Abstract
Spinal hematoma following neuraxial or perineural techniques is a rare but severe complication that can potentially lead to catastrophic consequences. The aim of this review is to analyze all reported cases of neuraxial or perineural bleeding after performance of a locoregional technique since the last guidelines update in 2018. We included articles indexed by MEDLINE, Scopus, and Google Scholar. We analyzed the patient’s age, surgical procedure, pre-operative anticoagulant and antiplatelet therapy, type of anesthetic procedure, vertebra level of the procedure, diameter and point type of the needle, hematoma type (spinal, subdural, epidural), signs and symptoms, time to imaging, and time to treatment and outcome. During our bibliographic research, we identified 5637 unique articles that were eligible according to our protocol criteria, identifying 18 separate cases of neuraxial bleeding. Although clinicians are usually aware of antiplatelet and anticoagulant perioperative management, a careful post-procedural observation and a detailed patient education are also imperative for the early detection of the symptoms of spinal cord ischemia.
Keywords: Epidural anesthesia, hemorrhage, patient safety, review, spinal anesthesia
Introduction
Spinal hematoma following neuraxial or perineural techniques is a rare [1–3] but severe complication that can potentially lead to catastrophic consequences and severely affect the patient’s quality of life.
In order to increase patient safety, the American Society of Regional Anesthesia and Pain Medicine (ASRA), in conjunction with the European Society of Anaesthesiology (ESA) developed guidelines to increase the safety of neuraxial and perineural techniques. These guidelines were published in the 1998 [4], 2003 [5], and 2010 [6] and updated in 2018 [7], specific guidelines for neuraxial pain procedure have also been published and updated [8].
The aim of this review is to retrieve all the cases of neuraxial or perineural bleeding after performance of a locoregional technique since the last guidelines update and to analyze the impact of ASRA guidelines on these cases.
Materials and Methods
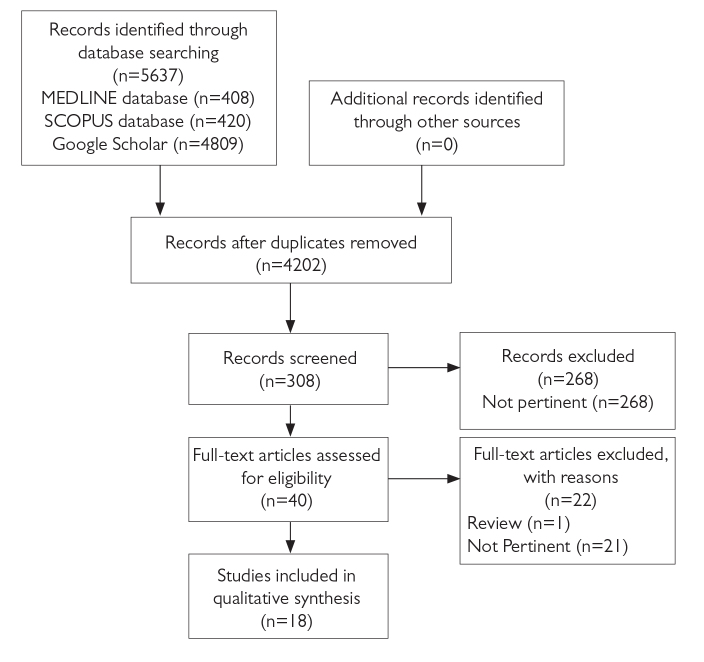
A review protocol was written before conducting this study, then we performed a systematic review of the medical literature following the PRISMA Statement Guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses) [9] for the identification, screening, and inclusion of articles (Figure 1). The bibliographic search was conducted on March 24th, 2019 by two of the authors (ADC and CC). In this review we included articles indexed by MEDLINE, Scopus, and Google Scholar. We used the following string as search method: (“hematoma” or “bleeding”) and (“subarachnoid” or “spinal” or “epidural”) not “spontaneous”. We did not apply any restrictions on the publication type, status, or language, however, we restricted publication period between March 2018 and March 2019. Furthermore, we searched other relevant reports by consulting references and citations. The following data were retrieved from the included reports: patient’s age, surgical procedure, pre-operative anticoagulant and antiplatelet therapy, type of anesthetic procedure, vertebra level of the procedure, diameter and point type of the needle, hematoma type (spinal, subdural, epidural), signs and symptoms, time to imaging, and time to treatment and outcome.
Figure 1.
PRISMA flowchart
Results
During our bibliographic research, we identified 5637 unique articles that were eligible according to our protocol criteria.
Two hundred and six articles were duplicate and hence were excluded, 2894 were not pertinent and therefore were excluded.
We read the abstract of the remaining 308 articles, 268 articles were not pertinent and hence were excluded; remaining 40 full-text articles were read and examined.
Among the examined articles, 22 were not pertinent to the review topic and were excluded. Another article was excluded because it was a review, the remaining 18 articles were eligible for this review [10–27]; among the eligible articles, 18 separate cases of neuraxial bleeding were described.
33% of the complications occurred after pain therapy maneuver, 17% during abdominal surgery, 11% in parturient patients, 11% in vascular surgery, 11% in orthopedic surgery, and 17% in other settings. 28% of the patients underwent spinal anesthesia while 72% of the patients received an epidural one. Type of surgery and anesthesiological technique characteristics are reported in Table 1.
Table 1.
Locoregional technique setting
| #Number | Age | Surgery | Indication | Technique | Level | Needle |
|---|---|---|---|---|---|---|
| #1[10] | 54 | Abdominal surgery | PO analgesia | Epidural | Thoracic | N |
| #2[11] | 74 | Abdominal surgery | PO analgesia | Epidural | T9-T10 | 18 G-Tuohy |
| #3[12] | 64 | Inguinal herniorrhaphy | Anesthesia | Spinal | Lumbar | 22 G-Quincke |
| #4[13] | 46 | Gynecologic surgery | PO analgesia | Epidural | T7-T8 | 16 G-Tuohy |
| #5[14] | 89 | Abdominal surgery | PO analgesia | Epidural | T8-T9 | N |
| #6[15] | 70 | - | Steroid injection | Epidural | L1-L2 | 22 G-Tuohy |
| #7[16] | 28 | - | Steroid injection | Epidural | Cervical | N |
| #8[17] | 31 | Cesarean delivery | Anesthesia | Spinal | L3-L4 | 26 G-Quincke |
| #9[18] | 75 | - | PRS | Epidural | Sacral | 22G |
| #10[19] | 56 | Orthopedic surgery | Anesthesia | Spinal | L3-L4 | 23 G-Quincke |
| #11[20] | 81 | - | Steroid injection | Epidural | L4-L5 | 22 G-Tuohy |
| #12[21] | 30 | - | Blood patch | Epidural | L5-S1 | N |
| #13 [22] | 35 | Labor | Analgesia | Epidural | L2 | 18 G-Tuohy |
| #14[23] | 59 | Vascular surgery | PO analgesia | Epidural | N | N |
| #15[24] | 59 | Orthopedic surgery | Anesthesia | Spinal | L3-L4 | 23 G-Quincke |
| #16[25] | 31 | Labor | Analgesia | Epidural | N | 17 G-Tuohy |
| #17[26] | 69 | Aortic aneurysm | Anesthesia | Spinal | L3-L4 | 27 G-Quincke |
| #18[27] | 64 | - | IL Steroid injection | Epidural | C7-T1 | 20 G-Tuohy |
PO: post-operative; IL: interlaminar; N: not clearly stated; G: gauge; PRS: pulsed radiofrequency stimulation
We identified possible predisposing factors and summarized them in Table 2. We defined “needle size (18G and above)” as a risk factor. 22% of the cases had none of the considered risk factors, 34% had one risk factor, 34% had two risk factors, 5% had three risk factors, and 5% had four risk factors. In seven cases, the use of post-operative anticoagulant was not clearly specified. Low weight molecular heparin (LWMH) was used for four patients during the post-operative period (case 1 [10], 2 [11], 4 [13], 8 [17]); however, for cases 1 and 2, the time interval between the performance of the neuraxial technique and first LWMH administration was not clear. For cases 4 and 8 [13], the physicians waited for six and nine hours, respectively. Unfractionated heparin was used in case 5 after more than five hours. In case 17 [26], 30 mg of intraoperative heparin was administered because patient was undergoing a vascular surgical procedure; however, the time fromthe neuraxial technique to heparin administration was not stated.
Table 2.
Perioperative risk factors
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | x | x | ||||||||||||||||
| Anatomic abnormalities | x | x | ||||||||||||||||
| Prev | ||||||||||||||||||
| Spine Surgery | x | x | ||||||||||||||||
| Difficult or repeated puncture | x | x | N | N | ||||||||||||||
| Needle size | N | x | x | N | N | N | x | N | x | |||||||||
| Indwelling Catheter | x | x | x | x | x | x | x | |||||||||||
| PrO-Antiplatelet | x | x | x | |||||||||||||||
| PrO-Anticoagulant | x | x | x | N |
N: not clearly stated;Prev: previous; PrO: pre-operative
We analyzed the type of complications and the associated symptoms that are summarized in Table 3. We retrieved 12 epidural hematoma cases, 4 subdural hematoma cases, and 2 subarachnoid hematoma cases. Patients with epidural hematomas developed headache (11%), back pain (39%), motor deficit (39%), and nausea or vomiting (5%); patients with subdural hematomas developed headache (75%), back pain (25%), motor deficit (25%), nausea or vomiting (25%), and fever (25%); patients with subarachnoid hematomas developed headache (100%), back pain (100%), nausea or vomiting (100%), and fever (100%).
Table 3.
Types of hematomas and associated symptoms
| #Number | Type | Headache | Back Pain | Motor deficit | N/V | Fever |
|---|---|---|---|---|---|---|
| #1[10] | Epidural | x | ||||
| #2[11] | Epidural | x | x | |||
| #3[12] | Subdural | x | x | x | ||
| #4[13] | Epidural | x | x | |||
| #5[14] | Epidural | x | ||||
| #6[15] | Epidural | x | ||||
| #7[16] | Epidural | x | x | |||
| #8[17] | Epidural | x | ||||
| #9[18] | Epidural | x | ||||
| #10[19] | Sub | x | x | x | x | |
| #11[20] | Epidural | x | ||||
| #12[21] | Subdural | x | x | |||
| #13 [22] | Subdural | x | x | |||
| #14[23] | Epidural | x | x | x | ||
| #15[24] | Sub | x | x | x | x | |
| #16[25] | Subdural | x | ||||
| #17[26] | Epidural | x | ||||
| #18[27] | Epidural | x |
N/V: nausea or vomiting; Sub: subarachnoid
In Table 4, we summarized time to imaging, type of imaging chosen, treatment, and outcome. The epidural procedure was found to the trigger for epidural hematomas in 75% of the cases (onset time thirty minutes to twenty-one days) while removal of the indwelling catheter was the trigger in 25% of the cases (onset time six hours to six days). 58% of the patients underwent laminectomy while the remaining patients underwent conservative treatment. Only two patients had no or minimal improvements, while the others had a full neurological recovery. It is important to highlight that all the patients who recovered completely and were undergoing conservative treatment had no motor deficit reported at any time. Only one patient with subdural hematoma underwent drainage while the other (75%) received conservative treatment with full recovery. All patients with subarachnoid hematomas were operated to remove the blood clot and had a complete restitutio ad integrum. The relationship between the papers and the ASRA guidelines can be found in Table 5; 45% of the papers mention Narouze’s guideline (11%) [28] or Horlocker’s guideline (17%) [6] or both (17%).
Table 4.
Timing to treatments and outcome
| #Number | Trigger | Time from trigger | Time to decision | Imaging | Treatment | Outcome |
|---|---|---|---|---|---|---|
| #1[10] | P | <24 h | Emergent | MRI | Laminectomy | No improvement |
| #2[11] | CR | 30 h | Emergent | MRI | Laminectomy | Full recovery |
| #3[12] | P | 1 d | 21 d | MRI | Conservative | N |
| #4[13] | CR | 6 h | <2 h | MRI | Laminectomy | Full recovery |
| #5[14] | P | 45 h | <4 h | MRI | Conservative | Minimal improvement |
| #6[15] | P | / | N | MRI | Laminectomy | Full recovery |
| #7[16] | P | / | N | MRI | Conservative | Full recovery |
| #8[17] | P | 3 d | 10 d | MRI | Conservative | Full recovery |
| #9[18] | P | 84 h | 84 h | MRI | Laminectomy | Improvement |
| #10[19] | P | 2d | 5d | MRI | Laminectomy and durotomy | Full recovery |
| #11[20] | P | 21 d | Immediate | MRI | Conservative | Improvement |
| #12[21] | P | 5 d | 8 d | MRI | Conservative | Full recovery |
| #13 [22] | N | / | 10 d | MRI | Drainage | Full recovery |
| #14[23] | CR | 6 d | 11 d | MRI | Conservative | N |
| #15[24] | P | 1 d | 4 d | MRI | Drainage | Full recovery |
| #16[25] | P | 6 h | N | CT | Conservative | N |
| #17[26] | P | 40 h | 40 h | MRI | Laminectomy | Full recovery |
| #18[27] | P | 30 min | 7 h | MRI | Laminectomy | Full recovery |
Trigger: bleeding trigger event; Time from trigger: time from trigger event to neurologic compromise; Time to treatment: time from neurologic compromise to treatment decision; CR: catheter removal; P: procedure; d: day; h: hour; min: minute; N: not clearly stated; CT: computed tomography; MRI: magnetic resonance imaging
Table 5.
Guideline reference
| #Number | Reference to GL | PrO GL followed | PO GL followed |
|---|---|---|---|
| #1[10] | Yes [28] | N | N |
| #2[11] | Yes [6] | Yes | Yes |
| #3[12] | No | / | / |
| #4[13] | Yes [6, 28] | N | Yes |
| #5[14] | Yes [6, 28] | Yes | Yes |
| #6[15] | Yes [6, 28] | Yes | Yes |
| #7[16] | No | / | / |
| #8[17] | Yes [6, 29] | Yes | Yes |
| #9[18] | No | No | No |
| #10[19] | No | / | / |
| #11[20] | Yes [6] | Yes | N |
| #12[21] | No | / | / |
| #13 [22] | No | / | / |
| #14[23] | No | / | / |
| #15[24] | No | / | / |
| #16[25] | No | / | / |
| #17[26] | No | / | / |
| #18[27] | Yes [28] | No | / |
PrO: pre-operative; PO: post-operative; N: not clearly stated; /: not in antiplatelet or anticoagulant therapy
Clinical and Research Consequences
Historically, neuraxial techniques were first developed more than one hundred years ago by August Bier [29]. Over the years, the practical applications of neuraxial techniques have progressively increased, ranging from perioperative analgesia to spinal cord stimulation implant. They are generally considered safe. However, there are a few risks associated with both spinal and epidural procedures.
Risk factors such as the age, the rising standards for perioperative venous thromboembolism prevention, and the introduction of new, potent antithrombotic medications, are increasing the risk of bleeding. This has led to the development of guidelines for higher safety standards in this complex field of anesthesia.
Although many societies provided their own guidelines [6, 28, 30] in this review we decided to consider the guidelines by Horlocker et al. [7] and the guideline by Narouze et al. [8] (especially for pain procedures) as reference.
In our series, we identified 18 different cases of hematomas: excluding 3 of them in which authors did not report the outcome, 87% of them had a good recovery.
This result is very optimistic; in a previous review, in fact, only 38% [31] of the patients had partial or full neurologic recovery, we may therefore suppose a publication bias, since authors are more likely to submit positive outcome results rather than negative ones.
The most frequently reported symptoms were headache, motor deficit, severe back pain, nausea or vomiting, and fever. Although bowel or bladder dysfunctions were not reported in this case study, they are not so rare symptoms after a spinal hematoma (8% of patients) [7]. It is important to notice that 44% of the patients presented only one of the above-mentioned symptoms, highlighting the importance of an accurate post-procedural surveillance.
17% of the patients took pre-operative antiplatelet medications and 17% of the patients took pre-operative anticoagulant drug. In all cases except one, physicians respected the suspension period for antiplatelet and anticoagulant drugs. In Case 9 [18], patient underwent an epidural pulsed radiofrequency stimulation under warfarin (INR 6) by mistake. In case 5 [14], an epidural hematoma patient was taking rivaroxaban (15 mg). Although the suspension time was higher than the recommended one (ninety-six hours versus the recommended sixty-six hours), it is particularly interesting being the first reported case of an epidural hematoma in a patient taking a new oral anticoagulant (NOA). Management of NOA could be challenging because the effective residual activity of the drug is not easy to predict. The only laboratory finding for evaluating the rivaroxaban residual activity is chromogenic anti-factor Xa assay calibrated for the drug, which might not be readily available in every hospital. Moreover, the residual anti-factor Xa activity considered acceptable for performing neuraxial anesthesiologic techniques remains unknown. However, considering that the patient had four different risk factors, it is difficult to understand the role of the anticoagulant in the hematoma development.
In case 18 [27], an epidural interlaminar steroid injection was performed on a patient consuming ibuprofen (after an adequate suspension period) and high dosage of fish oil (not suspended). Although the effect of fish oil on coagulation is not completely clear, caution suggests it should be managed like the other antiplatelet agents with a 6-day suspension prior to high-risk procedures, such as spinal cord or dorsal root ganglion stimulation. Epidural interlaminar steroid injection is an intermediate-risk procedure [8] for which there is no need to suspend fish oil consumption at normal daily doses (~1000 mg/d). In this case, the patient was taking twice the normal dose and this could have been a potential cause of bleeding, recommending caution.
In the discussion section of the articles, only 50% of the authors make reference to the guidelines. None of the authors referred to last published guidelines [7, 8]. This could easily explain the delay between writing and publishing of the article as the papers needed to be updated according to the guidelines update. 89% of the bleeding complications happened despite complying with the guidelines.
Guidelines [7] state that neurologic compromise is usually reversible in patients who undergo laminectomy within eight hours of onset of neurological dysfunction. However, only 17% of the papers clearly stated that patients received treatment within eight hours. In the remaining papers, 17% defined the treatment as “immediate” or “emergent” without a clear timing, 17% did not discuss the timing, and the remaining 49% of the patients were treated more than eight hours after the onset of neurologic compromise. Delays in treatment were mainly caused by underestimation of the symptoms by both physicians and patients. For example, in case 9 and 12, the patients observed symptoms at home but did not seek medical help for more than forty-eight hours.
In conclusion, Spinal hematomas are rare complications caused by neuraxial techniquesbut they can severely affect the quality of life of the patient.
Guidelines are crucial and therefore need to be reassessed and updated periodically. Although clinicians are usually aware of antiplatelet and anticoagulant perioperative management, a careful post-procedural observation and a detailed patient education must be undertaken and encouraged, in order to detect the symptoms of spinal cord ischemia early, preventing severe complications.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.D.C.; Design - A.D.C., L.S.; Supervision - A.D.C.; Data Collection and/or Processing - A.D.C., L.S., C.C.; Analysis and/or Interpretation - A.D.C., C.C., L.S.; Literature Search - A.D.C., L.S., C.C.; Writing Manuscript - A.D.C., L.S., C.C.; Critical Review - A.D.C., L.S., C.C.
Conflict of Interest: Authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Rocchi R, Lombardi C, Marradi I, et al. Intracranial and intraspinal hemorrhage following spinal anesthesia. Neurol Sci. 2009;30:393–6. doi: 10.1007/s10072-009-0103-1. [DOI] [PubMed] [Google Scholar]
- 2.Aromaa U, Lahdensuu M, Cozanitis D. Severe complications associated with epidural and spinal anaesthesias in Finland 1987–1993 A study based on patient insurance claims. Acta AnaesthesiolScand. 1997;41:445–52. doi: 10.1111/j.1399-6576.1997.tb04722.x. [DOI] [PubMed] [Google Scholar]
- 3.Auroy Y, Narchi P, Messiah A, et al. Serious complications related to Regional Anesthesia Results of a prospective survey in France. Anesthesiology. 1997;87:479–86. doi: 10.1097/00000542-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Horlocker TT, Wedel DJ. Neuraxial block and low-molecular-weight heparin: balancing perioperative analgesia and thromboprophylaxis. Reg Anesth Pain Med. 1998;23:164–77. doi: 10.1016/S1098-7339(98)90143-2. [DOI] [PubMed] [Google Scholar]
- 5.Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA consensus conference on neuraxial anesthesia and anticoagulation) Reg Anesth Pain Med. 2003;28:172–97. doi: 10.1053/rapm.2003.50046. [DOI] [PubMed] [Google Scholar]
- 6.Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition) Reg Anesth Pain Med. 2010;35:64–101. doi: 10.1097/AAP.0b013e3181c15dd0. [DOI] [PubMed] [Google Scholar]
- 7.Horlocker TT, Vandermeuelen E, Kopp SL, et al. Regional Anesthesia in the patient receiving Antithrombotic or Thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition) Reg Anesth Pain Med. 2018;43:263–309. doi: 10.1097/AAP.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 8.Narouze S, Benzon HT, Provenzano D, et al. Interventional Spine and pain procedures in patients on Antiplatelet and Anticoagulant Medications (Second Edition): Guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43:225–62. doi: 10.1097/AAP.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Lazar A, Anitescu M. Delayed diagnosis of an acute Epidural Hematoma due to MRI artifact from reinforced catheter. Pain Med. 2019 doi: 10.1093/pm/pnz018. pii: pnz018. [DOI] [PubMed] [Google Scholar]
- 11.De Cassai A. Thoracic epidural hematoma. Can J Anaesth. 2019;66:331–2. doi: 10.1007/s12630-018-01266-8. [DOI] [PubMed] [Google Scholar]
- 12.Issı Z, Öztürk V, İyilikçi L, et al. Spinal Epidural and Intracranial Subdural Haemorrhage that is a complication of Spinal Anaesthesia. Turk J AnaesthesiolReanim. 2018;46:319–22. doi: 10.5152/TJAR.2018.28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhorkar NM, Dhansura TS, Tarawade UB, et al. Epidural Hematoma: Vigilance beyond Guidelines. Indian J Crit Care Med. 2018;22:555–7. doi: 10.4103/ijccm.IJCCM_71_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burjorjee JE, Rooney R, Jaeger M. Epidural Hematoma following cessation of a direct oral anticoagulant: A case report. Reg Anesth Pain Med. 2018;43:313–6. doi: 10.1097/AAP.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 15.Sanders RA, Bendel MA, Moeschler SM, et al. Epidural Hematoma Following Interlaminar Epidural injection in patient taking aspirin. Reg Anesth Pain Med. 2018;43:310–2. doi: 10.1097/AAP.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 16.Ali SS, Shaw AE, Oselkin M, et al. Iatrogenic Spinal Epidural Hematoma associated with Intracranial Hypotension. Cureus. 2019;11:e4171. doi: 10.7759/cureus.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujic B, Holo-Djilvesi N, Djilvesi D, et al. Epidural hematoma following low molecular weight heparin prophylaxis and spinal anesthesia for cesarean delivery. Int J Obstet Anesth. 2019;37:118–21. doi: 10.1016/j.ijoa.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Chang MC. Epidural hematoma after caudal epidural pulsed radiofrequency stimulation: A case report. Medicine (Baltimore) 2018;97:e13090. doi: 10.1097/MD.0000000000013090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Ahn DK, Shin WS, et al. Spinal Subarachnoid Hematoma after Spinal Anesthesia: A Case Report. J Korean Soc Spine Surg. 2018;25:140–4. doi: 10.4184/jkss.2018.25.3.140. [DOI] [Google Scholar]
- 20.Kim SH, Han YJ, Kim YH, et al. Spontaneous absorption of a Lumbar Epidural Hematoma after Interlaminar Epidural Steroid injection in a patient with Spinal Stenosis: Close observation as a treatment strategy. Chin Med J (Engl) 2018;131:117–8. doi: 10.4103/0366-6999.221276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearsley R, McCaul C. Spinal subdural haematoma following an epidural blood patch. Anaesthesia Cases. 2018;6:29–33. doi: 10.21466/ac.SSHFAEB.2018. [DOI] [Google Scholar]
- 22.De Lipsis L, Belmonte R, Cusano M, et al. Subdural Hematoma as a consequence ofLabor Epidural Analgesia. Asian J Neurosurg. 2018;13:931–4. doi: 10.4103/ajns.AJNS_115_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh V, Patel S, Singh K. Unexpected intrathecal haemorrhage following uncomplicated placement and removal of an epidural catheter. BJR Case Reports. 2018;4:20170108. doi: 10.1259/bjrcr.20170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang W, Cho YH, Lee DH, et al. Subarachnoid hematoma after spinal anesthesia - A case report. Anesth Pain Med. 2018;13:154–7. doi: 10.17085/apm.2018.13.2.154. [DOI] [Google Scholar]
- 25.Szeto V, Kosirog J, Eilbert W. Intracranial subdural hematoma after epidural anesthesia: a case report and review of the literature. Int J Emerg Med. 2018;11:36. doi: 10.1186/s12245-018-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao YX, Li MX, Sun LJ. Good outcomes after the delayed removal of an epidural hematoma: A case report. Medicine (Baltimore) 2018;97:e0341. doi: 10.1097/MD.0000000000010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beasley D, Goree JH. Cervical epidural hematoma following interlaminar epidural steroid injection via the contralateral oblique view in patient taking omega-3 fatty acids. Reg Anesth Pain Med. 2019;44:253–5. doi: 10.1136/rapm-2018-000005. [DOI] [PubMed] [Google Scholar]
- 28.Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2015;40:182–212. doi: 10.1097/AAP.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 29.Goerig M, Agarwal K, Schulte am Esch J. The versatile August Bier (1861–1949), father of spinal anesthesia. J Clin Anesth. 2000;12:561–9. doi: 10.1016/S0952-8180(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 30.Leffert L, Butwick A, Carvalho B, et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928–44. doi: 10.1213/ANE.0000000000002530. [DOI] [PubMed] [Google Scholar]
- 31.Vandermeulen EP, van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165–77. doi: 10.1213/00000539-199412000-00024. [DOI] [PubMed] [Google Scholar]