Abstract
Introduction
Musculoskeletal (MSK) injuries are a frequent cause for emergency department (ED) visits in children. MSK injuries are associated with moderate-to-severe pain in most children, yet recent research confirms that the management of children’s pain in the ED remains inadequate. Clinicians are seeking better oral analgesic options for MSK injury pain with demonstrated efficacy and an excellent safety profile. This study aims to determine the efficacy and safety of adding oral acetaminophen or oral hydromorphone to oral ibuprofen and interpret this information within the context of parent/caregiver preference.
Methods and analysis
Using a novel preference-informed complementary trial design, two simultaneous trials are being conducted. Parents/caregivers of children presenting to the ED with acute limb injury will be approached and they will decide which trial they wish to participate in: an opioid-inclusive trial or a non-opioid trial. Both trials will follow randomised, double-blind, placebo-controlled, superiority-trial methodology and will enrol a minimum of 536 children across six Canadian paediatric EDs. Children will be eligible if they are 6 to 17 years of age and if they present to the ED with an acute limb injury and a self-reported verbal Numerical Rating Scale pain score ≥5. The primary objective is to determine the effectiveness of oral ibuprofen+oral hydromorphone versus oral ibuprofen+oral acetaminophen versus oral ibuprofen alone. Recruitment was launched in April 2019.
Ethics and dissemination
This study has been approved by the Health Research Ethics Board (University of Alberta), and by appropriate ethics boards at all recruiting centres. Informed consent will be obtained from parents/guardians of all participants, in conjunction with assent from the participants themselves. Study data will be submitted for publication regardless of results. This study is funded through a Canadian Institutes of Health Research grant.
Trial registration number
NCT03767933, first registered on 07 December 2018.
Keywords: pain management, paediatric orthopaedics, accident & emergency medicine
Strengths and limitations of this study.
This study employs a novel design involving two simultaneously run, complementary, randomised controlled trials.
Participating families will choose in which trial they wish to participate, thus engaging and empowering them as a key participant in healthcare research decision-making.
This study will collect preference and opinion data from families, in order to better understand their analgesic decision-making for their children.
We expect that some parents/caregivers will be hesitant to accept opioids thus leading to an imbalance in the pace of recruitment between the two trials.
Given the sample size, this study will not be able to provide definitive evidence regarding rare but serious adverse events.
Introduction
Musculoskeletal (MSK) injuries are very common and are associated with moderate-to-severe pain for most children.1 2 Despite three decades of research in this area, recent evidence confirms that paediatric pain management in the emergency department (ED) is still suboptimal.3–5 Previous studies have demonstrated that only 35% of children presenting to a paediatric ED with fractures or severe sprains received any analgesic.6 7
The American Academy of Pediatrics recommends acetaminophen, ibuprofen and opioids as the top three medication choices for the treatment of acute pain in children.8 These are also the top three most commonly used analgesics for children with MSK injury.3 4 6 9 10 However, there has recently been a concerted movement to limit opioid use in children, due, in large part, to the current Opioid Crisis.11 12 Clinicians are increasingly less likely to prescribe oral opioids to young children, and caregivers are increasingly less willing to administer them.5 The fear of adverse events, particularly respiratory depression and deep sedation, are other important reasons to explain the reluctance to prescribe an opioid to children with moderate-to-severe pain.13
Clinicians are currently seeking optimal (and for many, non-opioid) oral analgesic options with the best efficacy and safety profile. It is known that the under-treatment of children’s pain is partly due to a lack of evidence to support clinician decision-making in choosing the most effective medication.4 14 A recently published systematic review of MSK injury pain management concluded that an optimal analgesic approach could not be identified at this time.15 Very few paediatric studies of analgesic combination therapy for MSK injury exist, and extrapolation from adult data can be misleading, both in establishing the correct dose and in assessing effect.15–18 Research has demonstrated that a combination of oral morphine with ibuprofen was no more effective and was less safe than oral ibuprofen alone for children’s MSK pain.16 Two clinical trials of oral morphine versus ibuprofen have shown that oral morphine was not superior to ibuprofen alone.19 20 Similarly, oxycodone was no more effective and was less safe than ibuprofen for post-discharge fracture pain.21 Further, tramadol, hydrocodone and codeine are not recommended for widespread use in children due to safety concerns.22–25 There is some emerging work from non-ED settings to suggest that oral hydromorphone may be an effective alternative to oral morphine and oxycodone.26 27 Oral hydromorphone is a long-acting opioid analgesic with a duration of action up to 4 hours and is more potent than oral morphine, but with fewer side effects.28 Both oral hydromorphone and ibuprofen’s peak analgesic action occurs at 60 min post administration.
The proposed study aims to determine if acetaminophen or hydromorphone, when added to ibuprofen, offers more clinical pain relief than ibuprofen alone, for children with an acute MSK injury. Further, it will determine if the combination of hydromorphone and ibuprofen is more clinically effective than the combination of acetaminophen with ibuprofen. This study, which will consist of two clinical trials, will inform healthcare decisions by providing evidence for the effectiveness and safety of commonly prescribed analgesic agents, and compare them to the most commonly used monotherapy, ibuprofen.3 6
Methods and analysis
This study will be conducted with a novel preference-informed complementary trial design and is comprised of two simultaneous ‘parallel’ trials. Eligible parent/caregiver-child pairs will decide which trial they wish to participate in: a three-armed opioid-inclusive trial (the Opioid trial) or a two-armed non-opioid trial (the Non-Opioid trial). Once the parent/caregiver and child have chosen their preferred trial, conduct within each trial will follow traditional randomised, double-blind, parallel assignment, placebo-controlled superiority trial methodology. Study endpoints will be identical for both trials within this study. The study protocol is reported using the Standard Protocol Items: Recommendations for Interventional Trials-Patient-Reported Outcomes reporting guidelines.29 (see table 1)
Table 1.
WHO trial registration data set
| Data category | Information |
| Primary registry and trial identifying number | ClinicalTrials.gov, NCT03767933. |
| Date of registration in primary registry | 07 December 2018. |
| Secondary identifying numbers | University of Alberta Research Ethics Board # Pro00073476. |
| Source(s) of monetary or material support | Canadian Institutes of Health Research SPOR Innovative Clinical Trials Grant (MYG-151207). |
| Primary sponsor | University of Alberta. |
| Secondary sponsor(s) | – |
| Contact for public queries | Dr Samina Ali 780.248.5575 sali@ualberta.ca |
| Contact for scientific queries | Dr Samina Ali 780.248.5575 sali@ualberta.ca |
| Public Title | The No OUCH Study |
| Scientific Title | A Study of Non-Steroidal or Opioid Analgesia Use for Children with Musculoskeletal Injuries: The No OUCH Study |
| Countries of recruitment | Canada. |
| Health condition(s) or problem(s) studied | Acute musculoskeletal injury. |
| Intervention(s) | Opioid trial: (A) Oral hydromorphone (0.05 mg/kg, max 5 mg)+oral ibuprofen (10 mg/kg, max 600 mg). (B) Oral acetaminophen (15 mg/kg, max 1000 mg)+oral ibuprofen (10 mg/kg, max 600 mg). Non-Opioid trial: Oral acetaminophen (15 mg/kg, max 1000 mg)+oral ibuprofen (10 mg/kg, max 600 mg). (Comparator for both trials: Oral ibuprofen 10 mg/kg, max 600 mg) |
| Key inclusion and exclusion criteria | To be eligible to participate in this study, an individual must meet all of the following criteria: (1) Child aged 6 to 17 years, (2) Presenting to the emergency department with an acute limb injury (<24 hours old) that is neither obviously deformed nor having neurovascular compromise (as assessed by the triage nurse), (3) Self-reported pain score >5 on the 0 to 10 verbal Numerical Rating Scale at triage. |
| Exclusion criteria include: (1) Deemed to require immediate intravenous or intranasal pain medications by the clinical team, (2) Previously known hypersensitivity to study medications, (3) Acetaminophen or NSAID use within 3 hours prior to recruitment, (4) Opioid use within 1 hour prior to recruitment, (5) Caregiver and/or child cognitive impairment precluding the ability to self-report pain or respond to study questions, (6) Injury suspected to be due to non-accidental trauma/child abuse (as assessed by the triage nurse or reported by the family), (7) Suspected multi-limb fracture, (8) Chronic pain that necessitates daily analgesic use, (9) Hepatic or renal disease/dysfunction, (10) Bleeding disorder, (11) Known pregnancy, (12) Vomiting that precludes the ability to take oral medications (as determined by the family), (13) Caregiver and/or child’s inability to communicate fluently in English or French in the absence of a native language interpreter, (14) Caregiver unavailable for follow-up or (15) Previous enrolment in the No OUCH study. | |
| Study type | Randomised, double-blind, placebo-controlled superiority trials. |
| Date of first enrolment | 20 April 2019 |
| Sample size | 536 |
| Recruitment status | Actively recruiting. |
| Primary outcome(s) | The primary efficacy outcome will be the self-reported pain score at 60 min, using an 11-point, 0 to 10, verbal Numerical Rating Scale. |
| Key secondary outcomes | The principal safety endpoint will be the proportion of children with adverse events related to study drug administration. |
| Ethics review | University of Alberta Research Ethics Board # Pro00073476. |
| Completion date | – |
| Summary results | – |
| IPD sharing statement | De-identified data can be shared, on a case-by-case basis, on discussion with the principal investigator. |
IPD, Individual Participant Data; NSAID, non-steroidal anti-inflammatory drug.
Study setting
This study will be conducted in six paediatric EDs across Canada: (1) Stollery Children’s Hospital (Edmonton, Alberta) (coordinating site), (2) Alberta Children’s Hospital (Calgary, Alberta), (3) Winnipeg Children’s Hospital (Winnipeg, Manitoba), (4) Children’s Hospital at London Health Sciences Centre (London, Ontario), (5) CHEO (Ottawa, Ontario), and (6) Centre Hospitalier Universitaire Ste-Justine (Montreal, Quebec). The annual ED census for recruiting centres ranges from 30 000 to 80 000 patient visits. Study recruitment began on 20 April 2019 and is expected to be completed within 18 months.
Eligibility and exclusion criteria
Children will be eligible if they are 6 to 17 years, presenting to the ED with an acute limb injury (<24 hours old) that is neither obviously deformed nor having neurovascular compromise, and have a self-reported verbal Numerical Rating Scale pain score ≥5 at triage. This age group was chosen as fractures rarely occur under this age, and a consistent and validated pain measurement tool can be employed across this age range.
Children will be excluded if they meet any of the following criteria: (a) require immediate intravenous or intranasal pain medications, (b) have known hypersensitivity to study medications, (c) receive acetaminophen or non-steroidal anti-inflammatory drug within 3 hours prior to recruitment, (d) receive opioids within 1 hour prior to recruitment, (e) parent/caregiver or child cognitive impairment precluding the ability to self-report pain or respond to study questions, (f) injury suspected to be due to non-accidental trauma or child abuse, (g) suspected multi-limb fracture, (h) chronic pain that necessitates daily analgesic use, (i) known hepatic or renal disease/dysfunction, (j) known bleeding disorder, (k) known pregnancy, (l) vomiting that precludes the ability to take oral medications, (m) parent/caregiver and/or child’s inability to communicate fluently in English or French in the absence of a native language interpreter, (n) parent/caregiver unavailable for follow-up or (o) previous enrolment in this study.
Study Interventions and rescue medications
If a family chooses the Opioid trial, their child will be randomised to one of the three treatment arms: (a) oral ibuprofen+acetaminophen placebo+hydromorphone placebo, OR (b) oral ibuprofen+oral acetaminophen+hydromorphone placebo, OR (c) oral ibuprofen+acetaminophen placebo+oral hydromorphone.
If a family chooses the Non-Opioid trial, their child will be randomised to one of the two treatment arms: (a) oral ibuprofen+acetaminophen placebo, OR (b) oral ibuprofen+oral acetaminophen.
Ibuprofen will be dosed as 10 mg/kg (maximum 600 mg), acetaminophen as 15 mg/kg (maximum 1000 mg) and oral hydromorphone as 0.05 mg/kg (maximum 5 mg).
Given the consistent recommendations that ibuprofen will be the first-line therapy for acute MSK injury pain,15 30–32 and the fact that it is the medication of choice for triage-initiated pain protocols at most Canadian paediatric EDs,33 ibuprofen will serve as the comparator (standard of care) for both trials.
All study medications and placebos will be administered as a single oral dose in liquid form. No other medications will be administered as part of the study. However, enrolled patients will be eligible to receive additional analgesia at any time if requested and/or deemed necessary by the clinical team. The treating physician will order rescue analgesia at their discretion. Any such co-interventions, including non-pharmacologic interventions (eg, ice, splinting) will be documented.
Randomisation, allocation concealment and blinding
Randomisation will be determined using a secure online centralised randomisation tool hosted by the Women and Children’s Health Research Institute (WCHRI, University of Alberta).34 Participants will be allocated via a kit number. A statistician will oversee the generation of a randomised listing of the treatment by kit number using a 1:1:1 allocation scheme for the Opioid trial, and a 1:1 allocation scheme for the Non-Opioid trial. This will be further stratified by centre using block-randomisation with variable block sizes. These randomisation lists, which will be sent directly by the statistician to the participating site’s research pharmacy team, will be used by each participating site’s research pharmacy to create pre-packaged, sequential study kits for each trial. Research nurses will then allocate the kits to enrolled participants in a sequential fashion.
Study participants, research nurses (the outcome assessors), ED staff and data analysts will all be blinded with respect to the intervention. In the rare occurrence where a treating physician feels that knowing what the child has received will impact further clinical care, the study blind can be broken by the clinical team for patient safety. The protocol for unblinding will involve the research nurse logging in to a secure web-based unblinding system with REDCap. However, only the treating physician will ‘click’ on the button to reveal the study medications administered. Thus, parents/caregivers, children and research staff will remain blinded.
Recruitment and data collection
The patient’s initial assessment on arrival to the ED will be performed by a triage nurse. Triage nurses, research nurses or their designate will identify potentially eligible participants. Research nurses will be present in enrolling EDs up to 16 hours a day to screen children and assess eligibility based on the inclusion and exclusion criteria outlined above. Research nurses will follow site-specific Research Ethics Board (REB) guidelines regarding approaching families for research studies. Verbal consent for screening will be obtained from families and documented. For eligible parent/caregiver-child pairs who express interest in study participation, an ED physician will confirm eligibility, and the research nurse or designate will complete consent and assent, as appropriate (online supplementary appendix 1).
bmjopen-2019-035177supp001.pdf (4.9MB, pdf)
After obtaining written informed consent from the parent/caregiver, and assent from the child where appropriate, the research nurse will determine preference for study trial (ie, Opioid or Non-Opioid). In keeping with the ethical requirements of the involved Canadian institutions, we will have consent forms for parent/caregivers, assent forms for children and mature minor consent forms for both accompanied and unaccompanied youth who are deemed to be mature minors. All of these forms are written in a manner to reflect the reading and comprehension capacity of the target groups. If the parent/caregiver and child pair do not voice a trial preference, they will be enrolled in the Opioid trial as it contains all three possible medication combinations offered in the study, as outlined in the consent form. The research nurse will administer the study medications according to the randomisation scheme for that chosen trial (figure 1). If a participant vomits within 30 min of drug administration, it will be repeated once in accordance with current clinical and research practice.35 The parent/caregiver will be asked to complete a brief survey in the ED to explore their reasons for choosing their study trial (see online supplementary appendix 2).
Figure 1.
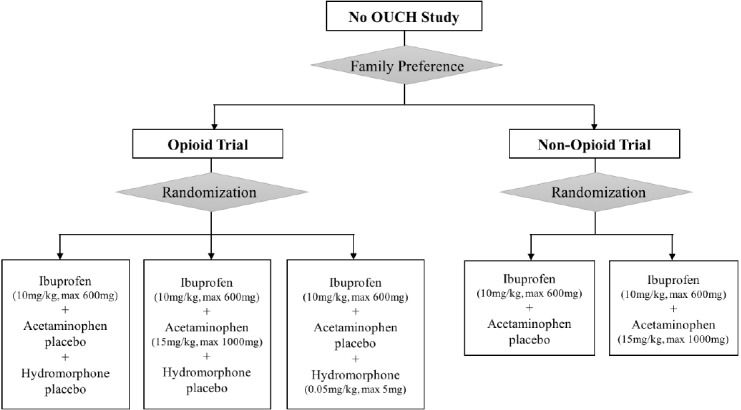
Study interventions.
bmjopen-2019-035177supp002.pdf (14.4MB, pdf)
Following study drug administration, the research nurse will monitor the participant for up to 120 min, with safety and efficacy measures recorded at the time of recruitment (T-R), time of study drug administration (T-0), at 30 min, 60 min, 90 min and 120 min post-study drug administration (T-30, T-60, T-90, T-120 respectively), at the time of medical examination (T-ME) and as soon as possible following X-ray (T-XR). All study measures at T-30, T-60, T-90 and T-120 will be collected within 15 min of the designated time point (ie, ±15 min). All study measures for T-ME and T-XR will be collected within 30 min of the designated time point. If a patient is discharged prior to T-120, the study measures will be recorded one last time at the time of discharge.
Pain scores will be measured on the verbal Numerical Rating Scale (vNRS), Visual Analogue Scale (VAS) and Faces Pain Scale-Revised (FPS-R) at each study time point.36 37 In addition, the research nurse will also evaluate the presence of adverse events (eg, nausea, vomiting), record vital signs (pulse, blood pressure, respiratory rate, oxygen saturation) and evaluate sedation level using the Ramsay Sedation Scale.38 Reporting of adverse events will be in keeping with Health Canada regulations and REB guidelines. Prior to their discharge from the ED, both the child and parent/caregiver will be asked to rate acceptability of the study medication received during the trial using a Likert scale. (figure 2)
Figure 2.
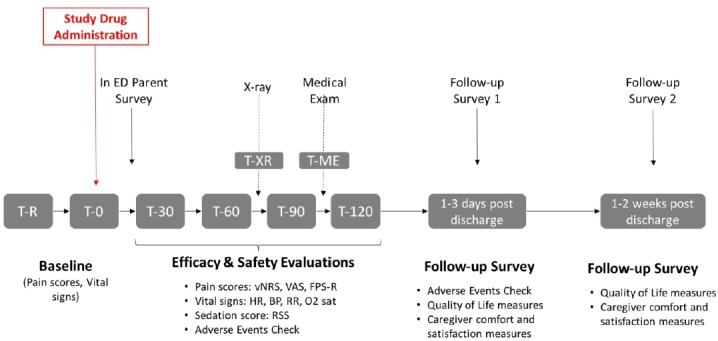
Schedule of study measures. BP, blood pressure; ED, emergency department; FPS-R, Faces Pain Scale-Revised; HR, heart rate; O2 sat, oxygensaturation; RR, respiratory rate; RSS, RamsaySedation Scale; T-ME, time of medical examination; T-R, time ofrecruitment; T-XR, time following X-ray; T-0, timeof study drug administration; T-30, T-60, T-90, T-120, 30 min,60 min, 90 minand 120 min, respectively; vNRS, verbal Numerical Rating Scale; VAS, VisualAnalogue Scale.
Two brief 10-min follow-up surveys will be completed with the parent/caregiver following their child’s discharge from the ED. Parents/caregivers will have the option of completing these over the phone or online via a secure email link. Non-responders to email contact and those who prefer phone follow-up will be called three to five times depending on local REB requirements. The first follow-up survey, conducted at 1 to 3 days post ED discharge, will determine the occurrence of any adverse events since discharge. The second follow-up survey will be completed at 1 to 2 weeks post ED discharge, to determine parent/caregiver comfort and satisfaction with at-home pain management and the extent of functional limitations for their child.
To achieve adequate participant enrolment to reach target sample size, we will monitor the monthly recruitment targets and have regular (every 4 to 8 week) team meetings to allow for timely implementation of procedural changes. There are no plans for patient follow-up beyond the 2-week study period, given that only one dose of study medications will be administered. All study scripts and data collection tools will be available in English and French.
Outcome measures
The Primary Efficacy Outcome will be the self-reported vNRS pain score at 60 min post study drug administration. The vNRS, ranging from 0 (no pain) to 10 (worst pain imaginable), is the most commonly used, responsive pain measurement tool for this study age group.39 It has been successfully employed in several children’s pain studies,40 41 and is validated for the age range of children included in this study.42 The 60 min primary outcome time point reflects the peak plasma concentration and clinical action of both oral hydromorphone and ibuprofen.28 43–45
The Principal Safety Endpoint will be the proportion of children with adverse events related to study drug administration. Medication safety profiles influence parent/caregiver and patient’s willingness to adhere to medication regimens.46 It has also been previously established that more safety data is urgently needed to inform clinical decision-making when using the study medications of interest.30
The Secondary Outcomes will include efficacy, safety and preference endpoints:
Secondary efficacy outcomes
A vNRS pain score <3 at T-60.
A vNRS pain score reduction of at least 2 points out of 10 at T-60.
Pain scores at study time-points (T-30, T-60, T-90, T-120, T-ME and T-XR).
ED length of stay, rescue analgesic in the 60 min following administration of study medication.
Time to effective analgesia, defined as the first vNRS pain score <3 post-intervention.
Children’s self-reported pain intensity on the VAS and the FPS-R at all study time-points.
Secondary safety outcomes
Any serious adverse events during the study period, including apnoea, cardiac arrest or death.
A Ramsay Sedation Score between 1 to 3.
Each specific adverse event type (eg, nausea, dizziness, itchiness) during the study period.
Missed fractures or dislocations.
Secondary preference outcomes
Parent/caregiver’s reasons for choosing the opioid or the non-opioid trial.
Self-reported parent/caregiver and child’s satisfaction with pain relief and acceptability of study medications, using a previously employed 5-point Likert scale.47
Physicians’ in-ED preference of analgesics for the patient.
Parent/caregiver’s comfort treating their child at home, as measured by a scale created by the study team.5
Sample size
The sample size for the three-armed opioid trial is 105 patients per arm, for a total of 315. The sample size for the two-armed non-opioid trial is 85 patients per arm, for a total of 170. Thus, the total for the No OUCH Study would be 485. To account for missing data for the primary outcome due to early withdrawal, the study will over-recruit by approximately 10%, for a target recruitment of approximately 540 patients. This sample size was determined based on a two-sided level of 0.05, a power of 0.95, a minimally clinically important difference of 1.5 on the vNRS, an estimate of the SD of the difference of 2.748 and a Bonferroni correction to adjust for the three treatment comparisons. Based on previously conducted survey work,49 an imbalance in recruitment pace between the opioid and non-opioid trials is expected. However, both trials will continue to recruit until the sample size is met for both. One trial will over-recruit to allow for completion of the other, without compromising the key preference-based study design. To ensure timely completion of the No OUCH Study, we will monitor the recruitment rates and potentially update the randomisation strategy if there is an extreme over-recruitment for one of the trials.
Statistical methods
All analyses will adhere to the principle of intention-to-treat. There will be three treatment comparisons: (1) ibuprofen versus ibuprofen plus acetaminophen, (2) ibuprofen versus ibuprofen plus hydromorphone, (3) ibuprofen plus acetaminophen versus ibuprofen plus hydromorphone. Due to homogeneity in the trial endpoints for the two complementary trials, we will consider a joint analysis across both the endpoints if the two patient populations are sufficiently similar. This will be determined using the following specified decision rules.
For each treatment comparison, the primary analysis will compare the mean vNRS reduction for pain scores at T-60. This comparison will be facilitated using a linear mixed model with the T-0 measure on the vNRS for pain as a covariate and a site-specific effect. We will consider whether the two trials can be analysed together used nested linear mixed models with and without a trial by treatment interaction term. If this interaction term is not significant then a single treatment effect will be estimated for each comparison. A two-sided level of 0.05 will be used to declare significance. A Bonferroni-Holm correction will be used to adjust for the three treatment comparisons. The proportion of children with a self-reported vNRS of less than 3 at 60 min, the proportion who require a rescue analgesic by 60 min and the proportion who experienced adverse events related to study drug administration will be analysed using a Mantel-Haenszel χ2 test, stratified by site. All other outcomes will be summarised using appropriate descriptive statistics.
There will be no interim analyses of the efficacy endpoints, as it is very difficult to change practice based on the results from small samples, regardless of the p value. The Data Safety Monitoring Board (DSMB) will be provided with a masked comparison between treatment groups with respect to the safety endpoints at the intervals of their choosing. The decision to stop the trial for safety reasons will be left to the discretion of the DSMB (see online supplementary appendix 3 for DSMB Charter). Interim analyses will also monitor the relative recruitment rate of the two trials. If insufficient participants are enrolled on either of the No OUCH trials, appropriate action will be taken to ensure sufficient power to conclude following the completion of the trials. Further information is available in the Statistical Analysis Plan, which will be published separately.
bmjopen-2019-035177supp003.pdf (18.8MB, pdf)
Health economic methods
The trial will also examine the relative cost-effectiveness of each of the medication options. The economic evaluation will take a healthcare perspective for the reference case, in line with Canadian Agency for Drugs and Technologies in Health guidance50 and in secondary analyses will consider societal costs. Information will be collected on interventions during ED visit, in hospital medication costs and follow-up care from other health services, as well as on costs incurred by families in interacting with health services. Quality of life will be measured by asking parents/caregivers to report their child’s quality of life using a 10-point numeric scale. The health economic analysis will estimate the expected cost per incremental change in quality of life and will use non-parametric bootstrapping methods to calculate uncertainty to assist in decision-making about the value of providing different treatment strategies.
Patient and public involvement
The team’s patient engagement partner (SH) has provided ongoing input on the study protocol and data collection tools. The study team was also supported by parent advisory groups at the ECHO (Evidence in Child Health to Enhance Outcomes) Research Program (Edmonton, Alberta) and TREKK (Translating Emergency Knowledge for Kids) (Winnipeg, Manitoba). Parent advisors reviewed and provided feedback on the wording, readability, sensitivity, flow and content of parent/caregiver surveys. Following recruitment completion, parent advisors will be engaged in focus groups to discuss study results and dissemination plans in the context of family-centred care.
Data management
Data management services will be provided by the WCHRI data coordinating centre. Study data will be entered and managed using REDCap (Research Electronic Data Capture) tools hosted and supported by WCHRI.51 WCHRI’s REDCap installation is a validated electronic, web-based data capture system housed in a secure data centre at the University of Alberta.
Data will be entered directly into the study database or, in case of technical failure, it may be collected on paper and then digitally recorded in REDCap. Selected data elements will be validated electronically on an ongoing basis throughout the study and any discrepancies will be assigned to members of the study team for resolution. REDCap includes internal quality checks, such as automatic range checks, to identify data that appear inconsistent, incomplete or inaccurate (see online supplementary appendix 4 for data management plan).
bmjopen-2019-035177supp004.pdf (527.2KB, pdf)
Only limited identifiable data will be stored in REDCap (eg, email address) for the purposes of completing follow-up surveys. Study participants’ contact information will be stored securely at each clinical site for internal use during the study. Paper records (eg, signed consent and assent forms) will be stored in a secure locked cabinet at each site, with limited access by the research team only. At the end of the study, all records will continue to be kept in a secure location for as long a period as dictated by the reviewing REB, institutional policies or sponsor requirements.
Monitoring
Monitoring for quality and regulatory compliance will be performed by the University of Alberta’s Quality Management in Clinical Research (QMCR) office. QMCR is an independent unit housed within the university’s central administration that provides arms-length review of all University of Alberta sponsored trials, at least three times per year. Details of clinical site monitoring will be documented in a Clinical Monitoring Plan.
Safety oversight will be under the direction of a DSMB which will function independently of the investigators. This committee will be chaired by Dr Garth Meckler and is composed of five individuals with expertise in trial methodology, epidemiology, biostatistics and paediatric emergency medicine. The DSMB will meet at least semi-annually to assess safety and efficacy data and will operate under the rules of an approved charter/terms of reference.
Ethics and dissemination
Based on previously conducted research with oral opioids,16 20 30 nausea, mild dizziness and drowsiness are expected to be possible non-serious adverse events in this study. There is a small potential risk of respiratory depression following the administration of any opioid, although the risk is notably greater with repeat dosing and intravenous administration. This risk will be minimised by using only a single oral dose and vigilantly monitoring the participant’s vital signs and level of sedation during the study period, which extends for 1 hour past the peak action point of the drugs.
This study will be federally monitored by Health Canada, and approval has been granted for the conduct of this study (HC6-24-c220455). The Research Ethics Board at the University of Alberta has further approved this study (Pro00073476). The five other participating centres acquired ethics approval from their local REBs prior to commencing recruitment. Any protocol amendments will be submitted for Health Canada review and REB approvals prior to implementation and will be added as an amendment to the ClinicalTrials.gov registration. Institutional approvals from each participating paediatric ED will be obtained prior to beginning recruitment.
Public opinion regarding opioids is notably negative at this time, thus there is a hesitancy to accept opioids, even when they are felt to be clinically indicated. As such, it is expected that some parents/caregivers will be hesitant to accept opioids.52–54 However, the study will leverage this opportunity to understand parent/caregiver’s perspectives and rationale for their decision-making. This valuable information can then inform knowledge translation of study results, educational initiatives and responsive healthcare provider prescribing of analgesia.
The study team plans to publish this trial in a high-impact, peer-reviewed journal and present the results at national and international meetings; authorship eligibility will be determined by employing the International Committee of Medical Journal Editors’ recommended guidelines.55 Statistical code and data set can be made available on request.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the iPCT SPOR team and our parent advisors, who provided valuable input on the study design and documents. The authors are also grateful to Dr Dennis Cote (College of Pharmacy, Rady Faculty of Health Sciences, University of Manitoba) for leading the development of the study placebos. The following are the members of the KidsCAN PERC Innovative Pediatric Clinical Trials No OUCH Study Team: Dr Amy Drendel, Dr Gareth Hopkin, Dr Jeff Round, Dr Martin Offringa, Dr Petros Pechlivanoglou, Dr Eleanor Pullenayegum, David Rios, Marie-Christine Auclair, Kelly Kim, Lise Bourrier, Lauren Dawson, Kamary Coriolano DaSilva, Pamela Marples, Rick Watts, Dr Jennifer Thull-Freedman, Dr Patrick McGrath, Dr Timothy AD Graham, Dr Lisa Hartling, Tannis Erickson, Brendon Foot, Kurt Schreiner and Julie Leung.
Footnotes
Twitter: @drsaminaali
Contributors: Dr SA developed and revised the protocol, co-drafted the protocol paper and will operationalise the study. She chose the previously validated tools for measuring the primary and secondary efficacy outcomes (vNRS, VAS and FPS-R). MR is the national study coordinator who contributed to study design, co-drafted the protocol paper and will operationalise the study. Dr LR and Dr CM co-developed the novel study methodology and contributed to protocol revision. Dr AR, AW, Dr MY and Dr AH led the statistical analysis planning and contributed to protocol revision. Dr ALD is a fracture outcomes expert who contributed to determining the secondary outcomes for the study; she contributed to the methodology and revised the protocol. Dr SG, Dr AS, Dr SS and Dr MB, as site leads for this study, reviewed and revised the protocol, with special input into the Methods section of the study. SH is a family representative who reviewed and provided input into the study protocol. She provided lived experience in patient-oriented outcomes. Dr NP, Dr MA and Dr TK co-developed the methodology and revised the protocol. All authors have approved this final version of the protocol. None of the authors have financial or other conflicts of interests as they pertain to this study and its involved recruitment sites.
Funding: This work is supported by an Innovative Clinical Trials Multi-year Grant from the Canadian Institutes of Health Research (funding reference number MYG-151207; 2017–2020), as part of the Strategy for Patient-Oriented Research and the Children’s Hospital Research Institute of Manitoba (Winnipeg, Manitoba), the Centre Hospitalier Universitaire Sainte-Justine (Montreal, Quebec), the Department of Pediatrics, University of Western Ontario (London, Ontario), the Alberta Children’s Hospital Research Institute (Calgary, Alberta), the Women and Children’s Health Research Institute (Edmonton, Alberta), the Children’s Hospital of Eastern Ontario Research Institute Inc (Ottawa, Ontario) and the Hospital for Sick Children Research Institute (Toronto, Ontario). This study is sponsored by The Governors of the University of Alberta (Suite 400, 8215–112 Street, Edmonton, Alberta, Canada T6G 2C8). Neither the study sponsor nor funders have any role in the collection, management, analysis or interpretation of data; writing of the report or the decision to submit the report for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Spady DW, Saunders DL, Schopflocher DP, et al. Patterns of injury in children: a population-based approach. Pediatrics 2004;113:522–9. 10.1542/peds.113.3.522 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information, (CIHI) National Trauma Registry Report: Major Injury in Canada. Canadian Institute for Health Information (CIHI), 2007. Available: https://www.cihi.ca/en/national-trauma-registry-report-major-injury-in-canada-2010-2011[Accessed 21 Dec 2018].
- 3.Kircher J, Drendel AL, Newton AS, et al. Acute pediatric musculoskeletal pain management in North America: a practice variation survey. Clin Pediatr 2014;53:1326–35. 10.1177/0009922814555972 [DOI] [PubMed] [Google Scholar]
- 4.Ali S, Chambers A, Johnson DW, et al. Reported practice variation in pediatric pain management: a survey of Canadian pediatric emergency physicians. CJEM 2014;16:352–60. 10.2310/8000.2013.131261 [DOI] [PubMed] [Google Scholar]
- 5.Whitson C, Ali S, Wright B, et al. Caregiver acceptance of analgesia for children in the emergency department: a multicentered study. CJEM 2017. [Google Scholar]
- 6.Kircher J, Drendel AL, Newton AS, et al. Pediatric musculoskeletal pain in the emergency department: a medical record review of practice variation. CJEM 2014;16:449–57. 10.1017/S1481803500003468 [DOI] [PubMed] [Google Scholar]
- 7.LeMay S, Johnston C, Choinière M, et al. Pain management interventions with parents in the emergency department: a randomized trial. J Adv Nurs 2010;66:2442–9. 10.1111/j.1365-2648.2010.05408.x [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. Committee on Psychosocial Aspects of Child and Family Health, Task Force on Pain in Infants, Children, and Adolescents . The assessment and management of acute pain in infants, children, and adolescents. Pediatrics 2001;108:793–7. 10.1542/peds.108.3.793 [DOI] [PubMed] [Google Scholar]
- 9.Drendel AL, Lyon R, Bergholte J, et al. Outpatient pediatric pain management practices for fractures. Pediatr Emerg Care 2006;22:94–9. 10.1097/01.pec.0000199564.64264.f4 [DOI] [PubMed] [Google Scholar]
- 10.Friday JH, Kanegaye JT, McCaslin I, et al. Ibuprofen provides analgesia equivalent to acetaminophen-codeine in the treatment of acute pain in children with extremity injuries: a randomized clinical trial. Acad Emerg Med 2009;16:711–6. 10.1111/j.1553-2712.2009.00471.x [DOI] [PubMed] [Google Scholar]
- 11.National Institute on Drug Abuse Opioid overdose crisis, 2019. Available: https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis [Accessed 03 Oct 2019].
- 12.Centers for Disease Control and Prevention Understanding the epidemic, 2018. Available: https://www.cdc.gov/drugoverdose/epidemic/index.html [Accessed 03 Oct 2019].
- 13.Alexander J, Manno M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann Emerg Med 2003;41:617–22. 10.1067/mem.2003.138 [DOI] [PubMed] [Google Scholar]
- 14.Ali S, Chambers AL, Johnson DW, et al. Paediatric pain management practice and policies across Alberta emergency departments. Paediatr Child Health 2014;19:190–4. 10.1093/pch/19.4.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le May S, Ali S, Khadra C, et al. Pain management of pediatric musculoskeletal injury in the emergency department: a systematic review. Pain Res Manag 2016;2016:4809394:1–10. 10.1155/2016/4809394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le May S, Ali S, Plint AC, et al. Oral analgesics utilization for children with musculoskeletal injury (OUCH trial): an RCT. Pediatrics 2017;140:peds.2017-0186. 10.1542/peds.2017-0186 [DOI] [PubMed] [Google Scholar]
- 17.Drendel AL, Gorelick MH, Weisman SJ, et al. A randomized clinical trial of ibuprofen versus acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med 2009;54:553–60. 10.1016/j.annemergmed.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Clark E, Plint AC, Correll R, et al. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics 2007;119:460–7. 10.1542/peds.2006-1347 [DOI] [PubMed] [Google Scholar]
- 19.Poonai N, Datoo N, Ali S, et al. Oral morphine versus ibuprofen administered at home for postoperative orthopedic pain in children: a randomized controlled trial. CMAJ 2017;189:E1252–8. 10.1503/cmaj.170017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poonai N, Bhullar G, Lin K, et al. Oral administration of morphine versus ibuprofen to manage postfracture pain in children: a randomized trial. CMAJ 2014;186:1358–63. 10.1503/cmaj.140907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali S, Drendel AL, Rosychuk RJ, et al. LO049: Ibuprofen or oxycodone? An observational cohort study of post-emergency department discharge management of children’s fracture pain. CJEM 2016;18:S47 10.1017/cem.2016.86 [DOI] [Google Scholar]
- 22.McGinley L. Fda warns against giving kids cough and cold medicines with codeine or hydrocodone. The Washington post., 2018. Available: https://www.washingtonpost.com/news/to-your-health/wp/2018/01/11/fda-warns-against-giving-kids-cough-and-cold-medicines-with-codeine-or-hydrocodone/ [Accessed 25 Feb 2020].
- 23.McGinley L. FDA warns of dangers of codeine and tramadol for children and breast-feeding mothers. The Washington Post, 2017. Available: https://www.washingtonpost.com/news/to-your-health/wp/2017/04/20/fda-warns-of-dangers-of-codeine-and-tramadol-for-children-and-breast-feeding-mothers/[Accessed 25 Feb 2020].
- 24.Tobias JD, Green TP, Coté CJ, et al. Codeine: Time to Say "No". Pediatrics 2016;138:peds.2016-2396. 10.1542/peds.2016-2396 [DOI] [PubMed] [Google Scholar]
- 25.Health Canada Health Canada’s review recommends codeine only be used in patients aged 12 and over, 2013. Available: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/33915a-eng.php [Accessed 25 Feb 2020].
- 26.Sharar SR, Bratton SL, Carrougher GJ, et al. A comparison of oral transmucosal fentanyl citrate and oral hydromorphone for inpatient pediatric burn wound care analgesia. J Burn Care Rehabil 1998;19:516–21. 10.1097/00004630-199811000-00010 [DOI] [PubMed] [Google Scholar]
- 27.Givens M, Rutherford C, Joshi G, et al. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med 2007;32:239–43. 10.1016/j.jemermed.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 28.Bissonnette B. Pediatric anesthesia. 2286 Shelton, CT: People's Medical Publishing House, 2014. [Google Scholar]
- 29.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartling L, Ali S, Dryden DM, et al. How safe are common analgesics for the treatment of acute pain for children? A systematic review. Pain Res Manag 2016;2016:5346819:1–15. 10.1155/2016/5346819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali S, Drendel AL, Kircher J, et al. Pain management of musculoskeletal injuries in children: current state and future directions. Pediatr Emerg Care 2010;26:518–28. 10.1097/PEC.0b013e3181e5c02b [DOI] [PubMed] [Google Scholar]
- 32.Morris L, Stulberg D, Stevermer JJ. Fracture pain relief for kids? ibuprofen does it better. J Fam Pract 2010;59:273–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Trottier ED, Ali S, Le May S, et al. Treating and reducing anxiety and pain in the paediatric emergency department: the trapped survey. Paediatr Child Health 2015;20:239–44. 10.1093/pch/20.5.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Women and Children's Health Research Institute, (WCHRI) Data coordinating centre.. Available: https://www.wchri.org/data-coordinating-centre [Accessed 21 Dec 2018].
- 35.Bjornson CL, Klassen TP, Williamson J, et al. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med 2004;351:1306–13. 10.1056/NEJMoa033534 [DOI] [PubMed] [Google Scholar]
- 36.Le May S, Ballard A, Khadra C, et al. Comparison of the psychometric properties of 3 pain scales used in the pediatric emergency department: visual analogue scale, faces pain Scale-Revised, and colour analogue scale. Pain 2018;159:1508–17. 10.1097/j.pain.0000000000001236 [DOI] [PubMed] [Google Scholar]
- 37.Tsze DS, von Baeyer CL, Pahalyants V, et al. Validity and Reliability of the Verbal Numerical Rating Scale for Children Aged 4 to 17 Years With Acute Pain. Ann Emerg Med 2018;71:691–702. 10.1016/j.annemergmed.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2:656–9. 10.1136/bmj.2.5920.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Baeyer CL. Children's self-report of pain intensity: what we know, where we are headed. Pain Res Manag 2009;14:39–45. 10.1155/2009/259759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miró J, Castarlenas E, de la Vega R, et al. Validity of three rating scales for measuring pain intensity in youths with physical disabilities. Eur J Pain 2016;20:130–7. 10.1002/ejp.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castarlenas E, Jensen MP, von Baeyer CL, et al. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents: a systematic review. Clin J Pain 2017;33:376–83. 10.1097/AJP.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 42.Castarlenas E, Miró J, Sánchez-Rodríguez E. Is the verbal numerical rating scale a valid tool for assessing pain intensity in children below 8 years of age? J Pain 2013;14:297–304. 10.1016/j.jpain.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 43.Vallner JJ, Stewart JT, Kotzan JA, et al. Pharmacokinetics and bioavailability of hydromorphone following intravenous and oral administration to human subjects. J Clin Pharmacol 1981;21:152–6. 10.1002/j.1552-4604.1981.tb05693.x [DOI] [PubMed] [Google Scholar]
- 44.Konstan MW, Hoppel CL, Chai BL, et al. Ibuprofen in children with cystic fibrosis: pharmacokinetics and adverse effects. J Pediatr 1991;118:956–64. 10.1016/S0022-3476(05)82218-8 [DOI] [PubMed] [Google Scholar]
- 45.Medical Economics Physician's desk reference. 56 edn Montvale, NJ: Medical Economics Company, Inc, 2002: 2002–5. [Google Scholar]
- 46.Ali S, Poonai N. Parents' preferences on pain treatment, even when faced with medication dilemmas, influence their decisions to administer opioids in children. Evid Based Nurs 2016;19:51–2. 10.1136/eb-2015-102164 [DOI] [PubMed] [Google Scholar]
- 47.Weingarten L, Kircher J, Drendel AL, et al. A survey of children's perspectives on pain management in the emergency department. J Emerg Med 2014;47:268–76. 10.1016/j.jemermed.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 48.Tsze DS, Hirschfeld L, Suarez L, et al. Clinical Interpretation of Changes in Self-Report Pain Scores in Children with Acute Pain & nbsp. Pediatric Academic Societies Annual Conference, 2017. [Google Scholar]
- 49.Jun E, Ali S, Yaskina M, et al. A two-centre survey of caregiver perspectives on opioid use for children’s acute pain management. Paediatr Child Health 2019:pxz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.CADTH Guidelines For the economic evaluation of health technologies:Canada. 4th ed Ottawa: CADTH, 2017. [Google Scholar]
- 51.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adhopia V. 'Our new normal': Hard-hit Ohio community fears opioid overdoses will be perpetual crisis.CBC News, 2017. Available: https://www.cbc.ca/news/health/ohio-overdoses-perpetual-crisis-1.4164136 [Accessed 25 Feb 2020].
- 53.Bearman D. When Pain Becomes Criminal: All About Opiate Phobia. Huffington Post, 2017. Available: https://www.huffpost.com/entry/when-pain-becomes-criminal-all-about-opiate-phobia_b_58c098b7e4b0c3276fb78137 [Accessed 25 Feb 2020].
- 54.Ubelacker S. The inside history of Canada’s opioid crisis: Canada is in the midst of an epidemic of opioid use and abuse. How did we get here? Maclean's, 2017. Available: https://www.macleans.ca/society/inside-the-history-of-canadas-opioid-crisis/ [Accessed 25 Feb 2020].
- 55.ICMJE International Committee of medical Journal editors, 2017. Available: http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html [Accessed 21 Dec 2018].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035177supp001.pdf (4.9MB, pdf)
bmjopen-2019-035177supp002.pdf (14.4MB, pdf)
bmjopen-2019-035177supp003.pdf (18.8MB, pdf)
bmjopen-2019-035177supp004.pdf (527.2KB, pdf)


