Abstract
Introduction
Low-molecular-weight heparin (LMWH) and vitamin K antagonists (VKA) are current treatment options for cancer patients suffering from acute venous thromboembolism (VTE). The role of direct-acting oral anticoagulants (DOACs) for the treatment of VTE in cancer patients, particular in comparison with the current standard of care which is LMWH, remains unclear. In this network meta-analysis, we compared the relative efficacy and safety of LMWH, VKA, and DOAC for the treatment of cancer-associated VTE.
Methods
A pre-specified search protocol identified 10 randomized controlled trials including 3242 cancer patients. Relative risks (RR) of recurrent VTE (efficacy) and major bleeding (safety) were analyzed using a random-effects meta-regression model.
Results
LMWH emerged as significantly superior to VKA with respect to risk reduction of recurrent VTE (RR=0.60, 95%CI:0.45-0.79, p<0.001), and its safety was comparable to VKA (RR=1.08, 95%CI:0.70-1.66, p=0.74). For the DOAC vs. VKA efficacy and safety comparison, the relative risk estimates were in favor of DOAC, but had confidence intervals that still included equivalence (RR for recurrent VTE=0.65, 95%CI:0.38-1.09, p=0.10; RR for major bleeding=0.72, 95%CI:0.39-1.37, p=0.32). In the indirect network comparison between DOAC and LMWH, the results indicated comparable efficacy (RR=1.08, 95%CI:0.59-1.95, p=0.81), and a non-significant relative risk towards improved safety with DOAC (RR=0.67, 95%CI:0.31-1.46, p=0.31). The results prevailed after adjusting for different risk of recurrent VTE and major bleeding between LMWH vs. VKA and DOAC vs. VKA studies.
Conclusion
The efficacy and safety of LMWH and DOACs for the treatment of VTE in cancer patients may be comparable.
Keywords: Cancer, Venous Thromboembolism, Treatment, Low-molecular-weight-heparin, Oral anticoagulants, Network Meta Analysis
Introduction
Venous thromboembolism (VTE) is a frequent complication and leading cause of death in patients with cancer.[1] The clinical course of cancer-associated VTE differs from VTE in non-cancer patients, most importantly because the risk of VTE recurrence and bleeding during anticoagulant therapy is substantially higher than in non-cancer patients.[2] Malignancy-associated morbidity and concurrent antineoplastic therapy further complicate the clinical management of VTE in patients with cancer.[3]
The question on the optimal anticoagulation therapy for cancer patients with VTE is an ongoing area of research and debate.[4, 5] Current guidelines of the major societies in the field agree in recommending a 3-6 months course of daily therapeutic doses of low molecular weight heparin (LMWH) as the first-line treatment for cancer-associated VTE.[3, 6–9] For patients, the administration of LMWH therapy via daily subcutaneous injections over a course of several months is associated with considerable burden. Guidelines further recommend vitamin K antagonists (VKA) in a target International normalized ratio (INR) range of 2.0 to 3.0 as an alternative therapy given LMWH is unavailable or not possible.[3] Here, the necessity for frequent INR monitoring and the potential interactions of VKA with patient diet and anti-cancer drugs are important limitations.[8, 10]
Recently, direct-acting oral anticoagulants (DOACs) that directly inhibit either factor Xa (apixaban, edoxaban, and rivaroxaban) or thrombin (dabigatran) have been introduced as novel agents for treatment of VTE.[11–14] Importantly, these drugs can be administered orally in a fixed dose without the need for laboratory monitoring, and appear to have less potential drug and dietary interactions than VKA.[15] In randomized controlled trials comparing standard VTE therapy (initial LMWH followed by long-term VKA) to DOACs, all DOACs were non-inferior with respect to efficacy (i.e. prevention of VTE recurrence), and tended to be associated with a smaller risk of bleeding.[4] While these studies included only a small proportion of cancer patients, several subgroup analyses and four recent meta-analyses in the cancer subpopulation suggest that the efficacy and safety patterns of DOACs in cancer patients may be comparable to the patterns observed in non-cancer patients.[4, 16–18] However, as head-to-head studies comparing DOACs with the currently recommended standard therapy for cancer-associated VTE, LMWH, have not been performed, the role of DOACs for the treatment of VTE in patients with cancer remains incompletely understood.[3, 4, 19]
In the absence of real-world head-to-head studies, network meta-analyses (NMA) can provide indirect estimates of comparative effectiveness, and thus identify important trends in the data relevant for guideline makers, clinical practice, and the design of future trials.[20] In this study, we report a network meta-analysis on the efficacy and safety of DOACs, LMWH, and VKA for the treatment of VTE in patients with cancer. By performing an indirect comparison between DOACs and LMWH, we aim to explore DOACs in relation to the current standard therapy for cancer-associated VTE in terms of recurrent VTE and major bleeding.
Methods
Definition of Study Question
To compare the relative efficacy and safety of VKA, DOAC, and LMWH for the long-term treatment of VTE in patients with cancer.
Definition of Study Population, Interventions, and Study Designs
Adult cancer patients with any type of solid or hematologic malignancy suffering from an objectively-confirmed acute episode of VTE (i.e. deep vein thrombosis [DVT] and/or pulmonary embolism [PE]) represent the study population of this analysis. Eligible interventions were pharmacological agents from the groups of VKAs, DOACs, and LMWHs. These interventions had to be tested in randomized controlled trials (RCTs) comparing two or more of the above interventions with a minimum treatment period of 3 months. Studies comparing the above interventions against placebo, unfractionated heparin (UFH), or pentasaccharides such as idra- or fondaparinux were ineligible.
Definition of Outcomes
The efficacy and safety outcomes of this analysis were recurrent VTE and major bleeding, respectively. Recurrent VTE (DVT and/or PE) was defined according to Carrier et al. as a new non-compressible segment on leg vein sonography, new filling defect on venography, new high probability ventilation/perfusion scan, or a new pulmonary artery filling defect on chest computed tomography or pulmonary angiography.[4] Major bleeding was defined according to ISTH criteria as a bleeding episode that was clinically overt and associated with one or more of the following criteria: (1) a fall in the hemoglobin level ≥2g/dL, (2) clinical indication for transfusion of ≥2 units of packed red blood cells, (3) bleeding located intracranially, in major joints, or the retroperitoneum, and (4) fatal bleeding.[21]
Search strategy and Study selection
A pre-specified online literature search protocol identified 840 articles, which were independently reviewed by two authors (FP and CA, Supplemental Table 1). One RCT that was not identified by the literature search but presented recently as an abstract (the CATCH trial) was manually added.[22] Finally, 10 studies were included in this meta-analysis (Table 1, Supplemental Figure 1). Cancer-specific data for 5 of these studies could be identified by including four congress abstracts and one published manuscript.[4, 23–25]
Table 1. Major characteristics of included studies.
Studies are sorted by date of publication and intervention type (LMWH and DOAC). *Study type: CPST – Acronym for “Cancer-population specific trial” (i.e. a trial exclusively in cancer patients), SA – Acronym for “Subgroup analysis” (i.e. results are subgroup data from a trial including cancer and non-cancer patients), Full report – Data taken from a published manuscript, Abstract – Data taken from a congress report (Abstract available online, full manuscript not yet published); †Follow-up - Event data reported below pertain to this timepoint of follow-up; ‡Six-month risks were calculated by transforming raw event data into a study-specific event rate ri (number of events per person-time at risk assuming that events occurred on average at half of follow-up(†)), and then transforming ri into a 6-month risk pi using the following equation: pi=1-exp^(-0.5*ri); **Weighted risks calculated by taking the sum of each study’s product of the 6-month risk pi and its individual weight wi (wi calculated as the ratio of patients in the study’s VKA arm divided by the total number of patients in the VKA arms of the LMWH or DOAC studies, respectively).
| Study acronym | CANTHANOX | CLOT | ONCENOX | LITE | Romera et al. | CATCH | RECOVER I+ II | HOKUSAI | AMPLIFY | EINSTEIN DVT+PE |
|---|---|---|---|---|---|---|---|---|---|---|
| First Author (Year of publication)Ref. | Meyer G (2002)[29] | Lee AYY (2003)[30] | Deitcher SR (2006)[28] | Hull RD (2007)[31] | Romera A (2008)[32] | Lee AYY (2014)[22] | Schulman S (2013)[24] | Raskob GE (2013)[25] | Agnelli G (2014)[23] | Prins MH (2014)[33] |
| Study type* (Publication status) | CPST (Full Report) | CPST (Full Report) | CPST (Full Report) | SA (Full Report) | SA (Full Report) | CPST (Abstract) | SA (Abstract) | SA (Abstract) | SA (Abstract) | SA (Full Report) |
| Intervention | LMWH (Enoxaparin) | LMWH (Dalteparin) | LMWH (Enoxaparin) | LMWH (Tinzaparin) | LMWH (Tinzaparin) | LMWH (Tinzaparin) | DOAC (Dabigatran) | DOAC (Edoxaban) | DOAC (Apixaban) | DOAC (Rivaroxaban) |
| Comparator | VKA (Warfarin) | VKA (Any type) | VKA (Warfarin) | VKA (Any type) | VKA (Aceno-coumarol) | VKA (Warfarin) | VKA (Warfarin) | VKA (Warfarin) | VKA (Warfarin) | VKA (Warfarin/Acenocoumarol) |
| Follow-up time† | 3 months | 6 months | 6 months | 3 months | 6 months | 6 months | 6 months | 7.3 months | 6 months | 6 months |
| Number of recurrent VTE events in intervention group (N total) | 2 (71) | 27 (336) | 4 (61) | 6 (100) | 2 (36) | 31 (449) | 10 (173) | 4 (109) | 3 (81) | 6 (258) |
| Number of recurrent VTE events in comparator group (N total) | 3 (75) | 53 (336) | 3 (30) | 10 (100) | 3 (33) | 45 (451) | 12 (162) | 7 (99) | 5 (78) | 8 (204) |
| Number of major bleedings in intervention group (N total) | 5 (71) | 19 (338) | 6 (67) | 7 (100) | N/A | 13 (449) | 6 (159) | 5 (109) | 2 (87) | 5 (257) |
| Number of major bleedings in comparator group (N total) | 12 (75) | 12 (335) | 1 (34) | 7 (100) | N/A | 12 (451) | 7 (152) | 3 (99) | 4 (80) | 8 (202) |
| Cancer status definition | Patients with solid or hematologic cancers, either active or in remission with ongoing antitumor treatment (no definition of active cancer reported) | Patients with active cancer (defined as recurrent or meta-static cancers, or cancer diagnosis or treatment within 6 months | Patients not being candidates for curative surgery with active cancer (defined as measurable disease or histo-cyto-logical diagnosis ± elevated tumor markers) | No specific definition reported | No specific definition reported | Patients with active cancer (no specific definition of active cancer reported) | Patients with active cancer (defined as a diagnosis of cancer, recurrent or metastatic disease, or cancer treatment within 5 years) | Patients with active cancer (judged at study entry by local investigators, specific definition for active cancer not reported) | Patients with active cancer (defined as cancer diagnosed or treated within the past 6 months) | Patients with active cancer (judged at study entry by local investigators, specific definition for active cancer not reported) |
| 6-month risk of recurrent VTE in VKA arm (%)‡ | 7.8% | 15.7% | 10.0% | 19.0% | 9.1% | 10.0% | 7.4% | 4.9% | 6.4% | 3.9% |
| 6-month risk of major bleeding in VKA arm (%)‡ | 29.4% | 3.6% | 2.9% | 13.5% | N/A | 2.7% | 4.6% | 2.1% | 5.0% | 4.0% |
| Weighted 6-month risk of recurrent VTE in VKA arm (%)** | 12.6% | 5.5% | ||||||||
| Weighted 6-month risk of major bleeding in VKA arm (%)** | 6.1% | 4.0% | ||||||||
Statistical analysis
All statistical analyses were performed using STATA (Windows Version 13.0, STATA Corp., TX, USA). The trial network was graphically visualized using the user-contributed networkplot function.[26] We expressed the efficacy and safety endpoints as relative risks with 95% CIs, and pooled them using a random-effects pairwise meta-analysis model (Stata routine metan). The I2 statistic was calculated as a quantitative measure of heterogeneity. The network meta-analysis (NMA) was carried out within a frequentist setting, using the multivariate random-effects meta-regression routine mvmeta.[27] Here, we compared strategies (i.e. LMWH vs. VKA) rather than individual drugs (e.g. tinzaparin vs. acenocoumarol). To gauge the potential results of future trials on VTE therapy in cancer, we calculated 95% predictive intervals and graphically presented them on forest plots in combination with meta-analysis estimates and their 95% CIs (Stata command intervalplot).[26] A surface under the cumulative ranking curve (SUCRA) analysis was performed to compare the ranks of the treatments with respect to efficacy and safety, with higher SUCRA values indicating better treatments (Stata command sucra).[26] To explore the extent of clinical heterogeneity resulting from differing between-study definitions of cancer status, we calculated the 6-month risk of recurrent VTE and major bleeding in the VKA arms of the included trials, and weighed them according to the total number of patients in the VKA arm (Table 1). We then used meta-regression to adjust our NMA and SUCRA results for each study’s six-month risk of VTE or bleeding in the VKA group, respectively. The dataset and full analysis code is available on request from the authors. Results are reported according to PRISMA criteria (Supplemental Table 4, Supplemental Figure 1).
Results
The evidence base
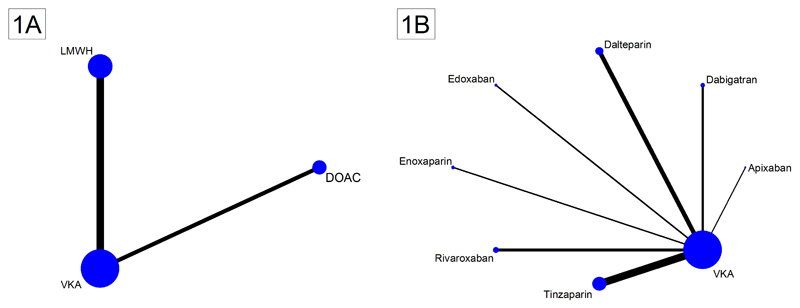
Three-thousand-two-hundred-forty-two cancer patients from 10 two-arm RCTs were included in this analysis (Table 1). Six studies compared VKA with LMWH (n=2078 patients), and five studies compared VKA with DOAC (n=1164 patients). Two network plots graphically represent the evidence base (Figures 1A+1B). Most evidence existed for VKA, followed by LMWH and DOAC.
Figure 1. Network plots of included studies on the treatment of cancer-associated VTE.
Nodes (blue dots) and edges (black connecting lines between nodes) are scaled according to the number of patients in the respective studies. Consequently, the larger the size of the respective trial(s), the larger the nodes and edges. (1A) Pooled network as analyzed in the network meta-analysis. As indicated by the size of the nodes and edges, most evidence exists for vitamin-K-antagonists (VKA), followed by low-molecular-weight heparin (LMWH) and non-vitamin-K-antagonist oral anticoagulants (DOAC). (1B) Full trial network showing individual LMWH and DOAC drugs. Again, the size of the nodes and edges is proportional to the size of the respective studies, and thus the amount of evidence for the drug within the trial network. Note: The length of the edges does not convey information, and differences in edge length is simply for better graphical presentation.
Assessment of bias and design differences in selected studies
The risk of bias in the selected studies was assessed using Cochrane criteria (Supplemental Table 2). While all 10 studies only included patients with objectively-confirmed acute symptomatic VTE, the criteria for defining patients’ cancer status at baseline were more heterogenic (Table 1). In comparison to the VKA arm of DOAC trials, the VKA arms of LMWH trials experienced both a higher risk of recurrent VTE (weighted 6-month risk: 12.6% vs. 5.5%) and major bleeding (6.1% vs. 4.0%, Table 1).
None of the selected studies actively screened for DVT and/or PE. Nine out of the 10 selected studies defined symptomatic recurrent VTE as the efficacy endpoint, while one study, the CATCH trial, also included incidental VTE events. The definition of the safety endpoint (major bleeding) appeared to be highly consistent across all 10 included studies.
Recurrent VTE and Major Bleeding – Pairwise Meta-Analysis
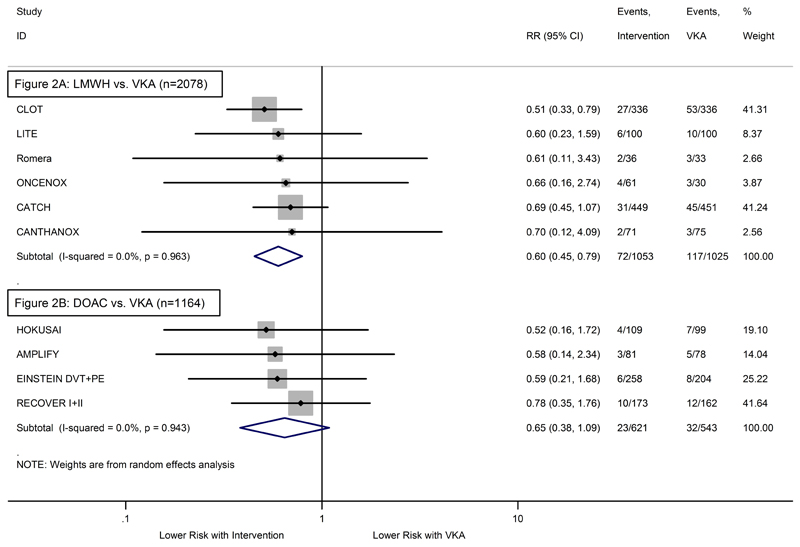
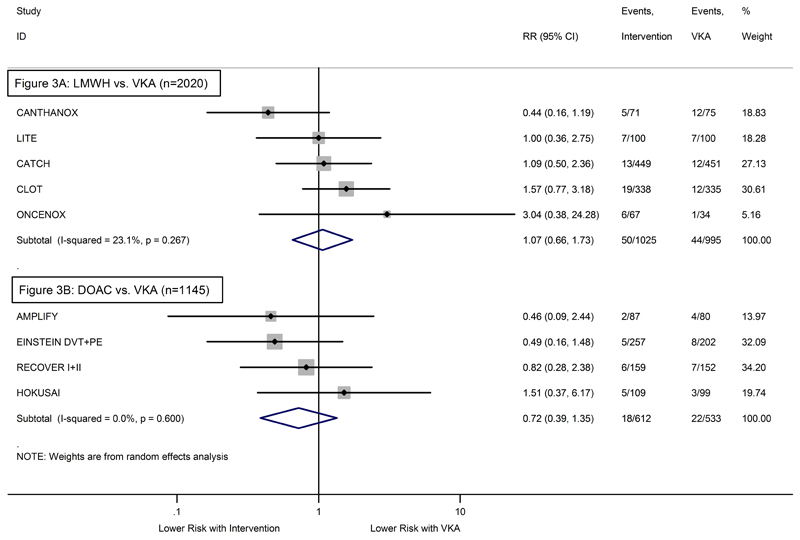
As compared to VKA, the relative risk of recurrent VTE was highly in favor of LMWH (Relative Risk (RR)=0.60, 95%CI: 0.45-0.79, p<0.001, Figure 2A). The risk of major bleeding did not differ significantly between LMWH and VKA (RR=1.07, 95%CI: 0.66-1.73, p=0.80, Figure 3A). Comparing DOACs to VKA, the relative risks were non-significantly in favor of DOACs for both recurrent VTE (RR=0.65, 95%CI: 0.38-1.09, p=0.10, Figure 3A) and major bleeding (RR=0.72, 95%CI: 0.39-1.35, p=0.31, Figure 3B). As indicated by the individual study weights, the pooled relative risks in LMWH studies were mainly determined by two trials (CLOT and CATCH account for more than 80% of the estimate), whereas the weightings for DOAC studies were more balanced. In LMWH studies, we observed low-level heterogeneity for the safety endpoint (I2=23.1%, p=0.27, Figure 3B). No evidence for statistical heterogeneity emerged in any of the three other comparisons (all I2=0.0%, Figures 2A+2B, Figure 3A).
Figure 2. Forest plot of the relative risks (RR) for recurrent VTE – Pairwise random-effects meta-analysis.
(2A) RCTs comparing LMWH with VKA. (2B) RCTs comparing DOAC with VKA. Grey boxes surrounding relative risk estimates are proportional to the weight of the respective study. P-value of I2 is from Q test.
Figure 3. Forest plot of the relative risks (RR) for major bleeding – Pairwise random-effects meta-analysis.
(3A) RCTs comparing LMWH with VKA. (3B) RCTs comparing DOAC with VKA. Grey boxes surrounding relative risk estimates are proportional to the weight of the respective study. P-value of I2 is from Q test.
Recurrent VTE and Major Bleeding – Network Meta-Analysis (NMA)
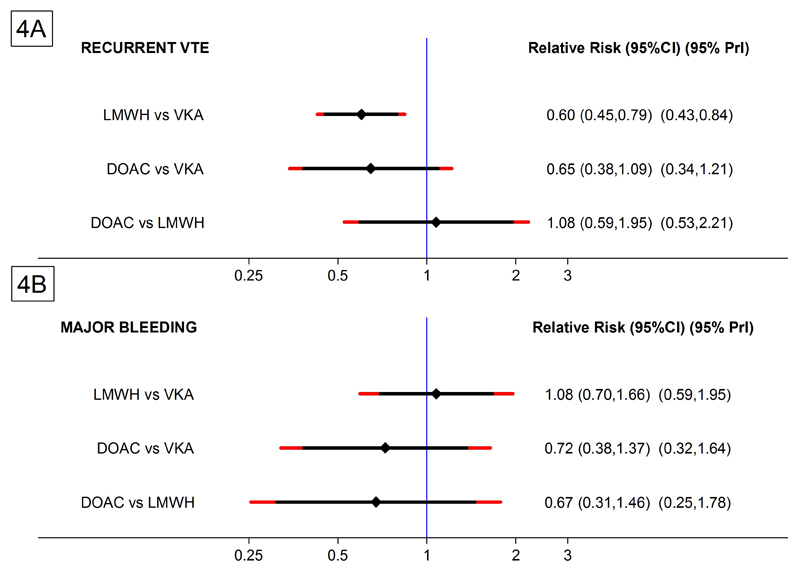
The risk of recurrent VTE was comparable between LMWH and DOAC (RR=1.08, 95%CI: 0.59-1.95, p=0.81, Figure 4, Table 2A). In terms of major bleeding, the indirect network comparison between DOAC and LMWH indicated a non-significant reduction of major bleeding with DOAC (RR=0.67, 95% CI: 0.31-1.46, p=0.31). The NMA estimates for the LMWH/VKA and DOAC/VKA comparisons were highly comparable to the estimates from the standard pairwise meta-analysis (Table 2).
Figure 4. Forest plot of the relative risks (RR) with 95% Predictive Intervals – Network meta-analysis (NMA).
95% Confidence Intervals (95% CI) are black, 95% Predictive Intervals (95% PrI) are red. (4A) Estimates for recurrent VTE. (4B) Estimates for major bleeding.
Table 2. Relative risks (RR) of recurrent VTE and major bleeding – Network meta-analysis.
Relative risks (with 95% CIs in round brackets) above the diagonal pertain to recurrent VTE (transparent background), whereas estimates below the diagonal pertain to major bleeding (gray background). The reference category for the respective comparison is always higher to the left, e.g. the RR of 0.72 (bottom left box) represents the comparison of VKA with DOAC using VKA as the reference. Here, an RR of 0.72 is in favor of DOAC. (2A) Estimates from the unadjusted network meta-analysis. (2B) Estimates from a network meta-analysis adjusted to a 10% six-month risk of recurrent VTE in the VKA arm (efficacy), and a 5% six-month risk of major bleeding in the VKA arm (safety).
| 2A. Unadjusted Network Meta-Analysis | ||
|---|---|---|
| VKA | 0.60 (0.45-0.79) | 0.65 (0.38-1.09) |
| 1.08 (0.70-1.66) | LMWH | 1.08 (0.59-1.95) |
| 0.72 (0.39-1.37) | 0.67 (0.31-1.46) | DOAC |
| 2B. Adjusted Network Meta-Analysis* | ||
|---|---|---|
| VKA | 0.67 (0.46-0.97) | 0.93 (0.20-4.40) |
| 1.30 (0.83-2.05) | LMWH | 0.71 (0.14-3.51) |
| 0.52 (0.21-1.29) | 0.40 (0.15-1.19) | DOAC |
The predictive interval (PrI) analysis for the DOAC vs. LMWH efficacy comparison was wide and symmetric around equivalence (95%PrI:0.53-2.21), suggesting that future head-to-head trials between DOACs and LMWH may have similar probabilities of being in favor of LMWH or DOAC with respect to recurrent VTE (Figure 4A). The predictive interval for the safety endpoint covered a wider range of relative risks in favor of DOAC, suggesting that results of a future trial comparing bleeding risk between DOAC and LMWH appears to have a higher probability of being in favor of DOAC (95%PrI:0.25-1.78, Figure 4B).
In the SUCRA analysis, LMWH emerged with the highest cumulative ranking probability for the efficacy endpoint, while DOAC had the highest cumulative ranking probability for the safety endpoint (Table 3).
Table 3. SUCRA values and ranking probabilities for VKA, DOAC, and LMWH – Unadjusted and Adjusted Network meta-analysis.
*Adjusted results are from a model with a 10% six-month risk of recurrent VTE in the VKA arm (efficacy), and a 5% six-month risk of major bleeding in the VKA arm (safety). Abbreviations: SUCRA – Surface under the cumulative ranking curve (higher values indicating a potentially better treatment), PrBest – Probability that the respective treatment is the best out of the three compared treatments (caution is warranted in not over-interpreting this measure due to its sensitivity to small and outlying studies), MeanRank - Mean of the distribution of ranking probabilities.
| Analysis | Treatment | Recurrent VTE | Major Bleeding | ||||
|---|---|---|---|---|---|---|---|
| SUCRA | PrBest | MeanRank | SUCRA | PrBest | MeanRank | ||
| Unadjusted NMA | VKA | 2.6 | 0.0% | 2.9 | 39.1 | 9.7% | 2.2 |
| DOAC | 68.0 | 41.2% | 1.6 | 84.6 | 79.0% | 1.3 | |
| LMWH | 79.4 | 58.7% | 1.4 | 26.2 | 11.3% | 2.5 | |
| Adjusted NMA* | VKA | 20.2 | 2.5% | 2.6 | 52.0 | 13.4% | 2.0 |
| DOAC | 84.5 | 74.7% | 1.3 | 89.7 | 84.1% | 1.2 | |
| LMWH | 45.2 | 22.8% | 2.1 | 8.2 | 2.5% | 2.8 | |
Next, we corrected the NMA comparisons of DOAC, LMWH, and VKA for potentially important clinical heterogeneity by including the six-month risks of recurrent VTE or bleeding in the studies’ VKA arms as a covariate in the NMA models (Supplemental Table 3). For the efficacy endpoint, we adjusted the comparison to a six-month VTE risk of 10%, while the safety comparison was adjusted to a six-month major bleeding risk of 5%. After this covariate adjustment, the relative risk of recurrent VTE for the DOAC/LMWH comparison was now non-significantly in favor of DOAC (RR=0.71, 95%CI: 0.14-3.51, p=0.68), while the relative risk of major bleeding moved further away from equivalence in favor of DOAC (RR=0.40, 95%CI: 0.15-1.19, p=0.08, Table 2B). In terms of the LMWHvs.VKA efficacy comparison, the adjusted relative risk was still significantly in favor of LMWH (RR=0.67, 95%CI: 0.46-0.97, p=0.04). Adjusting for the different VTE and bleeding risks exerted a strong influence on SUCRA results, with DOAC now being ranked with the highest SUCRA values for both efficacy and safety (Table 3).
Discussion
In this network meta-analysis we provided estimates of the relative efficacy and safety of DOACs, LMWH and VKA for the treatment of VTE in patients with cancer. Further, we updated a previous pair-wise meta-analysis comparing DOAC/LMWH and LMWH/VKA,[4] and identified issues relevant for the design of future real-world comparisons between DOAC and LMWH.
In terms of efficacy, LMWH emerged as significantly superior to VKA in both the pairwise and network meta-analysis, and its safety was comparable to VKA. For the DOAC vs VKA efficacy and safety comparison, the relative risk estimates were in favor of DOAC, but had confidence intervals that still included equivalence. Hence, our meta-analysis does not provide statistical evidence for superiority of DOACs over VKA with respect to efficacy and safety, and thus our results are consistent with current guidelines that recommend LMWH over VKA as the first-line treatment for VTE in patients with cancer.[3, 6–9]
The results of our analysis, which also included data from a most recently completed trial, the CATCH study,[22] are in line with four previous meta-analyses, of which three compared DOAC to VKA,[16–18] and one compared both LMWH and DOAC to VKA.[4] As indicated by its statistical weight, CATCH added much novel information towards the LMWH vs. VKA comparison, and our findings including CATCH are highly comparable to the results of the previous meta-analysis without CATCH.[4]
The main objective and added value of our NMA was to explore the comparative efficacy and safety between LMWH and DOAC for the treatment of VTE in cancer patients. LMWH, DOAC, and VKA formed a star-shaped network, which enabled a statistical comparison of LMWH with DOAC via their common comparator VKA. Here, the NMA indicated comparable efficacy, and a non-significant relative risk estimate towards improved safety with DOAC. Our SUCRA analysis of cumulative ranking probabilities suggested LMWH as superior treatment with respect to efficacy (followed by DOAC and VKA), and DOAC as the best treatment with respect to safety (followed by VKA and LMWH). Although NMAs can provide probabilities of “best treatment” (which is also reported in Table 3), we focus our interpretation on the SUCRA values, as they provide a fairer statistical comparison by being much more robust against the influence of small studies with outlying results (such as in ONCENOX).[26, 28]
In comparison to LMWH, DOACs have the a priori advantage of oral administration in a fixed dose without the need for laboratory monitoring, and potentially less interactions with patient diet and anti-cancer treatment.[8, 15] Additionally, this NMA suggests that DOACs may be similarly efficacious and safe as LMWH for the treatment of VTE in cancer patients. However, in comparison to LMWH, the current evidence for DOAC is exclusively derived from subgroup analyses. This discrepancy poses a difficult situation for authors of guidelines, who are confronted with assessing the level of recommendation for a treatment that appears to be comparable to the current standard of care, while the evidence for this treatment is not supported by trials specifically addressing the patient population in question. Several authors have thus urged the need for a real-world comparison between LMWH and DOAC in cancer patients with VTE,[4, 5, 19] and we believe that the current NMA highly supports this demand. Our analysis of predictive intervals aimed to explore where the results of such a future trial could lie. Here, the predictive interval of the LMWH vs. DOAC efficacy comparison were very wide and symmetric around equivalence, suggesting that a future trial may have similar probabilities of being in favor of LMWH or DOAC. For safety, the predictive interval was in favor of DOAC, but still included a wide probability of a future trial being in favor of LMWH. With current guidelines recommending LMWH as the first-line treatment for VTE in cancer patients, we believe that our predictive interval analysis implies that a future head-to-head comparison between LMWH and DOAC would be best designed as a non-inferiority trial.
A major challenge for this analysis was that inconsistent between-study definitions of cancer status may have led to clinical heterogeneity. While LMWH studies included patients with metastatic, recurrent, or recently diagnosed or treated cancers, one DOAC study defined “active cancer” as a diagnosis of or any treatment for cancer within the last 5 years (RE-COVER). Two of the four DOAC studies had relatively stringent cancer status criteria (EINSTEIN and AMPLIFY), and one DOAC (HOKUSAI) and three LMWH studies (CATCH, LITE, Romera et al.) did not report the exact cancer status definitions. Differing definitions of “active cancer” may have led to the inclusion of more high-VTE-risk patient in selected studies, an issue previously highlighted by Carrier et al.,[4] and di Minno et al.[5] Although the pairwise meta-analyses for recurrent VTE and major bleeding showed none or only low-level evidence for statistical heterogeneity of relative risks, we explored conceptual heterogeneity in more detail by calculating weighted six-month risks of recurrent VTE and LMWH in the VKA arms of LMWH and DOAC studies (as similarly performed by Carrier et al.[4]). Here, the risks varied between studies, and it emerged that the LMWH vs.VKA studies appeared to have higher risks of VTE and major bleeding in their VKA arms than DOAC vs.VKA studies. To us, this indicated the presence of clinical heterogeneity beyond what could be detected by statistical tests of heterogeneity. In this situation, the advantage of our network meta-regression framework was that we were able to adjust all comparisons for these imbalances by including six-month risks of recurrent VTE and major bleeding in the VKA arms as covariates, respectively. Such an adjusted analysis is not possible in a “traditional” pairwise meta-analysis, which underlines the advantages of the flexible NMA regression framework we used in this study.
Although this covariate-adjusted NMA model also did not provide clear statistical evidence for heterogeneity according to VTE and bleeding risk, it needs to be considered that meta-analytical tests for heterogeneity are known to have low power, and the calculated differences in the VKA arms were sufficient for us to anticipate the presence of heterogeneity. Using our meta-regression NMA model, we were then able to compare LMWH with DOAC at a common 10% risk of recurrent VTE and 5% risk of major bleeding in the VKA study arms. These two risks were selected because they (1) appeared to represent a meaningful balance between the two pooled and weighted VKA arm risks in LMWH and DOAC studies, (2) were covered by the distribution of VKA arm risks in the LMWH and DOAC studies, and (3) were clinically plausible. In this adjusted analysis, the efficacy comparison between LMWH and VKA was still significantly in favor of LMWH. However, the direction of the relative risk for the DOAC vs. LMWH efficacy analysis changed direction from 1.08 to 0.71 in favor of DOAC. Although the confidence intervals of this adjusted relative risk were still very wide, this can be interpreted in the sense that LMWH may be a little less effective in study populations at a lower a priori risk of recurrent VTE, and/or that DOAC may be a little more effective in study populations at a higher a priori risk of VTE. Comparing DOACs and LMWH with regard to major bleeding, the adjusted relative risk moved further away from equivalence in favor of DOAC, but the confidence interval of this estimate still included unity. We also observed changes in SUCRA results, with the adjusted analysis ranking DOAC with the highest cumulative ranking probabilities for both efficacy and safety (Table 3).
We would like to mention three limitations of this study. First, the consistency assumption could not be fully evaluated statistically, because the star-shaped network of the included VTE trials did not have any closed loops. Second, we did not include studies on unfractionated heparin (UFH) and pentasaccharides such as fonda- and indraparinux. Although UFH is an option for the initial treatment of VTE, UFH and pentasaccharides are not relevant today as a long-term cancer-associated VTE treatment, and further not recommended anymore by recent guidelines.[3] And thirdly, we want to explicitly state that network meta-analysis, although based on randomized evidence, does not represent randomized evidence. Indeed, the indirect comparisons provided by this NMA are purely observational, and thus subject to all potential types of selection and information bias routinely encountered in observational data. Although we tried to carefully adjust our indirect comparison for potential heterogeneity between LMWH and DOAC studies, we cannot exclude residual confounding. This residual confounding may have affected our results, and hence we urge the readership to interpret this article with the necessary caution. Although our analysis identifies a very important trend in the data, namely that LMWH and DOAC may be comparable regarding efficacy and safety for the treatment of cancer-associated VTE, this NMA does not represent a substitute for a properly conducted, cancer-population-specific randomized controlled trial between LMWH and DOAC.
Conclusion
In this network meta-analysis on the optimal treatment of VTE in cancer patients, LMWH and DOAC appeared to be comparable with respect to prevention of recurrent VTE and the risk of major bleeding. This finding prevailed after adjusting for potential heterogeneity between DOAC and LMWH trials. A future head-to-head comparison between LMWH and DOAC for the treatment of cancer-associated VTE is warranted, and may be best performed using a non-inferiority design.
Supplementary Material
Acknowledgements
This work was partly supported by an MD PhD studentship of the Austrian Science Fund (FWF-SFB-54 „Cellular Mediators linking Inflammation and Thrombosis – InThro“). Dr. Pabinger received honoraria for advisory board meetings and lectures from Bayer, Pfizer and Boehringer-Ingelheim. Dr. Ay received honoraria for lectures from Sanofi, Pfizer, Boehringer-Ingelheim, Bayer and Daiichi-Sankyo.
Funding
Austrian Science Fund (FWF-SFB-54)
Footnotes
Conflicts of Interest
The other authors have no conflicting interests to declare. The entities listed above had no role in the design, analysis, interpretation, or any other aspect of this study.
Author contributions
Conceived and designed the study: FP CA. Performed statistical analyses: FP. Conducted literature search and study selection: FP OK IP CA. Interpreted the results: FP OK CZ IP CA. Wrote the first draft of the manuscript: FP CA. Contributed to the writing of the manuscript: FP OK CZ IP CA. Agree with the manuscript’s results and conclusions: FP OK CZ IP CA. ICMJE criteria for authorship read and met: FP OK CZ IP CA.
References
- [1].Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of thrombosis and haemostasis : JTH. 2007;5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- [2].Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- [3].Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- [4].Carrier M, Cameron C, Delluc A, Castellucci L, Khorana AA, Lee AY. Efficacy and Safety of Anticoagulant Therapy for the Treatment of Acute Cancer-Associated Thrombosis: A Systematic Review and Meta-Analysis. Thrombosis research. 2014;134:1214–9. doi: 10.1016/j.thromres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- [5].Di Minno MN, Ageno W, Dentali F. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism: comment. Journal of thrombosis and haemostasis : JTH. 2014;12:2136–8. doi: 10.1111/jth.12746. [DOI] [PubMed] [Google Scholar]
- [6].Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed. Vol. 141. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest; 2012. pp. 7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS) European heart journal. 2014;35:3033–73. [Google Scholar]
- [8].Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2011;22(Suppl 6):vi85–92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- [9].Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, Brenner B, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Journal of thrombosis and haemostasis : JTH. 2013;11:56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- [10].Hull RD, Pineo GF, Brant R, Liang J, Cook R, Solymoss S, et al. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. The American journal of medicine. 2009;122:762–9 e3. doi: 10.1016/j.amjmed.2008.12.023. [DOI] [PubMed] [Google Scholar]
- [11].Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. The New England journal of medicine. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- [12].Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. The New England journal of medicine. 2013;369:1406–15. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- [13].Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis journal. 2013;11:21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- [15].Beyer-Westendorf J, Ageno W. Benefit-risk profile of non-vitamin K antagonist oral anticoagulants in the management of venous thromboembolism. Thrombosis and haemostasis. 2014;113 doi: 10.1160/TH14-06-0484. [DOI] [PubMed] [Google Scholar]
- [16].van der Hulle T, den Exter PL, Kooiman J, van der Hoeven JJ, Huisman MV, Klok FA. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. Journal of thrombosis and haemostasis : JTH. 2014;12:1116–20. doi: 10.1111/jth.12605. [DOI] [PubMed] [Google Scholar]
- [17].van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–75. doi: 10.1182/blood-2014-04-571232. [DOI] [PubMed] [Google Scholar]
- [18].Larsen TB, Nielsen PB, Skjoth F, Rasmussen LH, Lip GY. Non-vitamin k antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: a semi systematic review and meta-analysis of safety and efficacy outcomes. PloS one. 2014;9:e114445. doi: 10.1371/journal.pone.0114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ay C, Pabinger I. Treatment and secondary prevention of venous thromboembolism in cancer patients. Current strategies and new therapeutic options. Hamostaseologie. 2012;32:139–44. doi: 10.5482/ha-1168. [DOI] [PubMed] [Google Scholar]
- [20].Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ (Clinical research ed) 2013;346 doi: 10.1136/bmj.f2914. f2914. [DOI] [PubMed] [Google Scholar]
- [21].Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Journal of thrombosis and haemostasis : JTH. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- [22].Lee AYY, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA. A Randomized Trial of Long-Term Tinzaparin, a Low Molecular Weight Heparin (LMWH), Versus Warfarin for Treatment of Acute Venous Thromboembolism (VTE) in Cancer Patients - the CATCH Study. 2014 [Google Scholar]
- [23].Agnelli GBH, Cohen A, Curto M, Gallus AS, Pak R, Raskob GE, Weitz JI, Yamabe T. Abstract PP5527: Apixaban for the treatment of venous thromboembolism in cancer patients: data from the AMPLIFY trial. European heart journal. 2014;994 doi: 10.1111/jth.13153. [DOI] [PubMed] [Google Scholar]
- [24].Schulman S, Eriksson H, Goldhaber SZ, Kakkar S, Kearon C, Schellong SM, Feuring M, Peter N, Friedman J. Influence Of Active Cancer On The Efficacy and Safety Of Dabigatran Versus Warfarin For The Treatment Of Acute Venous Thromboembolism: A Pooled Analysis From RE-Cover and RE-Cover II. 2013 [Google Scholar]
- [25].Raskob GEBH, Angchaisuksiri P, Oh D, BOda Z, Lyons RM, Weitz JI, Zhang G, Lanz HJ, Mercuri M. Edoxaban For Long-Term Treatment Of Venous Thromboembolism In Cancer Patients. 2013 [Google Scholar]
- [26].Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS one. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].IR W. Multivariate random-effects meta-regression: Updates to mvmeta. The Stata Journal. 2011;11:255–70. [Google Scholar]
- [28].Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2006;12:389–96. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- [29].Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Archives of internal medicine. 2002;162:1729–35. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- [30].Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. The New England journal of medicine. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- [31].Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, et al. Self-managed long-term low-molecular-weight heparin therapy: the balance of benefits and harms. The American journal of medicine. 2007;120:72–82. doi: 10.1016/j.amjmed.2006.03.030. [DOI] [PubMed] [Google Scholar]
- [32].Romera A, Cairols MA, Vila-Coll R, Marti X, Colome E, Bonell A, et al. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2009;37:349–56. doi: 10.1016/j.ejvs.2008.11.030. [DOI] [PubMed] [Google Scholar]
- [33].Prins MH, Lensing AWA, Brighton TA, Lyons RM, Rehm J, Trajanovic M, Davidson BL, Beyer-Westendorf J, Pap FA, Berkowitz SD, Cohen AT, Kovacs MJ, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematology. 2014;1:e37–46. doi: 10.1016/S2352-3026(14)70018-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.