Abstract
Background
Immune checkpoint inhibitors (ICIs) are associated with rheumatic and musculoskeletal immune-related adverse events (irAEs) in 5%–20% of patients. Currently, patients refractory to corticosteroids and conventional disease-modifying antirheumatic drugs (cDMARD) are treated with biological DMARDs (bDMARDs) targeting tumor necrosis factor α (TNFα) and interleukin-6, although without a clear biological rationale. Synovial tissue (ST) biopsy presents a valuable opportunity to investigate irAE pathogenesis and appropriately stratify bDMARD use in refractory irAE patients.
Case presentation
We provide the first report of comparative, parallel ST and synovial fluid (SF) analyses of severe, cDMARD-refractory, seronegative polyarthritis, classified as a grade 3 irAE occurring in response to nivolumab treatment for metastatic squamous cell lung cancer, in comparison with ST and SF from patients with untreated rheumatoid arthritis (RA). We investigated immunohistochemical labeling of ST cytokine expression as a biological rationale for selecting therapy. Flow cytometric analysis of lymphocytes from ST, SF and blood collected before and after synovial biopsy-guided therapy, in comparison with RA, were evaluated for insights into the immunopathogenesis of irAE. Immunolabeling of ST demonstrated an excess of TNFα cytokine expression. Subsequent treatment with infliximab resulted in resolution of inflammatory symptoms and a significant reduction in C reactive protein levels. Flow cytometric analysis of synovial infiltrates indicated absence of programmed cell death protein-1 (PD-1) receptor positivity despite cessation of nivolumab approximately 200 days prior to the analyzes.
Conclusions
A deeper understanding of the immunopathogenetic basis of immune activation in irAEs is required in order to select therapy that is likely to be the most effective. This is the first report investigating parallel blood, ST and SF in ICI-induced severe rheumatic irAE. Use of a bDMARD directed by the dominant inflammatory cytokine achieved resolution of synovitis while maintaining cancer remission.
Keywords: rheumatology
Background
Recent success stories of treating cancer with immune checkpoint inhibitors (ICIs) illustrate the important roles that programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) play in regulating T-cell specific anti-tumor responses.1 Immunotherapy with monoclonal antibodies such as nivolumab or pembrolizumab (targeting PD-1), ipilimumab (targeting CTLA-4) or atezolizumab (targeting the ligand for PD-1, PD-L1) aims to attenuate ICI signaling in order to unleash potent T-cell mediated antitumor activity. However, ICI therapy is associated with inflammation that can recapitulate many features of autoimmunity.2 3 Approximately 50% of patients receiving ICIs experience immune-related adverse events (irAEs) that are most often reported to be gastrointestinal, dermatological or endocrine in nature.2 3 Rheumatological irAEs occur in 5%–20% of cases.4–6
Mild cases of rheumatic irAEs can be managed with corticosteroids and/or conventional disease-modifying antirheumatic drugs (cDMARDs), while refractory cases are often treated with biological DMARDs (bDMARDs) targeting tumor necrosis factor α (TNFα)7 and interleukin-6 (IL-6),8 although without any informed histopathological rationale. Critically, no clinical trials are currently in progress that will provide evidence for the preferential use of one bDMARD over another in corticosteroid and/or cDMARD-refractory irAEs. Here in, we describe a case of nivolumab-induced severe, cDMARD-refractory polyarthritis, successfully treated with synovial-biopsy informed bDMARD therapy. This is the first report characterizing parallel peripheral blood, synovial fluid (SF) and synovial tissue (ST) inflammatory infiltrates in a rheumatic irAE with comparison to equivalent samples from patients with early rheumatoid arthritis (RA).
Case presentation
Clinical presentation
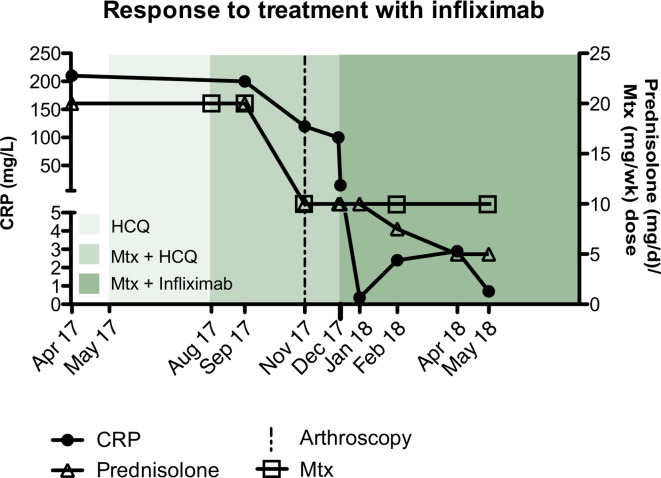
A 62-year-old man with metastatic stage IV squamous cell cancer (SCC) and no prior history of autoimmune disease was treated with nivolumab every 2 weeks (3 mg/kg) from July 2016 to April 2017, resulting in complete clinical remission of his SCC. Nivolumab was ceased in April 2017, after he developed musculoskeletal irAEs with disabling polyarthritis involving shoulders, elbows, proximal interphalangeal joints and right knee, classified as a grade 3 irAE. C reactive protein (CRP) was markedly elevated at 210 mg/L. Rheumatoid factor (RF), anticyclic-citrullinated peptide antibody (ACPA) and HLA-B27 were negative, and radiographs demonstrated no erosive changes. Despite prednisolone (20–25 mg daily), intra-articular corticosteroid and sequential hydroxychloroquine (200 mg daily) and methotrexate (20 mg weekly; figure 1), his synovitis remained active.
Figure 1.
Response to treatment with infliximab. Following development of severe inflammatory polyarthritis, elevated CRP at 210 g/L, and the subsequent cessation of nivolumab treatment in April 2017, this patient was commenced on high-dose prednisolone (20–25 mg daily) along with subsequent addition of hydroxychloroquine (200 mg daily, shown in light green) in may 2017 and then MTX (20 mg Weekly shown in green) in August 2017. Following failed attempts to taper prednisolone treatment, arthroscopic synovial biopsy and synovial biopsy-guided therapy with infliximab (shown in dark green) was initiated in quick succession (November 2017 and December 2017, respectively) in addition to holding HCQ. This was followed by a rapid decline in CRP simultaneous with effective tapering of prednisolone therapy. CRP, C reactive protein; HCQ, hydroxychloroquine; MTX, methotrexate.
Given the lack of definitive therapeutic guidelines for rheumatic irAEs, the emergence of synovial biopsy-guided therapeutic approaches in RA9 10 and following our experience with arthroscopic ST biopsies,11 12 the patient underwent arthroscopic ST biopsy of his right knee in November 2017 (200 days following cessation of nivolumab).
Sample acquisition and processing
At arthroscopy, nivolumab-induced synovitis was more severe macroscopically than the synovitis in comparator treatment-naïve early RA ST, with florid synovial hyperplasia and hypervascularization throughout (figure 2A, and see online supplementary additional movie file 1). Several ST biopsies were undertaken from areas of synovitis within the knee. Matched peripheral blood mononuclear cell (PBMC) and SF samples (Table 1) were collected. ST, SF and PBMC samples from this patient were compared with samples from three treatment-naïve patients with RF and/or ACPA-positive early RA (defined as within 12 months of onset of symptoms and fulfilling the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria),13 from whom samples were collected at the time of diagnosis. Additional PBMC and SF samples were collected from the irAE patient at the conclusion of 6 months infliximab therapy. All patients gave written informed consent.
Figure 2.
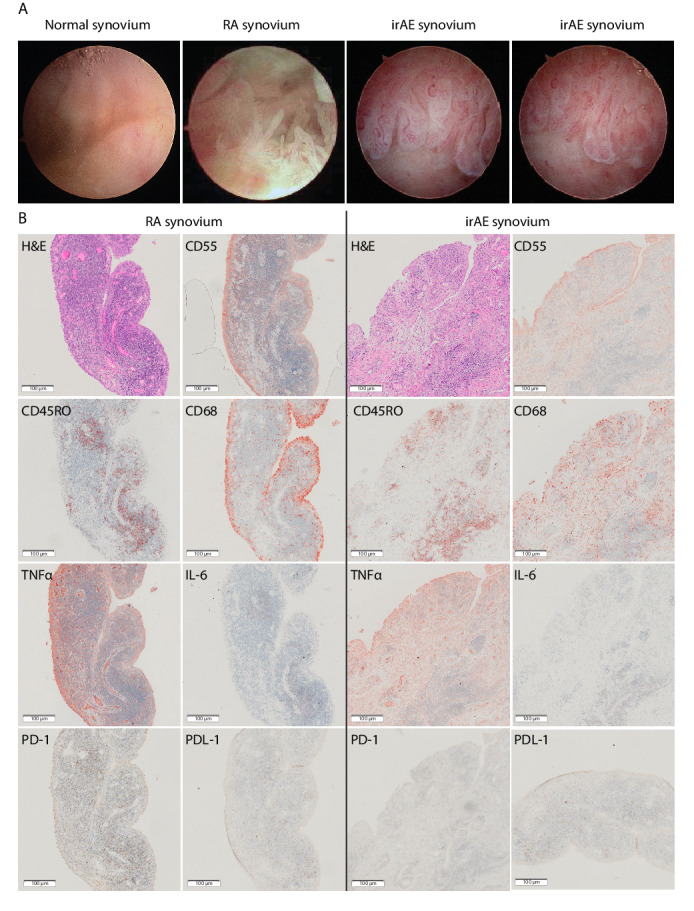
Rheumatic irAE recapitulates features of rheumatoid arthritis synovitis. (A) Camera-guided arthroscopic images show extensive hypervascularization and synovial hyperplasia in both irAE and early RA compared with the normal synovium. (B) Immunohistochemistry on synovial tissue sections (rheumatic irAE; left, RA; right) stained with: H&E; CD55 highlighting synovial membrane lining fibroblasts; CD68 identifying diffuse infiltration of macrophages in irAE compared with subsynovial lining localization in RA; CD45RO identifying abundant memory T cell infiltration; TNFα and IL-6 labeling showing differential proinflammatory cytokine expression in both samples; PD-1 demonstrating absent labeling in irAE compared with RA; and PDL-1 positive labeling in both irAE and RA. Single labeled slides were counter stained with Haematoxylin. Rheumatoid arthritis synovial tissue section images are representative of all 3 RA patients. All images were captured at ×20 magnification. IL-6, interleukin-6; irAE, immune-related adverse-events; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand 1; RA, rheumatoid arthritis; TNFα, tumour necrosis factor α.
Table 1.
Synovial fluid (knee) preinfliximab and postinfliximab therapy
| Pre-Infliximab | Post-Infliximab | |
| Polymorphonuclear cells (x10ˆ6) | 2800 | 3 |
| Red blood cells (x10ˆ6) | 70 | 150 |
| Lymphocytes (x10ˆ6) | 310 | Not reported |
| Mononuclear cells (x10ˆ6) | Not reported | 24 |
| Bacteria and culture | Absent; culture negative | Absent; culture negative |
| Crystals | Negative | Negative |
jitc-2019-000281supp001.mp4 (13.3MB, mp4)
ST samples were analyzed using histology, and flow cytometry following disaggregation. Histology was performed on serial 5m sections from formalin-fixed paraffin-embedded ST blocks. Sections were stained with H&E or labeled with the following primary antibodies for immunohistochemistry (IHC): CD45RO (Dako; UCHL1), CD55 (BioRad; C67), CD68 (Dako; KP1), TNFα (LifeSpan; C7952), IL-6 (Santa Cruz; 130326), and via a National Association of Testing Authorities approved protocol with PD-1 (CellMarque; MRQ22), PDL-1 (Ventana; SP263). Antibody-stained sections were incubated with horseradish peroxidase linked secondary antibody, followed by colorimetric visualization with either 3-amino-9-ethylcarbazole substrate-chromogen (for CD45RO, CD55, CD68, TNFα and IL-6) or 3,3′-Diaminobenzidine (for PD-1 and PDL-1). Nuclear counterstaining was performed with Mayer’s haematoxylin. Negative controls included the use of IgG isotype controls or omission of the primary antibody. Whole slide scans were captured on an Olympus VSI120 bright-field microscope at x20 magnification and sequential regions of interest compared for staining intensity. Semiquantative assessment of staining intensity was perfomed by two independent assessors using previously described techniques.14
For flow cytometry, fresh ST was dissociated using Milytenyi Tumour Dissociation Kit (Milytenyi; 130-095-929) and the gentleMACS dissociator as per manufacturer’s recommendations. Matched SF and whole blood was collected for each patient at arthroscopy and for irAE only, after 6 months of infliximab treatment. PBMCs were purified using Lymphoprep reagent. Cells were washed and counted prior to staining for flow cytometry with Zombie UV (BioLegend) in serum free PBS, followed by washing and staining with CD45RO-UV395 (BD; UCHL1), PD1-BV421 (BD; EH12.1), CD3-PerCP/Cy5.5 (BD; SK7), CD8-Alexa647 (BD; RPA-T8), CD4-Alexa700 (BD; SK3), CD20-APC/H7 (BD; L27) in 2% Fetal calf serum. Cells were resuspended in 2% fetal calf serum ready for FACS acquisition on a BD FACSAria Fusion flow cytometer. Analysis of cytometry data was performed using FlowJo 10.4
Results and patient progress
Analysis of the IHC inflammatory cell infiltrate pathotype revealed lymphoid (follicular) pattern of the patient with irAE and a similar pattern in one of the RA controls; of the two other RA controls, one had diffuse and the other low (or pauci) immune infiltration. IHC of ST from the patient with irAE revealed infiltrating memory T-cells (CD45RO+) and extensive CD68 expressing macrophages (lining and sublining) (figure 2B), and there were no detectable B cells; in comparison, two (of the 3) RA ST revealed presence of B cells within the lymphoid aggregates and slightly less extensive CD68 expressing lining/sublining macrophages. The irAE ST displayed abundant TNFα, compared with lower levels of IL-6 labeling (figure 2B). This differential cytokine expression was similar to that previously observed in a proportion of patients with RA.15 Finally, we detected abundant levels of PDL-1 labeling in both RA and irAE ST but there was no detectable PD-1 in ST from the patient with irAE (figure 2B). In summary, the synovial cellular infiltrate in ST was similar to the RA ST and was indicative of TNFα dominant RA-like disease. Critically, the presence of cellular intense and dominant staining of the proinflammatory cytokine TNFα in irAE ST (figure 2B) influenced our decision to prescribe Infliximab (an TNF antagonist) instead of an alternative bDMARD such as Tocilizumab (an IL-6R antagonist) for this irAE.
We then performed multiparameter flow cytometry to further characterize infiltrating T-cells in ST, SF and PBMCs in response to nivolumab-induced irAE, and, to identify changes in peripheral and SF T-cells after infliximab treatment.
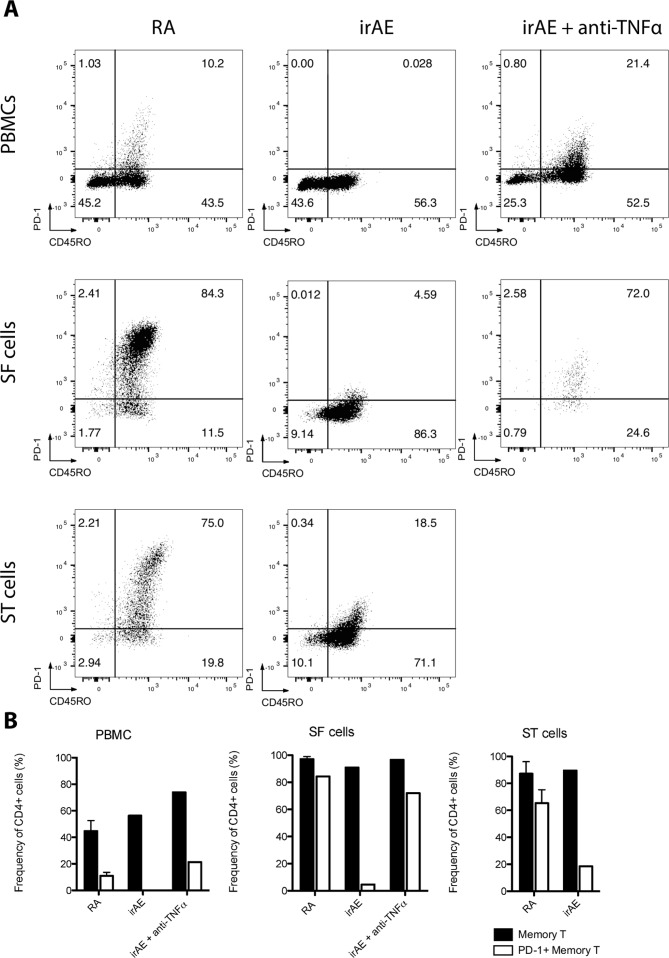
CD4 +T cells were gated (online supplementary additional figure 1) and CD45RO and PD-1 expressing cells were identified (figure 3A). Abundant memory T-cell infiltration was observed in both ST and SF compartments for RA and irAE samples, with samples from PBMCs exhibiting lower memory T-cell frequency. Importantly, we detected a reduction in PD-1 labeling on T-cells from irAE samples compared with early RA controls. In particular, prior to commencing infliximab, there was approximately a 20-fold reduction of PD-1 +memory T cells in the SF compartment and 4-fold reduction in the ST compartment when compared with RA (figure 3A). Furthermore, while early RA comparators exhibited fewer peripheral PD-1 +memory T-cells in PBMCs compared with RA ST and SF compartments, we observed complete ablation of PD-1 labeling in irAE PBMC memory T-cells (figure 3A).
Figure 3.
Abundant PD-1 +memory T cell infiltration resolved in infliximab treated irAE. (A) Peripheral blood mononuclear cells (PBMCs; top row), synovial fluid mononuclear cells (SF cells; middle row) and synovial tissue mononuclear cells (ST cells; bottom row) were prepared and labeled with Zombie UV live/dead stain, CD3, CD4, CD8, CD20, CD45RO and PD-1. CD4 +T cells were then presented on 2D plots identifying memory (CD45RO+) and PD-1 +memory T cells (PD1 +CD45RO+) in early RA (left column), ICI-irAE (middle column) and, infliximab-treated irAE (right column). Early RA plots are representative of all 3 RA samples. (B) Cumulative data showing the frequency of memory T cells and PD-1 +memory T cells for each sample type. 2D, two-dimensional; ICI, immune checkpoint inhibitorir; AE, immune-related adverse events; PD-1, programmed cell death protein-1; RA, rheumatoid arthritis.
jitc-2019-000281supp002.pdf (272.3KB, pdf)
Commencement of infliximab (5 mg/kg) led to a dramatic clinical response (figure 1), coupled with a precipitous fall in CRP to 0.36 mg/L. This improvement persisted despite weaning of prednisolone to 5 mg/d and methotrexate to 10 mg/week (figure 1). Infliximab was ceased after 6 months and at last follow-up—18 months after nivolumab cessation—he continues to have minimal synovitis, well controlled on low-dose methotrexate and prednisolone (5 mg daily) and, critically, no recurrence of his lung cancer.
Interestingly, in PBMCs and SF collected after 6 months of infliximab therapy, we identified a near restoration (compared with RA) of PD-1 expressing CD4 +memory T-cell frequency (figure 3A).
Discussion and conclusions
Checkpoint inhibitors have changed the therapeutic landscape for the treatment of metastatic cancers.1 However, irAEs result in reduced quality of life2 3 and in severe cases, cessation of a potentially lifesaving therapy.
While case series have reported success in treating rheumatologic irAEs with both corticosteroids and bDMARDs, both are thought to compromise ICI-mediated antitumor effects with prolonged use16 and long-term usage of corticosteroids can result in significant systemic toxicity.2 A better understanding of the immunopathogenesis of these events will allow greater confidence in selecting appropriate therapies. Furthermore, the synovial biopsy-guided approach we propose could be extrapolated to other irAEs including the more commonly seen colitis and dermatological complications.
ST research has increased our understanding of RA pathogenesis,11 12 17 resulted in the identification of new therapeutic targets18 19 and has the potential to stratify treatment.9 10 In this study, ST sampling provided a histopathological rationale for the choice of bDMARD to treat a severe treatment-refractory rheumatic irAE. irAE ST IHC was reminiscent of the RA synovium, demonstrating differentially expressed cytokines. This invited the option of targeting therapy to the dominant inflammatory cytokine, including TNFα and IL-67 8 for which antibodies have already been trialed empirically in irAEs. Abatacept, a CTLA-4 fusion protein targeting T-cells, may result in a loss of anti-tumor response20 and as a result is not routinely considered a treatment option.
Abundant macrophages and CD45RO+memory T-cell infiltration were observed in the ST of this patient with irAE synovitis. Importantly, while these features recapitulated those of RA, we also observed a noticeable increase in TNFα expression and absence of IL-6. Critically, the predominance of TNFα suggested that a TNFα inhibitor would be a more suitable bDMARD therapy than tocilizumab. Two (of the three) RA patients’ ST biopsies were positive for IL6, serving as positive control. Interestingly, the RA patient whose biopsy had more significantly positive IL6 also had lymphoid (follicular) pattern of infiltrate, similar to our patient with irAE.
Histological examinaton of the ST also indicated an absence of PD-1 labeling in the nivolumab-treated irAE patient compared with the ST from patients with early RA, despite significant positive labeling for PDL-1 expressing cells (figure 2). PD-1 expressing T-cells are considered key players in classic chronic autoimmune diseases like RA.21 Interestingly, flow cytometry of the abundant T-cells in the inflammatory synovium demonstrated PD-1 labeling was markedly lower in CD45RO+memory T-cells from irAE compared with RA. We hypothesize that this is due to sustained occupancy of the PD-1 receptor by nivolumab, even at the time of arthroscopy (~200 days after cessasion). This is considerably longer than the reported serum half-life of 12–20 days for nivolumab,22 23 and while prolonged occupancy on CD4 +T cells has been reported for nivolumab,23 24 this is the first indication of prolonged occupancy of PD-1 on CD4 +T cells in inflamed tissues in rheumatic irAEs and may influence ST T-cell infiltration and chronic inflammation. Indeed, it is plausible that the half-life of ICIs varies in different tissues, which may underpin both their antitumor effect as well as explaining the variation seen in the time of onset of organ-specific irAE.25
Using arthroscopic synovial biopsy, we were able to target a bDMARD to the dominant inflammatory cytokine resulting in resolution of severe synovitis in this patient. The presence of PD-1 staining in memory T-cells in both the peripheral blood and SF compartments following infliximab treatment is interesting and may indicate the natural attrition of nivolumab from the surface of T-cells, or indeed, the emergence of a new non-occupied T-cell population. It is interesting to speculate what the implications may be, of the re-emergence of this population of cells with respect to possible relapse of his SCC (this has not occurred thus far, 30 months following cessation of Nivolumab). A recently published26 case report of a synovial biopsy of a patient with irAE presenting as asymmetric inflammatory oligoarthritis and enthesitis also revealed a lymphoid pattern of infiltration. This patient responded only partially to adalimumab (in combination with methotrexate and corticosteroids), presumably owing to the fact that treatment was not directed by cytokine/cellular dominance; cytokine staining was not performed and the authors raise the possibility as to whether the patient may have responded better had an IL-6R inhibitor may have been used as reported in the study in RA by Dennis et al.10 In contrast, we believe the marked benefit to infliximab in our patient was as a result of dominance of TNF in his ST that underpinned this benefit.
A limitation of our study was the lack of interrogation of ST using molecular methods; it would have been ideal to further evaluate TNF and IL6/IL6R pathways, in particular. In addition, the reasons for selecting the cytokine/cellular staining that we did was also pragmatic: biological disease-modifying therapies are available (in the context of the inflammatory arthritides) against TNF, T-cell co-stimulation, IL6 and B-cells (anti-CD20) and hence we wished to use the information to specifically direct therapeutic choice.
In conclusion, our data indicate absence of PD1 positivity despite cessation of nivolumab 200 days prior, indicating a sustained tissue response much longer than the serum half-life of this medication. We demonstrate that a synovial biopsy-guided therapy approach using ST biopsy to inform biologic therapy is feasible, results in rapid resolution of disease and provides important information about the immunopathogenesis of the process. This synovial biopsy-guided therapy could be reasonably extended to other irAEs and may improve response rates over empirical therapy. Future investigations on irAE patients should aim to collect cells for single cell RNAseq, which could yield cellular information about irAE initiation, confirm PD-1 mRNA expression in infiltrating T-cells, and identify patterns in T-cell clonality that may help elucidate the mechanisms of disease and ultimately improve clinical outcomes; it is also probable that delineation of inflammatory pathways by such methods in future analyses will be productive with regards to more accurately identifying involved pathways and directing therapeutic choice.
jitc-2019-000281supp003.pdf (267.9KB, pdf)
Footnotes
Contributors: Conceptualisation: MDW, WM-B. Data acquisition and curation: WM-B, MDW, HW, TDW, SP, SS and SK. Methodology: MDW, WM-B, JGW, SK, HW and MDS. Drafting manuscript: MDW, WM-B, SP, JGW and MDS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: All protocols for collecting biopsies and blood were approved by the local Ethics Committee (Southern Adelaide Local Health Network Human Research Ethics Committee (SALHN HREC); 199.10 and 396.10.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Relevant material has been uploaded as additional material. Any other data required is available on request.
References
- 1.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29–39. 10.1016/j.intimp.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Chaudhary N, Garg M, et al. . Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49. 10.3389/fphar.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michot JM, Bigenwald C, Champiat S, et al. . Immune-Related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 4.Kostine M, Rouxel L, Barnetche T, et al. . Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell EL, Lau PKH, Khoo C, et al. . Rheumatic immune-related adverse events secondary to anti-programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: a case series. Eur J Cancer 2018;105:88–102. 10.1016/j.ejca.2018.09.027 [DOI] [PubMed] [Google Scholar]
- 6.Narváez J, Juarez-López P, LLuch J, et al. . Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev 2018;17:1040–5. 10.1016/j.autrev.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Calabrese C, Kirchner E, Kontzias A, et al. . Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 2017;3:e000412. 10.1136/rmdopen-2016-000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim ST, Tayar J, Trinh VA, et al. . Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76:2061–4. 10.1136/annrheumdis-2017-211560 [DOI] [PubMed] [Google Scholar]
- 9.Astorri E, Nerviani A, Bombardieri M, et al. . Towards a stratified targeted approach with biologic treatments in rheumatoid arthritis: role of synovial pathobiology. Curr Pharm Des 2015;21:2216–24. 10.2174/1381612821666150310145758 [DOI] [PubMed] [Google Scholar]
- 10.Dennis G, Holweg CTJ, Kummerfeld SK, et al. . Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 2014;16:R90. 10.1186/ar4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Walsh AM, Canavan M, et al. . Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One 2018;13:e0192704. 10.1371/journal.pone.0192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh AM, Wechalekar MD, Guo Y, et al. . Triple DMARD treatment in early rheumatoid arthritis modulates synovial T cell activation and plasmablast/plasma cell differentiation pathways. PLoS One 2017;12:e0183928. 10.1371/journal.pone.0183928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 14.Tak PP, van der Lubbe PA, Cauli A, et al. . Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum 1995;38:1457–65. 10.1002/art.1780381012 [DOI] [PubMed] [Google Scholar]
- 15.Ulfgren AK, Lindblad S, Klareskog L, et al. . Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis 1995;54:654–61. 10.1136/ard.54.8.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med 2017;376:1989–91. 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 17.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol 2013;25:334–44. 10.1097/BOR.0b013e32835fd8eb [DOI] [PubMed] [Google Scholar]
- 18.Cheung TT, McInnes IB. Future therapeutic targets in rheumatoid arthritis? Semin Immunopathol 2017;39:487–500. 10.1007/s00281-017-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenders MI, van den Berg WB. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol Sci 2015;36:189–95. 10.1016/j.tips.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 20.Naidoo J, Cappelli LC, Forde PM, et al. . Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 2017;22:627–30. 10.1634/theoncologist.2016-0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu M, Cavero V, Lu Q, et al. . Follicular helper T cells in rheumatoid arthritis. Clin Rheumatol 2015;34:1489–93. 10.1007/s10067-015-3028-5 [DOI] [PubMed] [Google Scholar]
- 22.Asmar R, Yang J, Carvajal RD. Clinical utility of nivolumab in the treatment of advanced melanoma. Ther Clin Risk Manag 2016;12:313–25. 10.2147/TCRM.S78039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Drake CG, Wollner I, et al. . Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol 2018;14:569–79. 10.1038/s41584-018-0074-9 [DOI] [PubMed] [Google Scholar]
- 26.Medina HA, Eickhoff J, Edison JD. Thinking inside the box: synovial tissue biopsy in immune checkpoint inhibitor-induced arthritis. J Clin Rheumatol 2019. 10.1097/RHU.0000000000001088. [Epub ahead of print: 24 May 2019]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000281supp001.mp4 (13.3MB, mp4)
jitc-2019-000281supp002.pdf (272.3KB, pdf)
jitc-2019-000281supp003.pdf (267.9KB, pdf)