Abstract
The immune system detects disturbances in homeostasis that occur during infection, sterile tissue damage and cancer. This initiates immune responses that seek to eliminate the trigger of immune activation and to reestablish homeostasis. At the same time, these mechanisms can also play a crucial role in the progression of disease. The occurrence of DNA in the cytosol constitutes a potent trigger for the innate immune system, governing the production of key inflammatory cytokines such as type I interferons and IL-1β. More recently, it has become clear that cytosolic DNA also triggers other biological responses, including various forms of programmed cell death. In this article we review the emerging literature on the pathways governing DNA-stimulated cell death and the current knowledge on how these processes shape immune responses to exogenous and endogenous challenges.
Keywords: Cell death, DNA sensing, Innate immunology, cGAS-STING pathway
Introduction
Danger sensing and signalling is essential for eradication of infections, resolution of sterile damage and elimination of neoplasia. This allows the organism to eliminate the microbial threat, initiate tissue repair, and to reestablish homeostasis. Danger-induced responses include the expression of type I and type III interferon (IFNs), the induction of pro-inflammatory genes, the activation of inflammasome cascades, the initiation of autophagy, and also the engagement of cell death pathways 1. One major danger signal is abnormal subcellular localization of DNA (Box 1) 2. DNA is normally compartmentalized to the nucleus and mitochondria, yet high levels of DNA in the cytoplasm or within the endolysosomal niche stimulates danger signalling. Normally, low levels of DNA occur in these compartments, for example, during genome replication, transcription of endogenous retroelements and phagocytosis of apoptotic cells. However, DNases located in the extracellular space (DNase I), endosomes (DNase II), and the cytoplasm (DNase III or TREX1) digest mislocalized DNA to levels below the threshold for danger signalling 3–5. Nevertheless, with the appearance of high amounts of exogenous DNA, for example during infections, certain thresholds seem to be passed so that DNA can initiate sensing and associated signalling cascades (Fig. 1A). This is also observed if the cellular DNases have reduced activity, or if this machinery is overwhelmed by endogenous substrates. In the past years it has emerged that the appearance of cytoplasmic DNA can also engage different types of programmed cell death (PCD) pathways and we now begin to understand the molecular basis for the interplay and cross-talk between cell death and other responses following sensing of DNA danger. In this review, we first describe key DNA sensors, and how they induce cytokine responses. This is followed by a detailed description of the current knowledge on DNA-stimulated cell death, and a discussion of how this may impact on defense and disease during infection, inflammatory diseases, and cancer.
DNA as a molecule stimulating innate immune responses.
DNA is the molecule carrying the genetic code of living organisms. However, DNA also has other properties, including being the main constituent of neutrophil extracellular traps 138, and being a danger signal for the innate immune system 6. DNA gains immunostimulatory activity when localized in abnormal cellular compartments, notably endosomes, the cytoplasm, or micronuclei 7,126,127,139,140. In the original definition by Charlie Janeway, pattern recognition receptors (PRRs) detect pathogen-associated molecular patterns (PAMPs), which are (i) conserved among entire classes of pathogens, (ii) essential for the survival of the pathogen, and (iii) different from “self” 141. In addition, the term danger-associated molecular patterns (DAMPs) has been coined, which is usually understood to represent endogenous molecules that gain PRR-agonistic activity upon cellular stress or damage. Based on these criteria, microbial DNA is not a bona fide PAMP, but falls into the broader category of microbe-associated molecular patterns. By contrast, host-derived DNA fulfils the criteria for the term DAMP.
Regarding the sensing of double-stranded (ds) DNA by PRRs at the molecular level, the cytosolic DNA sensors AIM2 and cGAS detect dsDNA through binding to the sugar-phosphate backbone in a sequence-independent manner 22,142–144. For AIM2, this is achieved through a positively charged surface in the carboxy terminal HIN domain, whereas cGAS uses a positively charged surface as well as the zinc thumb to interact with the DNA backbone. For both AIM2 and cGAS, immune activation is DNA length dependent 9,14,31,145, and in the case of cGAS it was recently reported that under conditions with low DNA concentrations, long dsDNA species (>1000 bps) are much more stimulatory and with much lower concentration thresholds 146. This argues for long DNA species being the physiological trigger of DNA-induced immune responses.
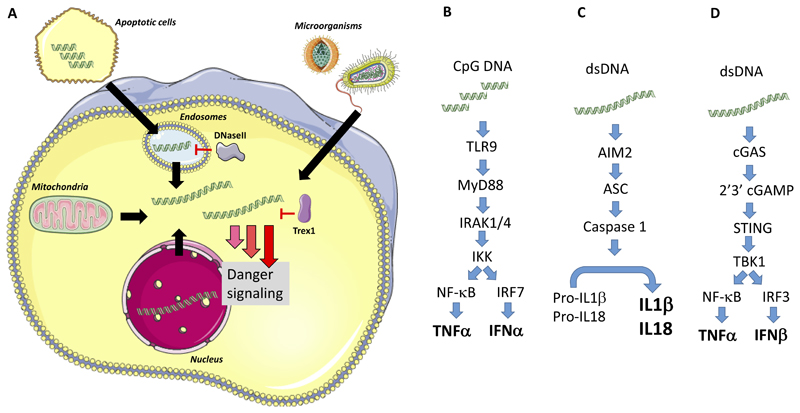
Figure 1. Hallmark cytokine responses induced by DNA-sensing PRRs.
(A) DNA originating from microbes, mitochondria, phagocytosed apoptotic cells, or leaked from the nucleus can accumulate in the cytoplasm where it is sensed by pattern recognition receptors. This initiates danger signaling. (B-D) Type I IFN and IL-1β production are the most studied immunological responses downstream of cytosolic DNA. (B) TLR9 senses CpG-rich DNA in endosomes and signals via MyD88 to activate IRF7, which is abundantly expressed in plasmacytoid dendritic cells. This leads to strong activation of genes encoding IFNα. (C) dsDNA in the cytoplasm is also sensed by AIM2, which promotes assembly of inflammasomes with caspase 1 activation, and downstream cleavage of pro-IL-1β to the bioactive cytokine. (D) dsDNA is sensed by cGAS, leading to enzymatic activation and synthesis of the STING agonist 2’3’cGAMP, thus leading to activation of the kinase TBK1, phosphorylation of the transcription factor IRF3, and transcriptional activation of the promotors for the type I (α/β) and type III (λ) IFN genes. Abbreviations not defined in the text: HTLV-I, Human T-lymphotropic virus I; IKK, IκB kinase; IRAK, Interleukin-1 receptor-associated kinase; Trex, Three prime repair exonuclease.
Immune stimulation by DNA
Focus on IFN and IL-1β
The immunostimulatory properties of DNA were first reported more than 50 years ago 6. In particular, the ability of DNA to induce production of type I and type III IFNs and IL-1β is a hallmark of DNA-stimulated immune responses. DNA is sensed in endolysosomes by Toll-like receptor 9 (TLR9) and in the cytoplasm by cyclic GMP-AMP (cGAMP) synthase (cGAS), and absent in melanoma 2 (AIM2), and under some circumstances also by RNA polymerase III 7–14.
TLR9
TLR9 was the first DNA-sensing pattern recognition receptor (PRR) to be identified 7. Like other nucleic acid-sensing TLRs, it localizes to the endolysosomal compartment and recognizes DNA at this site15. The most potent ligand for TLR9 is unmethylated CpG DNA, which occurs abundantly in bacteria 7,16. TLR9 signals through the common TLR adaptor myeloid differentiation primary response protein 88 (MYD88) and activates the transcription factor IFN regulatory factor 7 (IRF7), leading primarily to IFNα production 17 (Fig. 1B). TLR9 is expressed in a very cell-type restricted manner in humans, with B cells and plasmacytoid dendritic cells (pDCs) selectively expressing high levels of this PRR 18. As pDCs express high levels of IRF7, TLR9 is a potent inducer of type I IFN in this cell type 19.
AIM2
The second key DNA sensor to be identified was the Pyrin and HIN domain (PYHIN) family protein AIM2, which activates the inflammasome pathway 11–14. The inflammasome is a protein complex that initiates the cleavage of specific proteins to activate and regulate immune reactions. By far the best-studied inflammasome substrate is pro-IL-1β, which needs to be cleaved to render bioactive IL-1β – a central pro-inflammatory cytokine 20,21. AIM2 activates the inflammasome pathway in response to exogenous and endogenous DNA challenge (Fig. 1C) 11–14. Following DNA sensing, AIM2 recruits the adapter protein ASC (apoptosis-associated speck-like protein containing CARD), which in turn forms a filamentous structure that serves as a recruitment platform for pro-caspase-1. The recruitment of pro-caspase-1 to this structure results in its activation and thereby enables this protease to process its substrates 21–23. There are reports showing that another PYHIN protein IFI16, as well as NOD-, LRR- and pyrin domain-containing 3 (NLRP3), can also induce caspase-1 activation in response to DNA 24,25, the latter possibly through sensing of DNA-stimulated cell death as discussed below 26. These results may suggest species-specific and cell-specific pathways for IL-1β processing and release by DNA.
cGAS
Both of the above-mentioned DNA sensors are largely confined to the myeloid compartment, hence they cannot explain key parts of the immune response, for example, the sensing of DNA in virus-infected cells of non-myeloid origin. This notion led to an intense effort to identify cytosolic DNA sensors stimulating IFN expression, and ended with the identification of cGAS 8,27. Other cytosolic DNA sensors were also proposed, but were either not independently confirmed 28,29, were found to play more subordinate roles 9,10,30, or were shown to be accessory proteins in the cGAS signalling pathway 31–33. Upon DNA binding, the enzymatic activity of cGAS is initiated leading to production of the cyclic dinucleotide (CDN) 2’3’ cGAMP 8,27. This CDN is a ligand for the adaptor protein stimulator of IFN genes (STING), which in turn recruits TANK-binding kinase 1 (TBK1) 27. This leads to TBK1-mediated phosphorylation at serine 366 in the carboxy-terminal tail (CTT) of STING 34. IRF3 is then recruited to phospho-S366 of STING, positioning the latent transcription factor for TBK1-mediated phosphorylation and activation, eventually driving expression of IFNA and IFNB genes (Fig. 1D) 35. Consequently, the CTT of STING is essential for cGAS-STING-induced IFN expression. In addition to the IRF-IFN pathway, cGAS-STING signaling leads to activation of nuclear factor-κB (NF-κB), and induction of inflammatory cytokines26,36–38. The mechanism of STING-mediated activation of NF-κB remains is still poorly understood. Finally, it has recently emerged that the AIM2 pathway negatively regulates cGAS activity by, stimulating caspase 1-mediated cleavage of cGAS as well as depletion of intracellular potassium39,40. Thus, there is cross-talk between the DNA-stimulated pathways with impact on cytokine responses, but also PCD pathways as will be discussed below.
Biological importance of the DNA–IFN and DNA–IL-1β axes
It is well documented that DNA-driven cytokine responses are of critical biological importance. Mechanistically and functionally, one can distinguish DNA-driven type I IFN responses from DNA-triggered IL-1β maturation. For instance, control of herpes simplex virus 1 (HSV1) infection in the CNS is dependent on viral sensing and signalling by microglia through the cGAS–STING axis leading to type I IFN production 41–43. For bacterial infections, the role of STING-driven IFN responses differs significantly depending on the bacterial species. For instance, although both Legionella pneumophila and Listeria monocytogenes induce type I IFN expression in a STING-dependent manner, the downstream activity of type I IFNs is protective in the case of L. pneumophila infection, but deleterious in the case of L. monocytogenes infection in mice 44–47. There is also strong evidence for the importance of the IFN response triggered by endogenous DNA through the cGAS–STING pathway. For instance, individuals with TREX1 or DNase II deficiency develop autoinflammatory diseases 48,49, and murine studies have demonstrated this to be dependent on STING and the type I IFN receptor 4,50–52. Recently, it was reported that the release of endogenous DNA, as it occurs under acute conditions such as myocardial infarction, also triggers STING-dependent IFN responses, thereby augmenting the inflammatory pathology and decreasing survival rates 53. Finally, DNA-induced IFN responses also seem to promote control of cancer. The mechanisms involved include the activation of antigen presenting cells by tumour cell DNA, which promotes anti-tumour T cell responses 54,55.
DNA-induced IL-1β is also likely to play important roles in both defence and tissue damage. Studies from mouse disease models have shown similar phenotypes in Aim2-/- and Il1r-/- mice in several cases. For instance, mice deficient in AIM2 have reduced expression of IL-1β, and exhibit elevated susceptibility to Francisella tularensis infection, as is also observed in Ilr1-/- mice 56–59. Also, host DNA released from injured cells in the lungs during influenza A virus infections induces early AIM2-dependent IL-1β production 60,61. Accordingly, AIM2-deficiency ameliorated symptoms and lung pathology, similar to the phenotype of IL-1R-deficient mice 61,62. However, another study reported that AIM2-deficiency led to elevated inflammatory response to influenza A virus infection 60. This issue remains to be resolved. Regarding autoinflammatory diseases, there is evidence supporting the idea that the pathological IL-1β response in psoriasis is driven by the AIM2 inflammasome 63. Finally, chemotherapy causes severe gastrointestinal tract toxicity in a manner dependent on DNA damage and AIM2-induced IL-1β production, although the total dependence on AIM2 is somewhat surprising given the presumed release of multiple DAMPs during cell lysis 64. Collectively, the DNA–IFN and DNA–IL-1β axes play important roles in host defence and inflammatory diseases.
Pathways for DNA-stimulated cell death
As DNA sensors have primarily been regarded as PRRs, the main focus has been put on their cytokine outputs and other outcomes that depend on de novo gene expression. However, in recent years it has emerged that DNA sensing also induces additional cellular events, such as autophagy 65–67 (Fig. 2A). In addition, it has been shown that cytosolic DNA stimulates signalling pathways that lead to different types of PCD (Box 2). These include pathways that induce apoptosis, pyroptosis, necroptosis as well as lysosomal cell death12,14,68–70. The focus of this review is to describe the current knowledge on DNA-driven cell death (Fig. 2B), highlighting the pathways involved and their physiological relevance.
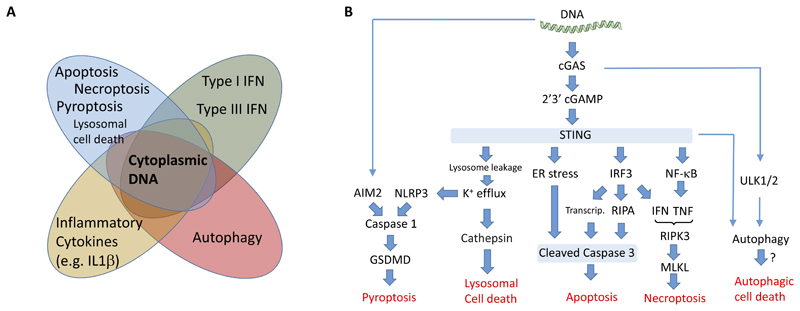
Figure 2. Cytoplasmic DNA activates a broad range of stress responses.
(A) The cellular responses induced by cytoplasmic DNA includes, in addition to production of type I IFN and IL1β, a range of other activities. This includes NF-κB-induced gene expression, autophagy, and a panel of PCD pathways, such as apoptosis, necroptosis, and pyroptosis. (B) Overview of the current knowledge on PCD induced by cytoplasmic DNA, including the interactions between the pathways. Abbreviations not defined in the text: ULK, Unc-51 like autophagy activating kinase.
Modalities of cell death induced by DNA.
Various forms of cell death can be induced in a programmed manner through specific signalling pathways initiated by a range of triggers, including DNA.
Apoptosis
Apoptosis was first observed more than 175 years ago by the zoologist Klaus Vogt studying development of the tadpole, and later named apoptosis by Kerr, Wyllie and Currie in 1972147. Apoptosis displays a number of biochemical and morphological characteristics, including cell shrinkage, membrane blebbing, chromatin fragmentation, and mRNA decay. Apoptosis can be induced through pathways relaying signals from plasma membrane receptors (extrinsic pathway) or from the mitochondria (intrinsic pathway)148. Both pathways converge in the activation of the effector caspases, caspase 3 and caspase 7, with the extrinsic pathway mainly utilizing the initiator caspase 8 and the intrinsic pathway caspase 9 149. Apoptotic cell death generates cell fragments called apoptotic bodies, which are phagocytosed and removed by macrophages. This functionality also, functions to hinder the spread of microorganisms and the release of immunostimulatory molecules 147. If not removed, apoptotic cells can undergo secondary necrosis 96,150.
Pyroptosis
One prominent form of programmed necrotic cell death is pyroptosis. The term was coined by Cookson and Brennan following the description of rapid caspase 1-dependent cell death in Salmonella-infected macrophages 151–153. Pyroptosis is initiated by so-called inflammatory caspases that encompass caspase-1, which is activated in the context of inflammasome complexes, or by caspases that are activated in response to cytosolic LPS, namely caspase 4 and caspase 5 in humans (or caspase 11 in mice). These proteases in turn cleave gasdermin D, thus generating an N-terminal fragment that localizes to the inner leaflet of the plasma membrane where it forms large pores. These pores result in osmotic swelling of cells and subsequent rupture of the plasma membrane 154,155. Thereby cytosolic content is released, which may act as DAMP molecules that trigger inflammation. Results from negative stain electron-, cryo electron, and atomic force-microscopy have shown that the Gasdermin pore has an inner diameter of 14-20 nm 156–158. Such a pore size allows passage of larger molecules, including mature IL-1β and caspase-1, with diameters of 4.5 and 7.5 nm, respectively.
While pyroptosis was initially considered to be restricted to the activation of the pro-inflammatory caspases, more recent evidence suggests that pyroptosis can also be executed in the context of apoptotic caspases. As such, the Gasdermin family member gasdermin E (also known as DFNA5) can be cleaved by the apoptotic effector caspase 3 to effectuate pyroptotic cell death in a manner analogous to gasdermin D 97,98. Consequently, pyroptosis has to be defined as a cell death that is carried out by gasdermin pores, rather than by a specific pattern recognition receptor (PRR) cascade or caspase159.
Necroptosis
Necroptosis is like pyroptosis a necrotic form of cell death that encompasses the disintegration of the plasma membrane and the release of cytosolic content. It was initially identified through studies where cells were treated with death receptor agonists in the presence of pan-caspase inhibitors 160. Necroptosis is induced by a number of pathways (for example, the RIPK1, TRIF or ZBP1 pathways) that converge upon the activation of receptor-interacting serine/threonine-protein kinase 3 (RIPK3). RIPK3, in turn, phosphorylates the pseudokinase protein MLKL (mixed lineage kinase domain-like protein) leading to a change in its conformation, translocation to the plasma membrane and subsequent membrane disruption 161–163. Activation of caspase 8 negatively regulates necroptosis 164, and thus necroptosis has been suggested to be a type of ‘back-up’ death pathway in cells where the apoptosis pathway is blocked, as can occur for instance during virus infections. It should be noted that most knowledge on necroptosis has been performed in vitro, and it remains to be thoroughly investigated to which extent necroptosis occurs in vivo.
Lysosomal cell death
Christian de Duve described lysosomal cell death more than 40 years ago. Lysosomal membrane permeabilization and the release of lysosomal hydrolases (e.g. cathepsins) occurs secondary to many forms of PCD. However, lysosomal membrane permeabilization can also function as the primary initiator of cell death. Here, the release of activated lysosomal hydrolases into the cytoplasm leads to the engagement of various redundant, yet ill-defined pathways that result in the effectuation of cell death 165. Indeed, attempts to define lysosomal cell death by genetic means have been unsuccessful, attributable to the redundancy of cell death inducing triggers originating from the leakage of lysosomal hydrolases into the cytoplasm. In this regard it is conceivable that lysosomal enzymes directly serve as the death-executing proteases. Lysosomal cell death typically displays a necrotic phenotype with plasma membrane permeabilization, with caspase inhibition showing only little effect. Nevertheless, despite the caspase-independent nature of lysosomal cell death, several characteristics of apoptosis, such as the cleavage of caspase 3 can be observed 26.
The AIM2 pathway in pyroptosis and apoptosis
One of the first reports linking DNA recognition to the induction of cell death was provided by Stacey and colleagues 71. These authors observed a dramatic decline in the viability of murine macrophages upon electroporation with different types of DNA molecules. Interestingly, the observed cell death did not show hallmarks of apoptosis, indicating that another cell death modality might be at play. In retrospect, this study provided a functional characterization of the first cytosolic DNA receptor to be discovered, namely AIM2. As outlined above, AIM2 is a cytosolic PYHIN protein that harbors an N-terminal Pyrin domain and a C-terminal HIN200 domain. AIM2 detects cytosolic DNA of more than 70-80 bp in length in a sequence-independent manner 22, resulting in the formation of a Pyrin-domain platform that functions as a seed to recruit the universal inflammasome adapter molecule ASC, and the protease caspase 1, which cleaves gasdermin D to induce pyroptosis (Fig. 3A). In mouse macrophages, cytosolic DNA delivery mainly triggers AIM2–ASC-dependent cell death, with caspase-1-initiated pyroptosis playing the predominant role. However, in the absence of caspase-1 and also when there are low amounts of DNA, ASC recruits and activates caspase-8, which then is the apical caspase in a subsequent apoptotic cell death response (Fig. 3A) 72. While this switches the quality of the cell death to apoptosis, it still represents an AIM2–ASC-dependent PCD. These findings extend the role of AIM2 and ASC beyond inflammasome activation, which might be relevant in cells types that do not express caspase-1, yet do express AIM2, ASC and caspase-8. The physiological importance of this alternative AIM2-driven PCD pathway has yet to be fully understood.
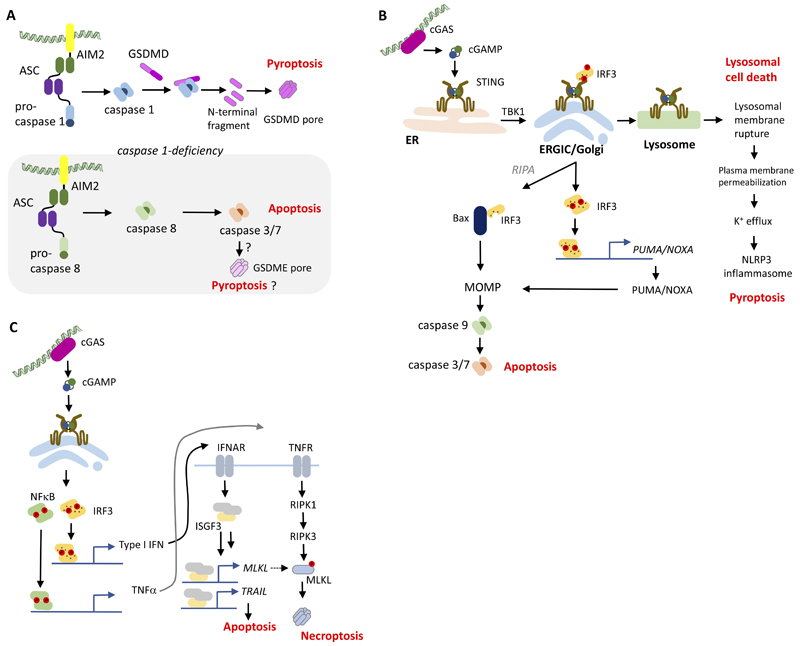
Figure 3. Pathways in DNA-stimulated cell death.
(A) Cell death pathways stimulated by AIM2 in response to DNA sensing. (B) Cell-autonomous death pathways stimulated through STING-dependent mechanisms. (C) Stimulation of apoptosis and necroptosis by STING-dependent paracrine signaling. Abbreviations not defined in the text: IFNAR, IFNα receptor; ISGF, IFN-stimulated gene factor; NF, nuclear factor; PUMA, p53 upregulated modulator of apoptosis; RIPA, RLR-induced IRF-3-mediated pathway of apoptosis; TNFR, TNF receptor.
STING and apoptosis
One of the first reports that implemented STING signalling in the triggering of cell death came from studies in T cells infected with human T cell leukemia virus type 1 73. Here, it was observed that primary T cells underwent apoptosis that was dependent on the formation of a complex between IRF3 and BAX (Bcl2-associated X gene), which promotes apoptosis through the mitochondrial pathway 73,74. Interestingly, this IRF3-mediated pathway of apoptosis (also known as RLR-induced IRF3-mediated pathway of apoptosis (RIPA)) operates independently of the transcriptional activity of IRF3, but still requires the presence of TBK1 74. This TBK1 requirement, yet the independence of IRF3 phosphorylation, might be attributed to the fact that TBK1-dependent phosphorylation of the adapter protein (STING or MAVS) is still required to allow IRF3 recruitment 34. Further studies revealed that linear polyubiquitination of IRF3 by linear ubiquitin chain assembly complex (LUBAC) played a critical role in this particular cell death pathway 75. In light of the fact that IRF3 is activated downstream of a number of signalling hubs, this type of cell death is not specific to cGAS–STING-dependent DNA recognition, but also seen in the context of RNA-activated RIG-I signalling. All in all, these results imply that cGAS–STING signalling triggers apoptosis independently of transcriptional activity through the mitochondrial apoptosis pathway (Fig 3B). Of note, most studies reporting on RIPA have been performed in fibroblast cell lines.
Another study that has reported on the capacity of STING to induce apoptosis proposed that the transcriptional activity of STING was required for apoptosis induction in T cells 76. To this end, it was shown that the de novo expression of the BH3-only proteins via STING signalling strongly correlated with the sensitivity of cells to succumb to apoptosis. This pro-apoptotic gene expression programme correlated with STING expression levels, serving as a possible explanation as to why mouse T cells (which show high STING expression), but not mouse macrophages (which show low STING expression) die of apoptosis upon STING activation. Again, since the induction of pro-apoptotic BH3-only proteins is also seen for other PRR pathways 77, this type of cell death induction is not unique to cGAS–STING signalling (Fig. 3B). Along these reports, additional studies have reported on the capacity of STING agonists to trigger apoptosis, yet mechanistic studies were not always performed that would allow the inference of the underlying mode of action. For example, prior to the discovery of STING, several studies documented the pro-apoptotic effects of the tumour vascular disrupting agent DMXAA (5,6-Dimethylxanthenone-4-acetic acid) in mice in vitro and in vivo 78,79. With the discovery of DMXAA being a direct and specific agonist for mouse STING 80,81, these studies retrospectively imply that STING-dependent effects were responsible for at least part of these observations.
Finally, cGAS-STING-induced type I IFN can stimulate genes that induce apoptosis in a paracrine manner82. Most notably, type I IFN stimulates expression of TNF-related apoptosis-inducing ligand (TRAIL), which act via death receptor 5 to induce apoptosis82,83 (Fig. 3C). This pathway has been demonstrated to be operative in vivo and to contribute to DNA-stimulated cell death and immunopathology82.
STING and lysosomal cell death
The CTT of STING and its capacity to recruit and activate TBK1 evolved along with the emergence of IRF3 and type I IFNs in the vertebrate system 84. In light of this observation, it seems plausible that certain STING functions do not require the CTT, the downstream kinase TBK1 or the functionality of the CTT to recruit IRF3 for its activation. For example, the profound lack of memory CD4+ T cells that is observed in patients with gain-of-function mutations in STING appears to be connected to such a CTT-independent STING functionality 85. To this end, transgenic expression of a constantly active STING molecule exerts a strong antiproliferative effect on CD4+ T cells and this functionality is also seen for a STING construct lacking the majority of the CTT that is incapable of activating TBK1 86. This indicates that canonical STING signal transduction is not required for this effect. Another, signaling-independent function of STING activation was observed in human myeloid cells. Here, STING activation leads to its translocation to lysosomes and the subsequent permeabilization of lysosomal membranes 26 (Fig. 3B). This in turn triggers a cell death pathway that is compatible with the concept of lysosomal cell death. STING-induced lysosomal cell death is observed in cells lacking TBK1 and IKKε and also when expressing a mutant version of STING that cannot facilitate IRF3 phosphorylation via TBK1. As such it is clearly distinct from the canonical signalling function of STING. Indeed, in human myeloid cells STING-dependent lysosomal cell death leads to the secondary activation of the NLRP3 inflammasome, which is sensitive to cell membrane perturbation that is triggered by lysosomal cell death 26. These results are in so far intriguing as human myeloid cells do not utilize the dedicated DNA-sensing inflammasome sensor AIM2 for this purpose. Of note, in mouse embryonic fibroblasts and macrophages, STING is also translocated to the lysosome upon activation 87, yet in these cells lysosomes function to degrade STING, but not to induce cell death. As such. It appears that cell-type specific properties dictate the involvement of lysosomes in STING biology.
Finally, although not demonstrated to be dependent on DNA and cGAS, STING is involved in ER stress 88. This leads to downstream apoptosis through a mechanism dependent on the ER-resident kinase PERK and the downstream target transcription factor CCAAT-enhancer-binding protein homologous protein 88.
STING and necroptosis
A third type of cell death stimulated by DNA is necroptosis 70 (Fig. 3C). In this regard, it was reported that DNA transfection as well as STING agonists — in the context of pan-caspase inhibition — activate necroptosis in mouse fibroblasts, as well as in bone marrow-derived macrophages 70,89. This DNA-induced necroptosis was dependent on STING-induced tumour necrosis factor (TNF) and IFNα/β, and could also be triggered by other TNF- and type I IFN-inducing stimuli, such as RIG-I or TLR3 agonists 70,89. As such, under these conditions, there seems to be no requirement for a STING-specific signal to induce necroptosis. Nevertheless, it has been shown that tonic type I IFN production, induced through the cGAS–STING pathway, generally lowers the threshold for necroptosis. This is governed by the expression of the key necroptosis executor mixed-linage kinase domain like (MLKL) 90. In this regard, also TLR4-triggered necroptosis was affected by cGAS and STING-deficiency, which could be explained by a loss of tonic type I IFN production and an associated decrease in MLKL expression 90. Of note, while all of these in vitro experiments require the concomitant inhibition of caspases to render cells sensitive to STING-dependent necroptosis, in vivo experiments suggest that necroptosis can indeed be achieved by single STING agonism. To this end, mice treated with high doses of a STING ligand succumbed to lethal shock associated with pro-inflammatory cytokine production, which could be in part rescued by deficiency of RIPK3 or MLKL 89. Future studies will be required to address the role of this pathway in the context of physiological cGAS–STING activation.
Role of cGAS–STING in autophagy-dependent cell death
Finally, it is also possible that cytosolic DNA can induce autophagy-dependent cell death, which is a form of cell death induced in an autophagy-dependent manner, notably in response to strong inducers of autophagy 91. The exact mechanisms that execute cell killing in autophagy-dependent cell death are not fully understood, but could give us key information on what governs the switch from healthy to deadly forms of autophagy. It is important to emphasize that there is currently no experimental evidence for DNA-activated autophagy-dependent cell death. However, since DNA strongly activates autophagy through both STING dependent and independent pathways 66,67,92 and bacteria-derived CDNs trigger extensive ER-phagy 88, it seems possible that DNA could induce cell death through autophagy under some conditions and in some cell types.
In summary, the accumulation of DNA in the cytoplasm can promote signalling leading to many distinct types of cell death. At this stage there is limited knowledge on the factors that determine which of the death pathways are activated in a given cell type and also how the DNA-stimulated death pathways interact with other DNA-stimulated reactions, and host responses in general.
Biological functions of different types of cell death
The different types of cell death differ with respect to how they impact on host responses to danger sensing (Table 1). Most notably, apoptosis is typically understood to be non-inflammatory in nature, due to the lack of release of cellular content, and the rapid elimination by phagocytic cells. Interestingly, the avoidance of DNA recognition plays an important role in this process. To this end, a recent study has shown that tissue-resident macrophages are programmed to clear apoptotic cells in an immunologically silent manner, due to several features of these cells, including high expression of apoptotic cell recognition receptors and low expression of TLR9 93. Moreover, also cell-intrinsic properties function to avoid activation of the immune system by DNA in the course of apoptosis. As such, the non-inflammatory nature of apoptosis proceeds despite the release of mitochondrial DNA into the cytoplasm in the context of mitochondrial outer membrane permeabilization, which could in principle be sensed by cGAS 94,95. Indeed, apoptotic caspases block induction of type I IFN through the cGAS–STING pathway following release of mitochondrial DNA 94,95, whereas the exact substrates in the cGAS–STING pathway that are targeted by the apoptotic caspases remain to be identified. Thus, apoptotic caspases both induce cell death and prevent the dying cells from activating a DNA-driven IFN response. Despite the generally non-inflammatory nature of apoptosis, it is important to note that apoptotic cells can undergo secondary necrosis 96. Thus, if not rapidly eliminated by phagocytes, apoptosis can lead to inflammation through secondary necrosis. Of note, recent studies have blurred the definitions of apoptotic signalling and programmed necrosis. For certain cell types it has been shown that caspase-3 can cleave gasdermin E (also known as DFNA5), which then results in pyroptotic cell death with the associated release of cytoplasmic content 97,98. Therefore, determined by multiple factors, including the expression of gasdermin E, it appears plausible that apoptotic signalling cascades can evoke mixed types of programmed cell death. Collectively, if apoptotic cells are not properly eliminated or if apoptotic cells do not prevent the cell-autonomous recognition of danger-associated molecular patterns (DAMPs), apoptosis can stimulate inflammation.
Table 1. Features of types of cell death induced by cytoplasmic DNA.
| Type of cell death | Kinetics | Plasma Membrane | Characteristics | Executor | Inflammatory |
|---|---|---|---|---|---|
| Apoptosis | Slow | Preserved integrity | DNA fragmentation Apoptotic bodies | Cleavage of caspase 3 and caspase 7 substrates | No |
| Pyroptosis | Fast | Rupture | Gasdermin cleavage | Gasdermin pores | Yes |
| Necroptosis | Slow | Rupture | MLKL phosphorylation | MLKL pores | Yes |
| Lysosomal cell death | Slow | Rupture1 | Death dependent on components released from lysosomes | Unknown | Yes |
| Autophagic cell death | Slow | Focal rupture | Cell death dependent on autophagy | Unknown | Yes/no |
The degree of plasma membrane rupture depends on the extent of lysosome lysis. Upon limited lysosomal rupture, the cells may be primed to caspase-independent apoptosis-like cell death
Although necroptosis and pyroptosis are induced by different pathways, they are both necrotic types of cell death with membrane rupture and release of intracellular content. One important difference between necroptosis and pyroptosis is that the latter is associated with release of inflammasome-cleaved bioactive cytokines, most notably IL-1β 20,21. Like pyroptosis and necroptosis, lysosomal cell death is also necrotic with plasma membrane rupture and release of cytoplasmic content, including DAMPs. It remains unknown whether cells dying through these different types of programmed necrosis differentially allow release of intracellular content, thereby resulting in a different repertoire of potential DAMP molecules being released.
Autophagy-dependent cell death is characterized by extensive cytoplasmic vacuolization eventually leading to phagocytic uptake and lysosomal degradation 91. As such, this type of cell death is most likely intermediate between apoptosis and different forms of necrosis with respect to stimulation of inflammation. Thus, DNA-stimulated cell death can contribute to both promotion and resolution of inflammation depending on the biological context.
Biological impact of DNA-stimulated PCD
Regarding the role of DNA-activated cell death pathways in defence and disease, there is now accumulating evidence for these pathways playing important roles in both the promotion and control of pathology (Fig. 4). For most studies, it is difficult to discern the contribution of DNA-driven cytokine responses from the PCD pathways being activated, as genetic tools to uncouple these two responses from one another are often not available. At the same time, it still remains challenging to ascertain a certain type of cell death in in vivo settings. In the following section, we try to provide examples in which the distinction between direct PRR signalling effects and PCD cascades was addressed.
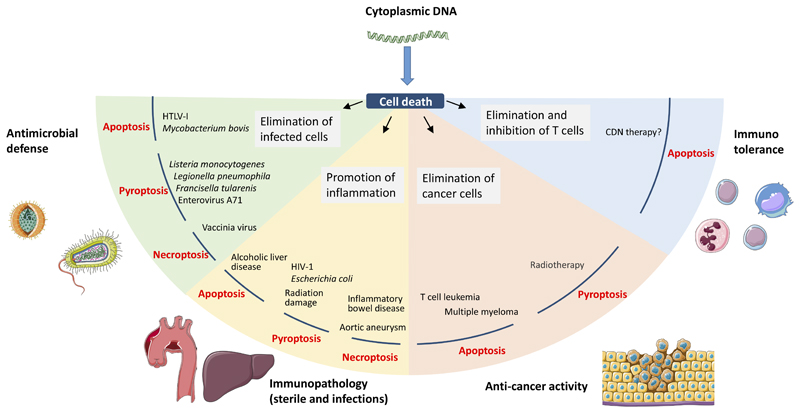
Figure 4. Role of DNA-stimulated programmed cell death in pathology and host protection.
Current understanding of the role of DNA-stimulated apoptosis, necroptosis, and pyroptosis in defense against infections and cancers, promotion of immunopathology, and regulation of immune responses. For more details see the text.
Microbial infections
In response to infections, human T cell leukemia virus type 1 induces apoptosis in human macrophages through STING-dependent triggering of IRF3–BAX complexes 73. Given the reported role for myeloid cells in spread of virus to CD4+ T cells 99, the depletion of the infected monocytes and myeloid precursor DCs via DNA-dependent PCD may function to prevent the spread of the virus and hence limit infection. In a study on Mycobacteroum bovis, it was found that the bacterium induces apoptosis in murine macrophages in a manner dependent on both ER stress and STING–TBK1–IRF3 100. Importantly, inhibiting TBK1 blocked mitochondrial apoptosis, and elevated bacterial replication, thus suggesting DNA induced PCD to block microbial spread. The cGAS–STING pathway was also demonstrated to mediate apoptosis-like cell death in human foreskin fibroblasts infected with HSV-1, as measured by PARP cleavage 101. However, since lysosomal cell death also leads to PARP cleavage, the published data did not resolve whether this occurs through the STING–IRF3–BAX pathway or the STING-induced lysosomal cell death pathway. It will be interesting to learn whether the cGAS–STING-dependent stimulation of apoptosis-like PCD impacts on control of herpesvirus infections. The observation that the HSV-1 ubiquitin E3 ligase ICP0 prevents cGAS–STING-dependent PARP cleavage 101 suggests this to be the case.
Necrotic types of PCD are also induced during infections through DNA-stimulated pathways. During infections with many bacteria, pyroptosis is induced by AIM2 and/or NLRP3 57,102–105. This includes infection with L. monocytogenes, L. pneumophila, Francisella tularenis, Streptococcus pneumonia and Brucella abortus. There is also evidence for activation of DNA-stimulated pyroptosis by viruses 106–108 and intracellular parasites 109. Although it has been reported that pyroptosis can be a central player in antibacterial defence in vivo 110, the importance of DNA-triggered pyroptosis in antimicrobial defense is still not fully established. To this end, it is currently difficult to discern the relevance of inflammasome-dependent cytokine maturation versus the induction of pyroptosis. Regarding viruses, one report has shown an antiviral role of AIM2-stimulated pyroptosis during infection with enterovirus A71 in a neuronal-like cell line 106. Many bacteria and viruses evade the DNA-stimulated inflammasomes, thus underscoring the importance of these pathways in antimicrobial defence. Examples of such infections include F. novicida, which uses a CRISPR/Cas system to maintain membrane integrity and limit AIM2 activation 111, and the HSV1 VP22 protein that binds AIM2 and inhibits downstream signalling 112. However, pyroptosis can also contribute to infection pathology. For instance, the observation that Casp1-/-Casp11-/- mice are protected against Escherichia coli sepsis whereas mice double-deficient in IL-1β and IL-18 are not, suggests that pyroptotic cell death amplifies the inflammatory response in a pathological manner 113. A DNA-dependent example is provided by HIV, which infects CD4+ T cells and eventually causes T cell depletion and immunodeficiency. Greene and coworkers demonstrated that the AIM2-family member IFI16 senses DNA in cells abortively infected with HIV, thus inducing pyroptosis, and potentially contributing to T cell depletion, chronic immune activation and disease pathogenesis 107.
Regarding the role of DNA-driven necroptosis during infections there is only limited information available. However, as outlined above, it has been reported that hyper-activation of STING in mice leads to a shock-like syndrome, which is dependent on TNF and IFNα/β, both of which are also required for STING-dependent necroptosis 89. These data suggest that STING signalling might also engage necroptosis under physiological conditions, such as infections with herpesviruses, which potently activate cGAS–STING signalling43,114. Importantly, murine gammaherpesvirus MHV68, which is closely related to Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus, induces necroptosis in a murine fibroblast cell line in a manner dependent STING and TNF70. Since several DNA viruses that are known to trigger the cGAS–STING pathway also encode proteins that can inhibit caspase 8, for example vaccinia virus 115, it is likely that DNA-driven necroptosis is of importance for control virus infections. Indeed, Ripk3-/- mice are more susceptible to vaccinia virus infection than wild-type mice 116.
Sterile inflammatory diseases
Given the potentially pro-inflammatory role of necrotic types of cell death, it would not be surprising if programmed DNA-stimulated cell pathways also contribute to the pathogenesis of sterile inflammatory diseases. This is indicated by the finding that the STING–IRF3–BAX apoptosis pathway is activated in alcoholic liver disease, and that Irf3-/- mice but not IFNAR-/- mice are protected from disease 117. In many inflammatory diseases where DNA-stimulated pathways have been reported to contribute to the pathogenesis, there is extensive cell death. This includes, systemic lupus erythematosus, Sjögren’s syndrome, and STING-associated vasculopathy with onset in infancy (SAVI) 85,118,119. However, at present there is limited data directly demonstrating that the cell death occurring in these pathologies is induced by DNA. Currently, the data available are indirect. For instance, STING agonists induce apoptosis in endothelial cells and disrupt vasculature 78, which is seen in the above-mentioned diseases. These diseases are also commonly referred to as type I interferonopathies, due to the high level of type I IFNs associated with them. Interestingly, DNA-induced IFN production is subject to negative regulation by AIM2-dependent pyroptosis 11,120. Therefore, lack of full AIM2 activity leads to augmented DNA-stimulated type I IFN responses due to prolonged activation of the cGAS–STING pathway. Two questions emerging from the discussion above are whether type I IFN is in fact the only main driver of the interferonopathies, and whether PCD may play a role. In favor of IFN-independent mechanisms also contributing to the pathogenesis, for at least some interferonopathies, is the finding that mice harbouring the SAVI-mimicking mutation N153S are not protected from disease in the absence of the central IFN-inducing transcription factor IRF3 121. This is, however, in contrast to Trex1-/-Irf3-/- mice, which are protected from cGAS–STING dependent disease 5,51,122. Since, accelerated cell death is observed in SAVI 85, it would be interesting to test whether STING N153S mice with selective defects in STING-dependent death pathways are protected against disease.
Despite the lack of solid evidence for DNA-driven cell death in human inflammatory diseases, the concept of DNA-stimulated cell death as a key driver in sterile inflammation has been demonstrated. Flavell and associates reported that radiation-induced tissue damage was dependent on DNA-induced cell death 123. Importantly Aim2-/- mice were protected against both subtotal body irradiation-induced gastrointestinal syndrome and total body irradiation-induced hematopoietic failure, and the effect of AIM2 was largely mediated by pyroptotic cell death. As another example of DNA-driven cell death in acute sterile inflammation, it was reported that Aortic aneurysm leads to STING-dependent activation of TBK1, RIPK3 and phospho-MLKL in smooth muscle cells. STING deficiency or TBK1 inhibition blocked these responses and improved the disease outcome in mice. These data suggest a role for STING dependent necroptosis in aortic aneurysm 124. Similar observation have been made in a mouse model for inflammatory bowel disease, where STING was found to drive type I IFN and TNFα-mediated necroptosis of intestinal epithelial cells125.
Cancer
Cancer is characterized by uncontrolled cell proliferation but also excessive cell turnover. This leads to accumulation of host DNA in the cytoplasm, which can stimulate beneficial and pathological signalling 37. In the process of proliferation of cancer cells, micronuclei are frequently formed in the cytoplasm, and these can be detected by cGAS 126,127. This could suggest a role for the cGAS–STING pathway, and maybe cytosolic DNA sensing in general in tumour immunity. In line with this, many cancer cells have lost STING expression, and clear effects of STING gene deletion or STING agonists on cancer development have been reported 54,55,128–130. On the other hand, cytosolic DNA may also drive innate immune responses that promote cancer progression 37,131.
The ability of the STING pathway to induce apoptosis or apoptosis-like cell death can directly target STING-expressing tumour cells for death following accumulation of DNA in the cytoplasm. In mouse models for T cell leukemia and multiple myeloma, treatment with STING agonists induces apoptosis in cancer cells and this promotes tumour control and improves disease outcome 76,132. These data reveal a therapeutic potential for CDNs as direct tumoricidal agents against STING-expressing tumour cells. It will be interesting to learn whether cell-autonomous apoptosis induced by endogenous DNA derived from tumour cells is involved in tumour immunosurveillance. On the other hand, since the T cell response is a major player in tumour immunity, apoptosis of lymphocytes may also hamper the anti-tumour immune response. To this end, it has been reported that STING agonists induce tolerogenic effects on T cell responses, so there is a need to understand this phenomenon in more detail133.
Many of the current cancer therapies target cancer cells through either irradiation or inhibition of DNA replication. As discussed above irradiation induces AIM2 activation and pyroptotic cell death 123. In addition, chemotherapeutic agents such as cisplatin and etoposide inhibit DNA replication37. Thus, the mechanisms through which conventional anti-cancer therapies kill cancer cells may involve DNA-activated PCD pathways. Therefore, altered expression of proteins in the DNA=sensing death pathway by cancer cells may not only affect tumour growth, but could also impact on the efficiency of therapy.
Mechanistic understanding of DNA-stimulated cell death pathways and their role in human pathologies may allow development of new treatments. There is currently a large interest in development and therapeutic use of agonists and antagonists for the cGAS–STING pathway, and significant progress has already been made 134–137. The field is thus in a favorable position to rapidly take advantage of such new discoveries, to potentially target DNA-triggered PCD in a number of diseases.
Concluding remarks and future outstanding questions
In recent years it has become clear that cells can succumb to cell death through a broad range of programmed mechanisms. At the same time, it has become apparent that these pathways constitute a central part of the immune response to infections and also the restoration of homeostasis under sterile conditions. Accumulation of cytoplasmic DNA, which occurs during infections, sterile tissue damage, autoimmune diseases, and cancer, is a very potent danger signal that has mainly been reported to induce the production of cytokines such as type I IFNs and IL-1β2. However, as outlined in this review, cytoplasmic DNA also induces a number of PCD pathways, which we are now starting to understand at the molecular level. Furthermore, there is accumulating evidence for these pathways being of relevance for the beneficial and pathological biological activities of cytoplasmic DNA. With the recent advances in this field, a number of outstanding questions have emerged. For instance, what determines whether DNA stimulates type I IFNs, IL-1β, apoptosis, autophagy, necroptosis, lysosomal cell death and/or pyroptosis? Possible influencing factors include; the strength of signal induced by DNA recognition, the duration of signalling, the cell type sensing DNA, the length and format of the DNA, whether there is microbial modulation of specific DNA-sensing pathways, the metabolic state of the host, and the local cytokine environment. Also, we still do not understand many of the molecular details of the pathways for DNA-stimulated PCD. In addition, much more work and better tools are required to fully characterize the roles of the different DNA-stimulated PCDs in diseases.
On a final note, the discovery that cytosolic DNA can induce multiple types of PCD has revealed the ironic phenomenon that the immune system uses ‘the molecule of life’ to induce death in many different ways.
Acknowledgments
SRP is funded by The European Research Council (ERC-AdG ENVISION; 786602); The Novo Nordisk Foundation (NNF18OC0030274), and the Lundbeck Foundation (R198-2015-171; R268-2016-3927); VH is funded by the The European Research Council (ERC-CoG GENESIS; 647858) and the DFG (CRC/Transregio 237; CRC 1335; SPP 1923).
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napirei M, et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 5.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs A, Cox RA, Rotem Z. Foreign nucleic acids as the stimulus to make interferon. Lancet. 1963;2:113–116. doi: 10.1016/s0140-6736(63)92585-6. [DOI] [PubMed] [Google Scholar]
- 7.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. doi:science.1232458 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogunjimi B, et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J Clin Invest. 2017;127:3543–3556. doi: 10.1172/JCI92280. doi:92280 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 14.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad-Nejad P, et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 18.Hornung V, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A. 2013;110:2969–2974. doi: 10.1073/pnas.1222694110. doi:1222694110 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 21.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. doi:nri.2015.7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin TC, et al. Structures of the HIN Domain: DNA Complexes Reveal Ligand Binding and Activation Mechanisms of the AIM2 Inflammasome and IFI16 Receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrone SR, et al. Assembly-driven activation of the AIM2 foreign-dsDNA sensor provides a polymerization template for downstream ASC. Nat Commun. 2015;6:7827. doi: 10.1038/ncomms8827. doi:ncomms8827 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerur N, et al. IFI16 Acts as a Nuclear Pathogen Sensor to Induce the Inflammasome in Response to Kaposi Sarcoma-Associated Herpesvirus Infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 26.Gaidt MM, et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell. 2017;171:1110–1124. doi: 10.1016/j.cell.2017.09.039. doi:S0092-8674(17)31133-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, et al. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. doi:science.1229963 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZQ, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nature Immunology. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu YH, Macmillan JB, Chen ZJ. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almine JF, et al. IFI16 and cGAS cooperate in activation of STING during DNA sensing in human keratinocytes. Nat Commun. 2017;8 doi: 10.1038/ncomms14392. 14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jønsson KL, et al. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat Commun. 2017;8 doi: 10.1038/ncomms14391. 14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015 doi: 10.1126/science.aaa2630. doi:science.aaa2630 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol. 2014;88:5328–5341. doi: 10.1128/JVI.00037-14. doi:JVI.00037-14 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn J, et al. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. doi: 10.1038/ncomms6166. doi:ncomms6166 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang R, et al. NEMO-IKKbeta Are Essential for IRF3 and NF-kappaB Activation in the cGAS-STING Pathway. J Immunol. 2017;199:3222–3233. doi: 10.4049/jimmunol.1700699. doi:jimmunol.1700699 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Inflammasome Activation Triggers Caspase-1-Mediated Cleavage of cGAS to Regulate Responses to DNA Virus Infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee I, et al. Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis. Immunity. 2018;49:413–426 e415. doi: 10.1016/j.immuni.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li XD, et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. doi:science.1244040 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinert LS, et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defense in the CNS. Nat Commun. 2016;7 doi: 10.1038/ncomms13348. 13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippmann J, et al. Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice. Cell Microbiol. 2011;13:1668–1682. doi: 10.1111/j.1462-5822.2011.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson RO, et al. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. doi:S1931-3128(15)00208-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen K, et al. Listeria monocytogenes induces IFNβ expression through an IFI16, cGAS, and STING dependent pathway. EMBO J. 2014;33:1654–1666. doi: 10.15252/embj.201488029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. PPATHOGENS-D-13-01967 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crow YJ, et al. Mutations in the gene encoding the 3 '-5 ' DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nature Genetics. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 49.Rodero MP, et al. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nat Commun. 2017;8:2176. doi: 10.1038/s41467-017-01932-3. 10.1038/s41467-017-01932-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J Virol. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gall A, et al. Autoimmunity Initiates in Nonhematopoietic Cells and Progresses via Lymphocytes in an Interferon-Dependent Autoimmune Disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. doi:1215006109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King KR, et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med. 2017;23:1481–1487. doi: 10.1038/nm.4428. doi:nm.4428 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. doi:S1074-7613(14)00393-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. doi:S1074-7613(14)00395-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Periasamy S, Le HT, Duffy EB, Chin H, Harton JA. Inflammasome-Independent NLRP3 Restriction of a Protective Early Neutrophil Response to Pulmonary Tularemia. PLoS Pathog. 2016;12:e1006059. doi: 10.1371/journal.ppat.1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schattgen SA, Gao G, Kurt-Jones EA, Fitzgerald KA. Cutting Edge: DNA in the Lung Microenvironment during Influenza Virus Infection Tempers Inflammation by Engaging the DNA Sensor AIM2. J Immunol. 2016;196:29–33. doi: 10.4049/jimmunol.1501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H, et al. AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality. J Immunol. 2017;198:4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dombrowski Y, et al. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lian Q, et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27:784–800. doi: 10.1038/cr.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen SB, et al. Activation of Autophagy by alpha-Herpesviruses in Myeloid Cells Is Mediated by Cytoplasmic Viral DNA through a Mechanism Dependent on Stimulator of IFN Genes. Journal of Immunology. 2011;187:5268–5276. doi: 10.4049/jimmunol.1100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prabakaran T, et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37:e97858. doi: 10.15252/embj.201797858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wenzel M, et al. Cytosolic DNA Triggers Mitochondrial Apoptosis via DNA Damage Signaling Proteins Independently of AIM2 and RNA Polymerase III. Journal of Immunology. 2012;188:394–403. doi: 10.4049/jimmunol.1100523. [DOI] [PubMed] [Google Scholar]
- 69.Chattopadhyay S, Yamashita M, Zhang Y, Sen GC. The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol. 2011;85:3708–3716. doi: 10.1128/JVI.02133-10. doi:JVI.02133-10 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schock SN, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24:615–625. doi: 10.1038/cdd.2016.153. doi:cdd2016153 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol. 1993;71(Pt 2):75–85. doi: 10.1038/icb.1993.8. [DOI] [PubMed] [Google Scholar]
- 72.Sagulenko V, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. doi:cdd201337 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sze A, et al. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe. 2013;14:422–434. doi: 10.1016/j.chom.2013.09.009. doi:S1931-3128(13)00329-6 [pii] [DOI] [PubMed] [Google Scholar]
- 74.Chattopadhyay S, et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. doi:emboj201050 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chattopadhyay S, Kuzmanovic T, Zhang Y, Wetzel JL, Sen GC. Ubiquitination of the Transcription Factor IRF-3 Activates RIPA, the Apoptotic Pathway that Protects Mice from Viral Pathogenesis. Immunity. 2016;44:1151–1161. doi: 10.1016/j.immuni.2016.04.009. doi:S1074-7613(16)30138-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gulen MF, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8:427. doi: 10.1038/s41467-017-00573-w. 10.1038/s41467-017-00573-w [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Besch R, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest. 2009;119:2399–2411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ching LM, et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-Dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86:1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ching LM, Zwain S, Baguley BC. Relationship between tumour endothelial cell apoptosis and tumour blood flow shutdown following treatment with the antivascular agent DMXAA in mice. Br J Cancer. 2004;90:906–910. doi: 10.1038/sj.bjc.6601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prantner D, et al. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem. 2012;287:39776–39788. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conlon J, et al. Mouse, but not Human STING, Binds and Signals in Response to the Vascular Disrupting Agent 5,6-Dimethylxanthenone-4-Acetic Acid. J Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. doi:jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Q, Man SM, Karki R, Malireddi RKS, Kanneganti TD. Detrimental Type I Interferon Signaling Dominates Protective AIM2 Inflammasome Responses during Francisella novicida Infection. Cell Rep. 2018;22:3168–3174. doi: 10.1016/j.celrep.2018.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. doi:ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margolis SR, Wilson SC, Vance RE. Evolutionary Origins of cGAS-STING Signaling. Trends Immunol. 2017;38:733–743. doi: 10.1016/j.it.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerboni S, et al. Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J Exp Med. 2017;214:1769–1785. doi: 10.1084/jem.20161674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonugunta VK, et al. Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-tumor Response. Cell Rep. 2017;21:3234–3242. doi: 10.1016/j.celrep.2017.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moretti J, et al. STING Senses Microbial Viability to Orchestrate Stress-Mediated Autophagy of the Endoplasmic Reticulum. Cell. 2017;171:809–823. doi: 10.1016/j.cell.2017.09.034. doi:S0092-8674(17)31128-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular Nucleic Acid Sensing Triggers Necroptosis through Synergistic Type I IFN and TNF Signaling. J Immunol. 2018;200:2748–2756. doi: 10.4049/jimmunol.1701492. doi:jimmunol.1701492 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarhan J, et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2018 doi: 10.1038/s41418-018-0122-7. 10.1038/s41418-018-0122-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–344. doi: 10.1038/nrm3999. doi:nrm3999 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang Q, et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. doi:S1931-3128(14)00032-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts AW, et al. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity. 2017;47:913–927 e916. doi: 10.1016/j.immuni.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. doi:S0092-8674(14)01514-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.White MJ, et al. Apoptotic Caspases Suppress mtDNA-Induced STING-Mediated Type I IFN Production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. doi:S0092-8674(14)01513-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 97.Rogers C, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8 doi: 10.1038/ncomms14128. 14128, doi:ncomms14128 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. doi:nature22393 [pii] [DOI] [PubMed] [Google Scholar]
- 99.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. doi: 10.1038/nm1745. doi:nm1745 [pii] [DOI] [PubMed] [Google Scholar]
- 100.Cui Y, et al. Mycobacterium bovis Induces Endoplasmic Reticulum Stress Mediated-Apoptosis by Activating IRF3 in a Murine Macrophage Cell Line. Front Cell Infect Microbiol. 2016;6:182. doi: 10.3389/fcimb.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diner BA, Lum KK, Toettcher JE, Cristea IM. Viral DNA Sensors IFI16 and Cyclic GMP-AMP Synthase Possess Distinct Functions in Regulating Viral Gene Expression, Immune Defenses, and Apoptotic Responses during Herpesvirus Infection. MBio. 2016;7 doi: 10.1128/mBio.01553-16. doi:mBio.01553-16 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim S, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomes MT, et al. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol. 2013;190:3629–3638. doi: 10.4049/jimmunol.1202817. doi:jimmunol.1202817 [pii] [DOI] [PubMed] [Google Scholar]
- 104.Sauer JD, et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. doi:S1931-3128(10)00110-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Man SM, et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016;167:382–396. doi: 10.1016/j.cell.2016.09.012. doi:S0092-8674(16)31245-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yogarajah T, Ong KC, Perera D, Wong KT. AIM2 Inflammasome-Mediated Pyroptosis in Enterovirus A71-Infected Neuronal Cells Restricts Viral Replication. Sci Rep. 2017;7:5845. doi: 10.1038/s41598-017-05589-2. 10.1038/s41598-017-05589-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monroe KM, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. doi:science.1243640 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eichholz K, et al. Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog. 2016;12:e1005871. doi: 10.1371/journal.ppat.1005871. PPATHOGENS-D-16-00373 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalantari P, et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2014;6:196–210. doi: 10.1016/j.celrep.2013.12.014. doi:S2211-1247(13)00759-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aachoui Y, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. doi:science.1230751 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampson TR, et al. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proc Natl Acad Sci U S A. 2014;111:11163–11168. doi: 10.1073/pnas.1323025111. 1323025111 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maruzuru Y, et al. Herpes Simplex Virus 1 VP22 Inhibits AIM2-Dependent Inflammasome Activation to Enable Efficient Viral Replication. Cell Host Microbe. 2018;23:254–265. doi: 10.1016/j.chom.2017.12.014. S1931-3128(17)30550-4 [pii] [DOI] [PubMed] [Google Scholar]
- 113.Sarkar A, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. 200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paijo J, et al. cGAS Senses Human Cytomegalovirus and Induces Type I Interferon Responses in Human Monocyte-Derived Cells. PLoS Pathog. 2016;12:e1005546. doi: 10.1371/journal.ppat.1005546. PPATHOGENS-D-15-02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tewari M, Dixit VM. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 116.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrasek J, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. 1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mistry P, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol. 2017;185:59–73. doi: 10.1016/j.clim.2016.08.010. doi:S1521-6616(16)30277-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nikolov NP, Illei GG. Pathogenesis of Sjogren's syndrome. Curr Opin Rheumatol. 2009;21:465–470. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Corrales L, et al. Antagonism of the STING Pathway via Activation of the AIM2 Inflammasome by Intracellular DNA. J Immunol. 2016;196:3191–3198. doi: 10.4049/jimmunol.1502538. doi:jimmunol.1502538 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Warner JD, et al. STING-associated vasculopathy develops independently of IRF3 in mice. J Exp Med. 2017;214:3279–3292. doi: 10.1084/jem.20171351. doi:jem.20171351 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci U S A. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. 1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu B, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science. 2016;354:765–768. doi: 10.1126/science.aaf7532. 354/6313/765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo W, et al. Activation of the STING Cytosolic DNA Sensor Signaling Contributes to Aortic Aneurysm, Dissection and Rupture in Mice. Circulation. 2018;136 A16944. [Google Scholar]
- 125.Aden K, et al. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med. 2018 doi: 10.1084/jem.20171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harding SM, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–470. doi: 10.1038/nature23470. doi:nature23470 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mackenzie KJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461–465. doi: 10.1038/nature23449. doi:nature23449 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350:568–571. doi: 10.1126/science.aab3291. doi:science.aab3291 [pii] [DOI] [PubMed] [Google Scholar]
- 129.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep. 2016;14:282–297. doi: 10.1016/j.celrep.2015.12.029. doi:S2211-1247(15)01453-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corrales L, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. doi:S2211-1247(15)00432-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bakhoum SF, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi: 10.1038/nature25432. doi:nature25432 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang CH, et al. Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res. 2016;76:2137–2152. doi: 10.1158/0008-5472.CAN-15-1885. 0008-5472.CAN-15-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang L, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. doi:jimmunol.1301419 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fu J, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa4306. 283ra252, 7/283/283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skouboe MK, et al. STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice. PLoS Pathog. 2018;14:e1006976. doi: 10.1371/journal.ppat.1006976. PPATHOGENS-D-17-01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vincent J, et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat Commun. 2017;8 doi: 10.1038/s41467-017-00833-9. 750, 10.1038/s41467-017-00833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holm CK, et al. Influenza A virus targets a cGAS independent STING pathway, which controls enveloped RNA viruses. Nat Commun. 2016;7 doi: 10.1038/ncomms10680. 10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 139.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 141.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt:11–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 142.Civril F, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–337. doi: 10.1038/nature12305. doi:nature12305 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao P, et al. Cyclic [G(2',5')pA(3',5')p] Is the Metazoan Second Messenger Produced by DNA-Activated Cyclic GMP-AMP Synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. doi:S0092-8674(13)00522-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kranzusch PJ, Lee AS, Berger JM, Doudna JA. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 2013;3:1362–1368. doi: 10.1016/j.celrep.2013.05.008. doi:S2211-1247(13)00225-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karayel E, et al. The TLR-independent DNA recognition pathway in murine macrophages: Ligand features and molecular signature. Eur J Immunol. 2009;39:1929–1936. doi: 10.1002/eji.200939344. [DOI] [PubMed] [Google Scholar]
- 146.Luecke S, et al. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18:1707–1715. doi: 10.15252/embr.201744017. doi:embr.201744017 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 149.Nagata S, Tanaka M. Programmed cell death and the immune system. Nat Rev Immunol. 2017;17:333–340. doi: 10.1038/nri.2016.153. doi:nri.2016.153 [pii] [DOI] [PubMed] [Google Scholar]
- 150.Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 2010;584:4491–4499. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 151.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 152.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 153.Hersh D, et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. doi:nature15514 [pii] [DOI] [PubMed] [Google Scholar]
- 155.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. doi:nature15541 [pii] [DOI] [PubMed] [Google Scholar]
- 156.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. doi:nature18590 [pii] [DOI] [PubMed] [Google Scholar]
- 158.Sborgi L, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. doi:embj.201694696 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. doi:nchembio711 [pii] [DOI] [PubMed] [Google Scholar]
- 161.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. doi:ncb2883 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen X, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. doi:cr2013171 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. doi:S1097-2765(14)00208-1 [pii] [DOI] [PubMed] [Google Scholar]
- 164.Gunther C, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. doi:nature10400 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aits S, Jaattela M. Lysosomal cell death at a glance. J Cell Sci. 2013;126:1905–1912. doi: 10.1242/jcs.091181. 126/9/1905. [DOI] [PubMed] [Google Scholar]