated for this and should contact their local specialist, with an interest in respiratory infection, to discuss the management of her condition.
Management
There are regional variations in how patients with NTM-PD are managed after their diagnosis, reflecting the lack of standardised pathways in the UK. A diagnosis does not necessarily translate into an immediate treatment requirement for the NTM, especially in patients with mild symptoms and/or an absence of radiological findings, but long-term follow-up is strongly advised. Currently, there is limited evidence and no clear consensus on when to start treatment, with the exception of severe disease, for example, the fibrocavitary phenotype of MAC.22 If treatment is deemed necessary, the regimen depends on the infecting NTM species and in some cases requires a combination of oral and intravenous or inhaled medications. In certain clinical scenarios, such as a symptomatic patient with M. abscessus infection and severe underlying lung disease, treatment is likely to be undertaken to control disease progression and to improve patient quality of life.
In addition, NTM have high levels of intrinsic resistance. While for some antibiotics, such as macrolides, in vitro drug susceptibility testing (DST) is associated with clinical efficacy, there is a generally inconsistent relationship between DST and clinical disease response.19 This makes the choice of antibiotics for NTM less straightforward than for most cases of TB. In addition, many pathogenic NTM species are slow-growing, leading to long treatment durations.26 Intolerance of therapies is common and options can be limited by drug–drug interactions and the patient’s comorbidities.26
Therefore, the decision to initiate treatment is complex and should consider the factors outlined in table 2.
Table 2.
Factors influencing the decision to initiate therapy for NTM-PD
| Host factors | Mycobacterial factors | Environmental factors |
| Severity of respiratory and constitutional symptoms (eg, cough, sputum production, breathlessness, fatigue and malaise) | Virulence/pathogenicity (usually identified from mycobacterial speciation) | Promotion of NTM persistence (eg, through the use of inhaled steroids) |
| Comorbidities | Drug resistance profile (note: there is usually only limited information available) | Ongoing exposure to mycobacteria |
| Degree of lung damage (severity and/or extent of disease) |
NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacterial pulmonary disease.
The surgical management of NTM-PD has been shown to result in favourable patient outcomes in specific cases.27 This requires multidisciplinary assessment and expert surgical input in a centre experienced in managing individuals with NTM-PD, alongside the careful selection of appropriate patients, such as those with focal lung disease. Patients should commence antibiotic treatment prior to surgery, which should be continued for 12 months after culture conversion.12
The complexities and chronicity of care argue for the development of an NTM specialist nurse role. Currently, most services rely on ad hoc support from TB, infectious disease, respiratory, COPD and bronchiectasis nurses; or have no specialist nurse input at all. The resulting regional variation in standards of care and competencies will not be resolved until a clear career pathway into NTM nursing is established. In the short term, local teams are best placed to drive service improvement by identifying and using available resource. For example, in some areas, TB nurses may be able to provide care via a case-management model similar to the established nursing pathway used for patients with TB.28 Elsewhere, it may be more appropriate for specialist nursing groups to take this on. Communication, education and shared examples of good practice between different nursing teams enable regional variation to be minimised over time.
Ongoing patient monitoring depends on available resource, as well as the patient’s specific management plan and needs. In general, we recommend that support is available on an ongoing basis, for example, via telephone contact with an NTM specialist nurse, while face-to-face clinical review occurs every 6 weeks to 6 months depending on need. Serial sputum cultures can be performed outside these assessments, although in practice, it is often easier for patients to provide samples at clinic visits.
Airway clearance, including physiotherapy, is a key component of NTM-PD care, which can counteract respiratory decline. Unfortunately, outside of specialist NTM centres, there are huge variations in the accessibility and responsiveness of physiotherapy services for patients with NTM.
We believe quality of life for patients with NTM-PD is a key consideration within any care plan. This may include an ongoing dialogue regarding the emergence and management of treatment-related side effects, and an assessment of social support needs, such as transport to appointments. Psychological interventions are also relevant for patients who live with a chronic illness that can seem mysterious, poorly understood and hard to explain to others.29 Nutritional status is important, given that it can be impaired by the disease itself, drug treatment and associated low mood. NTM-PD outcomes are worse in patients who are losing weight and poorly nourished.
A multidisciplinary team (MDT) management approach is recommended to ensure access to all specialties required to manage patients with NTM-PD.
NTM MDT composition
Other areas of lung disease, such as CF, bronchiectasis and TB, already have established and successful MDTs. Experience gained in these areas can be used as an excellent starting point for the development of NTM MDTs; the role of each specialist is considered in more detail as follows.
Respiratory physician
The respiratory physician is responsible for optimising a patient’s underlying disease and associated risk factors, plus providing ongoing monitoring.
It is imperative that the physician discusses treatment options with the patient before a decision is made, and the views of the patient are sought on the potential risks and benefits of starting treatment. This is emphasised in the 2017 BTS guidelines.12
Case: The Reference Laboratory reports that the M. avium isolated from our patient was phenotypically sensitive to macrolides. Following discussion of treatment options, the patient opts to start azithromycin, rifampicin and ethambutol. In an attempt to protect her lungs from further damage, a proton pump inhibitor is initiated to improve her GORD and her dose of inhaled corticosteroids is reduced. She is monitored for improvement, as well as possible worsening in her breathing following the reduction in inhaled corticosteroids.
Specialist pharmacist
The pharmacist has an important role in ensuring good patient outcomes in patients who often have comorbidities and are on complex long-term drug regimens. The pharmacist can provide advice on avoiding potential drug–drug interactions. For example, the case describes rifampicin, a potent inducer of certain cytochrome P450 enzymes, which can accelerate metabolism of multiple drugs, including corticosteroids. Pharmacists can help manage adverse events, for example, through patient education, the introduction of new medicines and therapeutic drug monitoring.
Specialist nurse
Nurses have knowledge of drugs, their side effects and interactions, have developed strategies to assist with management, and can provide support and advice, especially as a point of contact should any urgent issues arise. They also have a key role in risk assessment and adherence support through regular nurse-led follow-up.
Specialist physiotherapist
The main aim of the physiotherapist is to enhance the patient’s participation in everyday life, through assessment, advice, education and physical intervention. They have a key role in mobilising respiratory secretions through chest physiotherapy, reducing breathlessness and maintaining or improving exercise tolerance.
Radiologist
The radiologist has a vital role in the diagnosis and management of NTM-PD, and we advocate close working between the clinical and radiological teams to ensure that patients achieve the best possible outcomes.
Radiologists can provide diagnostic support, particularly where NTM-PD is not suspected, by recognising the CT imaging features of fibrocavitary and/or nodular–bronchiectatic NTM-PD, and advising the physician on target sites within the lungs or mediastinum for bronchoscopic lavage or biopsy to confirm a diagnosis. Serial CT imaging is also important for monitoring disease progression and response to treatment.
Case: Once established on treatment, our patient’s condition steadily improved. While she remained breathless on exertion, the number of exacerbations reduced and she was able to gain weight. Her sputum cultures for AFB became negative at 3 months and a follow-up CT scan at 6 months showed resolution of her nodules. She is scheduled to receive 12 months of treatment from when she became sputum culture negative.
Infection control
In general, NTM are not considered to be transmissible between patients.30 However, since 2012, there have been several concerning reports in CF centres of person-to-person transmission of M. abscessus. This is likely to be through fomite spread or the generation of long-lived infectious aerosols.30–32 In the setting of CF, particularly, it seems prudent that rigorous infection control measures are in place.
Another example of the importance of infection control is the 2013 outbreak of over 100 cases of life-threatening M. chimaera prosthetic valve endocarditis and disseminated disease in people with previous cardiac surgery. This was linked to contaminated heater–cooler units used during the patient’s surgery, with the factory manufacturing the units being the likely source. Surveillance and heightened clinician awareness are key to detect early transmission of NTM.33
Models of care
A lack of commissioned NTM services in the UK affects all aspects of care for patients with NTM-PD. A model of care is required that contains pathways providing seamless assessment, investigation and management across a number of disciplines and services in line with patients’ clinical presentation and need. This must include access to new and future high-cost treatments.
An attractive potential model is the commissioning of specialist centres that can advise and support other NTM services within their geographical region, similar to that used by the multidrug-resistant TB network.34 To support such a hub-and-spoke model of care, we recommend that local clinical networks are set up that link experts managing diseases associated with NTM, such as large CF units, TB clinics and bronchiectasis teams. This model should also include regular regional MDT meetings with a dedicated microbiologist and radiologist, where information can be shared in real time and local guidelines can be developed as required. Non-complex cases of NTM-PD could be managed within the disease-specific clinical team using an agreed NTM care plan.
Patient support
Currently, there is a distinct lack of support for patients diagnosed with NTM-PD in the UK. Many are unable to source accurate information on their condition outside of busy clinic appointments, and are left feeling isolated. In response to this unmet need, NTM Patient Care UK was founded by patients with NTM and clinicians in 2018. Its key aims are to provide education and information to increase understanding of NTM for both patients and clinicians, and to develop and implement a support network for NTM patient communities.
Research
We believe that research into NTM-PD can be strengthened in the UK in line with the findings of the recent US National Institute of Allergy and Infectious Diseases report.35 This will enable better understanding of who is at risk of NTM-PD, their clinical pathway and the natural history of NTM infection; this, in turn, will support the development of drugs that are more effective in treating the disease. There are several NTM research groups currently established in the UK, including NTM Network UK, the European Bronchiectasis Registry (EMBARC) and NTM-NET. NTM Network UK is an alliance of clinicians, public health scientists and research scientists and provides a framework within which NTM infections and disease can be systematically investigated, researched and managed. EMBARC launched the European NTM Registry in 2019 and aimsto gather key epidemiological data about the spectrum of patients with NTM-PD to stimulate greater research and interest in the field of NTM-PD. NTM-NET is an international network promoting clinical research in the field of NTM disease by sharing and developing ideas and research protocols. Increased collaboration and sharing of data will, we believe, ultimately contribute to improved patient management and outcomes for people living with NTM-PD.
Conclusions
Service optimisation and multidisciplinary working can improve the quality of care for patients with NTM-PD. The following strategies are key to achieve this goal:
Better identification of patients at risk of NTM-PD and modification of risk factors where applicable.
Standardisation of reference laboratory testing to offer clinicians access to accurate and prompt information on NTM species and drug sensitivities.
Development of recognised specialist NTM nursing care.
Standardisation of NTM-PD imaging strategies for monitoring of treatment and disease progression.
Establishment of a hub-and-spoke model of care to include clear referral and management pathways, dedicated NTM-PD MDTs to provide access to regional NTM experts and long-term patient follow-up.
Formation of clinical networks to bring together clinicians involved in the management of diseases associated with NTM, such as bronchiectasis and COPD.
Enabling patients to access relevant support groups that can provide information and support for their condition.
Development of NTM research groups to allow patient participation in clinical trials and to facilitate professional education.
Acknowledgments
The authors thank Dr Sarah Bryant of Axiom Health Ltd for medical writing and editorial support, which was funded by Insmed Limited.
Footnotes
Contributors: RvdL and ML conceived the manuscript. ML was responsible for drafting the manuscript for review and critical appraisal by the authors. All authors were responsible for revising the article critically for important intellectual content and for approving the final version to be published.
Funding: Insmed provided funding for medical writing and editorial support
Competing interests: JC, TF, BM, DP, PW and JW have nothing to disclose. ML reports that he is chair of NTM Network UK, which has received an unrestricted educational grant from Insmed for administrative Network support. RvdL is an Insmed employee.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing is not applicable as no datasets are generated and/or analysed for this study. There are no data in this work.
References
- 1.Moore JE, Kruijshaar ME, Ormerod LP, et al. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health 2010;10:612. 10.1186/1471-2458-10-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007-2012. BMC Infect Dis 2016;16:195. 10.1186/s12879-016-1521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England Tuberculosis in England 2019 report: Executive summary. Available: https://www.gov.uk/government/publications/tuberculosis-in-england-annual-report [Accessed 04 May 2020].
- 4.Forbes BA. Mycobacterial taxonomy. J Clin Microbiol 2017;55:380–3. 10.1128/JCM.01287-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedrizzi T, Meehan CJ, Grottola A, et al. Genomic characterization of nontuberculous mycobacteria. Sci Rep 2017;7:45258. 10.1038/srep45258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen MD, Herrmann J-L, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 2020. 10.1038/s41579-020-0331-1 [DOI] [PubMed] [Google Scholar]
- 7.Jones MM, Winthrop KL, Nelson SD, et al. Epidemiology of nontuberculous mycobacterial infections in the U.S. veterans health administration. PLoS One 2018;13:e0197976. 10.1371/journal.pone.0197976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wi YM. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect Chemother 2019;51:245–55. 10.3947/ic.2019.51.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017;49:1601855. 10.1183/13993003.01855-2016 [DOI] [PubMed] [Google Scholar]
- 10.Olivier KN, Weber DJ, Wallace RJ, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828–34. 10.1164/rccm.200207-678OC [DOI] [PubMed] [Google Scholar]
- 11.Schiff HF, Jones S, Achaiah A, et al. Clinical relevance of non-tuberculous mycobacteria isolated from respiratory specimens: seven year experience in a UK Hospital. Sci Rep 2019;9:1730. 10.1038/s41598-018-37350-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haworth CS, Banks J, Capstick T, et al. British thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72:ii1–64. 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- 13.Tebruegge M, Pantazidou A, MacGregor D, et al. Nontuberculous Mycobacterial Disease in Children - Epidemiology, Diagnosis & Management at a Tertiary Center. PLoS One 2016;11:e0147513. 10.1371/journal.pone.0147513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake MA, Ambrose LR, Lipman MCI, et al. '"Why me, why now?" Using clinical immunology and epidemiology to explain who gets nontuberculous mycobacterial infection. BMC Med 2016;14:54. 10.1186/s12916-016-0606-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Axson EL, Bual N, Bloom CI, et al. Risk factors and secondary care utilisation in a primary care population with non-tuberculous mycobacterial disease in the UK. Eur J Clin Microbiol Infect Dis 2019;38:117–24. 10.1007/s10096-018-3402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax 2015;70:677–82. 10.1136/thoraxjnl-2014-206470 [DOI] [PubMed] [Google Scholar]
- 17.Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol 2018;9:2901. 10.3389/fmicb.2018.02901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H, Zhao L, Xiao H, et al. Prevalence of nontuberculous mycobacteria in patients with bronchiectasis: a meta-analysis. Arch Med Sci 2014;10:661–8. 10.5114/aoms.2014.44857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park IK, Olivier KN. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 2015;36:217–24. 10.1055/s-0035-1546751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marras TK, Campitelli MA, Kwong JC, et al. Risk of nontuberculous mycobacterial pulmonary disease with obstructive lung disease. Eur Respir J 2016;48:928–31. 10.1183/13993003.00033-2016 [DOI] [PubMed] [Google Scholar]
- 21.Andréjak C, Nielsen R, Thomsen Vibeke Ø, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013;68:256–62. 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- 22.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 23.Cowman SA, Jacob J, Obaidee S, et al. Latent class analysis to define radiological subgroups in pulmonary nontuberculous mycobacterial disease. BMC Pulm Med 2018;18:145. 10.1186/s12890-018-0675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HS, Lee KS, Koh W-J, et al. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology 2012;263:260–70. 10.1148/radiol.12111374 [DOI] [PubMed] [Google Scholar]
- 25.Kuroishi S, Nakamura Y, Hayakawa H, et al. Mycobacterium avium complex disease: prognostic implication of high-resolution computed tomography findings. Eur Respir J 2008;32:147–52. 10.1183/09031936.00074207 [DOI] [PubMed] [Google Scholar]
- 26.Shulha JA, Escalante P, Wilson JW. Pharmacotherapy approaches in nontuberculous mycobacteria infections. Mayo Clin Proc 2019;94:1567–81. 10.1016/j.mayocp.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 27.Sakane T, Matsuoka K, Kumata S, et al. The outcomes of anatomical lung resection for nontuberculous mycobacterial lung disease. J Thorac Dis 2018;10:954–62. 10.21037/jtd.2018.01.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royal College of Nursing A case management tool for TB prevention, care and control in the UK. January, 2019. Available: https://www.rcn.org.uk/professional-development/publications/pub-006194 [Accessed 04 May 2020].
- 29.Henkle E, Aksamit T, Barker A, et al. Patient-Centered research priorities for pulmonary nontuberculous mycobacteria (NTM) infection. Ann Am Thorac Soc 2016;13:S379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aitken ML, Limaye A, Pottinger P, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 2012;185:231–2. 10.1164/ajrccm.185.2.231 [DOI] [PubMed] [Google Scholar]
- 31.Bryant JM, Grogono DM, Greaves D, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013;381:1551–60. 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous Mycobacterium. Science 2016;354:751–7. 10.1126/science.aaf8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ingen J, Kohl TA, Kranzer K, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis 2017;17:1033–41. 10.1016/S1473-3099(17)30324-9 [DOI] [PubMed] [Google Scholar]
- 34.BTS MDR-TB Clinical Advice Service Available: https://brit-thoracic.org.uk/quality-improvement/lung-disease-registries/bts-mdr-tb-clinical-advice-service [Accessed 04 May 2020].
- 35.Daniel-Wayman S, Abate G, Barber DL, et al. Advancing translational science for pulmonary nontuberculous mycobacterial infections. A road map for research. Am J Respir Crit Care Med 2019;199:947–51. 10.1164/rccm.201807-1273PP [DOI] [PMC free article] [PubMed] [Google Scholar]