Abstract
The left adrenal gland (LAG) is a common metastatic site in patients with non–small-cell lung cancer. In practice, staging mainly relies on radiologic studies and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Recently, a new technique using convex probe–endobronchial ultrasound (CP-EBUS) scope through the esophagus (EUS-B) has been introduced. A complete mediastinal staging and a reach for upper-abdominal structures in a single session naturally attract attention. However, scientific data are not sufficient to clearly judge the role of this technique in the cytological diagnosis of left adrenal lesions. Therefore, we present cases in which our patients have undergone EUS-B for LAG lesions to increase the data in the literature with regard to accessibility, diagnostic performance, and rate of complications.
Keywords: Adrenal tumor, endobronchial ultrasound-guided transbronchial needle aspiration, endoscopic ultrasound-guided fine-needle aspiration, non-small-cell lung cancer, staging procedures
INTRODUCTION
In patients with non-small-cell lung cancer, a proper staging should be done to manage the disease more efficiently. The techniques for mediastinal pathological staging have evolved to a relatively mature state with high sensitivity and specificity. However, the diagnosis of extra-thoracic lesions by means of the M descriptor of TNM staging may still be problematic and mainly relies on clinical measures and imaging modalities. Of these extra-thoracic lesions, the left adrenal gland (LAG) is of importance because an endosonographist can easily access the gland through a transgastric approach. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is commonly used for this purpose, but a recent technique using convex probe-endobronchial ultrasound (CP-EBUS) scope through the esophagus (transesophageal endobronchial ultrasound; EUS-B), has been introduced [1]. This new route for CP-EBUS is important because in select cases, the entire mediastinal and upper-abdominal sampling can be done by a single endosonographist in the same session, which shortens the time to diagnosis, avoids the risk of repeated interventions under sedation, and quite certainly brings patient satisfaction. Despite the simple rationale of EUS-B, the scientific data are scarce. Therefore, we present cases in which our patients have undergone EUS-B for LAG lesions to increase the data in the literature with regard to accessibility, diagnostic performance, and the rate of complications.
CASE PRESENTATIONS
Case 1
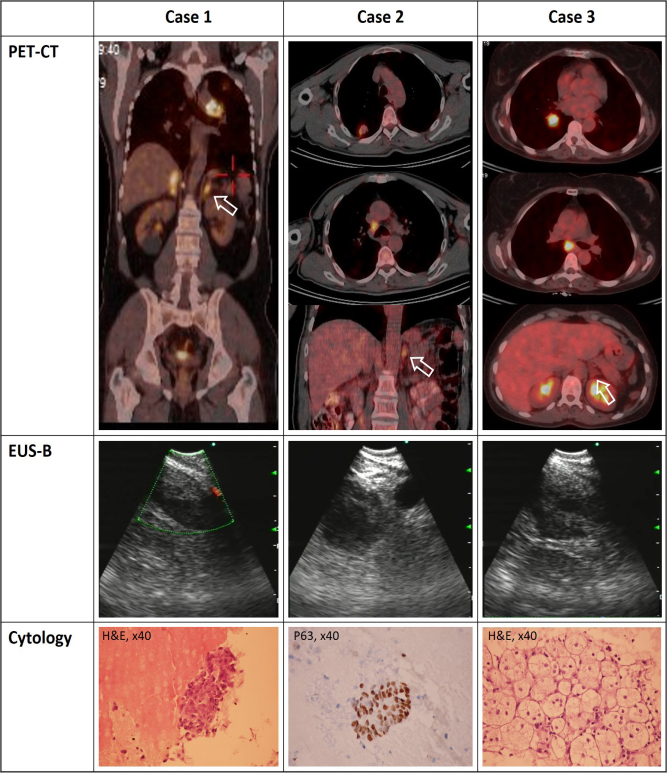
A 56-year-old male patient was admitted with chronic cough. The results of his physical examination were unremarkable. A posteroanterior chest roentgenogram revealed left hilar enlargement. Computed tomography (CT) revealed left hilar and bilateral adrenal masses, which was concluded with a high 18F-fluorodeoxyglucose (18F-FDG) uptake in positron emission tomography-computed tomography (PET-CT) (maximum standardized uptake value (SUVmax) of 16.2 and 12.4, respectively) (Figure). The tracheobronchial tree appeared normal in bronchoscopy, and transbronchial needle aspiration did not result in a diagnosis. Thus, CP-EBUS was performed for diagnostic and staging purposes. A mass of 35 mm invading the left pulmonary artery was seen and sampled after systematic contralateral hilar and mediastinal assessment. Subsequently, an EBUS scope was introduced to the esophagus and the LAG appeared as a round mass of 41-mm diameter on the left side of the abdominal aorta/celiac trunk. The lesion was sampled with a separate needle. No complications occurred. The cytologic material revealed a metastasis of lung adenocarcinoma. The patient was found to be inoperable and forwarded to a medical oncologist.
Figure 1.
Corresponding PET-CT, and sonographic and cytologic images of the cases.
EUS-B: transesophageal endobronchial ultrasound; H&E: hematoxylin & eosin dye; p63: tumor protein 63; TP63: PET-CT: positron emission tomography-computed tomography
Case 2
A 59-year-old man presented to an orthopedist with shoulder pain. A shoulder roentgenogram incidentally revealed a nodular lesion on the right upper lobe. CT showed two separate nodules at the right upper lobe (20×18 mm and 10×8 mm) and a right lower paratracheal (4R) lymphadenomegaly. PET-CT revealed high 18F-FDG uptakes in nodules, the right lower paratracheal lymphadenopathy (LAP), and the LAG (SUVmax of 4.2, 2.9, 9.4, and 5.9, respectively) (Figure). A videobronchoscopy was performed, and no endobronchial lesion was detected. Brushing (of the right upper lobe) and transbronchial needle aspiration (of the 4R lymph node) cytology materials did not result in a diagnosis. Thus, CP-EBUS was performed with systematic mediastinal staging followed by LAG sampling. The LAG was seen as a round mass with 31 mm diameter. The “seagull” appearance was completely lost. No complication occurred. The materials taken from 4R and LAG were diagnosed as squamous cell carcinoma of the lung. The patient received chemotherapy.
Case 3
A 54-year-old woman was admitted for cough and weight loss. Right hilar enlargement was observed in a chest roentgenogram, which was followed by a chest CT that showed a mass adjacent to the right lower lobe bronchus. A lymphadenomegaly at a subcarinal station and an enlarged LAG were clearly seen. PET-CT showed that these lesions were FDG avid (SUVmax of 12.9, 11.7, 3.7, respectively) (Figure). A videobronchoscopy and an EBUS were performed in the same session to minimize the time to diagnosis. An endobronchial lesion was seen penetrating the lateral wall of the right lower lobe bronchus. Endobronchial biopsy was performed and subsequently subcarinal lymphadenomegaly and LAG were sampled with CP-EBUS. The LAG resembled a seagull with an enlarged wing. No complication occurred. An endobronchial biopsy of the lesion and the cytologic material of the subcarinal lesion were diagnosed as squamous cell carcinoma. However, the LAG was normal and free of metastases. The patient was subjected to neoadjuvant treatment followed by restaging. After completing chemotherapy, repeated investigations revealed that the radiologic characteristics of the adrenal gland were stable, which meant that it was a true-negative result.
DISCUSSION
The adrenal gland is the fifth leading site of metastasis (8%) by lung cancer [2]. Median survival and adjusted hazard ratio after diagnosis are 5 months and 1.05 (95% confidence interval 0.83–1.32), respectively. As benign adrenal adenomas are common, an enlarged adrenal gland observed in CT results may require further investigations [3]. PET may be a great choice in this measure, which gives a high diagnostic performance [4, 5]. However, there is still a probability of receiving false-positive results [6]. In such cases, a physician may need a histological confirmation.
Traditionally, adrenal glands have been subjected to CT-guided percutaneous biopsy. However, the diagnostic yield and complication rates were not satisfying [1]. Regarding the close relationship of the stomach and adrenal glands, EUS-FNA has been used to sample LAGs for decades. Although both glands can be seen by an experienced endosonographist, it is most commonly used in the diagnosis of left adrenal lesions. There are several studies on the diagnostic performance of EUS-FNA showing a sensitivity of 42%–86% for the diagnosis of left adrenal lesions [7–9]. No complication was reported. Thus, EUS-FNA is now considered a minimally invasive, safe, and accurate technique for sampling the LAG [1].
In 2011, Buxbaum and Eloubeidi used an EBUS scope to sample paragastric structure in patients with esophageal strictures [10]. After that, the use of esophageal introduction of EBUS scope, which is called EUS-B, was started in paraesophageal and paragastric lesions. There were several case reports on the efficient and safe usage of EUS-B in the diagnosis of LAG [11, 12]. However, regarding the shortage of scientific data, the 2015 guidelines did not make a suggestion; instead, the guidelines called the procedure experimental [1]. In 2016, Crombag et al. [13, 14]. conducted a feasibility study that was followed by a prospective multicenter trial on the value of EUS-B in the diagnosis of left adrenal lesions in comparison with EUS-FNA. They reported that the success rate for LAG analysis (visualized, sampled, and adequate tissue obtained) was almost same with EUS-B-FNA and EUS-FNA (89% vs 93%, respectively). The sensitivity for LAG metastases for EUS-B-FNA and EUS-FNA was 87% and 83%, respectively. It is concluded that “LAG analysis by EUS-B shows a similar high success rate in comparison to conventional EUS” [14]. These results have encouraged us for EUS-B application.
In our experience, the step-by-step application was very similar with the literature [15]. After reaching the esophagus with screw motion, we pour 20 mL of tap water through the working channel of the scope while proceeding distally to dilate and lubricate the lumen in order to minimize the risk of esophageal wall damage. Using a liquid also improves the sonographic image because we only rely on sonographically seen paraesophageal structures and not fiberoptic images. When we reach the stomach, we aspirate the excess fluid back. The descending aorta and the celiac trunk can be easily seen through the fundus. Rotating the image clockwise makes the LAG visible. Doppler assessment should be cautiously done to prevent puncturing abdominal and intraparenchymal vessels because a case of adrenal hemorrhage has been reported [16].
There are some reports on the sonographic features of LAG lesions that may be related to malignancy. The risk of malignancy is increased when the “seagull” appearance is lost [7, 9]. There are inconsistent results, whether a large gland (≥3 mm) is related to malignancy or not [7, 9]. In our case, the LAG was enlarged and rounded and had lost the “seagull” appearance. These sonographic features were shown to be discriminative for malignancy [7, 9]. However, the same imaging findings may be seen in benign adenomas, highlighting the need for cytological sampling in select cases.
In general, mediastinal staging is performed with a systematic N3-N2-N1 order followed by sampling the mass itself when needed. So, after EBUS session is completed and LAG is visualized via transgastric approach, a separate needle shall be used to exclude the risk of contamination. We have used separate needles for all of the cases.
It seems that the need for LAG sampling in lung cancer staging is very rare. Between November 2017 and April 2019, the endosonographist in this study performed 122 EBUS operations for diagnostic and/or staging purposes for lung cancer. Three of them (2.4%) were suitable for concurrent LAG assessment. There should be an indication for mediastinal pathological staging and the results of LAG sampling should influence the management of the case. Also, the cost of a separate needle should be considered. In our cases, the clinical staging revealed a single station N2 or single central lesion with left adrenal lesions. The results had an impact on the clinical decision.
CONCLUSION
Left adrenal lesions can be accurately and safely sampled in the same session with mediastinal staging with CP-EBUS. Patient selection should be done carefully. The proper technique is already reported but the need for a detailed sonographic analysis and a separate needle should be highlighted.
MAIN POINTS.
Left adrenal lesions can be accurately and safely sampled in the same session with mediastinal staging with CP-EBUS.
Indication for EUS-B-FNA procedure is very limited. The results of LAG sampling should influence the management of the case.
There is a need for a separate needle to avoid contamination.
Footnotes
Informed Consent: Written informed consent was obtained from patients who participated in these cases.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - I.O.A.; Design - I.O.A.; Supervision - I.O.A., Z.A.; Resources - I.O.A., Z.A.; Materials - I.O.A., Z.A.; Data Collection and/or Processing - I.O.A.; Analysis and/or Interpretation- I.O.A.; Literature Search - I.O.A., Z.A.; Writing Manuscript - I.O.A., Z.A.; Critical Review - I.O.A., Z.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Vilmann P, Frost Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) European journal of cardio-thoracic surgery: official journal of the Eur J Cardiothorac Surg. 2015;48:1–15. doi: 10.1093/ejcts/ezv194. [DOI] [PubMed] [Google Scholar]
- 2.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Chang KJ, Erickson RA, Nguyen P. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration of the left adrenal gland. Gastrointest Endosc. 1996;44:568–72. doi: 10.1016/S0016-5107(96)70010-X. [DOI] [PubMed] [Google Scholar]
- 4.Koopman D, van Dalen JA, Stigt JA, et al. Current generation time-of-flight (18)F-FDG PET/CT provides higher SUVs for normal adrenal glands, while maintaining an accurate characterization of benign and malignant glands. Ann Nucl Med. 2016;30:145–52. doi: 10.1007/s12149-015-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jana S, Zhang T, Milstein DM, et al. FDG-PET and CT characterization of adrenal lesions in cancer patients. Eur J Nucl Med Mol Imaging. 2006;33:29–35. doi: 10.1007/s00259-005-1915-8. [DOI] [PubMed] [Google Scholar]
- 6.Erasmus JJ, Patz EF, McAdams HP, et al. Evaluation of adrenal masses in patients with bronchial carcinoma using 18F-Fluorodeoxyglucose positron emission tomography. Am J Roentgenol. 1997;168:1357–60. doi: 10.2214/ajr.168.5.9129444. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Seewald S, Tamhane A, et al. EUS-guided FNA of the left adrenal gland in patients with thoracic or GI malignancies. Gastrointest Endosc. 2004;59:627–33. doi: 10.1016/S0016-5107(04)00296-2. [DOI] [PubMed] [Google Scholar]
- 8.Schuurbiers OC, Tournoy KG, Schoppers HJ, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer. 2011;73:310–5. doi: 10.1016/j.lungcan.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Black KR, Tamhane A, et al. A large single-center experience of EUS-guided FNA of the left and right adrenal glands: diagnostic utility and impact on patient management. Gastrointest Endosc. 2010;71:745–53. doi: 10.1016/j.gie.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum JL, Eloubeidi MA. Transgastric endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) in patients with esophageal narrowing using the ultrasonic bronchovideoscope. Dis Esophagus. 2011;24:458–61. doi: 10.1111/j.1442-2050.2011.01179.x. [DOI] [PubMed] [Google Scholar]
- 11.Meena N, Hulett C, Jeffus S, et al. Left adrenal biopsy using the convex curvilinear ultrasound scope. Respiration. 2015;89:57–61. doi: 10.1159/000368370. [DOI] [PubMed] [Google Scholar]
- 12.Tamburrini M, Gothi D, Barbetta C, et al. Esophageal ultrasound with ultrasound bronchoscope (EUS-B) guided left adrenal biopsy: Case report with review of literature. Respir Med Case Rep. 2019;26:154–6. doi: 10.1016/j.rmcr.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crombag LM, Annema JT. Left adrenal gland analysis in lung cancer patients using the endobronchial ultrasound scope: A feasibility trial. Respiration. 2016;91:235–40. doi: 10.1159/000443991. [DOI] [PubMed] [Google Scholar]
- 14.Crombag L, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer. 2017;108:38–44. doi: 10.1016/j.lungcan.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Bugalho A, de Santis M, Slubowski A, et al. Trans-esophageal endobronchial ultrasound-guided needle aspiration (EUS-B-NA): A road map for the chest physician. Pulmonology. 2017;24:32–41. doi: 10.1016/j.rppnen.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Haseganu LE, Diehl DL. Left adrenal gland hemorrhage as a complication of EUS-FNA. Gastrointest Endosc. 2009;69:e51–2. doi: 10.1016/j.gie.2008.09.035. [DOI] [PubMed] [Google Scholar]