Abstract
OBJECTIVE
To develop a low-technology system that can be used by dog owners to obtain morphological and mobility measurements in companion dogs as candidate components of an eventual canine frailty scale.
ANIMALS
57 adult (≥ 1-year-old) dogs enrolled by 43 owners.
PROCEDURES
Morphological measurements of dogs were performed by investigators and dog owners. Dogs participated in timed in-clinic mobility trials across a flat surface (on-leash trial with the owner, on-leash trial with the investigator, and off-leash trial) and on stairs; each trial was repeated 3 times. Owners were asked to conduct a second stair trial at home 2 weeks later. Agreement between owner- and investigator-obtained measurements was assessed with Shrout-Fleiss intraclass correlation coefficients and paired t tests. Age, quartile of projected percentage of mean life span attained (adjusted for body weight), and height were evaluated as predictors of speed and stride length in mobility trials with linear regression and Spearman rank correlation analysis.
RESULTS
Agreement between owner- and investigator-obtained morphological measurements was strong. Age was a weak but significant predictor of decreased dog speed in mobility trials (adjusted R2, 0.10 to 0.23). Speed decreased significantly with increasing quartile of projected life span attained. A linear regression model that included height and age predicted dog speed better than models with age or height alone.
CONCLUSIONS AND CLINICAL RELEVANCE
Morphological and mobility trial measurements can be obtained by dog owners with minimal training. Low-technology measurements of mobility trial speed offer potential as components in a future scoring scale for canine frailty. (Am J Vet Res 2019;80:670–679)
Experts in human gerontology and geriatrics have identified physical frailty as a syndrome characterized by features such as diminished strength, diminished endurance, and physiologic malfunction that increases an individual’s vulnerability for developing increased dependency, death, or both.1 The use of various scales to characterize frailty is a focus of efforts in human medicine to increase healthy life expectancy.2–4 For instance, the Frailty Index is used to assess deficit accumulation (described as combinations of symptoms, diseases, conditions, and disability).4 Five measures are commonly used to define physical frailty in humans: unintentional weight loss, self-reported exhaustion, muscle weakness (eg, decreased grip strength), slow walking speed, and limited physical activity.5 Increased frailty correlates with risk of disability after major illness or surgery and with higher surgical complication and mortality rates.6–9 Slower walking speeds are predictive of postoperative outcomes (ie, poor recovery and increased morbidity and mortality rates).10–12 Ongoing efforts in human gerontology are focused on simplification of frailty measurement to facilitate widespread use in community settings.13 Identification of frailty in patients enables targeted interventions such as intensive monitoring, physical therapy, or nursing care that could delay or prevent adverse outcomes.13
Many dog owners and veterinarians have encountered signs of frailty in aging dogs (eg, decreased physical activity, limited mobility, and changes in cognition). However, this phenomenon has not been well described in the literature, and a distinction between an expected amount of age-associated functional decline and a frail phenotype that exceeds an expected decline has not been described.14–17 Identification of frailty in aging dogs could enable targeted intervention efforts; a quantitative frailty assessment could facilitate comparison of patients among diverse institutions and monitoring of individual patients over time. Ideally, a frailty assessment scale for aging dogs should be easily implemented and involve tools that are basic (ie, low-technology), portable, and widely available. Walking speed and unintentional weight loss are measures included in human frailty scales5 that could be easily evaluated in companion dogs.
Although scales have been developed for assessment of frailty in laboratory animals (ie, mice and rats),18,19 these scales include up to 31 criteria, some of which are invasive or require specialized equipment. A study20 of aging Golden Retriever and Labrador Retriever guide dogs defined a frailty-related phenotype by aligning physical examination findings with the presence or absence of the 5 measures (with some modification) commonly used to assess human frailty.5 The risk of death during the follow-up period was higher for dogs with ≥ 2 (vs ≤ 1) identified frailty measures. In that study,20 gait was only recorded as normal or abnormal (ie, marked stiffness, lameness, or ataxia), which is a narrower definition than that used for evaluating mobility with human frailty scales, and gait speed was not measured. In another study,21 dog owners who completed a questionnaire to assess their dog’s mobility identified decreased mobility in older dogs and in dogs with diagnosed orthopedic or neurologic disease, although this was a qualitative assessment that relied on owner-reported observations at a single time point.
There is a need for objective and quantitative measurement of frailty in dogs that can be easily performed in a wide range of breeds and settings without specialized equipment or skills. The purpose of the study reported here was to develop an easy-to-use method to assess mobility as a preliminary step in the eventual development of a comprehensive canine frailty scale. The primary goal of our study was to determine whether morphological and mobility measurements could be reliably collected by dog owners with minimal training in a low-technology environment. An additional goal was to evaluate morphological measurements and body weight as potential predictors of mobility trial speeds. We hypothesized that morphological measurements collected by minimally trained owners and trained investigators would be similar, regardless of the size, shape or breed of dogs. Additionally, we hypothesized that mobility trial speeds (on stairs, flat surfaces, or both) would correlate negatively with age and would correlate positively with ≥ 1 morphological measurement, body weight, or both. Finally, we hypothesized that minimally trained owners would not find it difficult to time their dogs during mobility trials in the clinic or at home.
Materials and Methods
A link to an online survey was emailed to all faculty, graduate and veterinary students, and staff of the College of Veterinary Medicine & Biomedical Sciences, Texas A&M University. Respondents who owned an adult (≥ 1-year-old) dog that was healthy and able to walk on a leash and safely and comfortably use stairs (in the owner’s judgment) were invited to participate in the study. Informed consent was obtained from all owners at the time of the first appointment. All study procedures were approved by the Texas A&M University Institutional Animal Care and Use Committee (2017–0087 CA). Because dog owner participation was required for the study, Institutional Review Board approval was sought, and the study was determined to be exempt (IRB2017–0140).
To ensure that investigators took measurements and recorded times in a standardized manner, 3 investigators’ dogs were used in a pilot investigation of the proposed procedures, which included morphological measurements, a timed on-leash walk across a flat surface (5-m distance), and a timed off-leash stair ascent. Four investigators (EMM, JCH, GJL, and KEC) rehearsed each step of the morphological measurement process, including verbal instructions to clients, use of the tape measure, and position of the investigator relative to the dog, until agreement on procedures and consistency in results was achieved among investigators. Each proposed mobility trial was performed and concurrently timed by 2 investigators until agreement and consistency were reached on start and stop time points and on instructions for the handler. The pilot resultsa were used to adjust study protocols. For instance, it was determined that morphological measurements were more consistent when obtained from the dog’s side, rather than by leaning over the dog, so the study protocol was modified to obtain unilateral measurements from the dog’s left side. For the mobility trials, the pilot study dogs tended to trot next to handlers when on leash, covering the 5-m distance more quickly than anticipated; accordingly, the distance was increased to 10 m. To decrease within-dog variation over repeated trials, the instructions were modified to have each handler move the dog along the course at a trot, and handlers were instructed to rehearse this task to determine what speed of their gait matched the dog’s natural trot. When it was recognized that pilot study dogs ascended the stairs at a run when released from their leashes, an off-leash speed trial across a flat surface was added. Lastly, because counting footfalls while operating the stopwatch was difficult, particularly for short-legged and long-haired dogs, a video recording of each dog performing an on-leash trial with its owner was added so that it could be replayed later at reduced speed to allow easier counting of footfalls. All these protocol changes were approved by an amendment submitted to the Institutional Animal Care and Use Committee.
Owners brought their dogs to a scheduled appointment at the veterinary teaching hospital for the in-clinic portion of the study. Sex, reproductive status, age, and breed (provided by the owner) were recorded for each dog. Dogs were weighed on a clinic floor scale that was calibrated at least every 3 months, and morphological measurements were obtained by an investigator using a cloth tape measure; morphological measurements included in the study reported here were selected from previous reports22–24 of canine morphometrics, with attention to those commonly measured by dog owners for agility sports or for fitting of wheeled carts for nonambulatory dogs. Height was measured from the highest point of the shoulders (ie, withers) to the floor; forelimb length was measured from the point of the elbow to the proximal edge of the carpal pad; torso length was measured from the greater humeral tubercle to the ischial tuberosity; and thigh circumference was measured around the proximal portion of each thigh. All measurements were recorded in centimeters. The owners observed the investigators making the measurements and then were given brief verbal instruction by an investigator before performing the same measurements on their own. Investigators observed the owners making measurements and provided clarification on the instructions if asked. Several lay terms were used to identify anatomic landmarks when instructing owners on measurements. The ischial tuberosity, greater humeral tubercle, and measurement site for circumference of the proximal aspect of the thigh were described as the point of the buttocks, point of the shoulder, and where the leg meets the body, respectively. During the trial, it became clear that the point of the buttocks was not well recognized by owners; accordingly, the instructions were amended to refer to the ischial tuberosity as the bony point on the end of the rump.
All in-clinic mobility trials were conducted with one of the investigators (EMM, JCH, GJL, or KEC). Each of the following in-clinic mobility trials was repeated 3 times in the same direction along the same flat 10-m indoor course: the dog was trotted on leash by its owner (on-leash trial with owner); the dog was trotted on leash by an investigator (on-leash trial with investigator); and the dog was allowed to walk, trot, or run toward the owner (off-leash trial). Trials were timed by the owner (on-leash trial with investigator) and an investigator (on-leash trial with owner and off-leash trial) using a smartphone stopwatch. One of the 3 repetitions of the on-leash trial with owner was video recorded by an investigator using a smartphone, and the recording was reviewed to count the number of footfalls of the left front paw over the course. For the off-leash trial, owners were allowed to call, clap, whistle, or use treats to motivate the dog to complete the course.
The following 2 stair-ascent mobility trials (with 3 repetitions each) were attempted: in the clinic, the dog was released at the bottom of a flight of closed-riser stairs (15 steps × 15-cm rise/step = total vertical gain of 2.33 m) by an investigator and allowed to ascend at its chosen pace toward the owner at the top of the stairs (stair trial 1); 2 weeks later, the owner was asked to repeat the stair trial at home (ie, in the dog’s daily environment), including timing the dog and recording the number of steps and the rise of 1 step (stair trial 2). For both stair trials, timing started when the first paw touched the bottom step and stopped when all 4 paws reached the top landing. The owner was again allowed to call, clap, whistle, or use treats to motivate the dog to complete the trial.
A link to an online follow-up survey (Supplementary Appendix S1, available at avmajournals.avma.org/doi/suppl/10.2460/ajvr.80.7.670) was emailed to each owner on the evening of the in-clinic visit date, with instructions to complete the survey within 2 weeks of the visit; owners who had > 1 dog participating in the study were emailed a separate survey for each participating dog. The owner was asked to provide the results from stair trial 2 and feedback about the level of difficulty encountered when performing the morphological measurements (with a tape measure in the clinic) and timing of dogs in the mobility trials (on-leash trial with investigator in the clinic and stair trial at home). Owners indicated the level of difficulty for these 3 tasks by selecting 1 of 4 options that ranged from not at all difficult to very difficult and were provided open fields in which they could suggest ways that each procedure could be made easier. Up to 3 reminders were sent at monthly intervals to owners who did not respond to the survey.
All times from the mobility trials were visually inspected to determine if there was a consistent pattern of increasing or decreasing completion times with subsequent repetitions. Because no patterns were detected, the times from the 3 repetitions were used to calculate the mean time, which was used in all subsequent analyses. If a dog did not complete one of the mobility trials (ie, completed < 3 repetitions), analysis of that trial was excluded, but the dog’s other mobility trials were analyzed. Physical examinations were not performed, but only dogs that were reported as being healthy by their owners were eligible to participate. Investigators noted on the data collection or sheet when a dog exhibited an abnormal gait (eg, lameness or weakness) during any of the trials; no further trials were attempted if it appeared that the abnormality might cause pain or pose a risk to the dog’s safety.
Statistical analysis
Statistical analyses of morphological measurements were carried out on untransformed values. Normality of the distribution of the values was assessed by visual inspection of histograms. The Shrout-Fleiss reliability test was used to evaluate agreement (ICC) between investigator- and owner-obtained measurements25; the ICC values were used to categorize agreement as weak (0.01 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), good (0.61 to 0.80), or strong (0.81 to 0.99).26 We evaluated whether there was significant directional bias in morphological measurements obtained by owners versus investigators by use of a paired t test; linear regression was used to obtain coefficient of determination (adjusted R2) values for owner versus investigator measurements. Correlations (adjusted R2) between morphological measurements and body weight were evaluated by linear regression.
For mobility trials on flat surfaces, speed was calculated (distance/time to cover distance). Pace was defined as the number of footfalls for the left front paw over the 10-m course; the inverse of pace was multiplied by 10 to obtain stride length (distance/footfall; for on-leash trial with owner only). Speed was calculated for the stair trials (vertical distance/time to cover distance). All times (flat surface and stair trials) were converted to speed to allow comparability with measurements obtained from other studies that might be carried out over different distances. We evaluated whether there was significant directional bias in owner- versus investigator-measured speed for each type of mobility trial by use of a paired t test. We used linear regression to evaluate body weight as a predictor of mobility trial measurements (ie, speed and stride length).
Because smaller dogs have longer life spans than larger dogs,27 we took the body weight of the dogs in this study into account to ensure that age data were comparable among dogs of different sizes. In brief, datab (Supplementary Table S1, available at avma journals.avma.org/doi/suppl/10.2460/ajvr.80.7.670) that were collected for a previous study27 on weight-based mean life span for dogs in England were used to determine the quartile of projected percentage of weight-based mean life span (ie, projected life span) attained for each dog in the study reported here (first [< 25%], second [25% to 49.9%], third [50% to 74.9%], or fourth [≥ 75%]). The association between quartile of projected life span attained and mobility trial speeds was evaluated by use of 1-way ANOVA and linear regression analysis. Spearman rank correlation coefficients were calculated to evaluate the association between mobility trial speeds and quartile of projected life span attained; the strength of association was categorized as weak (0.01 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), good (0.61 to 0.80), or strong (0.81 to 0.99).26
We used linear regression models to evaluate the ability of height and age to predict speed and stride length for each of the in-clinic mobility trials; models were fit with age alone, height alone, and age and height simultaneously. To determine the best predictive model, we used the Akaike information criterion and Akaike weights to assess the probability that a model provided the best fit among the candidate models.28
All statistical analyses were performed with standard software.c,d Values of P ≤ 0.05 were considered significant for all analyses.
Results
Fifty-seven dogs were enrolled by 43 owners (12 veterinarians, 8 veterinary technicians, and 23 other staff members or students). The dogs consisted of 32 spayed females, 24 neutered males, and 1 sexually intact female; the median age and body weight were 6 years (range, 1 to 16 years) and 22 kg (range, 1.8 to 51.8 kg), respectively. There were 27 mixed-breed dogs, 4 Labrador Retrievers, 4 Dachshunds, 3 Miniature Schnauzers, 2 Australian Shepherds, and 2 Miniature Australian Shepherds; 15 breeds were represented by 1 dog each (data not shown). There were 14 dogs in the first, 15 in the second, 14 in the third, and 14 in the fourth quartile of projected life span attained.
All morphological measurements (height, forelimb length, torso length, and left and right thigh circumferences) were obtained from 56 dogs; 1 dog attempted to bite when measured by the investigator, so only a height measurement was obtained for this dog. All owner-obtained morphological measurements agreed strongly with those obtained by investigators, with lowest agreement (ICC = 0.87) for right thigh circumference (Figure 1). The owner-obtained measurements were significantly greater for forelimb length (P = 0.02) and significantly (P < 0.001 for both comparisons) lower for right and left thigh circumference than those obtained by investigators. All morphological measurements were positively correlated with body weight (height [R2 = 0.84], forelimb length [R2 = 0.83], torso length [R2 = 0.84], left thigh circumference [R2 = 0.86], and right thigh circumference [R2 = 0.87]; P < 0.05 for all comparisons).
Figure 1—
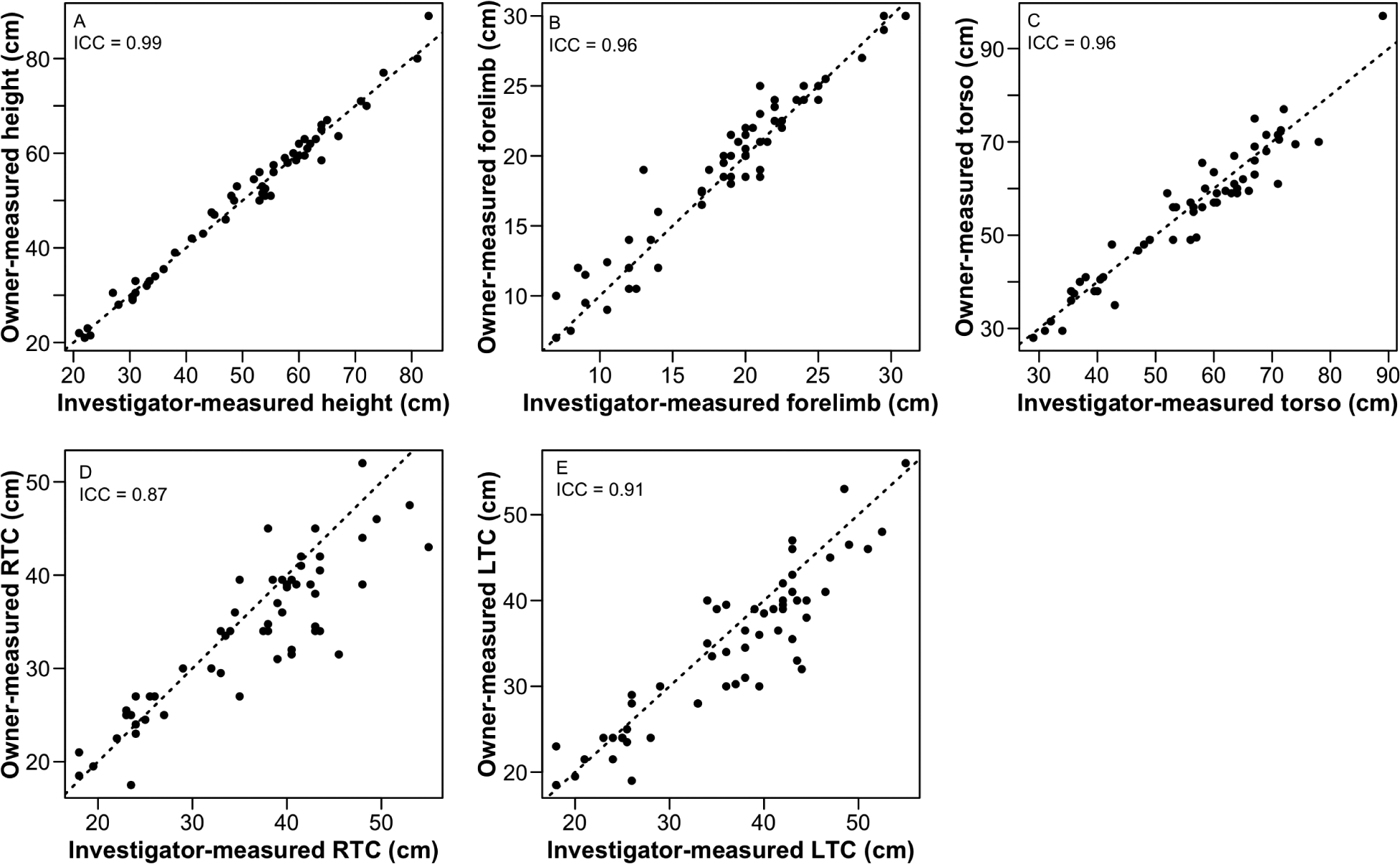
Results of analysis for agreement between owner-obtained and investigator-obtained measurements of height (A), forelimb length (B), torso length (C), and circumferences of the proximal aspect of the right (D) and left (E) thighs by use of the Shrout-Fleiss ICC for 57 adult (≥ 1-year-old) dogs in a study to develop a low-technology system that minimally trained dog owners could use to obtain morphological and mobility measurements in companion dogs and to evaluate age, body weight, and morphological measurements as predictors of measures of canine mobility. Data points represent results for individual dogs; dotted lines indicate perfect agreement between investigator- and owner-obtained values. All ICC values were significantly (P < 0.001) different from 0. LTC = Left thigh circumference. RTC = Right thigh circumference.
Fifty-four dogs completed all in-clinic mobility trials (on-leash trial with owner, on-leash trial with investigator, off-leash trial, and stair trial 1). Six dogs had visible gait abnormalities (eg, mild lameness or mild scuffing of paws or nails while walking) at the time of participation, of which 5 were able to complete all in-clinic mobility trials, but 1 was unable to complete stair trial 1. One blind dog without a gait abnormality was unable to navigate stairs and did not complete stair trial 1. Another dog without a gait abnormality did not complete stair trial 1 or the off-leash trial because the dog would not move in the desired direction when not on leash. Owners of dogs that did not complete stair trial 1 were instructed not to attempt stair trial 2.
Body weight was more predictive of stride length than of speeds from in-clinic mobility trials (Figure 2). Morphological measurements were also more predictive of stride length (height [R2 = 0.76], forelimb length [R2 = 0.73], torso length [R2 = 0.64], left thigh circumference [R2 = 0.62], and right thigh circumference [R2 = 0.66]; P < 0.001 for all comparisons) than of speeds from in-clinic mobility trials (Table 1).
Figure 2—
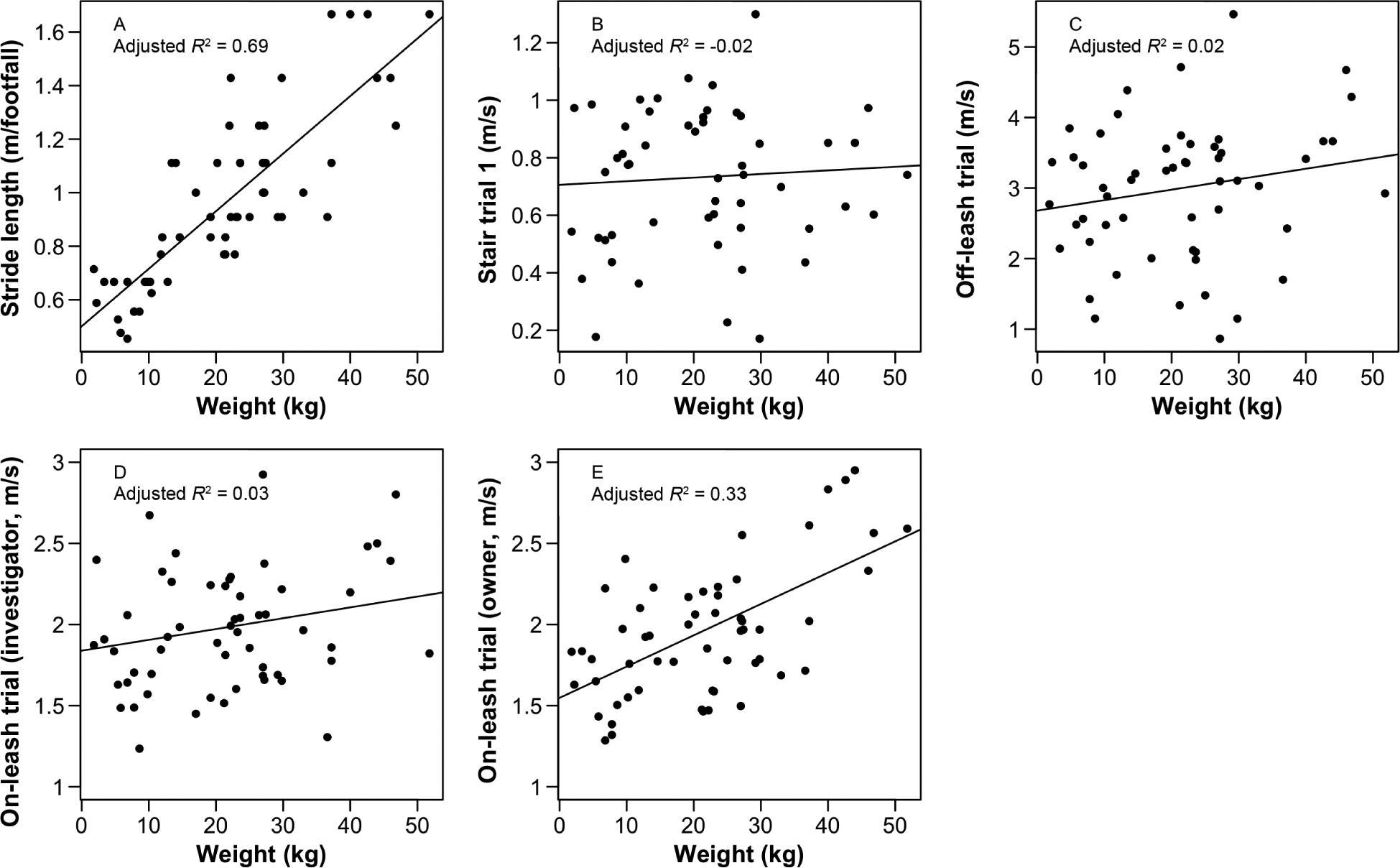
Results of linear regression analysis for body weight as a predictor of stride length (A) and in-clinic mobility trial speed in the stair trial 1 (B), off-leash trial (C), on-leash trial with the investigator (D), and on-leash trial with the owner (E) for the same dogs as in Figure 1. Each in-clinic mobility trial was repeated 3 times and was timed. In stair trial 1, the dog was released at the bottom of a flight of 15 closed-riser stairs by an investigator and allowed to ascend at any pace toward the owner at the top of the stairs. Remaining trials were all performed in 1 direction along the same flat 10-meter indoor course; the dog was allowed to move at any pace toward the owner in the off-leash trial and was trotted by the investigator or owner in the on-leash trials. Data points represent values for individual dogs, and solid lines represent the linear regression line (y = a + b•weight).
Table 1—
Correlation between investigator-obtained morphological measurements and measured speed for 57 dogs in a study to develop a low-technology system that minimally trained dog owners can use to obtain these measurements in companion dogs.
| Morphological measurement (cm) | On-leash trial with owner | On-leash trial with investigator | Off-leash trial | Stair trial 1* | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | P value | R2 | P value | R2 | P value | R2 | P value | |
| Height | 0.33 | < 0.001 | 0.08 | 0.02 | 0.04 | 0.085 | 0.03 | 0.11 |
| Forelimb length | 0.31 | < 0.001 | 0.08 | 0.02 | 0.04 | 0.08 | 0.03 | 0.10 |
| Torso length | 0.23 | < 0.001 | 0.01 | 0.22 | 0.001 | 0.34 | 0.01 | 0.61 |
| Right thigh circumference | 0.28 | < 0.001 | 0.03 | 0.11 | 0.04 | 0.07 | 0.01 | 0.25 |
| Left thigh circumference | 0.23 | < 0.001 | 0.03 | 0.12 | 0.03 | 0.12 | < 0.001 | 0.32 |
Stair trial 1 was conducted in the clinic on the same set of stairs for each dog; 3 of 57 (5%) dogs did not complete stair trial 1. A separate home stair trial (trial 2) was conducted by owners 2 weeks after the clinic visit (results not included).
There was no evidence of directional bias in measured speed between the on-leash trial with owner and the on-leash trial with investigator (P = 0.51). In intradog comparisons, speed for the off-leash trial was significantly (P < 0.001) faster than speed for the on-leash trial with owner (global mean ± SD for the study population, 2.99 ± 0.99 m/s and 1.96 ± 0.41 m/s, respectively). Age was a weakly negative predictor of speed for mobility trials on flat surfaces (on-leash trial with owner [R2 = 0.15], on-leash trial with investigator [R2 = 0.11], and off-leash trial [R2 = 0.17]; P < 0.02 for all comparisons). Quartile of projected life span attained was negatively correlated with speeds for all in-clinic mobility trials (ie, speed decreased as dogs attained a higher percentage of projected life span; Figure 3). The mean speed of dogs in the fourth quartile of projected life span was 60%, 63%, 82%, and 84% of that for younger dogs (first through third quartiles combined) in the stair trial 1, off-leash trial, on-leash trial with investigator, and on-leash trial with owner, respectively.
Figure 3—
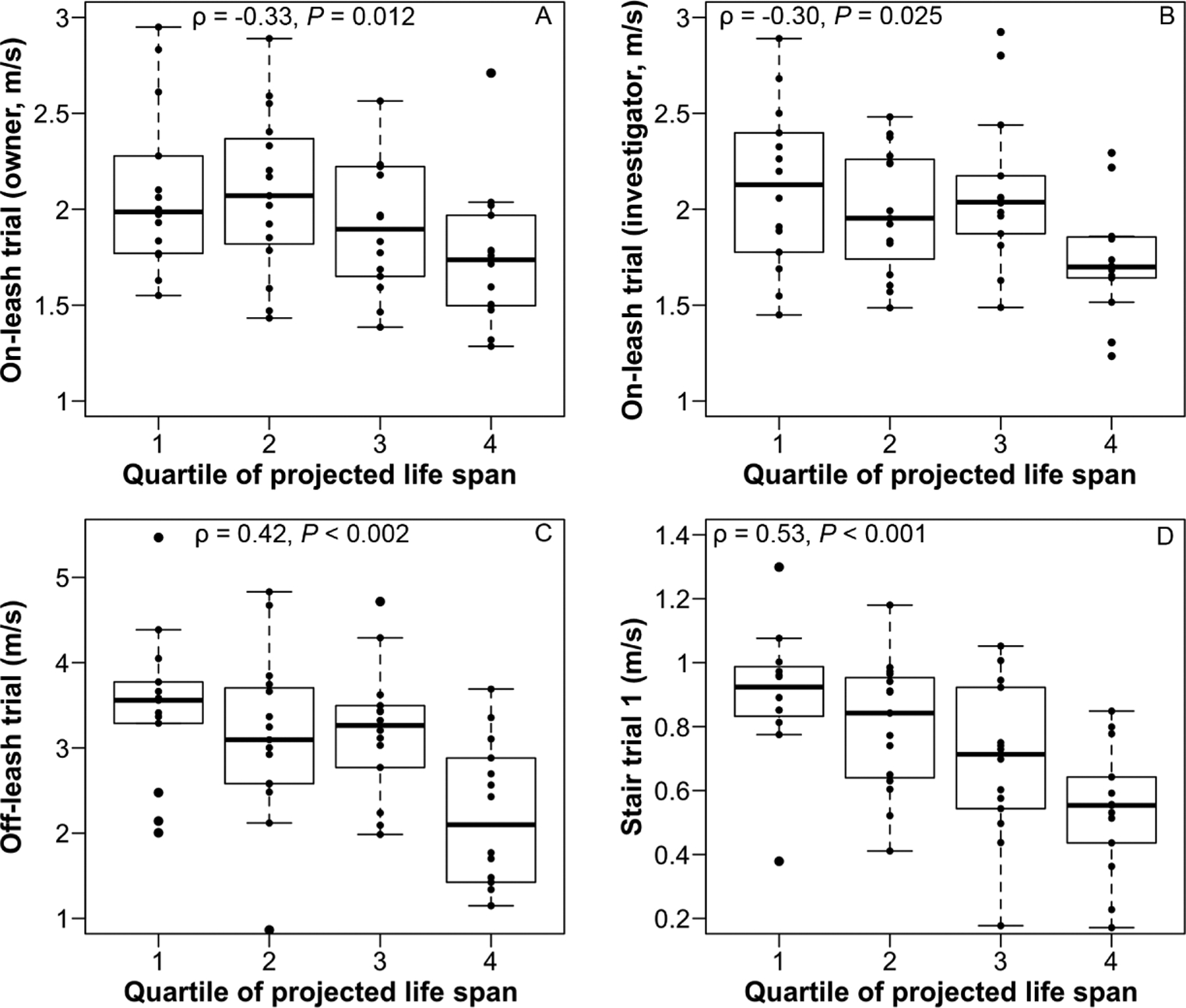
Box-and-whisker plots of speed during in-clinic mobility trials by quartile of projected weight-based mean life span attained for the same dogs as in Figure 1 during the on-leash trial with the owner (A), on-leash trial with the investigator (B), off-leash trial (C), and stair trial 1 (D) as measured by Spearman rank correlation coefficients (ρ). For each box, the upper and lower boundaries represent the 75th and 25th percentiles, respectively, and the horizontal line represents the median value. Upper whiskers represent the smaller of the following 2 values: maximum value for the variable or the 75th percentile value plus 1.5 times the difference between the values for the 75th and 25th percentiles. Similarly, lower whiskers represent the larger of the following 2 values: minimum value for the variable or the 25th percentile value minus 1.5 times the difference between the values for the 75th and 25th percentiles. Dots beyond the whiskers represent outlier values. Longevity datab by body weight classification were used to determine the quartile of projected weight-based mean life span attained for the dogs described here.
Height and age together were more predictive than either variable alone of a dog’s speed (for all in-clinic mobility trials) and stride length. For each outcome evaluated, the linear regression model that included both height and age (y = a + b1•height + b2•age) had the highest probability (range, 50.2% to 97.7%) of being the best fitting model, compared with models that included age or height alone (Table 2). Overall, the model results indicated that, for a given height, speed decreased with age.
Table 2—
Comparison of linear regression models to evaluate age and height as predictors of speed and stride length for the same 57 dogs as in Table 1.
| Outcome | Predictor | Adjusted R2* | AIC | Akaike weight† |
|---|---|---|---|---|
| Stride length | Age (y) | 0.035 | 33.828 | < 0.001 |
| Height (cm) | 0.76 | −45.87 | 0.198 | |
| Height + age | 0.78 | −48.67 | 0.802 | |
| Stair trial 1 | Age (y) | 0.23 | −6.37 | 0.497 |
| Height (cm) | 0.031 | 5.95 | 0.001 | |
| Height + age | 0.24 | −6.39 | 0.502 | |
| Off-leash trial | Age (y) | 0.19 | 150.49 | 0.386 |
| Height (cm) | 0.04 | 159.92 | 0.003 | |
| Height + age | 0.215 | 149.57 | 0.611 | |
| On-leash trial with investigator | Age (y) | 0.102 | 50.95 | 0.159 |
| Height (cm) | 0.080 | 52.37 | 0.078 | |
| Height + age | 0.165 | 47.81 | 0.763 | |
| On-leash trial with owner | Age (y) | 0.127 | 56.19 | < 0.001 |
| Height (cm) | 0.328 | 41.24 | 0.023 | |
| Height + age | 0.421 | 33.74 | 0.977 |
Adjusted R2 values indicate the proportion of variance in the outcome variable that is explained by the model, corrected for the number of predictor variables in the model.
Akaike weights indicate the relative probability of a given model being the best fitting among all models considered for a given outcome (eg, an Akaike weight of 0.977 for a given model was interpreted as meaning that there was a 97.7% probability it was the best fitting model among the other candidate models for a given outcome).
AIC = Akaike information criterion (measure of the quality of fit of each model, weighted by the number of factors in the model; smaller AIC values indicate a better fit).
Responses to the follow-up survey were received from 41 of 43 (95%) owners for 49 of 57 (86%) dogs. The home stair trial (stair trial 2) was completed by 43 of these dogs; 3 dogs (2 owners) did not have stairs in their daily environment and 3 dogs were excluded from participating in stair trial 2 because they had been unable to complete stair trial 1. Staircases used in stair trial 2 had a median of 12 steps (range, 11 to 15 steps; mode, 12 steps [17/42 responses]); the median step rise was 16.5 cm (range, 9.5 to 23 cm). One owner did not report the number of steps used in stair trial 2, and 1 owner did not report the step height. Of 41 dogs with complete data for both stair trials, 23 were faster in stair trial 1, and 18 were faster in stair trial 2.
Forty-one owners of 49 dogs answered the question of whether it was difficult to perform measurements of their dog with a tape measure at the clinic. Respondents indicated this was not at all difficult (n = 38 dogs); a little difficult, but not after the investigator showed them how (10); or somewhat difficult (1). Owners indicated (in response to open-ended questions) that including diagrams or drawings, possibly in advance of the study-related appointment, would have made it easier to take the measurements. Several owners of long-haired dogs commented that their dogs’ hair made it difficult to identify relevant landmarks when taking measurements, particularly for thigh circumference. Thirty-nine owners of 45 dogs indicated that it was not at all difficult to time the dog walking in the clinic, and 2 owners of 3 dogs indicated that it was a little difficult, but not after the investigator showed them how. One owner commented that that videotaping with slow-motion playback would have helped to mark the trial end time more accurately. Thirty-six owners of 43 dogs that completed stair trial 2 responded to the question of whether it was difficult to time the dog on stairs at home. Respondents indicated this was not at all difficult (n = 36 dogs), a little difficult (2), somewhat difficult (2), or very difficult (3). The most frequent comments indicated that an extra person was needed to help with at-home timing. In some instances, the owner attempted the trial alone and desired help from a second person; in other instances, the owner recruited a second person but desired help from a third or the owner simply suggested another person would have made it easier without specifying how many people had participated. One owner commented that slow-motion video playback would have helped to mark the times more accurately.
Discussion
The results of the present study indicated that minimally trained dog owners obtained morphological measurements that agreed strongly with those obtained by investigators. In addition, most dog owners (29/41 [71%]) reported that performing the required measurements for their dogs was not at all difficult, although owners of long-haired dogs reported that it was difficult to identify measurement landmarks, particularly at the proximal aspect of the thigh. Previous studies29,30 showed that muscle circumference can be used as an indirect measure of muscle strength and mass, although standardization of the measurement procedures (eg, location on the limb, limb position, and patient position) was recommended. Other tools used to indirectly measure muscle strength in dogs, such as MRI, CT, and dual-energy x-ray absorptiometry, are not feasible for routine serial monitoring.29,30
Owner-investigator agreement for morphological measurements was lowest for circumference of the proximal aspect of the thigh (particularly the right thigh). The owners who were unfamiliar with the use of a tape measure for circumference measurements may have found it difficult to measure thigh circumference. Investigators subjectively noted variation in how tightly owners pulled the tape measure when taking measurements and in owners’ interpretation of the anatomic description for this measurement (ie, where the leg meets the body). Because the study protocol specified that all measurements should be taken from the left side of the dog, most owners did not move (or move the dog) before performing right thigh measurements; it is possible that leaning over or around the dog to obtain this measurement may have influenced the degree of agreement. In retrospect, owners should have been instructed to move to the right side of the dog to measure the right thigh. Future studies are needed to evaluate the utility of instructional materials for owners on how to take measurements (ie, illustrative photographs or videos), and serial or comparative right-to-left measurements of proximal thigh region circumference to evaluate individual dogs.
In the present study, morphological measurements were positively correlated with dogs’ body weight and stride length; body weight was also predictive of stride length (ie, larger dogs take fewer but longer steps to traverse the same distance as smaller dogs). These findings suggested that stride length, as determined from footfall counts, may be a practical measure to include in a low-technology mobility assessment for dogs of all sizes. Because investigators found during the pilot phase that is was difficult to simultaneously count footfalls and time the dogs, a video recording for later counting of footfalls in slow motion was added. This was accomplished by use of a common smartphone, which was compatible with our goal of obtaining measurements in a low-technology environment.
Age was a weak but significant predictor of a dog’s speed in our mobility trials, indicating that speed may be a useful variable for defining frailty in dogs. In humans, an age-related decline in a number of functions (indicated by muscle weakness, low activity level, and more rapid onset of fatigue) is expected; however, markers of the frailty phenotype include a more marked or more rapid decline than that expected as part of the normal aging process.31 Simple measures of mobility that correlate with age offer promise as part of a future canine frailty scale. In the present study, when dog age was adjusted for size (body weight), dogs that had attained a higher percentage of their projected weight-based mean life span had slower speeds in the mobility trials. A previous study21 in dogs found that owner-assessed mobility (including walking, running, and climbing stairs) decreased with increasing age quartile; although dogs with orthopedic and neurologic disease may have been overrepresented in the higher age quartiles, it is interesting that the direction of the association with age quartiles was consistent with that for quartile of projected life span attained in the present study.
The stair trial was expected to be the most physically challenging task in this study, and we expected that older dogs would have slower stair ascent speeds than younger dogs. There are challenges associated with standardization of the mobility trials for dogs, given the difficulty in ensuring that companion dogs have a natural gait under evaluation conditions. To encourage a normal gait speed for dogs in the present study (to parallel conditions in human trials, in which subjects are instructed to walk naturally), handlers were instructed to move the dog along the flat course at a trot; owners could rehearse this task to determine what speed of their gait matched the dog’s natural trot. However, the potential influence of the handler on each dog’s speed prompted us to add the off-leash trial across a flat surface. The addition of the off-leash trial allowed for more nuanced results to emerge regarding the anticipated slower speed of older dogs on the stairs. For instance, when quartiles of projected weight-based mean life span attained were compared, there was a greater decrease in speed with age for off-leash trials (off-leash trial and stair trial 1) than for on-leash trials (on-leash trial with owner and on-leash trial with investigator). This suggested that the off-leash aspect of the trials may have had an impact on speed in addition to that of the stairs. Mobility trials in which dogs move at their own speed might identify subtle mobility changes in older dogs more efficiently than trials in which dogs need to keep up with a moving handler.
In the present study, the mobility trials included a timed flat-surface course (to parallel walking speed measurement in humans) and a timed stair ascent (to mimic the resistance portion of human frailty tests that often include stair climbing).4 In the authors’ experience, owners often report that their older dogs no longer use stairs, even when there is no known orthopedic or neurologic disease. In aging humans, the ability and willingness to use stairs are critical to assessment of frailty, risk of injury, and ability to maintain the activities of daily living, and difficulty with or reluctance to use stairs is commonly observed in older adults who walk without difficulty or reluctance on flat ground.32–36 Stair ascent involves multiple components (eg, isolated leg and back strength, proprioception, balance, vision, and range of motion), making it a more challenging mobility assessment for older adults than simply walking.37–41 It is reasonable to assume that the same is true for older companion dogs, and although limited work has been done in this area, the greater range of motion required for stair ascent, compared with trotting across a flat surface, has been confirmed in dogs.42,43
The results of the present study indicated that minimally trained owners were able to time mobility trials in the clinic or at home. However, owners reported that for 7 of 43 (16%) dogs, they encountered at least some difficulty when conducting the at-home stair trial (stair trial 2), with most indicating that an additional person (or 2) was needed to help with at-home measurements. A limitation to stair trial 2 was that the staircases in the home environment varied in number of steps and the height of each step; variation in other unidentified factors (eg, lighting and surface traction) was also likely. Low-technology mobility measurements that can be performed by owners on a large scale would by necessity take place in nonstandardized environments, and the present study provided insight into owners’ experiences with these tasks. Accordingly, the inclusion of an at-home stair ascent component in future canine frailty scales may need to be evaluated as a means of assessing changes over time in individual dogs rather than providing a single measurement. Future development of an at-home mobility trial should include assessment of the ideal number of people needed to accomplish the task and how feasible it is for owners to recruit the requisite number of participants.
A limitation of the present study was that all participants were affiliated with a veterinary teaching hospital, and these participants might have been more familiar with dog handling than dog owners in the general population. However, the tasks performed by participating dog owners (ie, obtaining morphological measurements with a tape measure and timing dogs during mobility trials) are not part of a routine veterinary physical examination; accordingly, the skill level of participants for performing these tasks did not likely differ from that expected among the general public. Another possible limitation was that dogs ≥ 1 year old were considered adult dogs, which may have resulted in the inclusion of dogs that were skeletally immature. Because large-breed dogs achieve full body size later than small-breed dogs, there is no standard definition of adult age that applies to all dogs. However, determinations of skeletal maturity, growth trajectory, or other markers of adult status were beyond the scope of this study. Only 4 dogs were < 2 years old at the time of enrollment, of which the largest weighed 20.2 kg and was 18 months old. If any of these 4 dogs were skeletally immature, it is unlikely that their inclusion would have appreciably changed the outcome of our study. Also, because not all dogs in the study were obedience trained, owners were allowed to use various methods to get their dogs’ attention and motivate them to perform the off-leash tasks. The use of low-technology mobility measurements by dog owners on a large scale would require the ability to engage dogs with various levels of training and temperament. This need further supports the utility of low-technology measurements that can be evaluated for changes over time, rather than on the basis of a single timed trial. Finally, we used the percentage of projected weight-based mean life span attainedb to allow age comparisons among dogs of different sizes. We recognize that there are limitations in the use of weight-based life span predictions for dogs (eg, cofactors such as breed are not accounted for), and definitions of canine life span and life expectancy will likely continue to be refined.
A primary goal of this study was to evaluate morphological and mobility measurements that could be easily reproduced by a dog owner without requirement of specialized skills or equipment. Such measures could eventually be incorporated into a more comprehensive canine frailty scale, with the aim of creating an objective tool that is predictive of morbidity and death in aging canine patients. Similar frailty assessment findings (eg, walking speed) in humans, mice, and rats are predictive of morbidity and death.6,8,12,18,19
Future studies of dogs are needed to define normal functional decline with age and to characterize a frailty phenotype; such studies should also evaluate the relationship between age and mobility trial times for specific breeds, evaluate trial times in individual dogs over time, and explore differences in outcome for various types of off-leash trials. In addition, studies are needed to evaluate whether slower speeds in aging dogs are attributable to shorter steps, slower turnover of steps of the same length, or a combination of these variables. Potential causes of such gait changes (eg, stiffness, pain, loss of muscle mass, mild incoordination, loss of balance, or other factors) should also be explored. The results of the present study indicated that it is possible to create a low-technology, reproducible assessment to detect differences in mobility among dogs by age category and size. Future development and refinement of this assessment may eventually allow owners and veterinarians to detect and document subtle mobility changes in aging companion dogs and to include mobility assessment in an eventual frailty scale for dogs.
Supplementary Material
Acknowledgments
Dr. Promislow was supported in part by the National Institute of Aging at the National Institutes of Health (R24 AG044284-01 and U19AG057377).
ABBREVIATIONS
- ICC
Intraclass correlation coefficient
Footnotes
The authors declare that there were no conflicts of interest.
Morgan EM, Heseltine JC, Levine GJ, et al. Pilot study of a low-tech mobility assessment for dogs (poster presentation). Vet Stud Res Day, Bristol, England, November 2017.
Original raw data from O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of owned dogs in England. Vet J 2013;198:638–643 was provided by DG O’Neill.
Excel for Mac 2011, version 14.7.3, Microsoft Corp, Redmond, Wash.
R: A language and environment for statistical computing, vesion 3.4.0. R Foundation for Statistical Computing, Vienna, Austria. Available at: www.R-project.org. Accessed Dec 10, 2017.
References
- 1.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Z, Lugtenberg M, Franse C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One 2017;12:e0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdullahi YS, Athanasopoulos LV, Casula RP, et al. Systematic review on the predictive ability of frailty assessment measures in cardiac surgery. Interact Cardiovasc Thorac Surg 2017;24:619–624. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc 2014;62:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 6.Ferrante LE, Pisani MA, Murphy TE, et al. Functional trajectories among older persons before and after critical illness. JAMA Intern Med 2015;175:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg L, Agrawal S, Pew T, et al. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol 2017;119:457–460. [DOI] [PubMed] [Google Scholar]
- 8.Oakland K, Nadler R, Cresswell L, et al. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann R Coll Surg Engl 2016;98:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buigues C, Juarros-Folgado P, Fernandez-Garrido J, et al. Frailty syndrome and pre-operative risk evaluation: a systematic review. Arch Gerontol Geriatr 2015;61:309–321. [DOI] [PubMed] [Google Scholar]
- 10.Afilalo J, Kim S, O’Brien S, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol 2016;1:314–321. [DOI] [PubMed] [Google Scholar]
- 11.Lee L, Patel T, Costa A, et al. Screening for frailty in primary care: accuracy of gait speed and hand-grip strength. Can Fam Physician 2017;63:e51–e57. [PMC free article] [PubMed] [Google Scholar]
- 12.Pamoukdjian F, Lévy V, Sebbane G, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: results from a prospective cohort study. J Nutr Health Aging 2017;21:202–206. [DOI] [PubMed] [Google Scholar]
- 13.Op het Veld LP, van Rossum E, Kempen GI, et al. Fried phenotype of frailty: cross-sectional comparison of three frailty stages on various health domains. BMC Geriatr 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies M Geriatric screening in first opinion practice—results from 45 dogs. J Small Anim Pract 2012;53:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999;214:1336–1341. [PubMed] [Google Scholar]
- 16.Salvin HE, McGreevy PD, Sachdev PS, et al. Under diagnosis of canine cognitive dysfunction: a cross-sectional survey of older companion dogs. Vet J 2010;184:277–281. [DOI] [PubMed] [Google Scholar]
- 17.Szabó D, Gee NR, Miklósi A. Natural or pathologic? Discrepancies in the study of behavioral and cognitive signs in aging family dogs. J Vet Behav 2016;11:86–98. [Google Scholar]
- 18.Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci 2017;72:885–891. [DOI] [PubMed] [Google Scholar]
- 19.Yorke A, Kane AE, Hancock Friesen CL, et al. Development of a rat clinical frailty index. J Gerontol A Biol Sci Med Sci 2017;72:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua J, Hoummady S, Muller C, et al. Assessment of frailty in aged dogs. Am J Vet Res 2016;77:1357–1365. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves L, Simoes A, Millis DL, et al. Development of a scale to evaluate mobility in dogs. Cienc Rural 2016;46:2210–2215. [Google Scholar]
- 22.Eddie’s Wheels for Pets. A visual guide to measuring your dog. Available at: eddieswheels.com/p/26/A-Visual-Guide-to-Measuring-Your-Dog. Accessed Jun 25, 2017.
- 23.Boyko AR, Quignon P, Li L, et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol 2010;8:e1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutter NB, Mosher DS, Gray MM, et al. Morphometrics within dog breeds are highly reproducible and dispute Rensch’s rule. Mamm Genome 2008;19:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 27.O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of owned dogs in England. Vet J 2013;198:638–643. [DOI] [PubMed] [Google Scholar]
- 28.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer, 2002;60–76. [Google Scholar]
- 29.Hesbach AL. Techniques for objective outcome assessment. Clin Tech Small Anim Pract 2007;22:146–154. [DOI] [PubMed] [Google Scholar]
- 30.Hesbach A A proposed canine movement performance test: the canine timed up and go test (CTUG). Orthoped Phys Ther Pract 2003;15:26. [Google Scholar]
- 31.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med 2011;27:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayagoitia RE, Harding J, Kitchen S. Identification of stair climbing ability levels in community-dwelling older adults based on the geometric mean of stair ascent and descent speed: the GeMSS classifier. Appl Ergon 2017;58:81–88. [DOI] [PubMed] [Google Scholar]
- 33.Herman T, Inbar-Borovsky N, Brozgol M, et al. The Dynamic Gait Index in healthy older adults: the role of stair climbing, fear of falling and gender. Gait Posture 2009;29:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamel KA, Cavanagh PR. Stair performance in people aged 75 and older. J Am Geriatr Soc 2004;52:563–567. [DOI] [PubMed] [Google Scholar]
- 35.Stessman J, Rottenberg Y, Jacobs JM. Climbing stairs, hand-rail use, and survival. J Nutr Health Aging 2017;21:195–201. [DOI] [PubMed] [Google Scholar]
- 36.Edwards N, Dulai J. Examining the relationships between walkability and physical activity among older persons: what about stairs? BMC Public Health 2018;18:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper NG, Wilken JM, Neptune RR. Muscle function and coordination of stair ascent. J Biomech Eng 2018;140:011001. [DOI] [PubMed] [Google Scholar]
- 38.Verghese J, Wang C, Xue X, et al. Self-reported difficulty in climbing up or down stairs in nondisabled elderly. Arch Phys Med Rehabil 2008;89:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Startzell JK, Owens DA, Mulfinger LM, et al. Stair negotiation in older people: a review. J Am Geriatr Soc 2000;48:567–580. [DOI] [PubMed] [Google Scholar]
- 40.Silverman AK, Neptune RR, Sinitski EH, et al. Whole-body angular momentum during stair ascent and descent. Gait Posture 2014;39:1109–1114. [DOI] [PubMed] [Google Scholar]
- 41.Bergland A, Sylliaas H, Jarnlo GB, et al. Health, balance, and walking as correlates of climbing steps. J Aging Phys Act 2008;16:42–52. [DOI] [PubMed] [Google Scholar]
- 42.Durant AM, Millis DL, Headrick JF. Kinematics of stair ascent in healthy dogs. Vet Comp Orthop Traumatol 2011;24:99–105. [DOI] [PubMed] [Google Scholar]
- 43.Carr JG, Millis DL, Weng HY. Exercises in canine physical rehabilitation: range of motion of the forelimb during stair and ramp ascent. J Small Anim Pract 2013;54:409–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


