Abstract
At least two distinct pluripotent states, referred to as naïve and primed, define the early mammalian embryo. In the mouse, the pluripotent epiblast cells in the pre/peri-implantation embryo are the source of naïve embryonic stem cells (ESCs). After the embryo implants, the epiblast lineage generates a restricted or primed population of stem cells, referred to as epiblast stem cells (EpiSCs). ESCs can be cultured in EpiSC media to generate epiblast-like cells (EpiLCs). The differentiation of naive ESCs into primed EpiLCs permits insights into the development and differentiation of the plurip otent epiblast lineage. This chapter describes the generation and characterization of EpiSCs as well as EpiLCs.
1. Introduction
Mammalian embryogenesis has been studied most intensively in the mouse. All cells of the very early mouse embryo are totipotent and can give rise to both extraembryonic (placental and yolk sac) and embryonic (epiblast and its derivatives) cells (Rossant, 1976; Tarkowski, 1959; Tarkowski & Wroblewska, 1967). The first morphological difference during embryogenesis is the formation of the trophectoderm in the blastocyst stage embryo. The remaining cells in the blastocyst form the inner cell mass, which subsequently separates into the primitive endoderm and the pluripotent epiblast cells. The trophectoderm and the primitive endoderm lineages generate the extra-embryonic cells of the placenta and the yolk sac. The epiblast, on the other hand, gives rise to somatic tissues and germ cells. The in vitro culture of epiblast-derived pluripotent stem cells has therefore garnered considerable interest both to glean insights into the early embryonic developmental program and as substrates for regenerative medicine.
Two types of stem cell lines have been derived from the mouse epiblast. The first is embryonic stem cells (ESCs) (Evans & Kaufman, 1981; Martin, 1981) and the second is epiblast stem cells (EpiSCs) (Brons et al., 2007; Guo et al., 2009; Tesar et al., 2007). Both ESCs and EpiSCs can be differentiated in vitro into the mesoderm, endoderm, ectoderm, as well as germ cells (Brons et al., 2007; Evans & Kaufman, 1981; Martin, 1981; Tesar et al., 2007). When injected into the blastocyst, ESCs can generate chimeric animals, but EpiSCs rarely can (Bradley, Evans, Kaufman, & Robertson, 1984; Brons et al., 2007; Tesar et al., 2007). EpiSCs express many (e.g., Oct4, Nanog, and Sox2), but not all (e.g., Rex1, Klf2, and Klf4) pluripotency-associated genes (Hayashi, Ohta, Kurimoto, Aramaki, & Saitou, 2011; Tesar et al., 2007). In the embryo, ESCs are in vitro analogs of the pre- or peri-implantation embryonic epiblast (Brook & Gardner, 1997; Evans & Kaufman, 1981; Martin, 1981). EpiSCs, on the other hand, are representatives of the early post-implantation epiblast (Gayen, Maclary, Buttigieg, Hinten, & Kalantry, 2015). In agreement, female ESCs contain two active X-chromosomes (Rastan, 1983; Rastan & Robertson, 1985), like in the epiblast cells of peri-implantation embryos (Gardner & Lyon, 1971; Mak et al., 2004), and female EpiSCs harbor an inactivated X-chromosome (Bao et al., 2009; Gayen et al., 2015; Guo et al., 2009), as in the epiblast cells of post-implantation embryos (McMahon, Fosten, & Monk, 1983; Monk & Harper, 1979). Due to their similarities to ESCs, EpiSCs are also considered pluripotent. But, due to their differences, ESCs are referred to as naïve pluripotent stem cells and EpiSCs as primed pluripotent stem cells, which are slightly differentiated cells relative to their ESC counterparts.
ESCs can be differentiated into cells that resemble epiblast-like stem cells (EpiLCs), which morphologically and transcriptionally mimic EpiSCs (Hackett & Surani, 2014; Hayashi et al., 2011; Nakamura et al., 2016). The derivation of EpiSCs and the stereotyped conversion of ESCs into EpiLCs provide a molecular dissection of naïve to primed pluripotency.
2. Materials
2.1. Mouse embryonic fibroblasts (MEFs)
2.1.1. MEF isolation
Pregnant mouse at E(15–17) (see Note 1).
Sterile dissection tools: scissors, blunt forceps, small blunt forceps, scalpels or oil-free razor blades.
Phosphate-buffered saline (PBS) without calcium and magnesium (Gibco#10010–023).
DNase I stock solution (13.33 mg/mL) (Roche#104159).
Trypsin-EDTA (0.25%) (Gibco#25300–062).
- MEF culture medium with antibiotic
- MEM Alpha (Gibco#12561–056).
- Fetal bovine serum (FBS) (Gibco#10437–028).
- Penicillin-streptomycin (100×) (Gibco#15070–063).
50 mL tubes.
15 mL tubes.
100 mm tissue culture dishes.
150 mm tissue culture dishes.
Sterile stir bar.
Sterile glycerol (80%).
Sterile sodium chloride solution (5 M).
2.1.2. Cryopreserving non-irradiated MEFs
Cryopreservation medium: Recovery Cell Culture Freezing Medium (Gibco#12648–010) (see Note 2).
Cryogenic vials (Corning#430659).
Cryogenic cooling container.
2.1.3. Irradiating of MEFs
Non-irradiated MEFs.
- MEF culture medium.
- MEM Alpha (Gibco#12561–056).
- Fetal bovine serum (FBS) (Gibco#10437–028).
Trypsin-EDTA (0.25%) (Gibco#25300–062).
Phosphate-buffered saline (PBS) without calcium and magnesium (Gibco#10010–023).
150 mm tissue culture dishes.
2.1.4. Cryopreserving irradiated MEFs
Cryopreservation medium: Recovery Cell Culture Freezing Medium (Gibco#12648–010).
Cryogenic vials (Corning#430659).
Cryo cooling container.
2.1.5. Thawing and culturing irradiated MEFs
MEF culture medium.
Different size tissue culture dishes.
2.2. Mouse embryonic stem cells (mESCs)
2.2.1. Derivation of mESCs
2.2.1.1. E3.5 blastocysts isolation
E3.5 pregnant mouse (see Note 1).
Irradiated mouse embryonic fibroblasts (MEFs) (see Note 3).
MEF culture medium.
- ESC derivation medium.
- KnockOut DMEM (Gibco#10829–018).
- KnockOut serum replacement (KSR) (Invitrogen#10828–028).
- l-glutamine (Gibco#25030).
- MEM non-essentials amino acids (Gibco#11140–050).
- β-Mercaptoethanol (Sigma#M7522) (see Note 4).
- Penicillin-streptomycin (100×) (Gibco#15070–063) (see Note 5).
- GSK3 inhibitor CHIR99021 (Stemgent#04–0004).
- MEK inhibitor PD0325901 (Stemgent#04–0006).
- LIF (107/mL) (Millipore#ESG1106).
Ethanol, 70% (v/v).
Sterile dissection tool: forceps and scissors (see Note 6).
35 mm tissue culture dishes.
3 mL syringe.
23-Gage needles.
4-Well tissue culture plates.
Phosphate-buffered saline (PBS) without calcium and magnesium (Gibco#10010–023).
Trypsin-EDTA (0.25%) (Gibco#25300–062).
DMSO (Sigma#D2650) (see Note 7).
2.2.1.2. Culturing ESCs
- ESC culture medium.
- KnockOut DMEM (Gibco#10829–018).
- Fetal bovine serum embryonic stem cell qualified (ES-FBS) (Bio-Techne#S10250).
- KnockOut serum replacement (KSR) (Invitrogen#10828–028).
- l-glutamine (Gibco#25030).
- MEM non-essentials amino acids (Gibco#11140–050).
- β-Mercaptoethanol (Sigma#M7522).
- GSK3 inhibitor CHIR99021 (Stemgent#04–0004).
- MEK inhibitor PD0325901 (Stemgent#04–0006).
- LIF (107/mL) (Millipore#ESG1106).
MEF feeder tissue culture plates.
Trypsin-EDTA (0.25%) (Gibco#25300–062).
DMSO (Sigma#D2650).
2.2.2. Cryopreserving ESCs
Cryopreservation medium: Recovery Cell Culture Freezing Medium (Gibco#12648–010).
Cryogenic vials (Corning#430659).
Cryo cooling container.
2.2.3. Thawing of ESCs
MEF medium.
ESC culture medium.
MEF feeder plates.
2.2.4. Characterization of ESCs
TRIzol (Life Technologies#15596018) (see Note 8).
SuperScript III One-Step RT-PCR System (Invitrogen#18080–044) (see Note 9).
- Primers for ESC-specific transcripts.
- Zfp42 (Rex1) forward: TGAAAGTGAGATTAGCCCCGAG
- Zfp42 (Rex1) reverse: GTCCCATCCCCTTCAATAGCAC
- Klf2 forward: TCGAGGCTAGATGCCTTGTGA
- Klf2 reverse: AAACGAAGCAGGCGGCAGA
- Klf4 forward: TGGTGCTTGGTGAGTTGTGG
- Klf4 reverse: GCTCCCCCGTTTGGTACCTT
- Stella forward: AGGCTCGAAGGAAATGAGTTTG
- Stella reverse: TCCTAATTCTTCCCGATTTTCG
- Stra8 forward: ACCCTGGTAGGGCTCTTCAA
- Stra8 reverse: GACCTCCTCTAAGCTGTTGGG
Materials for gel electrophoresis.
- Reagents for immunofluorescence (IF) for detection of ESC-specific protein expression.
- 1 × PBS.
- 6-Well dish, or similar chamber to be used for dehydration and for washing coverslips.
- Blocking buffer: 1 × PBS with 0.5 mg/mL BSA (NEB#B9000S), 50μg/mL tRNA, and 0.2% Tween 20 (make and use a 10% Tween 20 stock). Prewarm to 37°C.
- Primary antibody of choice (see Note 10): NANOG antibody (ReproCELL#RCAB002P-F), OCT4 antibody (Santa Cruz SC-5279), REX1 antibody (Thermo Scientific PA5–27567).
- Fluorescently conjugated secondary antibody: Alexa Fluor (Invitrogen) secondary antibodies work well with this protocol.
- Small glass plate (see Note 11).
- Parafilm.
- Forceps.
- IF chamber: A small humid chamber (see Note 12) for incubating slides, humidity provided by 1 × PBS.
- Incubator set to 37°C.
- 1 × PBS with 0.2% Tween 20 (Sigma-Aldrich#P9416).
- 4′,6-Diamidino-2-phenylindole, dihydrochloride (DAPI): dissolved in water at 5 mg/mL.
- Mounting medium (see Note 13).
- Microscope slides.
- Coverslips (see Note 14).
2.3. Mouse epiblast stem cells (EpiSCs)
2.3.1. Derivation of EpiSCs from preimplantation embryos
2.3.1.1. Epiblast isolation and plating
E3.5 timed pregnant female mouse (see Note 15).
Sterile dissection tool: forceps and scissors.
Syringe and needle (23 gage).
- K15F5 medium
- KnockOut DMEM (Gibco#10829–018).
- KnockOut serum replacement (KSR) (Invitrogen#10828–028).
- Fetal bovine serum embryonic stem cell qualified (ES-FBS) (Bio-Techne#S10250).
- L-Glutamine (Gibco#25030).
- MEM non-essentials amino acids (Gibco#11140–050).
- β-Mercaptoethanol (Sigma#M7522).
- Penicillin-streptomycin (100×) (Gibco#15070–063).
- EpiSC culture medium
- KnockOut DMEM (Gibco#10829–018).
- KnockOut serum replacement (KSR) (Invitrogen#10828–028).
- l-glutamine (Gibco#25030).
- MEM non-essentials amino acids (Gibco#11140–050).
- β-Mercaptoethanol (Sigma#M7522).
- FGF2 stock solution (10μg/mL) (R&D Systems 233-FB-025).
MEF feeder tissue culture plates.
Non-tissue culture dishes (e.g., 35 or 60 mm).
2.3.1.2. EpiSC culture
EpiSC passaging medium: Collagenase type IV (Invitrogen#17104–019) (1.5 mg/mL).
EpiSC culture medium.
MEF feeder plates.
2.3.2. Cryopreserving EpiSCs
Cryopreservation medium: Recovery cell culture freezing medium (Gibco#12648–010).
Cryogenic vials (Corning#430659).
Cryo cooling container.
2.3.3. Thawing of EpiSCs
MEF medium.
EpiSC culture medium.
MEF feeder plates.
2.3.4. EpiSC characterization
TRIzol (Life Technologies#15596018).
SuperScript III One-Step RT-PCR System (Invitrogen#18080–044).
- Primers for EpiSC-specific transcripts.
- Pou5f1 forward: CGTTCTCTTTGGAAAGGTGTTC
- Pou5f1 reverse: GAACCATACTCGAACCACATCC
- Fgf5 forward: CTGTACTGCAGAGTGGGCATCGG
- Fgf5 reverse: GACTTCTGCGAGGCTGCGACAGG
Materials for gel electrophoresis.
- Reagents for immunofluorescence (IF) for detection of proteins
- 1 × PBS.
- 6-Well dish, or similar chamber to be used for dehydration and washing coverslips.
- Blocking buffer: 1 × PBS with 0.5 mg/mL BSA (NEB#B9000S), 50μg/mL tRNA, and 0.2% Tween 20 (make and use a 10% Tween 20 stock). Prewarm to 37°C.
-
Primary antibody of choice (see Note 16):NANOG antibody (ReproCELL#RCAB002P-F), OCT4 antibody (Santa Cruz SC-5279), REX1 antibody (Thermo Scientific PA5–27567).
- Fluorescently conjugated secondary antibody: Alexa Fluor (Invitrogen) secondary antibodies work well with this protocol.
- Small glass plate.
- Parafilm.
- Forceps.
- IF chamber: A small humid chamber for incubating slides, humidity provided by 1 × PBS.
- Incubator set to 37°C.
- 1 PBS with 0.2% Tween 20 (Sigma-Aldrich#P9416).
- 4′,6-Diamidino-2-phenylindole, dihydrochloride (DAPI): dissolved in water at 5 mg/mL.
- Mounting medium.
- Microscope slides.
- Coverslips.
2.4. Epiblast like stem cells (EpiLCs)
2.4.1. Culturing ESCs
- ESC culture medium
- KnockOut DMEM (Gibco#10829–018).
- Fetal bovine serum embryonic stem cell qualified (ES-FBS) (Bio-Techne#S10250).
- KnockOut serum replacement (KSR) (Invitrogen#10828–028).
- L-Glutamine (Gibco#25030).
- MEM non-essentials amino acids (Gibco#11140–050).
- β-Mercaptoethanol (Sigma#M7522).
- GSK3 inhibitor CHIR99021 (Stemgent#04–0004).
- MEK inhibitor PD0325901 (Stemgent#04–0006).
- LIF (107/mL) (Millipore#ESG1106).
MEF feeder tissue culture plates.
Trypsin-EDTA (0.25%) (Gibco#25300–062).
DMSO (Sigma#D2650).
2.4.2. Differentiating ESCs into EpiLCs
- N2B27 medium
- DMEM/F12 (Gibco#11330–032).
- Neurobasal media (Gibco#21103–049).
- L-Glutamine (Gibco#25030).
- β-Mercaptoethanol (Sigma#M7522).
- N2 supplement (Invitrogen#17502048).
- B27 supplement (Invitrogen#17504–044).
- GSK3 inhibitor CHIR99021 (Stemgent#04–0004).
- MEK inhibitor PD0325901 (Stemgent#04–0006).
- LIF (107/mL) (Millipore#ESG1106).
FGF2 stock solution (10μg/mL) (R&D Systems 233-FB-025).
Activin A stock solution (10μg/mL) (R&D Systems 338-AC-005).
Accutase (Sigma#A6964).
Fibronectin (Sigma#F1141).
BSA fraction V solution in PBS (0.1%, w/v) (Sigma-Aldrich A3311 or A1470).
Gelatin (0.2%) (Sigma#G2500).
DMSO (Sigma#D2650).
2.4.3. Characterization of EpiLCs
TRIzol (Life Technologies#15596018).
SuperScript III One-Step RT-PCR System (Invitrogen#18080–044).
- Primers for ESC-specific transcripts.
- Zfp42 (Rex1) forward: TGGAAGCGAGTTCCCTTCTC
- Zfp42 (Rex1) reverse: GCCGCCTGCAAGTAATGAG
- Klf2 forward: TCGAGGCTAGATGCCTTGTGA
- Klf2 reverse: AAACGAAGCAGGCGGCAGA
- Klf4 forward: TGGTGCTTGGTGAGTTGTGG
- Klf4 reverse: GCTCCCCCGTTTGGTACCTT
- Stella forward: AGGCTCGAAGGAAATGAGTTTG
- Stella reverse: TCCTAATTCTTCCCGATTTTCG
- Stra8 forward: ACCCTGGTAGGGCTCTTCAA
- Stra8 reverse: GACCTCCTCTAAGCTGTTGGG
- Primers for EpiLC-specific transcripts.
- Fgf5 forward: CTGTACTGCAGAGTGGGCATCGG
- Fgf5 reverse: GACTTCTGCGAGGCTGCGACAGG
- Cer1 forward: CTCTGG GGAAGGCAGACCTAT
- Cer1 reverse: CCACAAACAGATCCGGCTT
- Primers for differentiated EpiLCs.
- Mesoderm
- Brachyury forward: CTTCCAGGTCCAACGCCTAC
- Brachyury reverse: GTCGTCGCTCATGTTCTTCAA
- Ectoderm
- β-III tubulin forward: TAGACCCCAGCGGCAACTAT
- β-III tubulin reverse: GTTCCAGGTTCCAAGTCCACC
- Endoderm
- FoxA2 forward: TCCGACTGGAGCAGCTACTAC
- FoxA2 reverse: GCGCCCACATAGGATGACA
- Materials for gel electrophoresis.
- Reagents for immunofluorescence (IF).
- 1 × PBS.
- 6-Well dish, or similar chamber to be used for dehydration and washing coverslips.
- Blocking buffer: 1 × PBS with 0.5 mg/mL BSA (NEB#B9000S), 50μg/mL tRNA, and 0.2% Tween 20 (make and use a 10% Tween 20 stock). Prewarm to 37°C.
- Primary antibody of choice (see Note 17): NANOG antibody (ReproCELL#RCAB002P-F), OCT4 antibody (Santa Cruz SC-5279), REX1 antibody (Thermo Scientific PA5–27567).
- Fluorescently conjugated secondary antibody: Alexa Fluor (Invitrogen) secondary antibodies work well with this protocol.
- Small glass plate.
- Parafilm.
- Forceps.
- IF chamber: A small humid chamber for incubating slides, humidity provided by 1 × PBS.
- Incubator set to 37°C.
- 1 × PBS with 0.2% Tween 20 (Sigma-Aldrich#P9416).
- 4′,6-Diamidino-2-phenylindole, dihydrochloride (DAPI): dissolved in water at 5 mg/mL. • Mounting medium.
- Microscope slides.
- Coverslips.
2.4.4. Cryopreservation of EpiLCs
Cryopreservation medium: Recovery cell culture freezing medium (Gibco#12648–010).
Cryogenic vials (Corning#430659).
Cryo cooling container.
2.5. Equipment
Incubator, humidified, 37°C; 5% CO2, 95% air.
Class II biosafety cabinet (tissue culture hood).
Automated cell counter.
Irradiation instrument.
Microscopes, inverted and stereomicroscope.
Pipettes.
Pipettors.
Vacuum Pump (Fisher Scientific, cat. no. 13-878-40 or equivalent).
Water bath (Thermo Scientific, model 2864 or equivalent).
3. Methods
Cell culture work should be performed under sterile conditions. All manipulation of cells and preparation of solutions should be done inside a certified tissue culture hood (class II biological safety cabinet). MEFs, ESCs, EpiSCs, and EpiLCs should be cultured at 37°C in a humid atmosphere with 5% CO2. MEFs can be derived from E(12.5–13.5) and E(15–17) mouse embryos. ESCs are derived from embryonic day (E) 3.5 mouse embryos. EpiSCs can be derived from E3.5, E4.5, and E5.5 mouse embryos.
3.1. Mouse embryonic fibroblasts (MEFs)
Mitotically inactive MEFs serve as feeder cells for the self-renewal of ESCs and EpiSCs.
3.1.1. MEF isolation
Warm MEF media (with penicillin-streptomycin) and PBS. Thaw trypsin and keep on ice. Prepare five 100 mm dishes containing sterile PBS.
Euthanize mouse at E(15–17) according to local animal care requirements. Remove uterine horns with embryos, cutting away the mesentery.
Cut along the entire length of the uterus with scissors, cut the placenta to remove each embryo, and place each embryo into a new 100 mm dish with PBS.
Remove the amniotic sac from each embryo and place each embryo in a new 100 mm dish.
Switch to a new set of sterile instruments (sterilize instruments by heating them in the “Germinator”); individually wash each embryo with PBS and place it in a new 100 mm dish.
Eviscerate and decapitate: Holding embryo by the head with abdomen facing up, use fine blunt forceps to puncture body wall and remove internal organs. Pinch off the head at the neck.
Place each carcass in a new 100 mm dish. Dispose of head and organs.
Take dish with embryos into tissue culture hood. Transfer embryos in a new 100 mm dish with 10 mL trypsin.
Mince carcasses with a sterile scalpel or oil-free razor blade in the hood. This should be thorough and take >5 min.
Use a 5 mL pipet to further homogenize the embryos. Do this quite thoroughly.
Use a 2 mL pipet to further break up the slurry. Do this quite thoroughly as well.
Use a 1 mL pipet and repeat step 10.
Use a 5 mL pipet to transfer entire slurry into a sterile 250 mL flask with a sterile stir bar.
Rinse plate with 10 mL trypsin to remove all remaining tissue and pipet into the 250 mL flask with the rest of the slurry.
Wash the 100 mm dish once more with 10 mL aliquot of trypsin.
The total volume in the flask should now exceed 30 mL.
OPTIONAL: Force tissue through collector screen in the cup with a sterile glass pestle. Continue to press tissue through the screen until as much as possible has gone through. Pipet 10 mL trypsin through screen, and also onto the bottom of the screen to remove all adhering tissue. Repeat with one more 10 mL aliquot of trypsin.
Add 0.3 mL DNase I stock solution to flask to reduce viscosity.
Place flask in 37°C incubator. Every 5 min, place on a stir plate and stir gently for 1 min. Total incubation time at 37°C should be approximately 30 min.
Pipet the entire solution into a 50 mL conical tube.
Pellet cells by centrifugation at 1500rpm for 5 min.
Remove media, leaving a small amount in the tube to resuspend the pellet.
Wash pellet twice with 30 mL MEF media.
Resuspend cells in 15 mL MEF media.
Dilute sample 1:10 (4.5 mL PBS + 0.5 mL cell suspension in 15 mL tube). Count viable cells (not debris). Expect 5 × 107−108 cells from 10 E15 fetuses.
Plate cells at 6 × 106 per 150 mm dish.
Change the media after 24h.
When confluent (3–5 days), split each 100 mm dish onto 5, 100 mm dishes. Freeze cells when the five dishes become confluent (freeze 6 × 106 cells per cryogenic vial with 1 mL freezing medium) or proceed with splitting once more 1:5 and follow the irradiation protocol (see Section 3.1.3).
3.1.2. Cryopreserving MEFs
Label cryogenic vials in the hood.
Aspirate media from the 150 mm dishes.
Wash cells in each dish with 15 mL room temperature PBS.
Trypsinize cells (10 mL 0.25% Trypsin-EDTA/1, 150 mm dish) at 37°C for 6 min.
Rinse vigorously after removal from the incubator to dislodge cells from the bottom of the plate.
Transfer cell suspension from two 150 mm dishes to one 50 mL tube containing 10 mL MEF media.
Spin down cells in the 50 mL tubes by centrifugation at 1500rpm for 5 min.
Remove the caps from labeled cryovials in the hood.
Aspirate trypsin solution from centrifuge tubes and tap each tube to loosen the pellet.
Combine the cells from all tubes into one common 50 mL tube using 10 mL MEF media.
Count the viable cells in the common 50 mL tube by automated cell counter and centrifuge (1500rpm for 5 min) to form a pellet.
Aspirate supernatant tap the tube to loosen the pellet and resuspend in recovery cell culture freezing medium. The amount of freezing media depends on the number of viable cells (6 × 106 cells/mL of cell suspension).
Working quickly, add 1 mL cell suspension to each cryovial, screw the cap on tightly, and place the vials in a freezing container in a −80°C freezer overnight.
Transfer vials from −80°C freezer to the liquid N2 freezer the next day. Keep vials on dry ice during the transfer.
Test thaw one vial (onto a gelatin-coated 150 mm dish) the day after transferring cells to the liquid N2 freezer to determine viability. Culture for 2–3 days in an antibiotic-free MEF medium to check for contamination.
3.1.3. Irradiating and cryopreserving MEFs
The volumes listed are for 150 mm dishes.
Thaw one vial of non-irradiated MEFs into one 150 mm dish. Use MEF media.
When confluent, aspirate media and wash the cells with 6 mL of sterile 1 × PBS and aspirate PBS. Add 9 mL of Trypsin-EDTA (0.25%) to the cells and incubate for ~5 min at 37°C. Then, pipette vigorously to break into single cells. Neutralize trypsin with 9 mL MEF media and pellet down the cells by centrifugation (1500rpm for 5 min at room temperature). Resuspend the cells in MEF media and split 1:6.
When cells are confluent, repeat step 2 above.
After two rounds of splitting and growth, the 36 plates of MEFs are ready for irradiation.
Harvest the cells by trypsinizing 5–6 plates at a time in 37 °C incubator for ~5 min.
Pipet up and down in trypsin to break clumps of cells apart. If they are sticking together, use a glass pipet to pipet cells up and down.
Neutralize trypsin with MEF media.
Combine dishes of trypsinized cells into 50 mL conical tubes.
Collect cells by centrifuging the tubes at 1500 rpm for 5 min. Aspirate trypsin and resuspend the cells in ~25 mL of MEF media.
Irradiate the cells with 6000rads.
Count the cells after irradiation with the automated counter.
Plate 2.5 × 106 cells/100 mm dish of irradiated MEFs for use within 1 week.
Collect the remaining irradiated MEFs via centrifugation.
Resuspend the cells in the freezing medium. Count the total number of viable cells and aliquot ~5 × 106 cells in 1 mL of the freezing medium per cryopreservation vial (see Note 18).
Tightly close the vials. Store vials in −80°C freezer for 1–3 days, then transfer to longer term storage in a liquid nitrogen tank.
3.1.4. Preparation of MEF feeder tissue culture dishes
Remove a vial from liquid nitrogen storage and thaw quickly in 37°C water bath until almost completely melted.
Gently add cell suspension to 10 mL of room temperature MEF medium in a 15 mL tube.
Spin tube down at 1500rpm for 5 min, remove supernatant and gently resuspend pellet in prewarmed MEF medium.
Plate irradiated MEFs on tissue culture dishes in MEF media (use 750μL MEF medium/4-well well; 1 mL/12-well well; 2 mL/6-well well; 10 mL/100 mm dish) (see Note 18).
After overnight plating, MEF feeders will be ready for culturing other cells.
3.2. Mouse embryonic stem cells (mESCs)
3.2.1. Derivation of ESCs
3.2.1.1. Preparation of MEF feeder plates
Thaw and plate irradiated MEFs onto 4-well tissue culture plates before the day of isolating embryos. The number of wells needed is equal to the number of embryos to be harvested and is thus dependent on the number of pregnant females used. The number of embryos received per female is highly variable but generally averages between 6 and 8.
On the day of embryo harvesting, remove the MEF media from 4-well plates and rinse the 4-well feeder plates with 1 × PBS. Replace the media with ES-derivation media.
3.2.1.2. Collecting mouse embryonic (E)3.5 mouse embryos
Dissect pregnant mouse in sterile conditions. Wipe the surrounding areas with 70% ethanol before beginning. Sterilize the scissors and forceps used for dissection with the dry-heat sterilization apparatus.
Remove the uteri of an E3.5 pregnant mouse. Trim away excess fat.
With a 3 mL syringe, collect 3 mL of ESC derivation media and attach a 23-gage needle to the syringe. While securing the uterine horn with forceps, insert the needle and flush the uterine horn with 1 mL ESC derivation media into a 35 mm dish. Repeat this process with the second uterine horn.
3.2.1.3. Plating and early culture
Using a P20 pipette set at 4μL, collect the flushed embryos and wash through several drops of ESC derivation media in a sterile 35 mm dish before placing them into a final drop of ESC derivation media.
Plate blastocysts in MEF-plated 4-well dishes containing 750μL ESC derivation media per well. Plate one blastocyst per well.
Incubate the plates at 37°C, 5% CO2. Do not disturb the plates for 48h to allow for the blastocysts to hatch and attach to the feeder layer. The majority (80–90%) of blastocysts should hatch and attach to the feeder layer.
On day 3 post-plating, replace the media with fresh ESC derivation media. Continue to feed in this manner every other day until disaggregation occurs.
After the initial 48h of incubation, monitor growth under a microscope daily. If outgrowths are prominent 4–5 days post-plating, proceed to the next step. If not, wait one or two more days before proceeding to the next step.
3.2.1.4. Disaggregation of blastocysts outgrowth
Aliquot 20μL trypsin into each well of a 96-well plate. Incubate at 37°C for at least 20 min.
Carefully wash each well with 500μL of 1 PBS and then add 500μL of 1 × PBS.
Use 1 or 2 sterile needles to scrape the blastocyst outgrowth away from the surrounding MEF cells. Do this under the microscope. Use a P20 pipet set at 3μL to place the outgrowth in one well of a 96-well plate containing prewarmed trypsin.
Once all the blastocyst outgrowths are in the 96-well dish, incubate in the 37°C incubator for 5–10 min.
Now dissociate each outgrowth individually using a P20 pipet set at 10μL. Vigorously pipet up and down to create a single cell suspension.Neutralize with 20μL MEF media/well.
Plate embryos individually into wells of a MEF-plated 96-well plate with ESC derivation media. Change media the next day as early as possible.
Mouse ES colonies will be evident over the next 2–3 days (see Note 19).
Passage and culture ES cells every alternative day (1:3–1:4) with ES culture media.
Change media daily.
3.2.2. Passaging ESCs
Aspirate media and wash the cells with room temperature 1 PBS.
Add 0.25% Trypsin-EDTA to cover the cell layer, usually 1/3 the amount used to culture the cells.
Return the cells to the incubator for 4–6 min. Observe cell dissociation with an inverted microscope.
As soon as colonies begin to dissociate, inactivate the trypsin by adding an equal amount of MEF medium.
Pipet up and down vigorously to dissociate into a single cell suspension.
Collect the cells in one tube and centrifuge for 5 min at 1500rpm. Resuspend the pellet in 1 mL of ESC medium, creating a single cell suspension through gentle trituration.
Plate the cells on MEF feeder plates (with 1:3 splitting).
3.2.3. Culturing ESCs
Culture ESCs on MEF feeders in ESC culture medium supplemented with 3μM GSK3 inhibitor CHIR99021 (Stemgent#04–0004), 1μM MEK inhibitor PD0325901 (Stemgent#04–0006), and 1000U/mL LIF (Millipore#ESG1106) (see Note 20).
When ~70–80% confluent, split ESCs with Trypsin-EDTA (0.25%) (see Section 3.2.2) into 1:3–1:4. ESCs. Change media daily (see Note 21).
3.2.4. Cryopreserving ESCs
When cells are 70–80% confluent, aspirate the media and wash the cells with 1 PBS.
Trypsinize the cells with 0.25% Trypsin-EDTA as mentioned above.
Pellet down the cells and resuspend in recovery cell culture freezing medium (Gibco#12648–010). Use 2 mL freezing medium per one ~70–80% confluent 6-well well and place into two cryogenic vials, each containing 1 mL cell suspension.
Put the vials in cryo cooling container and maintain at −80°C for 1 day.Then transfer vials to liquid nitrogen.
3.2.5. Thawing of ESCs
Remove a vial from liquid nitrogen storage and thaw quickly in 37°C water bath until almost completely melted.
Gently add cell suspension to 10 mL of room temperature MEF medium in a 15 mL tube.
Spin tube down at 1500rpm for 5 min, remove supernatant and gently resuspend pellet in 2 mL of prewarmed ESC culture medium.
Plate into one well of a 6-well plate. Place in a tissue culture incubator. Change media daily.
If recovery is adequate, colonies should be ready to passage in 2–3 days.
3.2.6. Characterization of ESCs
3.2.6.1. Morphological and molecular characterization of ESCs
The ESCs can be morphologically examined (Fig. 1) followed by profiling of the cells by RT-PCR and IF.
Fig. 1. Stereo micrographs of ESCs, EpilLCs, and EpiSCs highlighting differences in cel lular morphology.
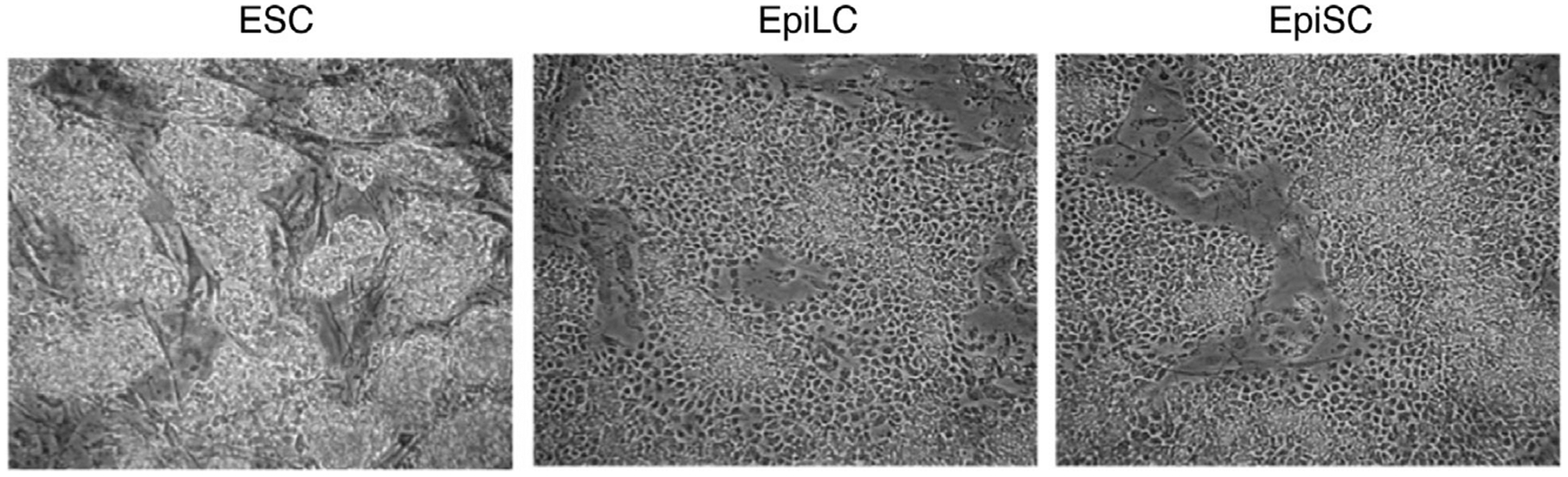
The figure was adopted from Gayen, S., Maclary, E., Buttigieg, E., Hinten, M., & Kalantry, S. (2015). A primary role for the Tsix lncRNA in maintaining random X-chromosome inactivation. Cell Reports, 11(8), 1251–1265. doi: 10.1016/j.celrep.2015.04.039.
For RT-PCR, prepare RNAs by aspirating media from one confluent 6-well well and resuspending the ESCs in 1 mL TRIzol reagent after washing with 1 × PBS. These samples can be stored in −80°C for later use.
Isolate RNA according to a standard TRIzol extraction protocol.
Analyze the expression of cell-specific markers by RT-PCR.
In addition to via RT-PCR, ESCs can be characterized through IF for proteins specific to ESCs.
3.2.6.2. IF-based detection of marker proteins in individual ESCs
Begin with fixed, permeabilized cells, plated on gelatinized glass coverslips and stored in 70% ethanol.
Make blocking buffer and warm to 37°C.
Place sample coverslip in a 6-well dish that contains 2 mL of 1 PBS in each well.
Wash briefly with three changes of 1 PBS to remove ethanol.
Wash with 1 PBS three times, 3 min each on a rocker.
Wrap a glass plate tightly with Parafilm for incubating coverslips for subsequent steps.
Block slides for 30 min at 37°C in 50μL prewarmed blocking buffer in a humid chamber: Place a 50μL drop of blocking buffer on the parafilm-wrapped glass plate and invert the coverslip, sample-side down, into the blocking buffer. Place the parafilm-wrapped plate in the humid chamber, and incubate for 30 min at 37°C. All incubations in blocking buffer, primary antibody, or secondary antibody should be set up in this manner.
Carefully lift coverslip from blocking buffer with forceps and place it into a 50μL droplet of diluted primary antibody on a parafilm-wrapped plate. Incubate with 50μL primary antibody diluted in prewarmed blocking buffer (dilution based on primary antibody you are using) in a humid chamber at 37°C for 1h (see Note 22).
Remove coverslip from primary antibody solution and place, sample-side up, in a 6-well dish. Wash three times with 1 PBS/0.2% Tween 20 for 3 min on a rocker.
Incubate coverslip in 50μL prewarmed blocking buffer on a parafilm-wrapped plate in a humid chamber for 5 min at 37°C.
Incubate coverslip with 50μL secondary antibody diluted in prewarmed blocking buffer in a humid chamber at 37°C for 30 min. Antibody dilution depends on the secondary antibody used; Alexa Fluor-conjugated secondary antibodies should be used at a 1:300 dilution.
Remove coverslip from secondary antibody and wash three times with 1 × PBS/0.2% Tween 20 for 3 min each on a rocker. The first wash should contain a 1:100,000–1:200,000 dilution of DAPI (5 mg/mL) (see Note 23). Then, rinse once briefly with PBS/0.2% Tween 20 and wash two more times for 5–7 min each while rocking to remove excess DAPI.
Remove coverslip from the dish, tap off excess liquid, and then mount on a slide, sample-side down, in mounting medium. Image samples or store at −20°C for later imaging.
3.3. Mouse epiblast stem cells (mEpiSCs)
3.3.1. Derivation of EpiSCs from E3.5 preimplantation embryos
3.3.1.1. Preparation of MEF feeder plates
Thaw and plate inactivated MEFs onto 4-well plates before the day of isolating embryos. The number of wells needed is dependent on the number of pregnant females. The number of embryos recovered per female can be variable but generally averages between 6 and 8 for most mouse strains. Blastocysts are plated in individual wells.
On the day of the embryo harvest, remove the MEF media and rinse the 4-well feeder plates with PBS. Replace the media with K15F5 media.
3.3.1.2. Collecting E3.5 blastocysts
Dissect the mice in a sterile condition. Wipe the surroundings with 70% ethanol before beginning. Sterilize the scissors and forceps used for dissection with the dry-heat sterilization apparatus.
Remove the uteri of a E3.5 pregnant mouse. Trim away excess fat.
With a 3 mL syringe, collect 3 mL of K15F5 medium and attach a 23-gage needle. While securing the uterine horn with forceps, insert the needle and flush the uterine horn with 1 mL of K15F5 medium into a 35 mm dish. Repeat this process with the second uterine horn.
3.3.1.3. Plating and early culture
Using a P20 pipette set at 4μL, collect the flushed embryos and wash through several drops of K15F5 medium before placing them into a final drop of K15F5 medium.
Plate E3.5 blastocysts in MEF-plated 4-well dishes containing 750μL K15F5 medium. Only plate one blastocyst per well in a 4-well MEF feeder plate. Always use fresh tips for plating each blastocyst.
Incubate the plates at 37°C, 5% CO2. Do not disturb the plates for 48h to allow for the blastocysts to hatch and attach to the feeder layer. The majority (80–90%) of blastocysts should hatch and attach to the feeder layer.
On day 3 post-plating, replace the medium with fresh medium. Continue to feed in this manner every day until disaggregation.
On day 5, begin to monitor the growth of the outgrowth under a microscope daily. If outgrowths are prominent at day 5 of plating, proceed to the next steps below. If not, wait one or two more days before proceeding to the next step.
3.3.1.4. Disaggregation of blastocysts outgrowth
Aliquot 20μL trypsin in each well of a 96-well plate. Incubate at 37°C for at least 20 min.
When the trypsin is warmed up, change media in each well of 4-well plates with sterile 1 × PBS. Repeat for a total of 2×.
Wipe down the surface around the microscope and the microscope itself with 70% ethanol.
Using a P20 pipet set at 3μL, scrape up the blastocyst outgrowth under the microscope and place in a 96-well well with prewarmed trypsin.Exercise as sterile technique as possible.
Process all of the blastocyst outgrowths as above.
Incubate in the 37°C incubator for 10 min.
Once all the blastocyst outgrowths are in the 96-well dish, pipet up and down very gently in each well using the P20 pipet set at 10μL.
Watch intermittently to make sure the blastocyst outgrowths are dissociating.
Check each of the 96-well wells under the microscope—the outgrowths should now be disaggregated in small clumps. Partial dissociation is important for EpiSC derivation (see Note 24).
Plate each of the partial dissociated embryos into a MEF-plated well of 24-well plate for an additional 6 days in K15F5 medium (750μL/well).
At this point, passage each culture by a brief exposure (2–3 min) to 0.25% trypsin/EDTA, inactivate trypsin with FBS-containing MEF medium, gently tritrate to prevent complete single cell dissociation of any pluripotent clusters, and plate into a 6-well plate containing feeders in K15F5 medium (2 mL/well).
Morphologically distinct mouse ES cell and/or EpiSC colonies will be evident over the next 4–8 days (16–20 total days from initial blastocyst explants). Separately expand these colony types in a medium designed to support either mouse ES cells or EpiSCs.
Pick individual EpiSC colonies and dissociate the EpiSC colonies manually into small clusters using a glass needle and plate into 24-well plate containing feeders in EpiSC culture medium. Passage EpiSCs every third day using 1.5 mg/mL collagenase type IV (Invitrogen) and tritu-rate into small clumps of 10–100 cells.
Continue growing EpiSC cells by passaging into 12-well (use 1.5 mL media) and 6-well (use 2 mL media) plates (MEF plated), respectively, with EpiSC culture medium.
3.3.2. Culturing EpiSCs
To passage EpiSCs, aspirate EpiSC culture medium from each well of 6-well plate, add 1 mL of EpiSC passaging medium (1.5 mg/mL collagenase type IV) (for one 6-well well), and incubate at 37°C for 8–12 min (see Note 25).
Add 1 mL of EpiSC culture medium to each well and dislodge colonies by gentle pipetting to create small clumps (not to single cells). Combine colony suspensions from each plate into one 15-mL conical tube.
Centrifuge at 1500rpm for 5 min. Gently resuspend the cell pellet in 2 mL EpiSC culture medium and perform another spin. Repeat the rinse/spin cycle one more time for a total of three. FGF2 is omitted from the EpiSC culture medium when used for rinsing.
Resuspend colonies in 2 mL of EpiSC culture medium and transfer to an individual well of a 6-well feeder plate.
Keep the plate in the incubator.
EpiSCs are typically split 1:4–1:6 every 2–3 days. The density at which each EpiSC line is grown is very important and must be determined empirically.
EpiSC culture medium should be changed daily.
3.3.3. Cryopreservation of EpiSCs
Isolate EpiSCs into small clumps after collagenase treatment as mentioned above.
Resuspend pellet from one 6-well well in 1 mL of recovery cell culture freezing medium (Gibco#12648–010) and place into two cryogenic vials each containing 1 mL cell suspension with freezing medium.
Place tubes in cryo cooling container and store at −80°C. The following day, transfer vials to a liquid nitrogen freezer for permanent storage.
3.3.4. Thawing of EpiSCs
Remove 1 vial from liquid nitrogen storage and thaw in 37°C water bath until almost completely melted.
Gently add cell suspension to 10 mL of room temperature MEF medium.
Spin down at 1500rpm for 5 min, remove supernatant and gently resuspend pellet in 2 mL of prewarmed EpiSC culture medium.
Remove MEF medium from MEF feeders and plate suspension into one well of 6-well plate. Place in a tissue culture 37°C incubator. Change media daily.
If recovery is adequate, colonies should be ready to passage in 2–3 days.
3.3.5. Characterization of EpiSCs
3.3.5.1. Morphological and molecular characterization of EpiSCs
The EpiSCs can be morphologically examined (Fig. 1) followed by profiling of the cells by RT-PCR and IF.
For RT-PCR, prepare RNAs by aspirating media from 1 confluent 6-well well of EpiSCs and by adding 1 mL of TRIzol reagent after washing with 1 × PBS. These samples can be stored in 1.5 mL tubes at −80°C for later use.
Isolate RNA according to a standard TRIzol extraction protocol.
Analyze the expression of cell-specific markers by RT-PCR.
In addition to via RT-PCR, the EpiLCs can be characterized through IF for proteins specific to EpiSCs.
In addition to RT-PCR and IF, female EpiSCs can also be characterized through RNA FISH to visualize expression of Xist RNA, a marker of X-chromosome inactivation, which should be expressed in EpiSCs.
3.3.5.2. IF-based detection of marker proteins in individual EpiSCs
Begin with fixed, permeabilized cells, plated on gelatinized glass coverslips and stored in 70% ethanol.
Make blocking buffer and warm to 37°C.
Place sample coverslip in a 6-well dish that contains 2 mL of 1 × PBS in each well.
Wash briefly with three changes of 1 × PBS to remove ethanol.
Wash again with 1 × PBS three times, 3 min each on a rocker.
Wrap a glass plate tightly with Parafilm for incubating coverslips in subsequent steps.
Block slides for 30 min at 37°C in 50μL prewarmed blocking buffer in a humid chamber: Place a 50μL drop of blocking buffer on the parafilm-wrapped glass plate and invert the coverslip, sample-side down, into the blocking buffer. Place the parafilm-wrapped plate in the humid chamber and incubate for 30 min at 37°C. All incubations in blocking buffer, primary antibody, or secondary antibody should be set up in this manner.
Carefully lift coverslip from blocking buffer with forceps and place into a 50μL droplet of diluted primary antibody on a parafilm-wrapped plate. Incubate with 50μL primary antibody diluted in prewarmed blocking buffer (dilution based on primary antibody you are using) in a humid chamber at 37°C for 1h (see Note 26).
Remove coverslip from primary antibody solution and place, sample-side up, in a 6-well dish. Wash three times with 1 × PBS/0.2% Tween 20 for 3 min on a rocker.
Incubate coverslip in 50μL prewarmed blocking buffer on a parafilm-wrapped plate in a humid chamber for 5 min at 37°C.
Incubate coverslip with 50μL secondary antibody diluted in prewarmed blocking buffer in a humid chamber at 37°C for 30 min. Antibody dilution depends on the secondary antibody used; Alexa Fluor-conjugated secondary antibodies should be used at a 1:300 dilution.
Remove coverslip from secondary antibody and wash three times with 1 × PBS/0.2% Tween 20 for 3 min each on a rocker. The first wash should contain a 1:100,000–1:200,000 dilution of DAPI (5 mg/mL) (see Note 23). Then, rinse once briefly with PBS/0.2% Tween 20 and wash two more times for 5–7 min each while rocking to remove excess DAPI.
Remove coverslip from the dish, tap off excess liquid, and then mount on a slide, sample-side down, in mounting medium. Image samples or store at −20°C for later imaging.
3.4. EpiLCs
3.4.1. Generating EpiLCs from ESC
3.4.1.1. Preparation of gelatin-coated tissue culture dishes
Prepare 0.2% gelatin with distilled water and autoclave. To prepare gelatin-coated tissue culture dishes, apply 0.2% gelatin for 10–15 min. Then, aspirate gelatin and dry gelatin by keeping the lid open inside tissue culture hood.
3.4.1.2. Preparation of fibronectin-coated tissue culture dishes
Prepare 10μg/mL fibronectin solution and apply to tissue culture dishes for 20 min. Aspirate the solution and let the dishes dry for 30 min by keeping the lid open inside the tissue culture hood.
3.4.1.3. Differentiating ESCs into EpiLCs
Culture ES cells on MEF feeders (6-well well) in 2i/LIF or serum/LIF conditions (see Note 27). When ESCs become confluent, add Trypsin-EDTA (0.25%) to the cells (700μL to 1 mL Trypsin/1, 6-well well) and incubate at 37°C for 5 min in the incubator. Pipet vigorously and neutralize trypsin with any media containing FBS. Then, pellet down the cells with centrifugation at 1500rpm for 5 min.
Resuspend the cells with 1 mL N2B27 media and again spin down the cells and aspirate media to remove any leftover trypsin with the cells.
Resuspend the cells in N2B27 medium supplemented with 3 μM GSK3 inhibitor CHIR99021 (Stemgent#04–0004), 1μM MEK inhibitor PD0325901 (Stemgent#04–0006), 1000U/mL LIF (Millipore#ESG1106) and plate into a gelatin-coated 6-well well (if necessary all the cells, plate onto 3, 6-well well from 1 confluent 6-well well; or maintain 1, 6-well well and discard the remaining cells). Change media daily.
After 2 days, add Accutase (see Note 28) and incubate for 5–10 min at room temperature. Check the flask frequently (in every 1–2 min) to see rounded cells while remaining attached to the bottom of the flask. Then, smack the flask against the palm of your hand to dislodge any adhered cells. Gently disperse the cells and take a sample of the cell suspension to determine the viable cell density.
Add an aliquot of the detached cells to fresh N2B27 media in new gelatin-coated dishes. Place the dishes into the incubator.
Culture the cells in gelatin-coated dishes for four passages. Each passage should be ~2 days and change media daily. At each passage, the cells should be split from 1:2 to 1:3. During culture at passages 1–4, colonies should look round and shiny.
To differentiate ESCs into EpiLCs, culture ESCs for 48h in N2B27 media supplemented with 10ng/mL FGF2 (R&D Systems#233-FB) and 20ng/mL Activin A (R&D Systems#338-AC) in Fibronectin (10μg/mL) (Sigma#F1141)-coated tissue culture dishes.
For further differentiation, culture the cells in N2B27 medium without FGF2 and Activin A for additional days.
3.4.2. Cryopreserving EpiLCs
As ESCs begin to differentiate into EpiLCs, the cells can be frozen at passages 1–4 before the generation of EpiLCs (see Note 29).
Dissociate cells using Accutase or 0.05% Trypsin-EDTA at passage 1–4 and pellet down the cells by centrifugation with 1500rpm at 37°C.
Resuspend pellet of one 6-well well in 1 mL of recovery cell culture freezing medium (Gibco#12648–010) and place into two cryogenic vials each containing 1 mL cell suspension with freezing medium.
Place tubes in cryo cooling container and store at −80°C. The following day, transfer vials to a liquid nitrogen freezer for permanent storage.
Use one cryogenic vial for thawing cells into one well of gelatin-coated 6-well plate.
3.4.3. Thawing cells for EpiLC generation
Prepare gelatin-coated tissue culture plate.
Remove one vial from liquid nitrogen storage and thaw in 37°C water bath until almost completely melted.
Gently add cell suspension to 10 mL of room temperature MEF medium.
Spin down at 1500rpm for 5 min, remove supernatant and gently resuspend pellet in 1 mL of prewarmed N2B27 medium.
Spin down cell again at 1500rpm for 5 min, remove supernatant and gently resuspend pellet in 2 mL of prewarmed N2B27 medium supplemented with 3μM GSK3 inhibitor CHIR99021 (Stemgent#04–0004), 1μM MEK inhibitor PD0325901 (Stemgent#04–0006), 1000 U/mL LIF (Millipore#ESG1106).
Plate suspension into one well of 6-well gelatin-coated plate. Place in tissue culture incubator. Change media daily.
If recovery is adequate, colonies should be ready to passage in 2 days.
Follow the protocol for the conversion of ESCs into EpiLCs as mentioned in Section 3.4.1.3.
3.4.4. Characterization of EpiLCs
3.4.4.1. Morphological and molecular characterization of EpiLCs
The differentiation of ESCs into EpiLCs can be morphologically examined (Fig. 1) followed by profiling of the cells by RT-PCR and IF.
For RT-PCR, prepare RNAs by aspirating media from one confluent 6-well well and resuspending the ESCs in 1 mL TRIzol reagent after washing with 1 × PBS. These samples can be stored in 80°C for later use.
Isolate RNA according to a standard TRIzol extraction protocol.
Analyze the expression of cell-specific markers by RT-PCR.
In addition to via RT-PCR, the EpiLCs can be characterized through IF for proteins specific for ESCs, EpiLCs, and differentiated EpiLCs.
In addition to RT-PCR and IF, female EpiLCs can also be characterized through RNA FISH for Xist RNA, a marker of X-chromosome inactivation, which is only expressed in EpiLCs and differentiated EpiLCs.
3.4.4.2. IF-based detection of marker proteins in individual EpiLCs
Begin with fixed, permeabilized cells, plated on gelatinized glass coverslips and stored in 70% ethanol.
Make blocking buffer and warm to 37°C.
Place sample coverslip in a 6-well dish that contains 2 mL of 1 × PBS in each well.
Wash briefly with three changes of 1 × PBS to remove ethanol.
Wash with 1 × PBS three times, 3 min each on a rocker.
Wrap a glass plate tightly with Parafilm for incubating coverslips in subsequent steps.
Block slides for 30 min at 37°C in 50μL prewarmed blocking buffer in a humid chamber: Place a 50 μL drop of blocking buffer on the parafilm-wrapped glass plate and invert the coverslip, sample-side down, onto the blocking buffer. Place the parafilm-wrapped plate in the humid chamber, and incubate for 30 min at 37°C. All incubations in blocking buffer, primary antibody, or secondary antibody should be set up in this manner.
Carefully lift coverslip from blocking buffer with forceps and place it into a 50μL droplet of diluted primary antibody on a parafilm-wrapped plate. Incubate with 50μL primary antibody diluted in prewarmed blocking buffer (dilution based on primary antibody you are using) in a humid chamber at 37°C for 1h (see Note 30).
Remove coverslip from primary antibody solution and place, sample-side up, in a 6-well dish. Wash three times with 1 × PBS/0.2% Tween 20 for 3 min on a rocker.
Incubate coverslip in 50μL prewarmed blocking buffer on a parafilm-wrapped plate in a humid chamber for 5 min at 37°C.
Incubate coverslip with 50μL secondary antibody diluted in prewarmed blocking buffer in a humid chamber at 37°C for 30 min. Antibody dilution depends on the secondary antibody used; Alexa Fluor-conjugated secondary antibodies should be used at a 1:300 dilution.
Remove coverslip from secondary antibody and wash three times with 1 × PBS/0.2% Tween 20 for 3 min each on a rocker. The first wash should contain a 1:100,000–1:200,000 dilution of DAPI (5 mg/mL). Then, rinse once briefly with PBS/0.2% Tween 20 and wash two more times for 5–7 min each while rocking to remove excess DAPI.
Remove coverslip from the dish, tap off excess liquid, and then mount on a slide, sample-side down, in mounting medium. Image samples or store at −20°C for later imaging.
4. Discussion
The slow and stepwise conversion of ESCs uniformly into EpiLCs permits the molecular dissection of transcriptional and epigenetic processes that tip the balance between self-renewal of naïve pluripotent stem cells vs differentiation of the naïve pluripotent stem cells into primed pluripotent stem cells. EpiLCs, however, are only transiently present in culture as ESCs differentiate. EpiSCs, on the other hand, are self-renewing primed pluripotent cells that can be stably propagated in culture.
Although EpiLCs morphologically and transcriptionally mimic EpiSCs, the two cell types are not identical. Both EpiLCs and EpiSCs share common expression patterns for some genes. For example, the pluripotency gene Oct3/4 (Pou5f1) is expressed in EpiSCs as well as in EpiLCs (Hayashi et al., 2011). Other pluripotency genes such as Sox2, Nanog, Klf5, however, are expressed in EpiSCs but downregulated in EpiLCs (Hayashi et al., 2011). Genes that characterize the postimplantation epiblast, e.g., Wnt3, Fgf5, and Dnmt3b, are upregulated in EpiSCs as well as EpiLCs (Hayashi et al., 2011). Endoderm marker genes such as Gata4, Gata6, and Sox17 are expressed highly in EpiSCs and a very low level in EpiLCs (Hayashi et al., 2011). Blimp1, a marker of primordial germ cells, is also expressed in a subset of EpiSCs but downregulated in EpiLCs (Hayashi et al., 2011). The differentiation of ESCs into EpiLCs may be a useful as a model system to investigate the transcriptional and epigenetic mechanisms underlying the ICM to epiblast differentiation. EpiLCs as well as EpiSCs may also serve as starting materials for the induction of other lineages derived from the epiblast.
5. Recipes
DNase I solution (13.33 mg/mL).
| Reagent | Amount |
|---|---|
| DNase I (Roche#104159) | 100 mg |
| Sterile sodium chloride (NaCl) (5 M) | 0.45 mL |
| Sterile 80% glycerol | 4.69 mL |
| Sterile distilled water | 2.36 mL |
Aliquots store at −20°C.
0.2% gelatin solution.
| Reagent | Amount |
|---|---|
| Gelatin | 200 mg |
| Distilled water | 100 mL |
Dissolve gelatin to distilled water and autoclave (final volume 100 mL). Keep it at 4 °C.
BSA fraction V solution in PBS (0.1%, w/v).
| Reagent | Amount |
|---|---|
| BSA fraction V (Sigma-Aldrich A3311 or A1470) | 10 mg |
| Phosphate-buffered saline (PBS) without Ca2+/Mg2+ | 10 mL |
Resuspend BSA in PBS. Filter through a 0.45-μm filter, aliquot, and store at −80°C.
FGF2 stock solution (10μg/mL).
| Reagent | Amount |
|---|---|
| Recombinant fibroblast growth factor-2 (FGF2; R&D Systems 233-FB-025) | 25 μg |
| BSA fraction V solution in PBS (0.1%, w/v) | 2.5 mL |
Resuspend lyophilized FGF2 in BSA/PBS, mix well, aliquot 50μL, and freeze at −80°C. Thaw each aliquot as needed and store it at 4°C. Do not refreeze the thawed aliquot.
Activin A stock solution (10μg/mL).
| Reagent | Amount |
|---|---|
| Recombinant Activin A (ActA; R&D Systems 338-AC-005) | 5 μg |
| BSA fraction V solution in PBS (0.1%, w/v) | 0.5 mL |
Resuspend lyophilized Activin A in its vial, mix well, aliquot 50μL, and freeze at −80°C. Thaw each aliquot as needed and store it at 4°C. Do not refreeze.
CHIR99021 stock solution (10 mM).
| Reagent | Amount |
|---|---|
| GSK3 inhibitor CHIR99021 (Stemgent#04–0004) | 5 mg |
| DMSO | 1.07 mL |
Protect from light, aliquots store at −20°C. The above data is based on the product molecular weight 465.34. Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which will affect the solvent volumes required to prepare stock solutions. Thaw each aliquot as needed and store it at 4°C. Do not refreeze.
MEK inhibitor PD0325901 (10 mM).
| Reagent | Amount |
|---|---|
| PD0325901 (Stemgent#04–0006) | 2 mg |
| DMSO | 414.8 μL |
Protect from light, aliquots store at −20°C. Thaw each aliquot as needed and store it at 4°C. Do not refreeze.
MEF medium.
| Reagent | Amount |
|---|---|
| MEM Alpha (1 ×) (Gibco#12561–056) | 90 mL |
| FBS (Gibco#10437–028) | 10 mL |
Filter and store the medium (final volume 100 mL) at 4°C and use within 2 weeks.
EpiSC dissociation medium: Collagenase type IV (1.5 mg/mL).
| Reagent | Amount | Final concentration |
|---|---|---|
| Collagenase type IV (Invitrogen#17104–019) | 0.5 mg | 1.5 mg/mL |
| EpiSC culture medium (without FGF2) | 333.33 mL |
Dissolve 0.5 mg of collagenase type IV in 333.33 mL of EpiSC medium. Filter, aliquot in 10 mL, and freeze at −80°C. Thaw each aliquot as needed and store it at 4°C. Do not refreeze.
ESC derivation medium.
| Reagent | Amount | Final concentration |
|---|---|---|
| KnockOut DMEM (Gibco#10829–018) | 76 mL | |
| KnockOut serum replacement (KSR) (Invitrogen#10828–028) | 20 mL | 20% |
| PeniciHin-streptomycin (100 x) (Gibco#15070–063) | 1 mL | 1 × |
| l-Glutamine (200 mM) (Gibco#25030) | 1 mL | 2 mM |
| MEM non-essentials amino acids (100 ×) (Gibco#11140–050) | 1 mL | 1 × |
| β-Mercaptoethanol (10 mM) (Sigma#M7522) | 1 mL | 0.1 mM |
| Supplements | ||
| GSK3 inhibitor CHIR99021 (10 mM) (Stemgent#04–0004) | 30 μL | 3 μM |
| MEK inhibitor PD0325901 (10 mM) (Stemgent#04–0006) | 10 μL | 1 μM |
| LIF (107/mL) (Millipore#ESG1106) | 10 μL | 103/mL |
Filter and store the medium (final volume 100 mL) at 4°C and use within 2 weeks. Add supplements (GSK3 inhibitor CHIR99021, MEK inhibitor PD0325901, and LIF) to the media during freshly during use of the media. Prepare β-mercaptoethanol 10 mM stock from 14.3 M stock (Sigma-Aldrich M7522) by adding 6.25μL to 9 mL of sterile distilled water.
ESC culture medium.
| Reagent | Amount | Final concentration |
|---|---|---|
| KnockOut DMEM (Gibco#10829–018) | 76 mL | |
| Fetal bovine serum embryonic stem cell qualified (ES-FBS) (Bio-Techne#S10250) | 5 mL | 5% |
| KnockOut serum replacement (KSR) (Invitrogen#10828–028) | 15 mL | 15% |
| l-glutamine (200 mM) (Gibco#25030) | 1 mL | 2 mM |
| MEM non-essentials amino acids (100 ×) (Gibco#11140–050) | 1 mL | 1 × |
| β-Mercaptoethanol (10 mM) (Sigma#M7522) | 1 mL | 0.1 mM |
| Supplements | ||
| GSK3 inhibitor CHIR99021 (10 mM) (Stemgent#04–0004) | 30 μL | 3 μM |
| MEK inhibitor PD0325901 (10 mM) (Stemgent#04–0006) | 10 μL | 1 μM |
| LIF (107/mL) (Millipore#ESG1106) | 10 μL | 103/mL |
Filter and store the medium (final volume 100 mL) at 4°C and use within 2 weeks. Add supplements (GSK3 inhibitor CHIR99021, MEK inhibitor PD0325901, and LIF) to the media during freshly during use of the media. ESC can be cultured in ESC media supplemented with LIF only. Prepare β-mercaptoethanol 10 mM stock from 14.3 M stock (Sigma-Aldrich M7522) by adding 6.25μL to 9 mL of sterile distilled water.
K15F5 medium.
| Reagent | Amount | Final concentration |
|---|---|---|
| KnockOut DMEM (Gibco#10829–018) | 76 mL | |
| KnockOut serum replacement (KSR) (Invitrogen#10828–028) | 15 mL | 15% |
| Fetal bovine serum embryonic stem cell qualified (ES-FBS) (Bio-Techne#S10250) | 5 mL | 5% |
| Penicillin-streptomycin (100 x) (Gibco#15070–063) | 1 mL | 1 × |
| l-Glutamine (200 mM) (Gibco#25030) | 1 mL | 2 mM |
| MEM non-essentials amino acids (100 ×) (Gibco#11140–050) | 1 mL | 1 × |
| β-Mercaptoethanol (10 mM) (Sigma#M7522) | 1 mL | 0.1 mM |
Filter and store the medium (final volume 100 mL) at 4°C and use within 2 weeks. Prepare β-mercaptoethanol 10 mM stock from 14.3 M stock (Sigma-Aldrich M7522) by adding 6.25μL to 9 mL of sterile distilled water.
EpiSC culture medium.
| Reagent | Amount | Final concentration |
|---|---|---|
| KnockOut DMEM (Gibco#10829–018) | 76 mL | |
| KnockOut serum replacement (KSR) (Invitrogen#10828–028) | 20 mL | 20% |
| l-Glutamine (200 mM) (Gibco#25030) | 1 mL | 2 mM |
| MEM non-essentials amino acids (100 ×) (Gibco#11140–050) | 1 mL | 1 × |
| β-Mercaptoethanol (10 mM) (Sigma#M7522) | 1 mL | 0.1 mM |
| FGF2 (R&D Systems) (10 μg/mL) | 100 μL | 10 ng/μl |
Filter and store the medium (final volume 100 mL) at 4°C and use it within 2 weeks. Add FGF2 supplement freshly.
N2B27 medium.
| Reagent | Amount | Final concentration |
|---|---|---|
| DMEM/F12 (Gibco#11330–032) | 47.5 mL | |
| Neurobasal media (Gibco#21103–049) | 47.5 mL | |
| l-Glutamine (200 mM) (GIBCO#25030) | 1 mL | 2 mM |
| β-Nercaptoethanol (10 mM) (Sigma#M7522) | 1 mL | 0.1 mM |
| N2 supplement (100 ×) (Invitrogen#17502048) | 1 mL | 1 × |
| B27 supplement (50 ×) (Invitrogen#17504–044) | 2 mL | 1 × |
Filter and store the medium (final volume 100 mL) at 4°C and use it within 2 weeks. Prepare β-mercaptoethanol 10 mM stock from 14.3 M stock (Sigma-Aldrich M7522) by adding 6.25μL to 9 mL of sterile distilled water.
6. Notes
All relevant governmental and institutional standard rules and regulations must be maintained during the experiments involving rodents. Timed natural matings should be used and 12h from the middle of the dark period indicate day 0.5 (E0.5).
Other freezing media can be used to freeze the cells.
Mitotically inactive MEFs served as feeders for ESC or EpiSC culture. Non-irradiated MEFs (Mitomycin-C treated) can be used as feeder cells.
This reagent is a biohazard; adequate safety instructions should be taken when handling.
We use penicillin-streptomycin in media only for the cell derivation and not for the cell culture.
Properly autoclaved and sharpened forceps and scissors should be used for any kind of dissection.
This reagent is a biohazard; adequate safety instructions should be taken when handling.
RNA purification methods other than TRIzol can be used in place of TRIzol.
Any robust RT-PCR system should work. For RT-PCR, random primers or gene specific reverse primer can be used for cDNA synthesis. Instead of RT-PCR, qPCR may be useful to detect the expression of specific transcripts in different cell lines of ESCs, EpiSCs, and EpiLCs.
Other primary antibodies specific to ESCs can be used if good antibodies exist.
Short glass plates designed for casting protein gels are a good size for hybridization. For example, Bio-Rad’s MiniPROTEAN Short Plates fit well in the humid chamber described in Note 12.
For humid chambers, a microscope slide box (i.e., one that holds 100 slides) works well. Place paper towels soaked in 1 × PBS at the bottom of the box to create a humid chamber for immunofluorescence.
For mounting medium, use Vectashield (Vector Labs) or similar anti-fade mounting medium and seal coverslips with clear nail polish after mounting on slides.
Use 22 × 22 mm coverslips, which fit well within a single well of a 6-well dish. All the dehydration and washing steps can be performed in the wells of this dish.
EpiSC can be derived from E3.5, E4.5, and E5.5 mouse embryos. E3.5 are preferable for EpiSC derivation to avoid microdissection of the embryo.
Other primary antibodies specific to EpiSCs can be used if good antibodies exist.
Other primary antibodies specific to EpiLCs can be used if good antibodies exist.
- The concentration of MEFs for plating should approximately be:
- 4-well plate: 2 × 105 cells/well; 8 × 105 cells/plate.
- 6-well plate: 8 × 105 cells/well; 5 × 106/plate.
- 10cm dish: 5 × 106/dish
The ES colonies are now in passage 1. Increase passage number by 1 with each subsequent exposure of trypsin and carefully track passage number by labeling plates and tubes.
ESCs can be cultured in 2i/LIF or in serum/LIF conditions.
Generally, ESCs become confluent in every 2–3 days. So, split ESCs after every 2–3 days.
In ESC, NANOG, OCT4, and REX1; all three proteins should be expressed.
Dilute 5 mg/mL DAPI 1:500 in RNase/DNase-free ultrapure water, and store in the dark at −20°C. Add 6–10μL of this dilution into each well while washing samples.
Caution should be taken not to produce a single cell suspension.
The time required for the collagenase to free EpiSC colonies from the plate can vary. Check the colonies in every few minutes. They are ready when the edges begin to retract from the plate.
NANOG and OCT4 should be expressed in EpiSCs but REX1 should not be expressed.
The ESCs can be cultured in serum/LIF conditions. The conversion of ESCs to EpiLCs can be performed starting from ESCs either cultured in 2i/LIF or in serum/LIF conditions. However, ESC to EpiLC conversion seems to be more robust and with lower levels of cell death starting with ESCs cultured in 2i/LIF.
Trypsin-EDTA (0.05%) can be used for splitting instead of Accutase. But, incubate the cells for a maximum of 2–3 min at 37°C after adding trypsin to the cells. Then, neutralize the trypsin by adding at least an equal volume of FBS-containing media. Pellet the cells by centrifugation at 1500rpm for 5 min at room temperature.
EpiLC cannot be preserved but it is possible to preserve the differentiating ESCs at passage numbers 1, 2, 3 or 4 before EpiLC conversion. However, it is recommended to continue the ESC to EpiLC conversion.
NANOG and OCT4 should be expressed in ESCs, EpiLCs and differentiated EpiLCs but REX1 will not.
Acknowledgments
We thank members of the Kalantry laboratory for discussions and help with the manuscript. We especially acknowledge Marissa Cloutier for proofreading and editing the manuscript. We also thank to Dr. Thomas L. Saunders for providing the protocol for derivation of MEF cells. Work in the Kalantry lab is funded by the NIH (R01GM124571 and R01HD095463) and March of Dimes (1-FY18-526).
References
- Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, et al. (2009). Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature, 461(7268), 1292–1295. 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, & Robertson E (1984). Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature, 309(5965), 255–256. 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 448(7150), 191–195. 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Brook FA, & Gardner RL (1997). The origin and efficient derivation of embryonic stem cells in the mouse. Proceedings of the National Academy of Sciences of the United States of America, 94(11), 5709–5712. 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, & Kaufman MH (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156. 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gardner RL, & Lyon MF (1971). X chromosome inactivation studied by injection of a single cell into the mouse blastocyst. Nature, 231(5302), 385–386. 10.1038/231385a0. [DOI] [PubMed] [Google Scholar]
- Gayen S, Maclary E, Buttigieg E, Hinten M, & Kalantry S (2015). A primary role for the Tsix lncRNA in maintaining random X-chromosome inactivation. Cell Reports, 11(8), 1251–1265. 10.1016/j.celrep.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, et al. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development, 136(7), 1063–1069. 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett JA, & Surani MA (2014). Regulatory principles of pluripotency: From the ground state up. Cell Stem Cell, 15(4), 416–430. 10.1016/j.stem.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, & Saitou M (2011). Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell, 146(4), 519–532. 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP, et al. (2004). Reactivation of the paternal X chromosome in early mouse embryos. Science, 303(5658), 666–669. 10.1126/science.1092674. [DOI] [PubMed] [Google Scholar]
- Martin GR (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America, 78(12), 7634–7638. 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Fosten M, & Monk M (1983). X-chromosome inactivation mosaicism in the three germ layers and the germ line of the mouse embryo. Journal of Embryology and Experimental Morphology, 74, 207–220. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6886595. [PubMed] [Google Scholar]
- Monk M, & Harper MI (1979). Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature, 281(5729), 311–313. 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, et al. (2016). A developmental coordinate of pluripotency among mice, monkeys and humans. Nature, 537(7618), 57–62. 10.1038/nature19096. [DOI] [PubMed] [Google Scholar]
- Rastan S (1983). Non-random X-chromosome inactivation in mouse X-autosome trans-location embryos—Location of the inactivation centre. Journal of Embryology and Experimental Morphology, 78, 1–22. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6198418. [PubMed] [Google Scholar]
- Rastan S, & Robertson EJ (1985). X-chromosome deletions in embryo-derived (EK) cell lines associated with lack of X-chromosome inactivation. Journal of Embryology and Experimental Morphology, 90, 379–388. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3834036. [PubMed] [Google Scholar]
- Rossant J (1976). Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs. Journal of Embryology and Experimental Morphology, 36(2), 283–290. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1033982. [PubMed] [Google Scholar]
- Tarkowski AK (1959). Experiments on the development of isolated blastomeres of mouse eggs. Nature, 184, 1286–1287. 10.1038/1841286a0. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, & Wroblewska J (1967). Development of blastomeres of mouse eggs iso lated at the 4- and 8-cell stage. Journal of Embryology and Experimental Morphology, 18(1), 155–180. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6048976. [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. (2007). New cell lines from mouse epiblast share defining features with human embry onic stem cells. Nature, 448(7150), 196–199. 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]


