Abstract
Objective
We aimed to evaluate the angiographic findings and outcomes of bronchial artery embolization in tuberculosis patients and to compare them with those of non-tuberculosis patients.
Materials and Methods
Patients who underwent bronchial artery embolization in a single interventional radiology department with hemoptysis were reviewed. A total of 89 patients (66 males and 23 females; mean age 52.71±15.37) were incorporated in the study. The patients were divided into two groups: tuberculosis group (n=36) and non-tuberculosis group (16 malignancy, 22 bronchiectasis, 6 pulmonary infection, 5 chronic obstructive pulmonary disease, 4 idiopathic; n=53). Angiography and embolization procedure were performed by interventional radiologists with 5, 10, and 20 years of experience. Angiographic findings were classified as tortuosity, hypertrophy, hypervascularity, aneurysm, bronchopulmonary shunt, extravasation, and normal bronchial artery. Chi-square test was used to compare angiographic findings between tuberculosis and non-tuberculosis patient groups.
Results
Bronchopulmonary shunt was found to be significantly higher in the tuberculosis group as compared to that in the non-tuberculosis group (p=0.002). Neither of the groups showed a statistically significant difference with respect to recurrence (p=0.436).
Conclusion
Bronchial artery embolization is a useful and effective treatment method of hemoptysis in tuberculosis. Evaluation of bronchopulmonary shunts in patients with tuberculosis is critical for the reduction of catastrophic complications.
Keywords: Embolization, digital subtraction angiography, hemoptysis, treatment outcome, tuberculosis
Introduction
Hemoptysis is a common and sometimes life-threatening symptom that may have many underlying etiologies. It is defined as the expectoration of blood from the respiratory system [1]. If hemoptysis is massive (>300 mL per day) and untreated, the mortality rates may rise to 50% [2]. Management of hemoptysis includes conservative treatment, surgery, and bronchial artery embolization (BAE). Patients admitted with massive hemoptysis are usually in poor condition medically and cannot tolerate surgery. In addition, serious complications such as asphyxia, bronchopleural fistula, and respiratory failure may occur in patients who undergo surgery [1]. Therefore, BAE has become the primary treatment method of massive or intermittent-moderate (>100 ml per day) hemoptysis [3, 4].
The causes of hemoptysis vary significantly between developed and non-developed countries. In non-developed countries, tuberculosis is the most frequent cause of massive hemoptysis [5]. Since Remy et al. [6] first described BAE for the management of hemoptysis, several studies have declared the efficacy of BAE in tuberculosis patients [7–11]. These studies have investigated the outcomes of BAE in tuberculosis patients and the risk factors that affect recurrence. However, angiographic findings and their influence on the embolization procedure are not reported in detail.
The aims of this study were to evaluate angiographic findings during BAE in tuberculosis patients and to compare the findings with those of non-tuberculosis patients. We also tried to reveal the effect of angiographic pattern on the success and technique of BAE.
Materials and Methods
Local ethics committee approval was obtained for this retrospective study. One hundred and five patients who underwent BAE between August 2015 and July 2018 in a single interventional radiology department with moderate (>100 mL per day) or severe (>300 mL per day) hemoptysis refractory for medical and bronchoscopic treatment were reviewed. In 16 patients, no pathologic artery was found during the angiography, so they were excluded from the study. A total of 89 patients (66 males and 23 females; mean age 52.71±15.37) were incorporated in the study. The patients were divided into two groups according to their diagnosis: tuberculosis group (n=36) and non-tuberculosis group (16 malignancy, 22 bronchiectasis, 6 pulmonary infection, 5 chronic obstructive pulmonary disease, 4 idiopathic; n=53). The diagnosis of tuberculosis was made by experienced pulmonologists after reviewing medical history, laboratory findings, acid-fast bacilli (AFB) smear, and a radiologic examination. Tuberculosis patients were subdivided into active and latent groups. Active disease was classified as primary and post primary (reactivation) tuberculosis [12]. Patients, who had not been previously exposed to Mycobacterium tuberculosis, with clinical (cough, hemoptysis, fatigue, malaise, weight loss, fever, night sweats), radiological (lymphadenopathy, consolidation, pleural effusion, miliary nodules), and laboratory (AFB-positivity) findings were considered as primary tuberculosis patients [13]. Patients who met the criteria for an active clinical case and AFB-positivity, with accompanying radiologic findings (consolidations predominant in the apical and upper lung zones, nodules, cavitations) were considered as reactivation tuberculosis patients [14]. Patients with AFB-negativity but having radiologic or clinical evidence of former tuberculosis were classified as latent tuberculosis patients [12]. Multi-drug resistant tuberculosis was defined as the resistance to isoniazid and rifampin therapy in culture studies [13].
Written informed consent was obtained from all patients prior to embolization. Angiography and embolization procedures were performed by interventional radiologists with 5, 10, and 20 years of experience with a classical method that has been previously described [15]. Before the procedure, a computed tomography (CT) of the thorax and a bronchoscopy were performed on all patients to find the pathologic lesion and artery. Common femoral artery was chosen for access under ultrasound guidance. The decision for embolization and selection of embolic agents were made by the operators during the procedure. After inserting a 5-French sheath into the common femoral artery, a thoracic aortogram was taken with a 5-French pigtail catheter to distinguish any abnormal sites and assess the origin of the bronchial and non-bronchial systemic arteries. In all patients, internal thoracic, subclavian, and intercostal arteriograms in addition to bronchial arteriograms were performed to observe any abnormal contrast filling. Simmons 1 and Cobra 2 catheters were used to find the origin of the pathologic arteries. Hand injection was used in selective bronchial or non-bronchial angiograms. After observing an abnormal angiographic finding, a microcatheter (Renegade microcatheter; Boston Scientific, Natick, Massachusetts) was advanced superselectively to the pathologic artery. Embolization was done after obtaining a superselective angiogram and after evaluating the angiographic findings. Microparticles ≥500 μm were used if there was a bronchopulmonary shunt. In other cases, embolization started with 350 μm sized microparticles to achieve complete embolization of the distal vascular territory. Microspheres (Embozene; Boston Scientific, Cork, Ireland) sized between 350–700 μm diameter were used as embolic agents. Embolization was ended when there were a significant contrast material stasis and no antegrade flow. Coils were not used to avoid any access difficulties in the case of possible recurrence.
We classified angiographic findings as tortuosity, hypertrophy, hypervascularity, aneurysm, bronchopulmonary shunt, extravasation, and normal bronchial artery (Figure 1) [16]. Tortuosity referred to more than two turns in opposite directions of the pathologic artery. Hypertrophy meant that the diameter of the anomalous artery is greater than 3 mm. Hypervascularity meant increased contrast filling with parenchymal blush and staining. Aneurysm referred to a localized dilation of the diseased artery. Bronchopulmonary shunt was described as contrast material flowing from systemic circulation into the pulmonary circulation. When no pathologic finding was seen, the artery was considered as “normal”.
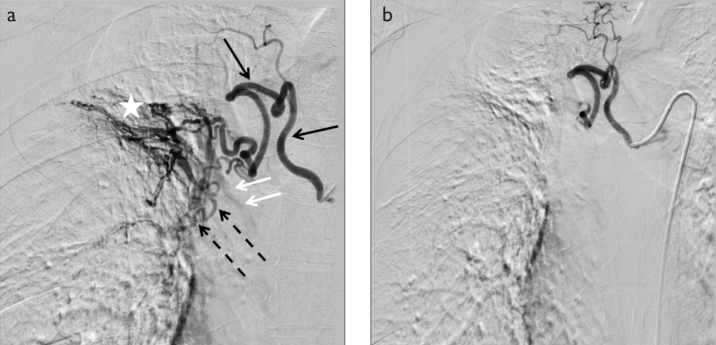
Figure 1. a, b.
a) Hypertrophy (black arrows), hypervascularity (star), tortuosity (dotted arrows), bronchopulmonary shunt (white arrows) are seen in the pathologic bronchial artery on a selective right intercostobronchial trunk angiogram. b) Postembolization angiogram demonstrates the cessation of antegrade flow.
Angiographic findings of the diseased arteries were evaluated by two interventional radiologists with 5 and 10 years of experience. All patient data were hidden during the analysis. In cases of disagreement between the two interventional radiologists, the images were reevaluated. Furthermore, a third interventional radiologist (U.B.) with 20 years experience reanalyzed the images, and the final decision was reached by consensus.
Technical success, clinical success, recurrence rates, and minor and major complication rates were considered during the outcome analysis. Technical success was described as rapid interruption of blood flow from the diseased artery [17]. Clinical success was defined as the total cessation of hemoptysis. Partial recovery that did not need any medication within a minimum of 30 days was also referred as clinical success [18]. The requirement of medical, surgical, or angiographic treatment for hemoptysis after embolization was regarded as recurrence. Follow-up information was obtained from inpatient and outpatient records retrospectively. Extended hospitalization, irreversible sequelae, or death were regarded as major complications. Minor complications such as hematoma at the access site were conditions that did not result in sequelae and needed only minimal care and observation [19].
Statistical Analysis
Statistical analysis was performed by using the Statistical Package for the Social Sciences version 22.0 (IBM Corp.; Armonk, NY, USA). Continuous data were expressed as means ± standard deviation (SD) and categorical variables as percentages. The distribution of continuous variables was evaluated by the Kolmogorov-Smirnov or the Shapiro-Wilk test. The independent t-test or Mann-Whitney U test was used for the comparison of age and the angiographic findings. The chi-square test or Fisher’s Exact test was used to compare angiographic findings between the tuberculosis and non-tuberculosis groups, between reactivation and latent groups of patients with tuberculosis, and to assess the relationship between recurrence rates and angiographic findings. P-values of less than 0.05 were considered statistically significant.
Results
Among 89 patients, 36 had tuberculosis (16 latent, 20 active) and 53 did not have tuberculosis. The tuberculosis group consisted of 34 men (94.4%) and 2 women (5.6%) with a mean age of 49.57±17.75. There were 32 males (60.4%) and 21 (39.6%) females in the non-tuberculosis group (mean age 54.57±13.45). There was a significant difference in the gender of patients between the two groups (p<0.001). All active tuberculosis patients consisted of reactivation tuberculosis patients. Among the reactivation group, two of the subjects were found to have multi-drug resistant tuberculosis.
A total of 98 embolization procedures were performed in 89 patients. The angiographic findings of all patients are presented in Table 1. Tortuosity and hypervascularity were the most common findings in the tuberculosis (both reactivation and latent) and non-tuberculosis groups. Among all patients, extravasation was the rarest observation, and it was observed in only one case with a reactivation tuberculosis patient (Figure 2). Aneurysm was the second least common observation in both the tuberculosis and non-tuberculosis groups. No significant correlation was observed between age or gender and any of the angiographic findings. Angiographic findings in tuberculosis and non-tuberculosis groups are summarized and compared in Table 1. No significant relationship was found between age or gender and angiographic findings in tuberculosis patients. Bronchopulmonary shunt was found to be significantly higher in tuberculosis patients compared to the non-tuberculosis group (p=0.002); 19 of 36 (52.8%) patients with tuberculosis had bronchopulmonary shunt; however, in the non-tuberculosis group, only 12 of 53 (22.6%) had a shunt. Angiographic findings of tuberculosis patients and comparisons are summarized in Table 2. No significant differences in angiographic findings were found between the tuberculosis reactivation and tuberculosis latent groups (p>0.05). The number of embolized arteries and origins of the embolized artery are shown in Table 3. In the tuberculosis group, 68.5% of the arterial abnormality was observed in the bronchial system. In the non-tuberculosis group, 76.1% of the pathologic arteries originated from the bronchial arteries.
Table 1.
Angiographic findings of tuberculosis and non-tuberculosis patients
| Findings | Total number of patients (n=89) | Tuberculosis group (n=36) | Non-tuberculosis group (n=53) | p value |
|---|---|---|---|---|
| Hypervascularity | ||||
| Absent | 4 (4.5%) | 1 (2.8%) | 4 (7.5%) | 0.537 |
| Present | 85 (95.5%) | 35 (97.2%) | 50 (92.5%) | |
| Hypertrophy | ||||
| Absent | 19 (21.4%) | 6 (17.1%) | 13 (24.5%) | 0.410 |
| Present | 70 (78.6%) | 30 (83.3%) | 40 (75.5%) | |
| Tortuosity | ||||
| Absent | 9 (10.2%) | 1 (2.8%) | 8 (15.1%) | 0.064 |
| Present | 80 (89.8%) | 35 (97.2%) | 45 (84.9%) | |
| Shunt | ||||
| Absent | 58 (65.2%) | 17 (47.2%) | 41 (77.4%) | 0.002 |
| Present | 31 (34.8%) | 19 (52.8%) | 12 (22.6%) | |
| Aneurysm | ||||
| Absent | 84 (94.4%) | 33 (91.7%) | 51 (96.2%) | 0.341 |
| Present | 5 (5.6%) | 3 (8.3%) | 2 (3.8%) | |
| Normal bronchial artery | ||||
| Absent | 80 (89.9%) | 35 (97.1%) | 45 (84.9%) | 0.064 |
| Present | 9 (10.1%) | 1 (2.9%) | 8 (15.1%) | |
| Extravasation | ||||
| Absent | 88 (98.9%) | 35 (97.3%) | 0 (0%) | N/A |
| Present | 1 (1.1%) | 1 (2.7%) | 0 (0%) | |
Figure 2.
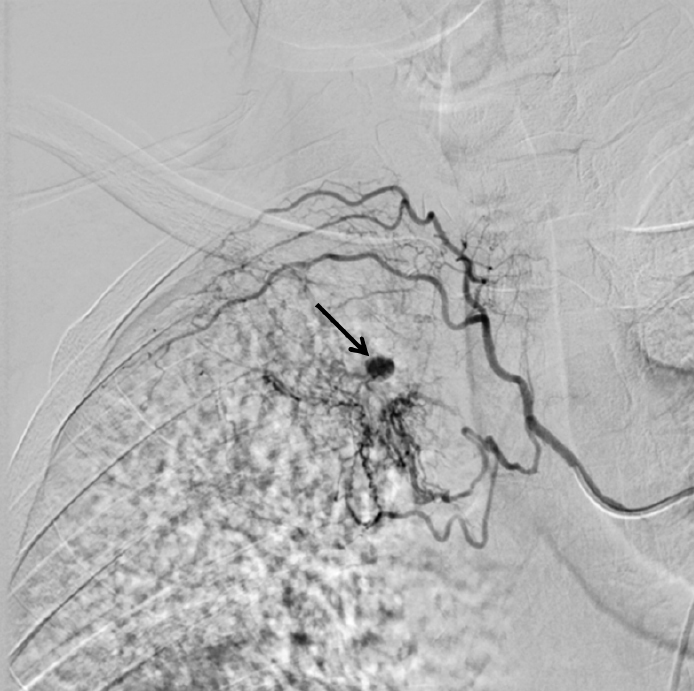
Right intercostobronchial arteriogram demonstrates hypervascular areas and extravasation (black arrow) in the right upper lobe
Table 2.
Angiographic findings of tuberculosis patients
| Findings | Reactivation tuberculosis (n=20) | Latent tuberculosis (n=16) | p value |
|---|---|---|---|
| Hypervascularity | |||
| Absent | 1 (5%) | 0 (0%) | 1 |
| Present | 19 (95%) | 16 (100%) | |
| Hypertrophy | |||
| Absent | 4 (20%) | 2 (12.5%) | 0.666 |
| Present | 16 (80%) | 14 (87.5%) | |
| Tortuosity | |||
| Absent | 1 (5%) | 0 (0%) | 1 |
| Present | 19 (95%) | 16 (100%) | |
| Shunt | |||
| Absent | 8 (40%) | 8 (50%) | 0.740 |
| Present | 12 (60%) | 8 (50%) | |
| Aneurysm | |||
| Absent | 19 (95%) | 14 (87.5%) | 0.582 |
| Present | 1 (5%) | 2 (12.5%) | |
Table 3.
Number of embolized arteries and origins of the pathologic artery in tuberculosis and non-tuberculosis group
| Origin of the embolized arteries | Tuberculosis group (n=57) | Non-tuberculosis group (n=71) | ||
|---|---|---|---|---|
|
|
|
|||
| n | % | n | % | |
| Bronchial | 39 | 68.5 | 54 | 76.1 |
| Intercostal | 11 | 19.3 | 12 | 16.9 |
| Subclavian | 4 | 7.0 | 2 | 2.8 |
| Inferior phrenic | 0 | 0 | 2 | 2.8 |
| Internal thoracic | 3 | 5.2 | 1 | 1.4 |
The mean follow-up period in this study was 17.09 months ±9.16 (range 1–36 months; median 18 months). A total of 128 arteries were embolized in 98 procedures; one artery in 55 patients (19 in tuberculosis, 36 in non-tuberculosis), two arteries in 29 patients (16 in tuberculosis, 13 in non-tuberculosis), three arteries in 5 patients (2 in tuberculosis, 3 in non-tuberculosis). Pathologic arteries could not be embolized in two procedures due to the challenging angles from the aorta. Thus, our overall technical success rate was 98% (98/100). Clinical success rate was recorded as 91.6% (33/36) among tuberculosis group and 98.1% (52/53) among non-tuberculosis group. The overall clinical success rate was 95.5% (85/89). No major complication was experienced. Short-lasting chest pain occurred in one patient (1/89, 1.1%) and stopped during monitoring. Bronchial artery spasm occurred in three patients (3/89, 3.3%) and bronchial artery dissection appeared in four patients (4/89, 4.4%).
Recurrence rates and the degree of hemoptysis are outlined in Table 4. Recurrence occurred in 12 of the 89 patients (13.4%). In the tuberculosis group, 6 patients (3 latent, 3 reactivation) required reembolization; and 6 patients in the non-tuberculosis group also underwent reembolization due to recurrent hemoptysis. No relationship was found between the severity of hemoptysis and recurrence (p>0.05). None of the groups showed a statistically significant difference with respect to recurrence (p=0.436). Also, neither reactivation nor latent groups were associated with significantly higher recurrence rates. Among the tuberculosis group, reembolization was performed after 8 months for one patient, after 4 months for another, after 3 months for the third, and within the first month for three patients. Clinical failure was seen in three patients with tuberculosis within one month. Of these three patients, two underwent reembolization due to recanalization of the same vessels. No abnormality was found during angiography in the last patient, thus medical treatment was given. Among the three patients who developed recurrence after one month had passed, recanalization with collaterals was observed in one (Figure 3). Pathologic arteries different from the ones observed in the first procedure were seen and embolized in the remaining two patients. There was no significant relationship between angiographic findings and recurrence in tuberculosis patients (p>0.05). Among non-tuberculosis recurrence group (n=6), reembolization was performed after 16 days (chronic obstructive pulmonary disease), 8 months (malignancy), 13 months (bronchiectasis), 18 months (bronchiectasis), 22 months (malignancy), and 31 months (bronchiectasis). In the clinical failure case within the first month, recanalization of the same vessel was observed during the procedure. Different arteries were the cause of hemoptysis in the remaining five cases.
Table 4.
Degree of hemoptysis and recurrence rates
| Tuberculosis group (n=36) | Non-tuberculosis group (n=53) | |
|---|---|---|
| Degree of hemoptysis | ||
| Moderate | 17 (47.2%) | 31 (58.5%) |
| Massive | 19 (52.8%) | 22 (41.5%) |
| Recurrens | ||
| <30 days | 3 (8.3%) | 1 (1.9%) |
| >30 days | 3 (8.3%) | 5 (9.4%) |
Figure 3.
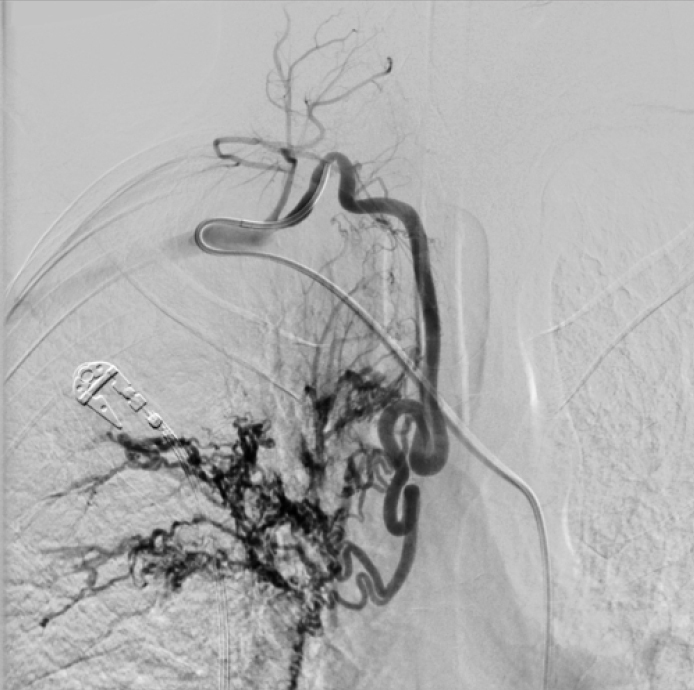
Patient referred with hemoptysis after right intercostobronchial trunk embolization. Right costocervical trunk angiogram shows the hypervascular lesion with hypertrophy and tortuosity
Discussion
Our study demonstrated that hypervascularity and tortuosity were the most common angiographic findings in tuberculosis patients. Bronchopulmonary shunt was significantly higher in the tuberculosis group compared to that in the non-tuberculosis group.
Continuous airway inflammation, bacterial superinfection, cavities, scar lesions, bronchiectasis, and erosion of the adjacent vessels may be possible reasons of vascular rupture in tuberculosis [13]. Chronic inflammation induces localized hypoxia and subsequent reduction in the pulmonary flow. Various angiogenic growth factors occur to supply adequate perfusion to the lungs. Neovascularization aggravates the fragility of vessels and eventually hemorrhaging occurs [20].
As a consensus, tortuosity, hypervascularity, hypertrophy, bronchopulmonary shunt, bronchial artery aneurysm, and active contrast extravasation are the main pathologic signs that need to be carefully examined during embolization in patients with pulmonary tuberculosis. In patients with tuberculosis, tortuosity and hypervascularity were the most prevalent results similar to those obtained by Dabo et al. and Anuradha et al. [10, 21]. Contrast extravasation, which is a direct sign of bleeding, is a rare angiographic finding in BAE [5]. In parallel with this condition, we only observed contrast extravasation in one patient during the procedure. Bronchopulmonary shunt seemed to be a common angiographic finding in tuberculosis similar to what was observed by Shin et al. [9]. There was no statistically significant difference in the angiographic findings between the tuberculosis and non-tuberculosis groups, except for bronchopulmonary shunting.
Bronchopulmonary shunts are connections between bronchial and pulmonary arteries or bronchial arteries and pulmonary veins. Vascular proliferation, remodeling, and collaterals can be seen between bronchial and non-bronchial systemic arteries occasionally. However, bronchopulmonary shunts may occur only in some conditions such as chronic inflammatory processes, malignancies, or decreases in the pulmonary flow (chronic thromboembolic pulmonary hypertension and congenital pulmonary stenosis) [22, 23]. As a consequence of shunting, pressure and circulation in the affected area augments and leads to hemoptysis [24].
The detection of bronchopulmonary shunts is vital to prevent the passage of the embolic agent into the pulmonary system in the presence of a bronchopulmonary shunt [25]. To avoid either pulmonary or systemic infarction, >325 μm sized particles are suggested for use [5]. During BAE in patients with tuberculosis, the presence of a bronchopulmonary shunt should be scrutinized to avoid severe complications. Furthermore, if a bronchopulmonary shunt is seen in an urgent BAE procedure with massive hemoptysis and unknown diagnosis, tuberculosis may be considered as the underlying cause. The operator may suggest the clinician search for tuberculosis in such conditions. Anuradha et al. [10] reported that bronchopulmonary shunts were common in patients admitted with re-bleeding. No statistical significance was observed between bronchopulmonary shunt and recurrence in our study. However, manifested bronchopulmonary shunts were detected in two clinical failure cases in the tuberculosis group.
In the tuberculosis group, 32% of the bleeding source was the non-bronchial system. Intercostal (19.3%) and subclavian (7%) arteries were the leading origins in the non-bronchial system. Ramakantan et al. [7] reported similar rates in intercostal arteries. We found higher rates of pathologic subclavian and internal thoracic arteries with rates of 7.0% and 5.2%, respectively. According to our results, searching for potential non-bronchial systemic vessels is critical for appropriate treatment as there is a high rate of non-bronchial origin. Additionally, it was reported that embolization of non-bronchial systemic collaterals decreased the recurrence rates [10].
We showed that BAE was a secure, efficient, and functional method for controlling hemoptysis in both tuberculosis or other underlying diseases with a clinical success rate of 91.6% and 98.1%, respectively. These rates were similar to those of Lee et al. and Swanson et al. [26, 27]. The clinical success rates ranged from 70–99% in the literature [28]. Technical success rates of BAE had a wide range between 81–100% [21, 29, 30]. Clinical success rates were higher in the non-tuberculosis group than the tuberculosis group in the present study. This may be due to inadequate embolization of collaterals, rapid recanalization of the embolized artery, or conservatively unstoppable progression of the disease. Although angiographic findings were not associated with clinical success or recurrence, they manipulated the technique of embolization. Greater than 350 μm sized particles were used when bronchopulmonary shunts were observed.
The main reasons of recurrence are speculated to be incomplete embolization, recanalization of the embolized arteries, development of new collaterals, or progression of the underlying disease [9, 26, 29]. The studies investigating recurrence associated factors in tuberculosis after BAE had conflicting results. Hwang et al. [31] showed a statistical difference in bronchopulmonary shunt between the recurrence and non-recurrence groups. Contrary to this, Shin et al. [9] assessed the relationship between angiographic findings and recurrence in tuberculosis patients but found no statistical significance. Furthermore, Anuradha et al. [10] did not notice any significant difference between recurrence and any angiographic or clinical feature in tuberculosis. Similarly, no significant relationship was found between angiographic findings and recurrence in the present study. Some studies showed that the recurrence rate was significantly higher in reactivation tuberculosis [9, 26]. However, we did not observe any difference between the reactivation and latent groups.
Major complications usually occur because of unintentional embolization of spinal arteries, manipulation of subclavian arteries, or transition of embolic agents through bronchopulmonary shunts. Agmy et al. reported monoparesis in 2 out of 348 patients [32]. Fruchter et al.[33] declared transient neurological complication rates as 4.4%. No neurologic symptoms occurred after embolization in our cases. The incidence of minor complications such as transient chest pain, arterial vasospasm, and dissection were compatible with the findings in the literature. Although groin hematoma and femoral artery pseudoaneurysms were also reported after BAE procedures [34, 35], we did not observe these problems. It may be due to the ultrasound-guided puncture of the common femoral artery.
The study had some limitations. The retrospective study design was a major limitation of the present study. Additionally, because of the relatively small sample size of the tuberculosis group, the number of patients with tuberculosis who had re-bleeding after embolization was too small. Further prospective studies with a larger number of patients are advisable.
In conclusion, BAE is a useful and effective treatment method for moderate and massive hemoptysis in tuberculosis. The occurence of bronchopulmonary shunts seemed to be significantly higher in patients with tuberculosis. A detailed investigation of bronchopulmonary shunts in patients with tuberculosis during BAE is critical for both effective treatment and reduction of catastrophic complications.
Main Points.
Bronchial artery embolization is a safe and effective treatment method for hemoptysis in patients with tuberculosis.
Bronchopulmonary shunt was significantly higher in tuberculosis patients compared to the non-tuberculosis group.
There was no difference in the angiographic findings between reactivation and latent tuberculosis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from Tepecik Education and Research Hospital (14/11/2018, 2018/ 13-6).
Informed Consent: Written Informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.S., A.E.C.; Design- O.S., U.B.; Supervision- U.B.; Resources - O.S., A.E.C., M.Y.Y; Materials - O.S., U.B; Data Collection and/or Processing - A.E.C., M.Y.Y.; Analysis and/or Interpretation - O.S., M.Y.Y.; Literature Search - O.S., A.E.C., M.Y.Y.; Writing Manuscript - O.S; Critical Review - U.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: Authors have no conflicts of interest to declare.
References
- 1.Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Interv Radiol. 2010;33:240–50. doi: 10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- 2.Larici AR, Franchi P, Occhipinti M, et al. Diagnosis and management of hemoptysis. Diagn Interv Radiol. 2014;20:299–309. doi: 10.5152/dir.2014.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uflacker R, Kaemmerer A, Picon PD, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985;157:637–44. doi: 10.1148/radiology.157.3.4059552. [DOI] [PubMed] [Google Scholar]
- 4.Syha R, Benz T, Hetzel J, et al. Bronchial artery embolization in hemoptysis:10-year survival and recurrence- free survival in benign and malignant etiologies-a retrospective study. Rofo. 2016;188:1061–6. doi: 10.1055/s-0042-112227. [DOI] [PubMed] [Google Scholar]
- 5.Yoon W, Kim JK, Kim YH, et al. Bronchial and nonbronchial systemic artery embolization for lifethreatening haemoptysis: a comprehensive review. Radiographics. 2002;22:1395–409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- 6.Rémy J, Arnaud A, Fardou H, et al. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–7. doi: 10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Ramakantan R, Bandekar VG, Gandhi MS, et al. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200:691–4. doi: 10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- 8.Sanyika C, Corr P, Royston D, et al. Pulmonary angiography and embolization for severe hemoptysis due to cavitary pulmonary tuberculosis. Cardiovasc Intervent Radiol. 1999;22:457–60. doi: 10.1007/s002709900432. [DOI] [PubMed] [Google Scholar]
- 9.Shin BS, Jeon GS, Lee SA, et al. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011;15:1093–8. doi: 10.5588/ijtld.10.0659. [DOI] [PubMed] [Google Scholar]
- 10.Anuradha C, Shyamkumar NK, Vinu M, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101. doi: 10.4261/1305-3825.DIR.3876-11.2. [DOI] [PubMed] [Google Scholar]
- 11.Pei R, Zhou Y, Wang G, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis secondary to tuberculosis. PLoS One. 2014;9:e115956. doi: 10.1371/journal.pone.0115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diagnostic standards and classification of tuberculosis in adults and children: official statement of the American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 13.Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary Tuberculosis: Role of Radiology in Diagnosis and Management. Radiographics. 2017;37:52–72. doi: 10.1148/rg.2017160032. [DOI] [PubMed] [Google Scholar]
- 14.Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings-early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–60. doi: 10.1148/radiology.186.3.8430169. [DOI] [PubMed] [Google Scholar]
- 15.Yu-Tang Goh P, Lin M, Teo N, et al. Embolization for hemoptysis: a six -year review. Cardiovasc Intervent Radiol. 2002;25:17–25. doi: 10.1007/s00270-001-0047-1. [DOI] [PubMed] [Google Scholar]
- 16.Kamble AN, Gangawani G, Chauhan C. A retrospective study for identifying signs of diseased bronchial artery in bronchial artery embolization(BAE) ECR C-1841. 2018 [Google Scholar]
- 17.Dave BR, Sharma A, Kalva SP, et al. Nine-year single center experience with transcatheter arterial embolization for hemoptysis: medium term outcomes. Vasc Endovascular Surg. 2011;45:258–68. doi: 10.1177/1538574410395036. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Kim YI, Kim HY, et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc Intervent Radiol. 2007;30:638–43. doi: 10.1007/s00270-007-9034-5. [DOI] [PubMed] [Google Scholar]
- 19.Drooz AT, Lewis CA, Allen TE, et al. Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol. 2003;14:S237–42. [PubMed] [Google Scholar]
- 20.Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol. 2011;28:48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabó H, Gomes R, Marinho A, et al. Bronchial artery embolisation in management of hemoptysis--A retrospective analysis in a tertiary university hospital. Rev Port Pneumol (2006) 2016;22:34–8. doi: 10.1016/j.rppnen.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ley S, Kreitner KF, Morgenstern I, et al. Bronchopulmonary shunts in patients with chronic thromboembolic pulmonary hypertension: evaluation with helical CT and MR imaging. AJR Am J Roentgenol. 2002;179:1209–15. doi: 10.2214/ajr.179.5.1791209. [DOI] [PubMed] [Google Scholar]
- 23.Ustünsöz B, Bozlar U, Ors F, et al. Bronchial artery embolization: experience with 10 cases. Diagn Interv Radiol. 2006;12:43–6. [PubMed] [Google Scholar]
- 24.Marshall TJ, Jackson JE. Vascular intervention in the thorax: bronchial artery embolization for haemoptysis. Eur Radiol. 1997;7:1221–7. doi: 10.1007/s003300050279. [DOI] [PubMed] [Google Scholar]
- 25.Cody O'Dell M, Gill AE, Hawkins CM. Bronchial Artery Embolization for the Treatment of Acute Hemoptysis. Tech Vasc Interv Radiol. 2017;20:263–5. doi: 10.1053/j.tvir.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Chan JW, Chan SC, et al. Bronchial artery embolization can be equally safe and effective in the management of chronic recurrent hemoptysis. Hong Kong Med J. 2008;14:14–20. [PubMed] [Google Scholar]
- 27.Swanson KL, Johnson CM, Prakash UB, et al. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121:789–95. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- 28.Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23:307–17. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhalla A, Kandasamy D, Veedu P, et al. A retrospective analysis of 334 cases of hemoptysis treated by bronchial artery embolization. Oman Med J. 2015;30:119–28. doi: 10.5001/omj.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucukay F, Topcuoglu OM, Alpar A, et al. Bronchial Artery Embolization with Large Sized (700–900 μm) Tris-acryl Microspheres (Embosphere) for Massive Hemoptysis: Long-Term Results (Clinical Research) Cardiovasc Intervent Radiol. 2018;41:225–30. doi: 10.1007/s00270-017-1818-7. [DOI] [PubMed] [Google Scholar]
- 31.Hwang HG, Lee HS, Choi JS, et al. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013;74:111–9. doi: 10.4046/trd.2013.74.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agmy GM, Wafy SM, Mohamed SAA, et al. Bronchial and nonbronchial systemic artery embolization in management of hemoptysis: experience with 348 patients. Int Sch Res Not. 2013;26:e263259. doi: 10.1155/2013/263259. [DOI] [Google Scholar]
- 33.Fruchter O, Schneer S, Rusanov V, et al. Bronchial artery embolization for massive hemoptysis: long-term follow-up. Asian Cardiovasc Thorac Ann. 2015;23:55–60. doi: 10.1177/0218492314544310. [DOI] [PubMed] [Google Scholar]
- 34.Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602. doi: 10.1148/radiol.13130046. [DOI] [PubMed] [Google Scholar]
- 35.Tom LM, Palevsky HI, Holsclaw DS, et al. Recurrent bleeding, survival, and longitudinal pulmonary function following bronchial artery embolization for hemoptysis in a U.S. adult population. J Vasc Interv Radiol. 2015;26:1806–13.e1. doi: 10.1016/j.jvir.2015.08.019. [DOI] [PubMed] [Google Scholar]