Abstract
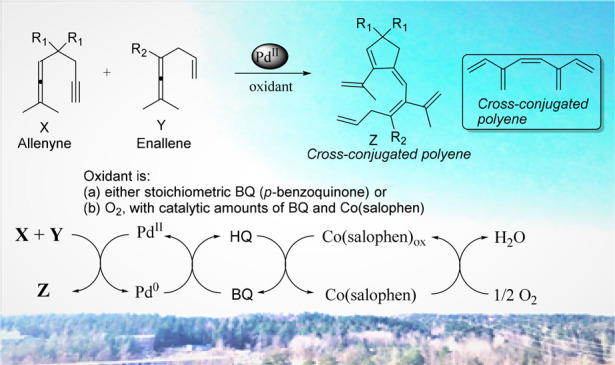
An efficient palladium-catalyzed oxidative C–C bond forming cascade reaction of allenes involving a coupling between an enallene and an allenyne followed by a carbocyclization of the generated Pd-intermediate was developed. This cascade reaction afforded functionalized cross-conjugated polyenes. The enallene is initially activated by palladium and reacts with the allenyne to give the cross-conjugated polyenes.
Introduction
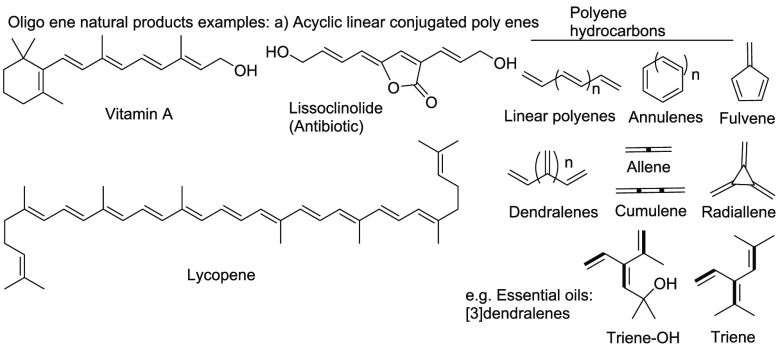
Stereo- or regiocontrolled selective construction of unsaturated molecular scaffolds through sequential multiple carbon–carbon (C–C) bond formation remains one of the major challenges in organic chemistry. In particular, in cascade reactions, transition-metal-catalyzed cyclizations of allenes provide efficient and atom-economical routes to polyunsaturated molecules.1 Polyenes and oligoenes occur as structural elements in pharmaceutically active compounds and important natural products such as Vitamin A, Lycopene, β-carotene, Lutein, lissoclinolide, naturally occurring [3]dendralenes, etc. (Scheme 1).2 The synthesis of such nonaromatic cross-conjugated [3]dendralenes have recently attracted considerable interest.3,4
Scheme 1. Examples of Polyene Compounds.
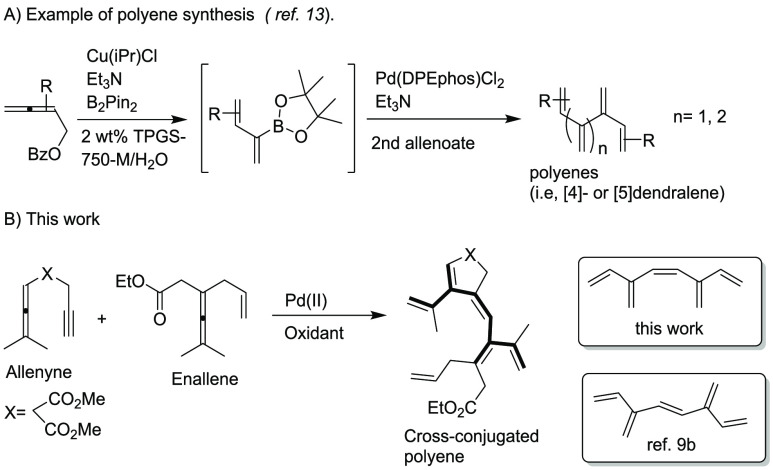
In recent years, acyclic cross-conjugated polyenes (dendralenes) have been used in diene transmissive Diels–Alder (DTDA) sequences for rapid generation of complex scaffolds bearing multiple stereogenic centers (Scheme 2).5 Due to regioselective functionalization of the multiple olefinic sites, other applications of dendralenes are found in the synthesis of ivyane family compounds,6 vinylogous Nazarov reactions,7 organocatalytic domino cyclizations,8 oxidative reactions,9a and metathesis of [3]dendralene–Fe(CO)3 complexes.9b However, synthetic methods for the preparation of higher cross-conjugated polyenes are quite limited as one-step reactions. In this respect, Hopf, Sherburn, Shimizu and their co-workers reported on acyclic cross conjugated polyenes.3b,10−12 Recently, the Lipshutz group reported a tandem borylation/Suzuki–Miyaura reaction for the synthesis of cross-conjugated polyenes such as [4]- and [5]dendralene (Scheme 3A).13
Scheme 2. Examples of Cross-Conjugated Polyenes Applications.
Scheme 3. Example of Polyene Synthesis and Proposal for this Work.
In the past decade, our research group has focused on Pd(II)-catalyzed oxidative carbocyclization reactions of allenes1h and we reported the synthesis of various types of [3]dendralenes via C–C bond formation.3d Compared to intramolecular reactions of allenes, intermolecular couplings of allenes are more challenging and would provide an array of novel conjugated structures. To the best of our knowledge, intermolecular cascade reactions between allenes with C–C bond formation for the synthesis of cross-conjugated polyenes have not yet been reported in a one-pot reaction. We therefore decided to study palladium(II)-catalyzed oxidative coupling and carbocyclization reactions of enallenes with allenynes for the synthesis of polyenes (Scheme 3 B). These cross-conjugated polyenes will have the (Z)-configuration at the middle double bond. The synthesis of the unsubstituted (E)-isomer of the corresponding polyene has been reported by Paddon-Row and Sherburn (lower box, Scheme 3B).9b
In this report, palladium-catalyzed intermolecular carbocyclization cascade reactions provide a wide variety of interesting polyene products in high yield and with excellent regio- and stereoselectivity (Scheme 3, B).
Results and Discussion
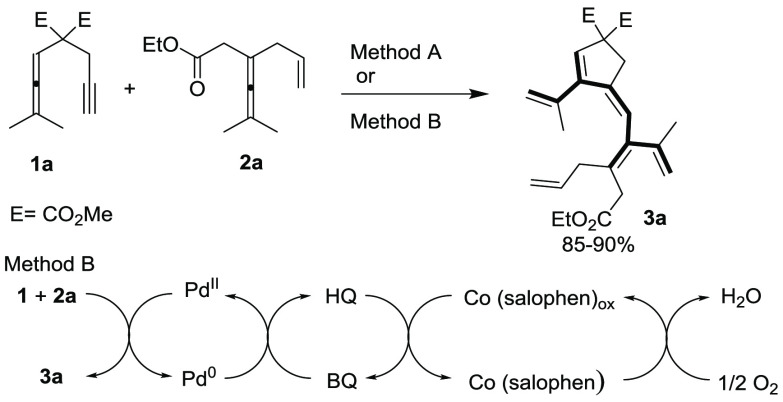
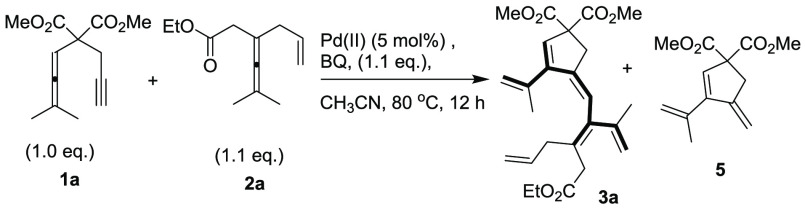
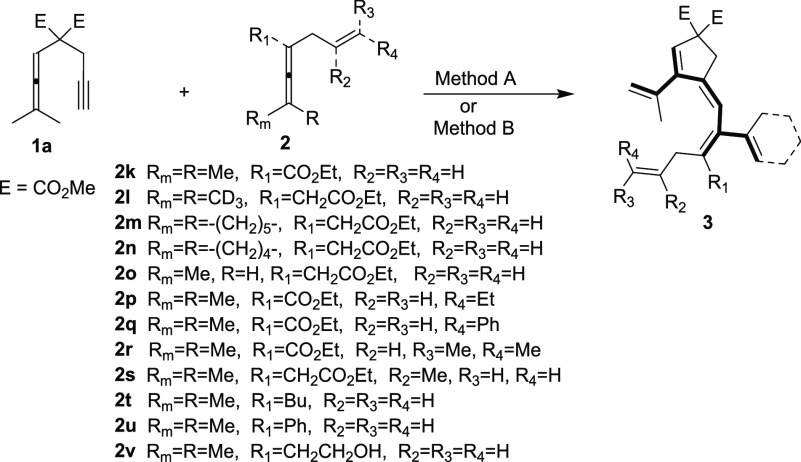
In our initial investigation, allenyne 1a and enallene 2a were chosen as the substrates for this challenging transformation (Scheme 4).
Scheme 4. Cascade Reaction.
Method A: 2a (2 equiv), Pd(OAc)2 (5 mol %), BQ (2 equiv), Na2CO3 (20 mol %), CH3CN, 80 °C, 24 h. Method B: Pd(OAc)2 (5 mol %), BQ (20 mol %), Co(salophen) (5 mol %), Na2CO3 (20 mol %), CH3CN, 80 °C, O2 balloon, 24 h.
Preparation of Starting Materials
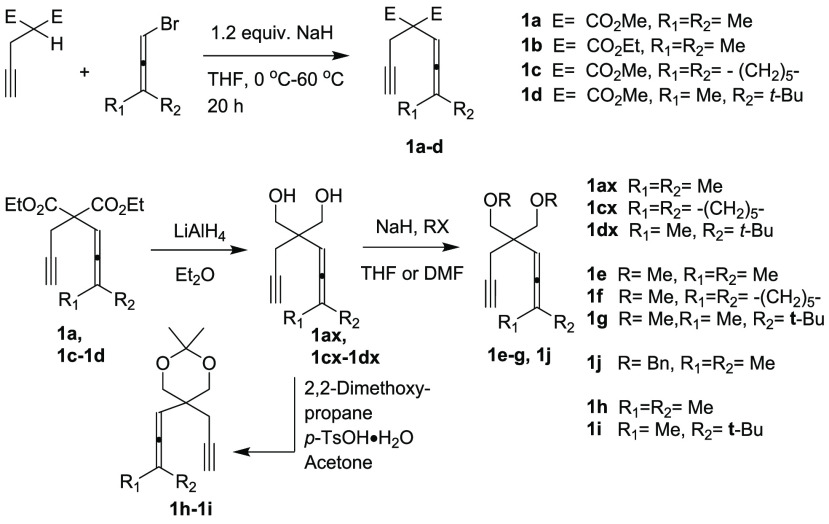
All allenynes 1 were prepared from propargyl malonate and the corresponding bromoallenes (Scheme 5 and Experimental Section). The eneallenes 2 were synthesized from propargyl alcohol derivatives and subsequent 1,3-rearrangement or iron-catalyzed SN2′ Grignard reaction (see Supporting Information).
Scheme 5. Preparation of Allenynes.
In preliminary experiments, we observed that treatment of 1a and 1.1 equiv of 2a with 5 mol % of Pd(OAc)2 and 1.1 equiv of benzoquinone (BQ) in DCE at 80 °C gave a 64% NMR yield of the regio- and stereodefined dendralene derivative 3a together with 6% of cycloisomerization product 5 (Table 1, entry 1).14 With these inspiring results in hand, we set out to determine the role of the solvent and the Pd(II) catalyst. In a screening of various solvents in the presence of Pd(OAc)2 catalyst (Table 1, entries 1–8), CH3CN was found to be the best solvent, which delivered the product 3a in 75% yield without formation of side product 5 (entry 8). Furthermore, palladium catalyst screening showed that Pd(TFA)2, Pd(PPh3)2Cl2, and Pd(PhCN)2Cl2 were not suitable for the reaction and in these cases the allenyne 1a and enallene 2a were recovered (Table 1, entries 9–11). However, Pd(acac)2 catalyzed the reaction efficiently to give the product 3a in 60% yield. (Table 1, entry 12). In further optimizations, treatment of 1a and 1.5 equiv of 2a with 5 mol % of Pd(OAc)2, and 1.5 equiv of BQ afforded 3a in 80% yield (Table 1, entry 13). Increasing 1a and BQ to 2.0 equiv each with 1 mol % of Pd(OAc)2 improved the yield of 3a to 83% (Table 1, entry 14). The yield of 3a further increased with the addition 0.2 equiv of Na2CO3 (Table 1, entry 15). With an increased reaction time, we found that treatment of 1a and 2.0 equiv of 2a with 5 mol % of Pd(OAc)2, and 2.0 equiv of BQ in CH3CN at 80 °C for 24 h afforded 3a in 94% yield (Method A) (Table 1, entry 16). Additives such as Et3N or AcOH did not improve the yield of 3a (Table 1, entries 17–18).
Table 1. Optimizatiion of the Reactiona.
| Yield
(%)b |
|||||
|---|---|---|---|---|---|
| Entry | Cat. (Pd) | Solvent | 3a | 5 | Recovery of 1a/2a (%)b |
| 1 | Pd(OAc)2 | DCE | 64 | 6 | – |
| 2 | Pd(OAc)2 | Dioxane | 51 | 6 | – |
| 3 | Pd(OAc)2 | DMSO | 55 | – | – |
| 4c | Pd(OAc)2 | THF | 43 | – | – |
| 5c | Pd(OAc)2 | MeOH | 50 | 4 | – |
| 6c | Pd(OAc)2 | Acetone | 33 | – | – |
| 7 | Pd(OAc)2 | Toluene | 65 | 7 | – |
| 8 | Pd(OAc)2 | MeCN | 75 | – | – |
| 9 | Pd(TFA)2 | MeCN | – | – | 94/91 |
| 10 | Pd(PPh3)2Cl2 | MeCN | – | – | 70/71 |
| 11 | Pd(MeCN)2Cl2 | MeCN | – | – | 71/47 |
| 12 | Pd(acac)2 | MeCN | 60 | 6 | – |
| 13g | Pd(OAc)2 | MeCN | 80 | – | – |
| 14d,f | Pd(OAc)2 | MeCN | 83 | – | – |
| 15d,e | Pd(OAc)2 | MeCN | 88 | – | – |
| 16d,e,h | Pd(OAc)2 | MeCN | 94 | – | – |
| 17d,h,i | Pd(OAc)2 | MeCN | 91 | – | – |
| 18d,h,j | Pd(OAc)2 | MeCN | 90 | – | – |
The reaction was conducted in the indicated solvent (1 mL) at 80 °C using 1a (0.1 mmol), allene-ene 2a (0.11 mmol), and BQ (1.1 equiv) in the presence of palladium catalyst (5 mol %).
Yield determined by 1H NMR analysis using anisole as the internal standard.
The reaction was run at 60 °C.
2a (2.0 equiv) and 2.0 equiv of BQ was used.
0.2 equiv of Na2CO3 was used.
1 mol % Pd(OAc)2 was used.
2a (1.5 equiv) and 1.5 equiv of BQ was used.
Reaction time 24 h.
Et3N (20 mol %).
AcOH (20 mol %).
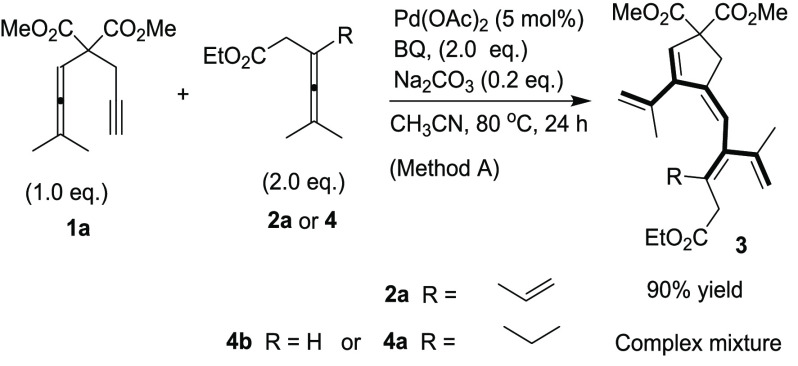
To demonstrate the necessity of the olefin group in the enallene 2a,15 comparative experiments with allenes lacking the pending olefin were carried out. Without the pending olefin, these reactions failed to give any cross-conjugated polyene product 3 with Method A in CH3CN (Scheme 6). Thus, when 4a and 4b were allowed to react with 1a, no detectable amounts of 3 were formed. These results are in accordance with previous results that the olefin group of 2a is an indispensable assisting/directing group for activation of the allene.15
Scheme 6. Comparative Experiment.
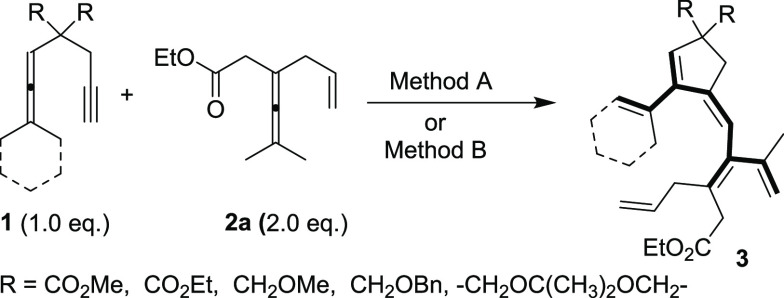
In further studies, we investigated Pd(II)-catalyzed aerobic oxidative coupling–carbocyclization reactions between allenyne 1a and enallene 2a for the synthesis of 3a (Scheme 4, Method B). We have previously developed various biomimetic methods for palladium-catalyzed aerobic oxidation of unsaturated substrates.16 The employment of an aerobic biomimetic oxidation system is an environmentally benign process associated with high atom economy.17 A key feature of Scheme 4, Method B is the multistep electron transfer occurring, which enables a mild aerobic oxidation. This multistep electron transfer system involves three redox pairs: PdII/Pd0, (BQ)/HQ, and Co(salophen)ox/Co(salophen). The BQ and Co(salophen) are used as electron transfer mediators (ETMs), and molecular oxygen is applied as the oxidant. We found that reaction of 1a with 2a in the presence of catalytic amounts of Pd(OAc)2 (5 mol %), BQ (20 mol %), and Co(salophen) (5 mol %) in CH3CN at 80 °C under molecular oxygen (1 atm) for 24 h afforded 3a in 85% yield (Method B). Under optimized reaction conditions Methods A and B, we investigated the scope of the reaction by using different allenyne substrates (Table 2, 1a–1j).
Table 2. Scope of Substrate 1a.
Isolated yield after column chromatography Method A: 2a (2 equiv), Pd(OAc)2 (5 mol %), BQ (2 equiv), Na2CO3 (20 mol %), CH3CN, 80 °C, 24 h. Method B: Pd(OAc)2 (5 mol %), BQ (20 mol %), Co(salophen) (5 mol %), Na2CO3 (20 mol %), CH3CN, 80 °C, O2 balloon, 24 h.
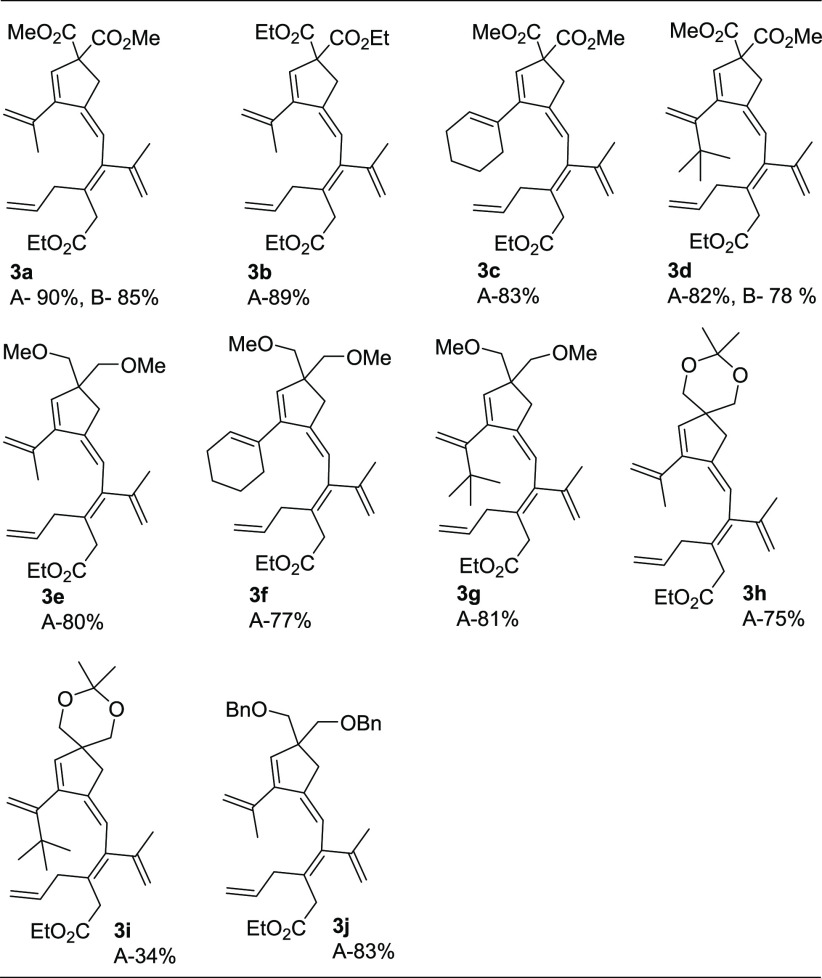
Under standard nonaerobic conditions (Method A), with methyl groups at the terminal position of the allene moiety of the allenenyne or when these methyl groups were changed to cyclohexylidene or one of them to t-Bu, the reaction with 2a gave the corresponding cross-conjugated polyene (Table 2, 3a–3d) in good yields (82–90%). The use of either stoichiometric amounts of BQ (Method A) or catalytic amounts of BQ under aerobic conditions (Method B) afforded similar results, as shown in Table 2 from the examples 3a and 3d. It is worth noting that the reaction of allenyne substrates 1e–1j (Table 2) having two methyl ethers, a 1,3 dioxane, or two benzyl ethers in place of the two carboalkoxy groups, along with cyclohexylidene or tertiary butyl on the allene moiety, afforded the corresponding polyene derivatives (Table 2, 3e–3j) selectively in good yields (70–83%), except for 1i, which afforded 3i in 34% yield. These results show that the malonate group of the tether is not necessary for a successful transformation.
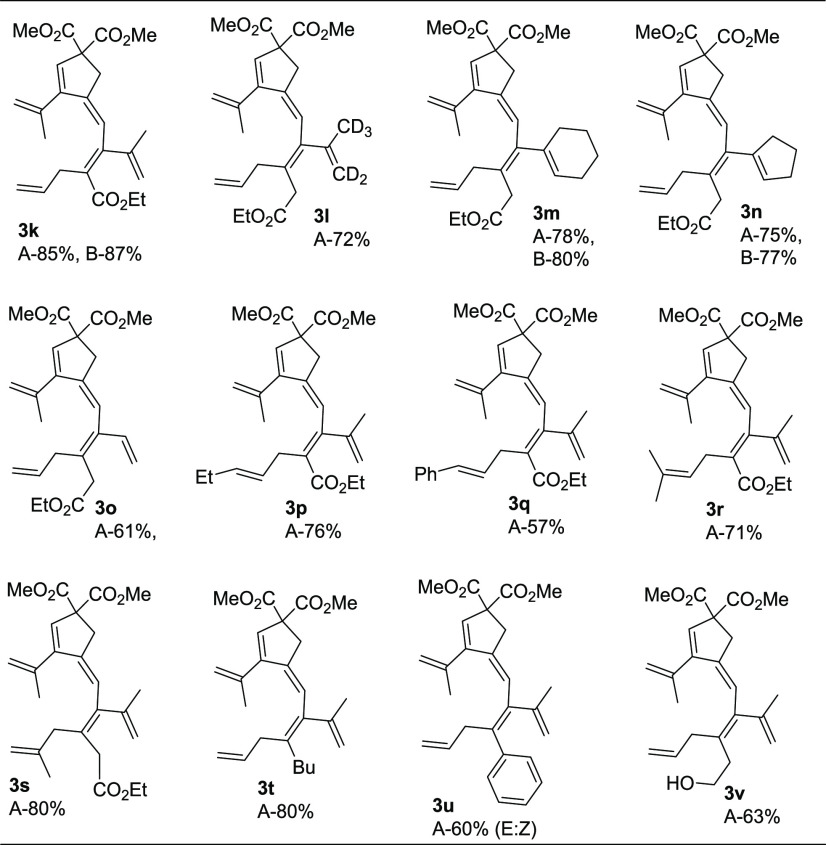
To expand the scope of the method, we tested differently substituted enallenes 2 for the coupling–carbocyclization cascade reaction using allenyne 1a as the cosubstrate. Under standard conditions (Method A), a number of functionalized enallenes 2 served as excellent candidates for formation of cross-conjugated polyenes in good yields. When allyl-substituted 2,3-dienoate (2k) was employed, the reaction gave the desired product 3k in 85% yield. Variation of the methyl groups on the allene moiety of 2, e.g. deuterated methyls (2l), cyclohexylidene group (2m), cyclopentylidene group (2n), or one methyl presence (2o), in the reaction with 1a provided the corresponding cross-conjugated polyenes in good yields (Table 3, 3l–3o). Moreover, substrates 2p–2s with methyl, ethyl, or phenyl substitution on the olefin moiety afforded 3p–3s (Table 3) in moderate to good yields (57–80%). Reaction of substituted enallenes 2t–2v with 1a afforded the desired products 3t–3v in 60–80% yield (Table 3). As shown in Table 3, the reaction also works under aerobic conditions with catalytic amounts of BQ together with Co(salophen) in catalytic amounts (Method B). Thus, reaction of enallenes 2k, 2m–2n with 1a using molecular oxygen as the oxidant afforded products 3k, 3m–3n in 77–85% yield (Table 3).
Table 3. Scope of Enallene Substrates 2a.
Isolated yield after column chromatography. Method A: 2a (2 equiv), Pd(OAc)2 (5 mol %), BQ (2 equiv), Na2CO3 (20 mol %), CH3CN, 80 °C, 24 h. Method B: Pd(OAc)2 (5 mol %), BQ (20 mol %), Co(salophen) (5 mol %), Na2CO3 (20 mol %), CH3CN, 80 °C, O2 balloon, 24 h.
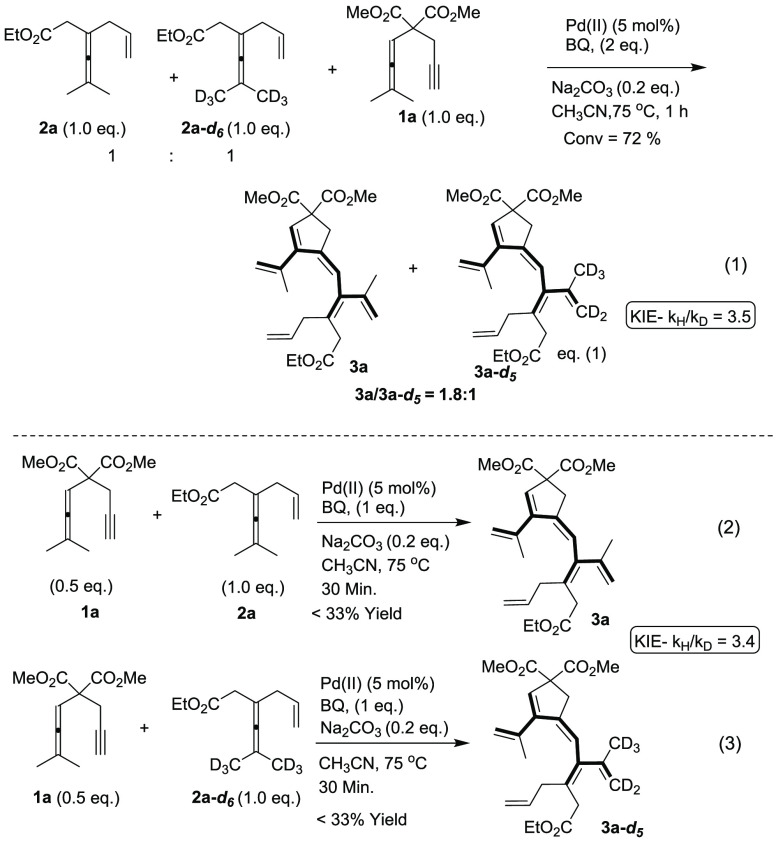
To gain further insight into the reaction mechanism, the deuterium kinetic isotope effects (KIE) were studied (eqs 1–3). An intermolecular competition experiment was conducted at 75 °C using a 1:1 mixture of 2a and 2a-d6 (eq 1). The products ratio 3a and 3a-d5 was measured as 1.8:1, from which the competitive KIE was determined to be kH/kD = 3.5 (see Supporting Information). Furthermore, parallel kinetic experiments afforded a KIE (kH/kD from initial rate) value of 3.4 (eqs 2 and 3) which indicates the initial allenylic C(sp3)–H bond cleavage is involved in the rate-determining step in the reaction. The large competitive KIE (kH/kD = 3.5) in C–H bond cleavage requires that this step is the first irreversible step.
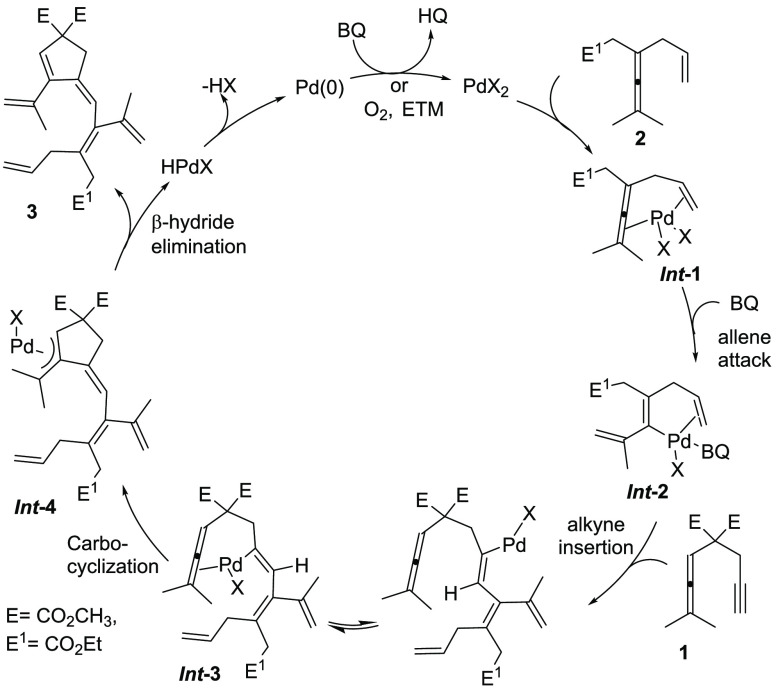
Based on the mechanistic studies including the KIE measurements (eqs 1–3) and our previous work,18 we propose the mechanism as shown in Scheme 7. The large deuterium isotope effect found for the C–H bond cleavage of the enallene 2a indicates that the enallene is the compound first activated and not the allenyne.19 Initial reaction of Pd(OAc)2 with enallene 2 would give dienyl–PdII complex Int-2 via allenic C–H bond cleavage of chelated π-complex Int-1 (Scheme 7). This activation of the allene is triggered by the coordination of the assisting olefin.15 Vinylpalladium intermediate Int-2 would then undergo an insertion of the vinylpalladium bond into the alkyne of allenyne 1, which leads to Int-3. Subsequent intramolecular insertion of the vinylpalladium bond of Int-3 into the allene would lead to (π-allyl)-palladium intermediate Int-4. Subsequent β-hydride elimination via C(sp3)–H bond cleavage would provide the cross-conjugated polyene 3 and release Pd0 for the next cycle.
Scheme 7. Proposed Mechanism for the Formation of 3.
 |
1 |
Conclusion
We have developed an efficient one-pot PdII-catalyzed oxidative coupling–carbocyclization cascade reaction for the synthesis of cross-conjugated polyene via intermolecular C–C bond formation and subsequent carbocyclization. This transformation allows highly regio- and stereoselective formation of cross-conjugated polyenes using enallene and allenyne under aerobic conditions with environmentally friendly O2 as the terminal oxidant. These important cross-conjugated polyenes, which are readily obtained in a one-pot cascade reaction in the present work, are difficult to prepare by other methods. Further studies on the scope of natural product synthesis and other synthetic application of this new cascade reaction are currently underway in our laboratory.
Experimental Section
General Information
For the synthesis of complex molecules, unless otherwise noted, all reagents were used as received from the commercial suppliers. Pd(OAc)2 was obtained from Pressure Chemicals and used without further purification. Alkynes were commercially available from Sigma-Aldrich or Acros. The palladium-catalyzed cascade reactions could be performed without any efforts to exclude moisture. DCE was distilled using CaH2, Dry THF and toluene, were obtained froma VAC Solvent Purifier. The other dry solvents were purchased from Sigma-Aldrich. Reactions were monitored using thin-layer chromatography (TLC) (SiO2). TLC plates were visualized with UV light (254 nm) or KMnO4 stain. Flash chromatography was carried out with 60 Å (particle size 35–70 μm) normal flash silica gel. NMR spectra were recorded at 400 MHz (1H) or 500 MHz (1H) and at 100 MHz (13C) or 125 MHz (13C), respectively. Chemical shifts (δ) are reported in ppm, using the residual solvent peak in CDCl3 (H = 7.26 and C = 77.0 ppm) as the internal standard, and coupling constants (J) are given in Hz. HRMS data were recorded using ESI-TOF techniques.
Allenynes 1a(20) and 1c(21) were prepared as described in literature. Allenynes 1b and 1d were prepared from propargylmalonate and the corresponding bromoallene in a similar manner.20 All allene derivatives 2a, 2k–2v, and 4a–4b were prepared according to a previously described procedures.3d,15,18c,22
Representative Procedure for the Synthesis of 1b and 1d: Synthesis of 1b
To a suspension of NaH (60% in mineral oil, 0.456 g, 11.4 mmol) in anhydrous THF (60 mL) was added a solution of diethyl propargylmalonate (2.0 g, 8.83 mmol) in anhydrous THF (5 mL) at 0 °C. After the addition, the mixture was stirred for another 20 min at room temperature Then a solution of bromoallene (2.6 g, 17.6 mmol) in anhydrous THF (5 mL) was added at room temperature and the resulting mixture was refluxed for 20 h. After the reaction was complete as monitored by TLC, it was cooled to room temperature. Most of the solvent was removed under vacuum, and then the reaction mixture was diluted with 50 mL of Et2O and quenched with 10 mL of water. The organic layer was separated, and the aqueous layer was extracted with diethyl ether (2 × 20 mL). The combined organic layers were dried over Na2SO4. Evaporation and column chromatography on silica gel (pentane/ethyl acetate = 30/1) afforded 1b (0.71 g, 31%).
Characterization of Allenynes 1b and 1d
Diethyl-2-(3-methyl-2λ5-buta-1,2-dien-1-yl)-2-(prop-2-yn-1-yl)malonate (1b)
1H NMR (400 MHz, CDCl3) δ = 4.94 (sept, J = 2.9 Hz, 1H), 4.25–4.15 (m, 4H), 2.86 (d, J = 2.7 Hz, 2H), 1.95 (t, J = 2.7 Hz, 1H), 1.71 (d, J = 2.9 Hz, 6H), 1.25 (t, J = 7.1 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 201.7, 169.2, 100.5, 87.7, 79.6, 70.4, 61.7, 57.5, 24.3, 19.9, 13.9; HRMS (ESI) m/z: [M + Na]+ calcd for C15H20O4Na, 287.1254; found, 287.1263. Isolated yield: 31% (0.71 g), as a liquid. Column chromatography on silica gel (pentane/ethyl acetate = 30/1).
In the same manner 1d was obtained from 1-bromo-3,4,4-trimethyl-1,2-pentadiene.
Dimethyl-2-(prop-2-yn-1-yl)-2-(3,4,4-trimethyl-2λ5-penta-1,2-dien-1-yl)malonate (1d)
1H NMR (400 MHz, CDCl3) δ = 5.64 (q, J = 2.9 Hz, 1H), 3.74 (d, J = 9.5 Hz, 6H), 2.87 (d, J = 2.7 Hz, 2H), 1.97 (t, J = 2.7 Hz, 1H), 1.71 (d, J = 2.8 Hz, 3H), 1.02 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 200.0, 169.6, 114.2, 89.2, 79.5, 70.7, 57.7, 52.8, 52.7, 33.7, 28.8, 24.6, 14.5; HRMS (ESI) m/z: [M + Na]+ calcd for C16H22O4Na, 301.1410; found, 301.1421. Isolated yield: 32% (0.79 g), as a liquid. Column chromatography on silica gel (pentane/ethyl acetate = 30/1).
Representative Procedure for the Synthesis of 1ax, 1cx, and 1dx: Synthesis of 1ax
To a suspension of LiAlH4 (171 mg, 4.5 mmol) in anhydrous Et2O (15 mL) was added a solution of 1a (0.34 g, 1.5 mmol) in anhydrous Et2O (10 mL) at 0 °C. After the addition, the mixture was stirred for another 2 h at rt and carefully quenched with H2O (5 mL). The resulting mixture was extracted with diethyl ether (2 × 30 mL). The combined organic layers were washed with H2O (20 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel, pentane/ethyl acetate = 1:3) to yield 1ax (203 mg, 79% yield) as a white solid.
Compounds 1cx and 1dx were prepared from 1c and 1d, respectively, in the same manner.
Characterization of Products 1ax, 1cx, and 1dx
2-(3-Methyl-2λ5-buta-1,2-dien-1-yl)-2-(prop-2-yn-1-yl)propane-1,3-diol (1ax)
1H NMR (400 MHz, CDCl3) δ = 4.94 (sept, J = 2.9 Hz, 1H), 3.69 (s, 4H), 2.40 (d, J = 2.7 Hz, 2H), 2.04 (bs, 2H), 2.02 (d, J = 2.7 Hz, 1H), 1.71 (d, J = 3.0 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 201.7, 98.3, 90.0, 81.1, 70.6, 67.4, 44.5, 22.9, 20.6; HRMS (ESI) m/z: [M + Na]+ calcd for C11H16O2Na, 203.1043; found, 203.1049. Isolated yield: 79% (203 mg), as a semi-solid. Column chromatography (silica gel, pentane/ethyl acetate = 1:3).
2-(2-Cyclohexylidene-2λ5-vinyl)-2-(prop-2-yn-1-yl)propane-1,3-diol (1cx)
1H NMR (400 MHz, CDCl3) δ = 4.94 (quint, J = 2.9 Hz, 1H), 3.67 (d, J = 1.0 Hz, 4H), 2.39 (d, J = 2.7 Hz, 2H), 2.39 (bs, 2H), 2.16–2.06 (m, 4H), 2.00 (t, J = 2.7 Hz, 1H), 1.42–1.68 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 198.3, 105.5, 89.8, 81.1, 70.6, 67.2, 44.4, 31.6, 27.3, 25.9, 22.9; HRMS (ESI) m/z: [M + Na]+ calcd for C14H20O2Na, 243.1356; found, 243.1362. Isolated yield: 81% (268 mg), as a semi-solid. Column chromatography (silica gel, pentane/ethyl acetate = 1:2.5).
2-(Prop-2-yn-1-yl)-2-(3,4,4-trimethyl-2λ5-penta-1,2-dien-1-yl)propane-1,3-diol (1dx)
1H NMR (400 MHz, CDCl3) δ = 5.01 (q, J = 2.9 Hz, 1H), 3.70 (s, 4H), 2.41 (d, J = 2.7 Hz, 2H), 2.17 (bs, 2H), 2.02 (t, J = 2.7 Hz, 1H), 1.71 (d, J = 2.9 Hz, 3H), 1.05 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 200.0, 111.6, 91.7, 81.2, 70.7, 67.5, 67.4, 44.3, 33.1, 29.0, 29.6, 22.8, 15.4; HRMS (ESI) m/z: [M + Na]+ calcd for C14H22O2Na, 245.1512; found, 245.1521. Isolated yield: 97% (323 mg), as a semi-solid. Column chromatography (silica gel, pentane/ethyl acetate = 1:3).
Representative Procedure for the Synthesis of 1e, 1f, and 1g: Synthesis of 1e
To a suspension of 1ax (160 mg, 0.89 mmol) in THF (10 mL) was added NaH (60% in mineral oil (142 mg, 3.6 mmol)) at 0 °C. The reaction mixture was subsequently stirred for 30 min at the same temperature, and then MeI (0.45 mL, 7.1 mmol) was added. The reaction mixture was warmed to room temperature and stirred for 1 h. The reaction mixture was quenched by addition of water and extracted with Et2O (2 × 20 mL). The combined organic layers were washed with brine (20 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (silica gel, pentane/ethyl acetate = 98:2) to afford 1e (150 mg, 81% yield) as a colorless oil.
In the same manner, 1f and 1g were obtained from 1cx and 1dx, respectively.
Characterization of Products 1e, 1f, and 1g
4,4-Bis(methoxymethyl)-7-methyl-6λ5-octa-5,6-dien-1-yne (1e)
1H NMR (400 MHz, CDCl3) δ = 5.00 (sept, J = 2.9 Hz, 1H), 3.36–3.31 (m, 10H), 2.32 (d, J = 2.5 Hz, 2H), 1.94 (t, J = 2.5 Hz, 1H), 1.68 (d, J = 2.7 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 201.5, 97.4, 90.7, 81.6, 75.4, 69.7, 59.3, 43.4, 23.4, 20.5; HRMS (ESI) m/z: [M + Na]+ calcd for C13H20O2Na, 231.1356; found, 231.1367. Isolated yield: 81% (150 mg), as a colorless oil. Flash column chromatography (silica gel, pentane/ethyl acetate = 98:2).
(3,3-Bis(methoxymethyl)-1λ5-hex-1-en-5-yn-1-ylidene)cyclohexane (1f)
1H NMR (400 MHz, CDCl3) δ = 5.00 (quint, J = 2.1 Hz, 1H), 3.34 (d, J = 2.9 Hz, 4H), 3.33 (s, 6H), 2.34 (d, J = 2.7 Hz, 2H), 2.19–2.05 (m, 4H), 1.94 (t, J = 2.7 Hz, 1H), 1.42–1.67 (m, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 198.0, 104.7, 90.5, 81.5, 75.5, 69.8, 59.4, 43.2, 31.5, 27.3, 26.1, 23.4; HRMS (ESI) m/z: [M + Na]+ calcd for C16H24O2Na, 271.1669; found, 271.1670. Isolated yield: 95% (209 mg), as a colorless oil. Flash column chromatography (silica gel, pentane/ethyl acetate = 98:2).
4,4-Bis(methoxymethyl)-7,8,8-trimethyl-6λ5-nona-5,6-dien-1-yne (1g)
1H NMR (400 MHz, CDCl3) δ = 5.08 (q, J = 2.9 Hz, 1H), 3.36–3.29 (m, 10H), 2.32 (d, J = 2.6 Hz, 2H), 1.94 (t, J = 2.6 Hz, 1H), 1.68 (d, J = 2.7 Hz, 3H) 1.04 (s, 9H); 113C{1H} NMR (100 MHz, CDCl3) δ = 199.7, 110.7, 92.5, 81.7, 75.5, 69.9, 59.3, 43.3, 33.1, 29.0, 23.3, 15.2; HRMS (ESI) m/z: [M + Na]+ calcd for C16H26O2Na, 273.1825; found, 273.1829. Isolated yield: 85% (189 mg), as a colerless oil. Flash column chromatography (silica gel, pentane/ethyl acetate = 98:2).
Representative Procedure for the Synthesis of 1h and 1i: Synthesis of 1h
To a suspension of 1ax (108 mg, 0.60 mmol) in acetone (5 mL) was added 2,2-dimethoxypropane (72 mg, 0.69 mmol) and p-TsOH·H2O (5.7 mg, 0.03 mmol). The reaction mixture was stirred at room temperature for 3 h and quenched with sat. NaHCO3 (aq.). Acetone was evaporated, and the residue was extracted with Et2O (2 × 20 mL). The combined organic layers were washed with brine (20 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (silica gel, pentane/ethyl acetate = 97:3) to yield 1h (111 mg, 84% yield) as a colorless oil.
In the same manner, 1i was obtained from 1dx.
Characterization of Products 1h and 1i
2,2-Dimethyl-5-(3-methyl-2λ5-buta-1,2-dien-1-yl)-5-(prop-2-yn-1-yl)-1,3-dioxane (1h)
1H NMR (400 MHz, CDCl3) δ = 4.88 (sept, J = 2.9 Hz, 1H), 3.70 (qt, J = 14.5 Hz, J = 11.6 Hz, 4H), 2.53 (d, J = 2.6 Hz, 2H), 1.99 (t, J = 2.7 Hz, 1H), 1.40 (d, J = 3.0 Hz, 6H), 1.39 (d, J = 8.1 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 201.7, 98.2, 97.9, 89.9, 81.4, 70.4, 66.9, 36.9, 27.6, 23.8, 20.5, 19.8; HRMS (ESI) m/z: [M + Na]+ calcd for C14H20NaO2, 243.1356; found, 243.1364. Isolated yield: 84% (111 mg), as a colorless oil. Flash column chromatography (silica gel, pentane/ethyl acetate = 97:3).
2,2-Dimethyl-5-(prop-2-yn-1-yl)-5-(3,4,4-trimethyl-2λ5-penta-1,2-dien-1-yl)-1,3-dioxane (1i)
1H NMR (400 MHz, CDCl3) δ = 4.98 (q, J = 2.9 Hz, 1H), 3.77–3.61 (m, 4H), 2.54 (qd, J = 13.0 Hz, 2.6 Hz, 2H), 1.99 (t, J = 2.7 Hz, 1H), 1.70 (d, J = 3.0 Hz, 3H), 1.40 (d, J = 6.5 Hz, 6H), 1.04 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 199.9, 111.6, 97.9, 91.5, 81.5, 70.6, 66.9, 66.8, 36.8, 33.0, 29.0, 27.9, 24.0, 19.5, 15.3; HRMS (ESI) m/z: [M + Na]+ calcd for C17H26O2Na, 285.1825; found, 285.1822. Isolated yield: 81% (128 mg), as a colorless oil. Flash column chromatography (silica gel, pentane/ethyl acetate = 97:3).
Synthesis and Characterization of 1j
(((2-(3-Methyl-2λ5-buta-1,2-dien-1-yl)-2-(prop-2-yn-1-yl)propane-1,3-diyl)bis(oxy))bis(methylene))dibenzene (1j)
To a suspension of NaH (60% in mineral oil, 124 mg, 3.1 mmol) in anhydrous DMF (3 mL) was added a solution of 1ax (140 mg, 0.78 mmol) in anhydrous DMF (2 mL) at 0 °C. After the addition, the mixture was stirred for another 1 h at 0 °C. Then BnBr (170 μL, 1.4 mmol) was added at 0 °C, and the resulting mixture was stirred at rt overnight. Then the reaction mixture was diluted with 20 mL of Et2O and quenched with 5 mL of water. The organic layer was separated, and the aqueous layer was extracted with diethyl ether (2 × 20 mL). The combined organic layers were washed with H2O (10 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (silica gel, pentane/diethyl ether = 100:1) to yield 1j (221 mg, 79% yield) as a liquid; 1H NMR (400 MHz, CDCl3): δ = 7.34–7.26 (m, 10H), 5.12 (sept, J = 2.9 Hz, 1H), 4.54 (s, 4H), 3.53 (s, 4H), 2.45 (d, J = 2.7 Hz, 2H), 1.95 (t, J = 2.7 Hz, 1H), 1.70 (d, J = 2.9 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3): δ = 201.5, 138.7, 128.2, 127.3, 97.4, 90.9, 81.7, 73.3, 73.1, 69.8, 43.8, 23.5, 20.5; HRMS (ESI) m/z: [M + Na]+ calcd for C25H28O2Na, 383.1982; found, 383.1987.
Method A: Nonaerobic Oxidative Coupling-Carbocyclization for Preparation of 3
Representative Procedure: Synthesis of 3a
In a sealable microwave tube were placed 1a (47.2 mg, 0.2 mmol), Pd(OAc)2 (2.2 mg, 0.01 mmol), 1,4-benzoquinone (BQ) (43.2 mg, 0.4 mmol), anhydrous Na2CO3 (4.2 mg, 0.04 mmol), and 2a (77 mg, 0.4 mmol). To this mixture, 2.0 mL of anhydrous CH3CN solvent were added and the tube was sealed with the cap. The reaction was stirred at 80 °C in an oil bath for 24 h. After full consumption of starting material 1a as monitored by TLC, the reaction mixture was concentrated in vacuo and purified via short column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95) affording 77 mg (90%) of 3a as a liquid. The reaction was also run on a 1.3 mmol scale using 1a (0.307 g, 1,3 mmol), which afforded 0.45g (81%) of 3a after short column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Method B: Aerobic Oxidative Coupling-Carbocyclization for Preparation of 3
Representative Procedure: Synthesis of 3a
In a sealable microwave tube were placed 1a (47.2 mg, 0.2 mmol), Pd(OAc)2 (2.2 mg, 0.01 mmol), Cobalt(salophen) (3.7 mg, 0.01 mmol), 20 mol % of 1,4-benzoquinone (BQ) (4.4 mg, 0.04 mmol), anhydrous Na2CO3(4.2 mg, 0.04 mmol) and 2a (77 mg, 0.4 mmol). The tube was sealed with the cap. To this mixture was added 2.0 mL of anhydrous CH3CN via syringe. Then the reaction was stirred at 80 °C in an oil bath under an oxygen atmosphere using molecular oxygen balloon connected via a needle for 24 h. After full consumption of starting material 1a as monitored by TLC, the reaction mixture was concentrated in vacuo and purified via short column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95) afforded 73 mg (85%) of 3a as a liquid.
Characterization of Products 3
Dimethyl-(Z)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3a)
1H NMR (400 MHz, CDCl3) δ = 6.24 (s, 1H), 5.94 (s, 1H), 5.76–5.66 (m, 1H), 5.15 (s, 1H), 5.10 (s, 1H), 5.06 (s, 1H), 5.02 (d, J = 4.9 Hz, 1H), 4.98 (s, 1H), 4.85 (d, J = 1.2 Hz, 1H), 4.09 (q, J = 7.1 Hz, 2H), 3.71 (s, 6H), 3.19 (d, J = 4.0 Hz, 4H), 2.93 (d, J = 6.4 Hz, 2H), 1.92 (s, 3H), 1.81 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.9, 170.9, 151.1, 144.2, 142.3, 139.9, 138.0, 135.0, 128.8, 119.5, 116.3, 116.0, 115.9, 63.8, 60.4, 52.7, 38.1, 36.9, 36.7, 23.2, 23.0, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C25H32O6Na, 451.2091; found, 451.2069. Isolated yield: Method A, 90% (77 mg); Method B, 85% (73 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Diethyl-(Z)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3b)
1H NMR (400 MHz, CDCl3) δ = 6.24 (s, 1H), 5.95 (s, 1H), 5.76–5.66 (m, 1H), 5.15 (s, 1H), 5.10 (s, 1H), 5.06 (s, 1H), 5.02 (d, J = 6.6 Hz, 1H), 4.98 (s, 1H), 4.86 (s, 1H), 4.22–4.04 (m, 6H), 3.19 (s, 4H), 2.94 (d, J = 6.1 Hz, 2H), 1.93 (s, 3H), 1.82 (s, 3H), 1.23 (t, J = 7.1 Hz, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.9, 170.5, 151.1, 144.3, 142.6, 140.0, 138.1, 135.0, 129.2, 128.7, 119.4, 116.1, 115.9, 115.8, 64.0, 61.5, 60.4, 38.2, 36.8, 36.7, 23.2, 23.1, 14.1, 13.9; HRMS (ESI) m/z: [M + Na]+ calcd for C27H36O6Na, 479.2404; found, 479.2382. Isolated yield: Method A, 89% (82 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-3-(cyclohex-1-en-1-yl)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)cyclopent-2-ene-1,1-dicarboxylate (3c)
1H NMR (400 MHz, CDCl3) δ = 6.21 (s, 1H), 5.86 (s, 1H), 5.82–5.80 (m, 1H), 5.78–5.68 (m, 1H), 5.11–5.09 (m, 1H), 5.04–5.02 (m, 1H), 4.99 (s, 1H), 4.85 (d, J = 1.2 Hz, 1H), 4.09 (q, J = 7.1 Hz, 2H), 3.71 (s, 6H), 3.20 (s, 2H), 3.18 (d, J = 2.2 Hz, 2H), 2.94 (d, J = 6.4 Hz, 2H), 2.14–2.10 (m, 4H), 1.82 (s, 3H), 1.72–1.56 (m, 4H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.0, 171.2, 152.0, 144.2, 142.8, 140.2, 135.0, 131.4, 128.5, 128.1, 127.5, 119.2, 115.9, 115.8, 63.8, 60.4, 52.7, 38.2, 36.9, 36.8, 28.6, 25.3, 23.3, 22.7, 22.0, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C28H36O6Na, 491.2404; found, 491.2392. Isolated yield: Method A, 83% (77 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-3-(3,3-dimethylbut-1-en-2-yl)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)cyclopent-2-ene-1,1-dicarboxylate (3d)
1H NMR (400 MHz, CDCl3) δ = 6.07 (s, 1H), 5.80 (s, 1H), 5.76–5.63 (m, 1H), 5.25 (d, J = 1.4 Hz, 1H), 5.11 (s, 1H), 5.02–4.97 (m, 2H), 4.87–4.86 (m, 1H), 4.74 (d, J = 1.4 Hz, 1H), 4.09 (q, J = 7.1 Hz, 2H), 3.73 (s, 6H), 3.19 (s, 2H), 3.17 (d, J = 2.2 Hz, 2H), 2.91 (d, J = 6.4 Hz, 2H), 1.79 (s, 3H), 1.23 (t, J = 7.1 Hz, 3H), 1.06 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.0, 171.2, 152.0, 144.3, 142.8, 140.1, 135.0, 131.4, 128.5, 128.1, 127.5, 119.2, 115.9, 115.8, 63.8, 60.4, 52.7, 38.2, 36.9, 36.8, 28.7, 25.3, 23.3, 22.7, 22.0, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C28H38O6Na, 493.2561; found, 493.2573. Isolated yield: Method A, 82% (78 mg); Method B, 78% (74 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Ethyl-(Z)-3-allyl-4-((Z)-(4,4-bis(methoxymethyl)-2-(prop-1-en-2-yl)cyclopent-2-en-1-ylidene)methyl)-5-methylhexa-3,5-dienoate (3e)
1H NMR (400 MHz, CDCl3) δ = 6.18 (s, 1H), 5.85 (s, 1H), 5.78–5.66 (m, 1H), 5.09 (s, 1H), 5.05–4.98 (m, 4H), 4.82 (s, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.31 (s, 6H), 3.26 (s, 4H), 3.19 (s, 2H), 2.94 (d, J = 6.4 Hz, 2H), 2.43 (d, J = 2.1 Hz, 2H), 1.91 (s, 3H), 1.79 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.2, 148.8, 145.0, 144.6, 140.6, 138.9, 136.1, 135.3, 127.2, 118.1, 115.8, 115.3, 115.0, 60.3, 59.3, 52.2, 38.0, 36.9, 36.4, 23.3, 23.2, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C25H36O4Na, 423.2506; found, 423.2494. Isolated yield: Method A, 80% (64 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 4:96).
Ethyl-(Z)-3-allyl-4-((Z)-(2-(cyclohex-1-en-1-yl)-4,4-bis(methoxymethyl)cyclopent-2-en-1-ylidene)methyl)-5-methylhexa-3,5-dienoate (3f)
1H NMR (400 MHz, CDCl3) δ = 6.14 (s, 1H), 5.83–5.68 (m, 3H), 5.06–4.98 (m, 3H), 4.81 (dd, J = 2.4 Hz, 0.9 Hz, 1H), 4.10 (q, J = 7.1 Hz, 2H), 3.31 (s, 6H), 3.26 (s, 4H), 3.19 (s, 2H), 2.94 (d, J = 6.4 Hz, 2H), 2.40 (d, J = 2.1 Hz, 2H), 2.16–2.07 (m, 4H), 1.79 (s, 3H), 1.71–1.57 (m, 4H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.2, 149.8, 145.5, 144.7, 140.8, 135.3, 134.5, 132.1, 126.9, 126.9, 117.7, 115.8, 115.2, 60.3, 59.3, 52.1, 38.1, 36.9, 36.4, 28.7, 25.3, 23.2, 22.9, 22.2, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C28H40O4Na: 463.2819; found, 463.2825. Isolated yield: Method A, 77% (68 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Ethyl-(Z)-3-allyl-4-((Z)-(2-(3,3-dimethylbut-1-en-2-yl)-4,4-bis(methoxymethyl)cyclopent-2-en-1-ylidene)methyl)-5-methylhexa-3,5-dienoate (3g)
1H NMR (400 MHz, CDCl3) δ = 5.97 (s, 1H), 5.76–5.68 (m, 1H), 5.66 (s, 1H), 5.20 (d, J = 1.7 Hz, 1H), 5.07–4.97 (m, 3H), 4.82 (dd, J = 2.4 Hz, 0.9 Hz, 1H), 4.69 (d, J = 1.7 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.32 (s, 6H), 3.28 (s, 4H), 3.18 (s, 2H), 2.91 (d, J = 6.4 Hz, 2H), 2.39 (d, J = 2.3 Hz, 2H), 1.76 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H), 1.06 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.3, 152.7, 148.7, 148.5, 144.4, 140.7, 136.5, 135.2, 127.1, 117.8, 115.6, 115.5, 112.2, 60.3, 59.3, 52.8, 38.1, 36.8, 35.8, 35.2, 29.5, 23.3, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C28H42O4Na, 465.2975; found, 465.2985. Isolated yield: Method A, 81% (71 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 4:96).
Ethyl-(Z)-3-allyl-4-((Z)-(8,8-dimethyl-3-(prop-1-en-2-yl)-7,9-dioxaspiro[4.5]dec-3-en-2-ylidene)methyl)-5-methylhexa-3,5-dienoate (3h)
1H NMR (400 MHz, CDCl3) δ = 6.21 (s, 1H), 5.96 (s, 1H), 5.77–5.66 (m, 1H), 5.11 (s, 1H), 5.07 (s, 1H), 5.04–4.99 (m, 3H), 4.84 (d, J = 1.3 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.69 (d, J = 11.3 Hz, 2H), 3.59 (d, J = 11.3 Hz, 2H), 3.20 (s, 2H), 2.93 (d, J = 6.4 Hz, 2H), 2.49 (d, J = 2.2 Hz, 2H), 1.92 (s, 3H), 1.79 (s, 3H), 1.43 (d, J = 5.8 Hz, 6H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.1, 149.1, 144.5, 144.1, 140.4, 138.8, 135.2, 135.1, 127.8, 118.6, 115.9, 115.6, 115.2, 97.5, 68.4, 60.4, 46.5, 38.1, 37.5, 36.8, 24.0, 23.5, 23.3, 23.3, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C26H36O4Na, 435.2506; found, 435.2497. Isolated yield: Method A, 75% (62 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Ethyl-(Z)-3-allyl-4-((Z)-(3-(3,3-dimethylbut-1-en-2-yl)-8,8-dimethyl-7,9-dioxaspiro[4.5]dec-3-en-2-ylidene)methyl)-5-methylhexa-3,5-dienoate (3i)
1H NMR (400 MHz, CDCl3) δ = 6.00 (s, 1H), 5.77 (s, 1H), 5.77–5.66 (m, 1H), 5.21 (s, 1H), 5.09 (s, 1H), 5.02 (d, J = 6.8 Hz, 1H), 4.97 (s, 1H), 4.85 (s, 1H), 4.69 (d, J = 1.5 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.72 (d, J = 11.3 Hz, 2H), 3.60 (d, J = 11.3 Hz, 2H), 3.19 (s, 2H), 2.91 (d, J = 6.4 Hz, 2H), 2.48 (d, J = 2.2 Hz, 2H), 1.77 (s, 3H), 1.43 (d, J = 4.3 Hz, 6H), 1.25 (t, J = 7.1 Hz, 3H), 1.06 (s, 9H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.3, 152.5, 149.1, 147.4, 144.4, 140.5, 135.5, 135.0, 127.7, 118.7, 115.8, 115.7, 112.5, 97.5, 68.6, 60.4, 47.1, 38.2, 36.8, 36.6, 35.7, 29.6, 24.3, 23.4, 23.3, 14.2;; HRMS (ESI) m/z: [M + Na]+ calcd for C29H42O4Na, 477.2975; found, 477.2977. Isolated yield: Method A, 34% (31 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Ethyl(Z)-3-allyl-4-((Z)-(4,4-bis((benzyloxy)methyl)-2-(prop-1-en-2-yl)cyclopent-2-en-1-ylidene)methyl)-5-methylhexa-3,5-dienoate (3j)
1H NMR (400 MHz, CDCl3) δ = 7.34–7.23 (m, 10H), 6.19 (s, 1H), 5.93 (s, 1H), 5.80–5.68 (m, 1H), 5.11–4.99 (m, 5H), 4.84 (s, 1H), 4.51 (s, 4H), 4.10 (q, J = 7.1 Hz, 2H), 3.43 (s, 4H), 3.21 (s, 2H), 2.95 (d, J = 6.4 Hz, 2H), 2.50 (d, J = 2.1 Hz, 2H), 1.93 (s, 3H), 1.79 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.2, 148.9, 145.2, 144.7, 140.7, 139.0, 138.8, 136.4, 135.3, 128.2, 127.3, 127.3, 127.2, 117.9, 115.8, 115.3, 115.0, 73.5, 73.2, 60.4, 52.4, 38.0, 36.9, 36.6, 23.4, 23.3, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C37H44O4Na, 575.3132; found, 575.3125. Isolated yield: Method A, 83% (91 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 3:97).
Dimethyl-(Z)-4-((Z)-3-(ethoxycarbonyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3k)
1H NMR (400 MHz, CDCl3) δ = 6.19 (t, J = 2.2 Hz, 1H), 6.03 (s, 1H), 5.82–5.72 (m, 1H), 5.19 (s, 1H), 5.07–4.99 (m, 3H), 4.95 (s, 1H), 4.87 (d, J = 0.8 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.73 (s, 6H), 3.22 (d, J = 2.2 Hz, 2H), 3.07 (d, J = 6.1 Hz, 2H), 1.93 (d, J = 4.0 Hz, 6H), 1.23 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.7, 169.9, 150.9, 145.8, 145.6, 144.7, 137.6, 134.2, 130.5, 129.8, 118.3, 116.6, 116.0, 115.2, 63.9, 60.3, 52.9, 37.0, 34.4, 23.0, 22.7, 13.8; HRMS (ESI) m/z: [M + Na]+ calcd for C24H30O6Na, 437.1940; found, 437.1950. Isolated yield: Method A, 85% (70 mg); Method B, 87% (72 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-(prop-1-en-2-yl-d5)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3l)
1H NMR (400 MHz, CDCl3) δ = 6.26 (s, 1H), 5.95 (s, 1H), 5.73–5.69 (m, 1H), 5.16 (s, 1H), 5.07–4.99 (m, 3H), 4.11 (q, J = 7.0 Hz, 2H), 3.72 (s, 6H), 3.20 (s, 4H), 2.95 (d, J = 5.7 Hz, 2H), 1.94 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.0, 171.0, 151.2, 144.0, 142.3, 139.9, 138.0, 135.0, 128.9, 128.8, 119.6, 116.3, 116.0, 63.9, 60.4, 52.8, 38.2, 36.9, 36.8, 23.1, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C25H27D5O6Na, 456.2410; found, 456.2393. Isolated yield: Method A, 72% (62 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-2-(cyclohex-1-en-1-yl)-3-(2-ethoxy-2-oxoethyl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3m)
1H NMR (400 MHz, CDCl3) δ = 6.27 (t, J = 2.2 Hz, 1H), 5.92 (s, 1H), 5.77–5.67 (m, 1H), 5.55 (sept, J = 2.2 Hz, 1H), 5.16–5.15 (m, 1H), 5.06–4.98 (m, 3H), 4.11 (q, J = 7.1 Hz, 2H), 3.73 (s, 6H), 3.22 (d, J = 2.2 Hz, 2H), 3.17 (s, 2H), 2.94 (d, J = 6.1 Hz, 2H), 2.11–2.09 (m, 2H), 1.98–1.93 (m, 5H), 1.60–1.70 (m, 4H), 1.23 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.2, 171.1, 151.4, 141.9, 140.5, 138.1, 137.1, 135.2, 129.1, 128.5, 126.9, 120.1, 116.2, 115.8, 63.9, 60.4, 52.8, 38.3, 37.0, 36.8, 28.9, 25.3, 23.1, 22.7, 21.9, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C28H36O6Na, 491.2404; found, 491.2421. Isolated yield: Method A, 78% (74 mg); Method B, 80% (76 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-2-(cyclopent-1-en-1-yl)-3-(2-ethoxy-2-oxoethyl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3n)
1H NMR (400 MHz, CDCl3) δ = 6.27 (s, 1H), 5.93 (s, 1H), 5.77–5.67 (m, 1H), 5.58 (s, 1H), 5.15 (s, 1H), 5.07 (s, 1H), 5.03–4.98 (m, 2H), 4.11 (q, J = 7.1 Hz, 2H), 3.72 (s, 6H), 3.17 (s, 2H), 3.09 (d, J = 2.2 Hz, 2H), 2.94 (d, J = 6.1 Hz, 2H), 2.36 (dt, J = 40.2 Hz, 7.1 Hz, 4H), 1.94–1.88 (m, 5H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.0, 171.0, 150.8, 142.3, 142.1, 137.9, 135.2, 135.0, 130.0, 129.4, 128.7, 120.2, 116.2, 116.0, 63.7, 60.4, 52.8, 38.3, 37.2, 36.7, 36.2, 32.9, 23.4, 23.0, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C27H34O6Na, 477.2248; found, 477.2249. Isolated yield: Method A, 75% (69 mg); Method B, 77% (71 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-2-vinylhexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3o)
1H NMR (400 MHz, CDCl3) δ = 6.64 (dd, J = 17.1 Hz, 10.5 Hz, 1H), 6.13 (s, 1H), 6.02 (s, 1H), 5.74–5.63 (m, 1H), 5.20–5.13 (m, 4H), 5.03 (d, J = 6.1 Hz, 1H), 5.00 (s, 1H), 4.13 (q, J = 7.1 Hz, 2H), 3.71 (s, 6H), 3.25 (s, 2H), 2.98–2.96 (m, 4H), 1.98 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.2, 170.9, 149.1, 144.0, 137.6, 135.2, 134.9, 132.3, 130.4, 129.6, 119.3, 116.8, 116.6, 116.4, 63.3, 60.7, 52.8, 39.3, 37.1, 36.5, 22.9, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C24H30O6Na, 437.1940; found, 437.1931. Isolated yield: Method A, 61% (50 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((2Z,5E)-3-(ethoxycarbonyl)-2-(prop-1-en-2-yl)octa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3p)
1H NMR (400 MHz, CDCl3) δ = 6.21 (t, J = 7.1 Hz, 2H), 6.03 (s, 1H), 5.52–5.45 (m, 1H), 5.37–5.30 (m, 1H), 5.19 (pent, J = 1.4 Hz, 1H), 5.07 (s, 1H), 4.95 (pent, J = 1.6 Hz, 1H), 4.87 (dd, J = 1.0 Hz, 1.9 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.73 (s, 6H), 3.22 (d, J = 2.2 Hz, 2H), 3.01 (d, J = 6.1 Hz, 2H), 1.95–1.93 (m, 8H), 1.23 (t, J = 7.1 Hz, 3H), 0.93 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 170.7, 170.1, 151.0, 145.7, 145.5, 143.7, 137.7, 134.0, 130.9, 130.3, 124.4, 118.5, 116.6, 115.2, 64.0, 60.3, 52.9, 37.0, 33.4, 25.5, 23.0, 22.7, 13.9, 13.7; HRMS (ESI) m/z: [M + Na]+ calcd for C26H34O6Na, 465.2248; found, 465.2239. Isolated yield: Method A, 76% (67 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 7:93).
Dimethyl-(Z)-4-((2Z,5E)-3-(ethoxycarbonyl)-6-phenyl-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3q)
1H NMR (400 MHz, CDCl3) δ = 7.33–7.18 (m, 5H), 6.40 (d, J = 15.8 Hz, 1H), 6.27 (s, 1H), 6.18–6.10 (m, 1H), 6.05 (s, 1H), 5.22–5.20 (m, 1H), 5.09 (s, 1H), 4.97 (s, 1H), 4.90 (s, 1H), 4.13 (q, J = 7.1 Hz, 2H), 3.72 (s, 6H), 3.26–3.23 (m, 4H), 1.96 (s, 6H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 170.7, 169.9, 150.9, 146.0, 145.6, 144.9, 137.7, 137.4, 131.3, 130.6, 129.8, 128.4, 127.0, 126.2, 126.1, 118.4, 116.7, 115.2, 64.0, 60.4, 52.9, 37.1, 33.8, 23.1, 22.7, 13.8; HRMS (ESI) m/z: [M + Na]+ calcd for C30H34O6Na, 513.2253; found, 513.2260. Isolated yield: Method A, 57% (56 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-3-(ethoxycarbonyl)-6-methyl-2-(prop-1-en-2-yl)hepta-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3r)
1H NMR (400 MHz, CDCl3) δ = 6.20 (t, J = 2.2 Hz, 1H), 6.02 (s, 1H), 5.19–5.17 (m, 1H), 5.07–5.04 (m, 2H), 4.93 (s, 1H), 4.86 (s, 1H), 4.10 (q, J = 7.1 Hz, 2H), 3.73 (s, 6H), 3.21 (d, J = 2.2 Hz, 2H), 3.01 (d, J = 6.9 Hz, 2H), 1.92 (d, J = 12.5 Hz, 6H), 1.66 (s, 3H), 1.60 (s, 3H), 1.23 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 170.7, 170.2, 150.9, 145.7, 145.3, 142.9, 137.7, 133.1, 131.6, 130.2, 120.2, 118.5, 116.6, 115.2, 63.9, 60.2, 52.9, 37.0, 29.4, 25.6, 23.0, 22.6, 17.8, 13.8; HRMS (ESI) m/z: [M + Na]+ calcd for C26H34O6Na, 465.2253; found, 465.2238. Isolated yield: Method A, 71% (63 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((Z)-3-(2-ethoxy-2-oxoethyl)-5-methyl-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3s)
1H NMR (400 MHz, CDCl3) δ = 6.26 (s, 1H), 5.94 (s, 1H), 5.16–5.12 (m, 2H), 5.06 (s, 1H), 4.87 (s, 1H), 4.76 (s, 1H), 4.66 (s, 1H), 4.10 (q, J = 7.1 Hz, 2H), 3.72 (s, 6H), 3.21 (d, J = 2.1 Hz, 2H), 3.19 (s, 2H), 2.90 (s, 2H), 1.93 (s, 3H), 1.84 (s, 3H), 1.65 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 172.0, 171.0, 151.2, 144.4, 142.7, 142.2, 140.6, 138.0, 129.0, 128.7, 119.8, 116.2, 115.9, 111.7, 63.9, 60.4, 52.8, 40.4, 38.0, 36.9, 23.3, 23.1, 22.3, 14.1; HRMS (ESI) m/z: [M + Na]+ calcd for C26H34O6Na, 465.2248; found, 465.2240. Isolated yield: Method A, 80% (71 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 5:95).
Dimethyl-(Z)-4-((E)-3-allyl-2-(prop-1-en-2-yl)hept-2-en-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3t)
1H NMR (400 MHz, CDCl3) δ = 6.26 (s, 1H), 5.89 (s, 1H), 5.77–5.67 (m, 1H), 5.15 (s, 1H), 5.08 (s, 1H), 5.06 (s, 1H), 5.03–4.96 (m, 2H), 4.80 (s, 1H), 3.72 (s, 6H), 3.21 (d, J = 2.1 Hz, 2H), 2.86 (d, J = 6.1 Hz, 2H), 2.13–2.09 (m, 2H), 1.93 (s, 3H), 1.84 (s, 3H), 1.36–1.27 (m, 5H), 0.88 (t, J = 7.1 Hz, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.2, 151.7, 144.7, 140.3, 138.2, 137.5, 136.5, 136.1, 127.7, 120.5, 116.2, 115.1, 63.9, 52.8, 36.9, 36.0, 32.9, 31.5, 23.9, 23.2, 23.0, 13.9; HRMS (ESI) m/z: [M + Na]+ calcd for C25H34O4Na, 421.2349; found, 421.2353. Isolated yield: Method A, 80% (64 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 3:97).
Dimethyl-(Z)-4-((Z)-3-phenyl-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3u)
1H NMR (400 MHz, CDCl3) δ = 7.27–7.15 (m, 10H), 6.42 (t, J = 2.2 Hz, 1H), 5.98 (s, 1H), 5.91 (t, J = 2.2 Hz, 1H), 5.83 (s, 1H), 5.74–5.65 (m, 2H), 5.21 (s, 2H), 5.15 (s, 2H), 4.90–4.85 (m, 6H), 4.73 (s, 1H), 4.70 (s, 1H), 3.73 (d, J = 4.8 Hz, 12H), 3.27–3.20 (m, 8H), 1.98 (d, J = 14.8 Hz, 6H), 1.65 (d, J = 17.4 Hz, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.1, 171.0, 152.0, 151.2, 144.4, 143.9, 143.1, 142.5, 141.6, 140.3, 139.0, 138.4, 138.0, 137.8, 137.5, 136.7, 135.0, 129.4, 128.7, 128.6, 127.8, 127.6, 127.5, 126.6, 126.3, 121.9, 121.2, 117.9, 116.3, 116.0, 115.9, 115.5, 115.4, 63.9, 63.9, 52.8, 52.8, 39.7, 39.4, 37.1, 36.8, 23.8, 23.5, 23.1, 22.8; HRMS (ESI) m/z: [M + Na]+ calcd for C27H30O4Na, 441.2036; found, 441.2034. Isolated yield: Method A, 60% (50 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 3:97).
Dimethy-(Z)-4-((Z)-3-(2-hydroxyethyl)-2-(prop-1-en-2-yl)hexa-2,5-dien-1-ylidene)-3-(prop-1-en-2-yl)cyclopent-2-ene-1,1-dicarboxylate (3v)
1H NMR (400 MHz, CDCl3) δ = 6.27 (s, 1H), 5.93 (s, 1H), 5.81–5.71 (m, 1H), 5.17 (s, 1H), 5.13 (s, 1H), 5.06–5.01 (m, 3H), 4.84 (s, 1H), 4.45 (s, 1H), 3.73 (s, 6H), 3.67 (q, J = 7.1 Hz, 2H), 3.22 (d, J = 2.1 Hz, 2H), 2.91 (d, J = 6.1 Hz, 2H), 2.46 (t, J = 7.1 Hz, 2H), 1.94 (s, 3H), 1.86 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3) δ = 171.1, 151.6, 149.4, 144.5, 141.3, 139.4, 138.1, 135.8, 132.6, 128.4, 119.9, 116.3, 116.1, 115.8, 115.6, 63.9, 61.8, 52.9, 36.9, 36.6, 36.2, 24.0, 23.2; HRMS (ESI) m/z: [M + Na]+ calcd for C23H30O5Na, 409.1985; found, 409.1986. Isolated yield: Method A, 63% (49 mg), as a liquid. Column chromatography on silica gel (eluent: ethyl acetate/pentane, 10:90).
Acknowledgments
Financial support from the Swedish Research Council (2016-03897), the European Union, and the Olle Engqvist Foundation is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c00186.
General preparation of starting materials, mechanistic study, kinetic isotope effect (KIE) experiments, and 1H/13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews on allene reactions, see:; a Lechel T.; Pfrengle F.; Reissig H.-U.; Zimmer R. Three Carbons for Complexity! Recent Developments of Palladium-Catalyzed Reactions of Allenes. ChemCatChem 2013, 5, 2100–2130. 10.1002/cctc.201200875. [DOI] [Google Scholar]; b Yu S.; Ma S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]; c Krause N.; Winter C. Gold-Catalyzed Nucleophilic Cyclization of Functionalized Allenes: A Powerful Access to Carbo- and Heterocycles. Chem. Rev. 2011, 111, 1994–2001. 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]; d Ye J. T.; Ma S. Palladium-Catalyzed Cyclization Reactions of Allenes in the Presence of Unsaturated Carbon-Carbon Bonds. Acc. Chem. Res. 2014, 47, 989–1000. 10.1021/ar4002069. [DOI] [PubMed] [Google Scholar]; e Lledo A.; Pla-Quintana A.; Roglans A. Allenes, versatile unsaturated motifs in transition-metal-catalysed [2 + 2+2] cycloaddition reactions. Chem. Soc. Rev. 2016, 45, 2010–2023. 10.1039/C5CS00535C. [DOI] [PubMed] [Google Scholar]; f Beccalli E. M.; Broggini G.; Martinelli M.; Sottocarnola S. C-C, C-O, C-N Bond Formation on sp2 Carbon by Pd(II)-Catalyzed Reactions Involving Oxidant Agents. Chem. Rev. 2007, 107, 5318–5365. 10.1021/cr068006f. [DOI] [PubMed] [Google Scholar]; g Hong X.; Stevens M. C.; Liu P.; Wender P. A.; Houk K. N. Reactivity and Chemoselectivity of Allenes in Rh(I)-Catalyzed Intermolecular (5 + 2) Cycloadditions with Vinylcyclopropanes: Allene-Mediated Rhodacycle Formation Can Poison Rh(I)- Catalyzed Cycloadditions. J. Am. Chem. Soc. 2014, 136, 17273–17283. 10.1021/ja5098308. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Yang B.; Qiu Y.; Bäckvall J.-E. Control of Selectivity in Palladium(II)-Catalyzed Oxidative Transformations of Allenes. Acc. Chem. Res. 2018, 51, 1520–1531. 10.1021/acs.accounts.8b00138. [DOI] [PubMed] [Google Scholar]
- For polyenes, see:; a Woerly E. M.; Roy J.; Burke M. D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 2014, 6, 484–491. 10.1038/nchem.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Goodman G. E.; Thornquist M. D.; Balmes J.; Cullen M. R.; Meyskens F. L.; Omenn G. S.; Valanis B.; Williams J. H. The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping β-Carotene and Retinol Supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- a Hopf H. Forgotten hydrocarbons prepared. Nature 2009, 460, 183–184. 10.1038/460183a. [DOI] [PubMed] [Google Scholar]; b Hopf H.; Sherburn M. S. Dendralenes Branch Out: Cross-Conjugated Oligoenes Allow the Rapid Generation of Molecular Complexity. Angew. Chem., Int. Ed. 2012, 51, 2298–2338. 10.1002/anie.201102987. [DOI] [PubMed] [Google Scholar]; c Wang H.; Beiring B.; Yu D.; Collins K. D.; Glorius F. [3]Dendralene Synthesis: Rhodium(III)- Catalyzed Alkenyl C-H Activation and Coupling Reaction with Allenyl Carbinol Carbonate. Angew. Chem., Int. Ed. 2013, 52, 12430–12434. 10.1002/anie.201306754. [DOI] [PubMed] [Google Scholar]; d Qiu Y.-A.; Posevins D.; Bäckvall J.-E. Selective Palladium-Catalyzed Allenic C-H Bond Oxidation for the Synthesis of [3]Dendralenes. Angew. Chem., Int. Ed. 2017, 56, 13112–13116. 10.1002/anie.201706211. [DOI] [PubMed] [Google Scholar]; e George J.; Ward J. S.; Sherburn M. S. A General Synthesis of Dendralenes. Chem. Sci. 2019, 10, 9969–9973. 10.1039/C9SC03976G. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Horvath K. L.; Newton C. G.; Roper K. A.; Ward J. S.; Sherburn M. S. A Broad-Spectrum Synthesis of Tetravinylethylenes. Chem. - Eur. J. 2019, 25, 4072–4076. 10.1002/chem.201900550. [DOI] [PubMed] [Google Scholar]
- Sherburn M. S. Preparation and Synthetic Value of π-Bond-Rich Branched Hydrocarbons. Acc. Chem. Res. 2015, 48, 1961–1967. 10.1021/acs.accounts.5b00242. [DOI] [PubMed] [Google Scholar]
- a Lindeboom E. J.; Willis A. C.; Paddon-Row M. N.; Sherburn M. S. Tetravinylethylene. Angew. Chem., Int. Ed. 2014, 53, 5440–5443. 10.1002/anie.201402840. [DOI] [PubMed] [Google Scholar]; b Saglam M. F.; Alborzi A. R.; Payne A. D.; Willis A. C.; Paddon-Row M. N.; Sherburn M. S. Synthesis and Diels-Alder Reactivity of Substituted [4]Dendralenes. J. Org. Chem. 2016, 81, 1461–1475. 10.1021/acs.joc.5b02583. [DOI] [PubMed] [Google Scholar]; c Naidu G. S.; Singh R.; Kumar M.; Ghosh S. K. Tuning the Stability and the Reactivity of Substituted [3]Dendralenes for Quick Access to Diverse Copiously Functionalized Fused Polycycles with Step and Atom Economy. J. Org. Chem. 2017, 82, 3648–3658. 10.1021/acs.joc.7b00169. [DOI] [PubMed] [Google Scholar]
- Bojase G.; Nguyen T. V.; Payne A. D.; Willis A. C.; Sherburn M. S. Synthesis and properties of the ivyanes: the parent 1,1-oligocyclopropanes. Chem. Sci. 2011, 2, 229–232. 10.1039/C0SC00500B. [DOI] [Google Scholar]
- Rieder C. J.; Winberg K. J.; West F. G. Cyclization of Cross-Conjugated Trienes: The Vinylogous Nazarov Reaction. J. Am. Chem. Soc. 2009, 131, 7504–7505. 10.1021/ja9023226. [DOI] [PubMed] [Google Scholar]
- Green N. J.; Lawrence A. L.; Bojase G.; Willis A. C.; Paddon-Row M. N.; Sherburn M. S. Domino cycloaddition organocascades of dendralenes. Angew. Chem., Int. Ed. 2013, 52, 8333–8336. 10.1002/anie.201302185. [DOI] [PubMed] [Google Scholar]
- a Mao M.; Zhang L.; Chen Y.-Z.; Zhu J.; Wu L. Palladium-Catalyzed Coupling of Allenylphosphine Oxides with N-Tosylhydrazones toward Phosphinyl [3]Dendralenes. ACS Catal. 2017, 7, 181–185. 10.1021/acscatal.6b02972. [DOI] [Google Scholar]; b Toombs-Ruane H.; Osinski N.; Fallon T.; Wills C.; Willis A. C.; Paddon-Row M. N.; Sherburn M. S. Synthesis and Applications of Tricarbonyliron Complexes of Dendralenes. Chem. - Asian J. 2011, 6, 3243–3250. 10.1002/asia.201100455. [DOI] [PubMed] [Google Scholar]
- Fielder S.; Rowan D. D.; Sherburn M. S. First Synthesis of the Dendralene Family of Fundamental Hydrocarbons. Angew. Chem., Int. Ed. 2000, 39, 4331–4333. . [DOI] [PubMed] [Google Scholar]
- Shimizu M.; Kurahashi T.; Shimono K.; Tanaka K.; Nagao I.; Kiyomoto S.-I.; Hiyama T. Facile Synthesis and Palladium-Catalyzed Cross-Coupling Reactions of 2,3- Bis(pinacolatoboryl)-1,3-butadiene. Chem. - Asian J. 2007, 2, 1400–1408. 10.1002/asia.200700120. [DOI] [PubMed] [Google Scholar]
- Bojase G.; Payne A. D.; Willis A. C.; Sherburn M. S. One-step synthesis and exploratory chemistry of [5]dendralene. Angew. Chem., Int. Ed. 2008, 47, 910–912. 10.1002/anie.200704470. [DOI] [PubMed] [Google Scholar]
- Lippincott D. J.; Linstadt R. T. H.; Maser M. R.; Lipshutz B. H. Synthesis of Functionalized [3]-, [4]-, [5]-, and [6]Dendralenes through Palladium-Catalyzed Cross-Couplings of Substituted Allenoates. Angew. Chem., Int. Ed. 2017, 56, 847–850. 10.1002/anie.201609636. [DOI] [PubMed] [Google Scholar]
- For a similar gold-catalyzed reaction, see:; Cheong P. H.-Y.; Morganelli P.; Luzung M. R.; Houk K. N.; Toste F. D. Gold-Catalyzed Cycloisomerization of 1,5-Allenynes via Dual Activation of an Ene Reaction. J. Am. Chem. Soc. 2008, 130, 4517–4526. 10.1021/ja711058f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.; Yang B.; Jiang T.; Bäckvall J.-E. Olefin-Directed Palladium-Catalyzed Regio- and Stereoselective Oxidative Arylation of Allenes. Angew. Chem., Int. Ed. 2015, 54, 9066–9069. 10.1002/anie.201502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For aerobic biomimetic palladium-catalyzed oxidations, see:; a Piera J.; Bäckvall J.-E. Catalytic Oxidation of Organic Substrates by Molecular Oxygen and Hydrogen Peroxide by Multistep Electron Transfer-A Biomimetic Approach. Angew. Chem., Int. Ed. 2008, 47, 3506–3523. 10.1002/anie.200700604. [DOI] [PubMed] [Google Scholar]; b Bäckvall J.-E.; Hopkins R. B.; Grennberg H.; Mader M.; Awasthi A. K. Multistep electron transfer in palladium-catalyzed aerobic oxidations via a metal macrocycle quinone system. J. Am. Chem. Soc. 1990, 112, 5160–5166. 10.1021/ja00169a025. [DOI] [Google Scholar]; c Piera J.; Närhi K.; Bäckvall J.-E. Pd(II)- Catalyzed Aerobic Allylic Oxidative Carbocyclization of Allene-Substituted Olefins: Immobilization of an Oxygen- Activating Catalyst. Angew. Chem., Int. Ed. 2006, 45, 6914–6917. 10.1002/anie.200602421. [DOI] [PubMed] [Google Scholar]; d Johnston E. V.; Karlsson E. A.; Lindberg S. A.; Åkermark B.; Bäckvall J.-E. Efficient Reoxidation of Palladium by a Hybrid Catalyst in Aerobic Palladium-Catalyzed Carbocyclization of Enallenes. Chem. - Eur. J. 2009, 15, 6799–6801. 10.1002/chem.200900980. [DOI] [PubMed] [Google Scholar]; e Naidu V. R.; Posevins D.; Volla C. M. R.; Bäckvall J.-E. Selective Cascade Reaction of Bisallenes via Palladium-Catalyzed Aerobic Oxidative Carbocyclization-Borylation and Aldehyde Trapping. Angew. Chem., Int. Ed. 2017, 56, 1590–1594. 10.1002/anie.201610524. [DOI] [PubMed] [Google Scholar]
- For direct reoxidation of Pd(0) by O2, in palladium-catalyzed oxidations, see:; a Stahl S. S. Palladium oxidase catalysis: selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem., Int. Ed. 2004, 43, 3400–3420. 10.1002/anie.200300630. [DOI] [PubMed] [Google Scholar]; b Trend R. M.; Ramtohul Y. K.; Stoltz B. M. Oxidative Cyclizations in a Nonpolar Solvent Using Molecular Oxygen and Studies on the Stereochemistry of Oxypalladation. J. Am. Chem. Soc. 2005, 127, 17778–17788. 10.1021/ja055534k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang Y. H.; Yu J. Q. Pd(II)-Catalyzed Hydroxylation of Arenes with 1 atm of O2 or Air. J. Am. Chem. Soc. 2009, 131, 14654–14655. 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]
- For allenyne and enallene, see:; a Deng Y.; Bäckvall J.-E. Palladium-Catalyzed Oxidative Acyloxylation/Carbocyclization of Allenynes. Angew. Chem., Int. Ed. 2013, 52, 3217–3221. 10.1002/anie.201208718. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bartholomeyzik T.; Pendrill R.; Lihammar R.; Jiang T.; Widmalm G.; Bäckvall J.-E. Kinetics and Mechanism of the Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes. J. Am. Chem. Soc. 2018, 140, 298–309. 10.1021/jacs.7b10267. [DOI] [PubMed] [Google Scholar]; c Qiu Y.; Yang B.; Zhu C.; Bäckvall J.-E. Palladium-Catalyzed Oxidative Carbocyclization-Borylation of Enallenes to Cyclobutenes. Angew. Chem., Int. Ed. 2016, 55, 6520–6524. 10.1002/anie.201601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In experiment where an enallene and an allenyne in a 1:1 ratio were allowed to compete with one another in the activation by palladium(II) it was shown that the enallene reacts faster than the allenyne. These studies involved Pd-catalyzed coupling of the competing substrates with B2pin2 or PhB(OH)2 (see Supporting Information).
- Deng Y.; Bartholomeyzik T.; Persson A. K. Å.; Sun J.; Bäckvall J.-E. Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes. Angew. Chem., Int. Ed. 2012, 51, 2703–2707. 10.1002/anie.201107592. [DOI] [PubMed] [Google Scholar]
- Pardo-Rodríguez V.; Marco-Martínez J.; Buñuel E.; Cárdenas D. J. Pd-Catalyzed Borylative Cyclization of Allenynes and Enallenes. Org. Lett. 2009, 11, 4548–4551. 10.1021/ol9017694. [DOI] [PubMed] [Google Scholar]
- a Zhu C.; Yang B.; Bäckvall J.-E. Highly Selective Cascade C-C Bond Formation via Palladium-Catalyzed Oxidative Carbonylation-Carbocyclization-Carbonylation-Alkynylation of Enallenes. J. Am. Chem. Soc. 2015, 137, 11868–11871. 10.1021/jacs.5b06828. [DOI] [PubMed] [Google Scholar]; b Zhang H.; Fu X.; Chen J.; Wang E.; Liu Y.; Li Y. Generation of Allenic/Propargylic Zirconium Complexes and Subsequent Cross-Coupling Reactions: A Facile Synthesis of Multisubstituted Allenes. J. Org. Chem. 2009, 74, 9351–9358. 10.1021/jo9020419. [DOI] [PubMed] [Google Scholar]; c Kessler S. N.; Bäckvall J.-E. Iron-catalyzed Cross-Coupling of Propargyl Carboxylates and Grignard Reagents: Synthesis of Substituted Allenes. Angew. Chem., Int. Ed. 2016, 55, 3734–3738. 10.1002/anie.201511139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.