Abstract
Purpose
Tryptophan 2,3-dioxygenase (TDO), encoded by the gene TDO2, is an enzyme that catalyses the first and rate-limiting step of tryptophan (Try) degradation in the kynurenine (Kyn) pathway in the liver. Recently, TDO has been demonstrated to be expressed in various human tumours, especially hepatocellular carcinoma (HCC). However, the role of TDO in HCC is still not very clear. Here, we studied the role of TDO in HCC.
Methods
We demonstrated that TDO is overexpressed in human HCC tissues and is significantly correlated with malignant phenotype characteristics, including tumour size, tumour differentiation, vascular invasion, etc. Kaplan–Meier analysis showed a poor overall survival rate in patients with TDO-overexpressing tumours. In addition, the effects of TDO on HCC tumour growth and metastasis were detected both in vivo and in vitro. TDO overexpression facilitated HCC cell growth, invasion and migration.
Conclusion
Our results suggest that TDO positively regulates HCC proliferation and invasion and acts as a new prognostic biomarker of HCC.
Keywords: TDO, tryptophan 2, 3-dioxygenase, hepatocellular carcinoma, tryptophan
Introduction
Cancer death rates have continuously declined over 20 years, with an overall drop of 25%; however, the incidence rates and death rates of liver cancer continue to increase rapidly.1,2 HCC accounts for 90% of liver cancer, and even the incidence and death rates of HCC in China still ranked fourth and third among tumour diseases in 2015, respectively.3 Clinically, alpha fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen (CA199) are commonly used to predict the occurrence and prognosis of HCC; however, they are not sensitive and specific enough for the early detection of HCC and postoperative recurrence.4,5 Thus, there is an urgent need to better understand the biology of HCC and develop sensitive biomarkers for HCC.
TDO is a homotetrameric cytosolic enzyme that was thought to be expressed only in the liver. TDO is the rate-limiting enzyme in the first step of Try metabolism in mammals and converts Try to produce Kyn.6,7 In addition, TDO has been implicated as a key regulator of neurotoxicity involved in neurodegenerative diseases and ageing.8,9 Schmidt et al found that HeLa cells expressing recombinant TDO were capable of inhibiting the growth of bacteria, parasites and viruses.10 TDO-positive cells were capable of inhibiting anti CD3-driven T-cell proliferation and IFN-γ production.10 TDO was first found to be expressed in human glioma cells and then in many human tumours, such as hepatocarcinomas, melanomas, bladder carcinomas, breast cancer, and other tumour tissues, where it regulates the tumour immune response.11 Pharmacological inhibition of TDO prevents tumoural immune resistance and promotes tumour rejection.12,13 TDO overexpression results in resistance to immune rejection by T cells in a P815 mouse tumour model, and mice grafted with TDO-overexpressing tumour cells developed progressive tumours and died faster.12,14 Overexpression of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein theta in primary tumours was linked to poor prognosis of patients with HCC.15 However, whether TDO is upregulated or downregulated in HCC tissue is still unclear. In addition, the role of TDO in the tumorigenesis of HCC has still not been fully explored.
In this study, we examined TDO expression in an HCC clinical tissue microarray (TMA) by using immunohistochemical (IHC) analysis to explore whether the expression of TDO is correlated with the pathological features and clinical prognosis of HCC. We report that TDO is overexpressed in human HCC tissues, which is significantly correlated with malignant phenotype characteristics. Kaplan–Meier survival analysis showed a poor overall survival rate in patients with TDO-overexpressing tumours. The effects of TDO on cell functional assays were further assessed in vitro and in vivo. Our results demonstrate that the overexpression and knockdown of TDO promoted and inhibited HCC cell proliferation, respectively.
Patients and Methods
Patients and Tissue Specimens
A total of 93 patients with primary HCC tissues and matched adjacent normal tissues who underwent curative resection at Shanghai General Hospital (Shanghai, China) between January 2010 and December 2015 were reviewed. There were 83 males and 10 females with a mean age of 60 years (range 38–78 years). Primary fresh cancer tissues and matched normal adjacent tissues were obtained from 40 hepatocellular carcinoma patients (35 males and 5 females) who had not received chemotherapy, radiotherapy or other related anti-tumour therapies prior to surgery at Shanghai General Hospital. All these tissues were collected following surgical resection and stored at −80°C immediately for further RNA and protein extraction. Informed consent was obtained from the patients, and this research was approved by the Institutional Research Ethics Committee of Shanghai General Hospital and the ethical guidelines of Helsinki. All the diagnoses were confirmed by at least two certified pathologists who did not know the patients’ information. The tumour grade and stage classification were based on the international union against cancer guidelines.16 Disease-free survival (DFS) and overall survival (OS) rates were defined as the interval from initial surgery to clinically or radiologically proven recurrence/metastasis and death, respectively. Follow-up occurred for at least 4 years or until patient death.
Quantitative Real-Time PCR
Total RNA was isolated from liver tissues and cells by TRIzol reagent (Takara, Japan). After RNA intensity and purity were checked, cDNA was synthesized from 2 mg total RNA using the Prime Script RT reagent kit (Takara, Japan). Then, 1 mg cDNA was used as a template for qRT-PCR with SYBR Premix Ex Taq II (Takara, Japan) according to the manufacturer’s instructions. The primers used for qRT-PCR were as follows: TDO, forward: 5′-CACCGTGTGGTGGTCATCTT-3′ and reverse 5′-GGAAGCCTGATGCTGGAGAC-3′; GAPDH, forward: 5′-GGGAAGGTGAAGGTCGGAGT-3′ and reverse 5′-GGGGTCATTGATGGCAACA-3′. Normalization with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to obtain the relative quantities [Δ cycle threshold (Ct) values]. The copy number of each PCR product was three, and the relative mRNA levels were calculated by the 2−ΔΔCt method.
Western Blot Analysis
Tissues or cells were lysed by RIPA lysis buffer (Beyotime Biotechnology, China), and the protease inhibitor phenylmethanesulfonyl fluoride was added for 30 min at 4°C. According to the reagent instructions, the protein concentration was assayed by a BCA protein assay kit (Beyotime Biotechnology, China). The total protein was mixed with SDS-PAGE sample loading buffer (Beyotime Biotechnology, China) in a 4:1 proportion, incubated at 100°C for denaturation, and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the proteins were transferred onto PVDF membranes (Millipore, Billerica, MA) after electrophoresis in a 4°C environment. Membranes were blocked in 5% milk-TBST (0.05% Tween-20, pH=7.4) for 2 h at normal temperature, washed with TBST three times, five min each time, and incubated with primary antibodies at 4°C overnight. The primary antibodies included TDO2 antibody (1:500, Novus, USA) and GAPDH monoclonal antibody (1:2000, ProteinTech, USA). After incubation with primary antibody, the membranes were washed three times with TBST; each wash was 10 min. Then, secondary antibody was added and incubated for 2 h at normal temperature. The secondary antibodies included goat anti-rabbit IgG (1:2000, ProteinTech, USA) and goat anti-mouse IgG (1:2000, ProteinTech, USA). After washing three times, proteins were detected by ECL reagent (Millipore, Billerica, MA).
Immunochemistry
Before antigen retrieval in citrate buffer, TMAs were dewaxed and rehydrated and a graded series of ethanol. Then, the cells were incubated with anti-TDO2 antibody (Novus) overnight at 4°C and incubated with an HRP-conjugated secondary antibody for 30 min at room temperature. The staining intensity for TDO was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (intense). The staining area was scored as 0 (0), 1 (1~25%), 2 (26~50%), 3 (51~75%), or 4 (76~100%). After multiplying the staining intensity score by the staining area score, the total score index was designated follows: 0~3, negative expression; 4~6, weak expression; and 8~12, strong expression, according to the intensity and total area of the stain, The score product of staining intensity and percentage by IHC were analyzed the Semi-quantitative scoring method.17,18 The intensity and extent of staining were scored independently by two experienced pathologists who did not know the patients’ information.
Cell Culture and Establishment of Stable Cells with TDO Knockdown or Overexpression
The hepatocellular carcinoma cell lines LM3, HepG2, Huh-7, SMMC-7721 (7721), and MHCC-97H (97H) and the normal liver cell line Lo2 were obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cell culture was performed using Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY) containing 10% foetal bovine serum and 1% penicillin streptomycin (Gibco, USA). Cells were grown under a moist atmosphere containing 5% CO2 at 37°C. Lentivirus preparation and transduction were performed according to the manufacturer’s protocol to construct stable cells. Short hairpin RNA (shRNA) plasmids for TDO and the control plasmid were purchased from Ribobio (Guangzhou, China). Control, shRNA (TDO), or TDO plasmids were cotransfected with the packaging plasmids into 293T cells using Lipofectamine 2000 (Invitrogen, CA). The efficiency of TDO knockdown or overexpression was validated by qRT-PCR and immunoblot analyses.
Cell Proliferation
Cells were seeded in 96-well plates (2000 cells per well), with each well containing 100 mL medium. After culturing cells for 6, 24, 48, 72, and 96 h, 10 mL Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) was added to each well; cells were then incubated for an additional 2 h. Finally, the absorbance was measured at 450 nm by Varioskan LUX (Thermo Fisher, CA, USA). The experiments were performed as previously described and run three times.19
Plate Colony Formation Assays
Experimentally transfected cells were seeded in six-well plates and cultured for 14 days. The cells were then fixed with methyl alcohol for 15 min and stained with crystal violet solution for 15 min. Then, colony cells were counted, and the plates were photographed. The experiments were performed as previously described and run three times.20
Transwell Assays
The transwell 24-well Boyden chamber (Corning, USA) with 8.0 μm pore size polycarbonate membrane was used for the cell invasion assays (with Matrigel) according to the manufacturer’s protocol. Briefly, each group of cells (5 ×104/chamber) was plated in the upper chambers in 200 ul serum-free media for 36 h, while the bottom chambers contained 600 ul media supplemented with 10% fetal bovine serum (FBS) as a chemoattractant. Cells that migrated and invaded to the reverse side of chamber inserts were fixed by methyl alcohol and stained with 0.1% crystal violet. The experiments were performed as previously described and run in three times.21
Tumor Growth Assay in vivo
All animal experiments were approved by the Animal Care Committee of Shanghai Jiao Tong University Affiliated Shanghai General Hospital and performed according to the animal welfare guidelines of the People’s Republic of China National Standard (GB/T 35,892‐2018). Four-week-old male BALB/c nude mice were used and randomly divided into two groups and each group had five mice. For the subcutaneous tumour growth assay, experimental or control cells (1 × 107/200 μL) were injected subcutaneously into the groin of nude mice. Tumour diameters were measured every 3 days after the injection 1 week. Tumour volume was calculated according to the formula: volume = length × width 2× 1/2. Three weeks later, all mice were sacrificed, and tumours were removed and fixed with formalin.
Statistical Analysis
Data analysis were carried out using the SPSS 22.0 statistical software package (SPSS, Chicago, IL, USA). Differences of TDO mRNA expression between hepatocellular carcinoma tissues and normal adjacent tissues were estimated by the Student’s T-test. The χ2 test was appropriately used to determine the statistical significance between TDO expression and clinicopathological variables. Survival curves were calculated by the Kaplan–Meier method with the Log rank test employed for the comparison of differences. For all tests, P value<0.05 considered to be statistically significant.
Results
TDO Is Overexpressed in HCC Tissues, Which Predicts Poor Clinical Outcomes in HCC Patients
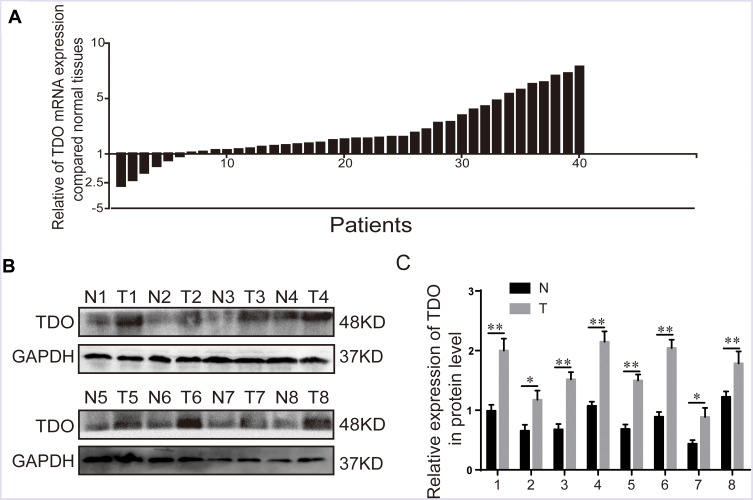
Forty paired specimens were selected to investigate the TDO transcript levels in HCC tissues and matched normal adjacent tissues by quantitative real-time PCR. HCC samples had significantly higher levels of TDO transcript than normal adjacent tissues (Figure 1A). Western blot analysis further confirmed that TDO expression was increased in HCC tissues compared with normal adjacent tissues (Figure 1B and C).
Figure 1.
The expression of TDO in HCC tissues and paired normal adjacent tissues. Quantitative real-time PCR detection of relative TDO expression in 40 human HCC tissue specimens (T=40) and paired adjacent normal tissues (N=40) (A). The logarithmic scale 2ΔΔCt was used to calculate the relative TDO2 expression. Western blot analysis was used to detect TDO protein expression in 8 representative paired HCC tissue specimens (B and C). Error bars represent the SD. *P < 0.05 and **P < 0.01.
Correlation Between TDO Expression and Clinical Pathological Characteristics in HCC
We further explored whether HCC tissues had higher TDO expression than adjacent normal tissues by immunohistochemistry (IHC). A tissue microarray was performed in 93 pairs of HCC and matched normal adjacent tissues. We observed that TDO expression was mainly located in the cytoplasm. We divided TDO expression of the 93 HCC patients into high (77/93) (Figure 2A and E) and low level (16/93) (Figure 2C and G) groups by using the staining intensity in the corresponding normal adjacent tissues as a cut-off point (Figure 2B and F, D and H) (Table 1). The results confirmed that TDO was overexpressed in tumour tissues (P<0.001). The relationship between TDO expression and clinicopathological characteristics is shown in Table 2. The results demonstrated that TDO was obviously upregulated in HCC tissues compared to normal adjacent tissues (p<0.001). Moreover, TDO overexpression was highly correlated with clinicopathological characteristics. We found that TDO overexpression was associated with tumour size (P=0.006), tumour differentiation (P=0.043), and vascular invasion (P=0.022), but there was no evidence that high TDO expression was associated with sex, age, tumour number, liver cirrhosis and hepatitis virus (P>0.05). The relationship between TDO, overall survival (OS) or disease-free survival (DFS) was detected by Kaplan–Meier survival analysis with Log rank testing. Patients with high TDO expression had poorer OS (P=0.001) (Figure 3A) and DFS (P=0.002) (Figure 3B) than those with low TDO expression. Similar phenomena were observed between the tumour differentiation UICC-stage I–II (P=0.027, Figure 3C) and UICC-stage III–IV (P=0.045) (Figure 3D), in which the higher the stage was, the worse the prognosis. There was a similar trend towards shorter OS in patients with high TDO-expressing tumours than in those with low TDO-expression tumours without relapse (P=0.001) (Figure 3E) or with relapse (P=0.037, Figure 3F). In conclusion, high expression of TDO can act as a novel independent prognostic biomarker for shorter overall survival independent of advanced clinical stage and tumour relapse.
Figure 2.
Immunohistochemical staining for TDO expression in HCC tissues and paired normal adjacent tissues. TDO high expression of TDO in HCC (A and E) and TDO expression in normal adjacent tissues (B and F). Low expression of TDO in HCC tissues (C and G) and in normal adjacent tissues (D and H). (A–D) Original magnification: 100×; (E–H) original magnification: 200×.
Table 1.
Expression of TDO in 93 Cases of HCC and Adjacent Normal Liver Tissues (χ2-Test)
| Tissue Sample | N | TDO | Expression | P value |
|---|---|---|---|---|
| High (%) | Low (%) | |||
| Normal tissue | 93 | 55 (59.1) | 38 (40.9) | <0.001 |
| Tumor tissue | 93 | 77 (82.8) | 16 (17.2) |
Table 2.
Correlation Between Clinicopathologic Features and the Expression of TDO in 93 Cases of HCC Tissues (χ2-Test)
| N | TDO | Expression | P value | |
|---|---|---|---|---|
| High (%) | Low (%) | |||
| Age (year) | 0.094 | |||
| <60 | 68 | 59 (86.8%) | 9 (13.2%) | |
| ≥60 | 25 | 18 (72.0%) | 7 (28.0%) | |
| Gender | 0.523 | |||
| Male | 83 | 68 (81.9%) | 15(18.1%) | |
| Female | 10 | 9 (90.0%) | 1 (10.0%) | |
| Tumor number | 0.091 | |||
| Single | 80 | 65 (81.3%) | 15 (18.8%) | |
| Multiple | 13 | 12 (92.3%) | 1 (7.7%) | |
| Tumor size | 0.006 | |||
| <5 cm | 41 | 29 (70.7%) | 12 (29.3%) | |
| ≥5cm | 52 | 48 (92.3%) | 4 (7.7%) | |
| Tumor differentiation | 0.043 | |||
| I–II | 61 | 47 (77.0%) | 14 (23.0%) | |
| III–IV | 32 | 30 (93.8%) | 2 (6.3%) | |
| Tumor location | 0.056 | |||
| Left liver | 34 | 24 (70.6%) | 10 (29.4%) | |
| Right liver | 57 | 51 (89.5%) | 6 (10.5%) | |
| Whole liver | 2 | 2 (100.0%) | 0 (0.0%) | |
| Vascular invasion | 0.022 | |||
| Yes | 28 | 27 (96.4%) | 1 (3.6%) | |
| No | 65 | 50(76.9%) | 15 (23.1%) | |
| Liver cirrhosis | 0.268 | |||
| Yes | 36 | 32 (88.9%) | 4 (11.1%) | |
| No | 57 | 45 (78.9%) | 12 (21.1%) | |
| Hepatitis virus | 0.448 | |||
| Yes | 11 | 10 (90.9%) | 1 (9.1%) | |
| No | 82 | 67 (81.7%) | 15 (18.3%) |
Figure 3.
Kaplan–Meier analysis with Log rank testing of survival in HCC patients. Kaplan–Meier analysis with Log rank testing of survival was performed in HCC patients with different TDO expression levels. Overall survival (A) and disease-free survival (DFS) (B), comparisons of OS between HCC with strong TDO expression and low TDO expression and normal adjacent tissues in early UICC stage (I–II) (C) and advanced UICC stage (III–IV) (D) and without or with relapse (E and F). Error bars represent the SD.
Overexpression of TDO Promotes HCC Cell Proliferation, Migration, and Invasion in vitro
TDO mRNA and protein expression were detected in 5 different human HCC cell lines compared to the normal liver cell line Lo2. We observed higher TDO expression in the Huh7 cell line and lower expression in the LM3 cell line (Figure 4A and B). We next used shRNA constructs to knockdown TDO expression in Huh7 cells, and LM3 cells with low TDO expression were transfected with the TDO plasmid. Then, TDO expression was evaluated by qRT-PCR and WB (Figure 4C and D). CCK-8 assays showed that overexpression of TDO significantly promoted HCC cell proliferation, while TDO knockdown evidently inhibited this phenomenon (Figure 4E and F). Transwell assays showed that TDO knockdown inhibited cell invasion in Huh7 cells. Moreover, wound healing assays showed that overexpression and knockdown of TDO accelerated and delayed HCC cell wound healing (Figure 4G and H). TDO upregulation increased the invasion ability of LM3 cells (Figure 4I and J). TDO overexpression or knockdown in HCC cells increased or reduced the colony formation ability of HCC cells, respectively, compared with that in the control cells (Figure 4K and L). In conclusion, our data indicate that TDO enhances HCC cell proliferation, invasion in vitro.
Figure 4.
TDO expression in cell lines and in vitro HCC cell functional assays. Relative expression of TDO protein in normal liver and 5 other HCC cell lines (A). Relative expression of TDO mRNA in normal liver and 5 other HCC cell lines (B). TDO in LM3 and Huh7 cells transfected with TDO overexpression or downregulation vectors. Quantitative real-time PCR and Western blotting were used to detect TDO expression in the HCC cell lines Huh7 and LM3 (C and D). Overexpression or knockdown of TDO suppressed or elevated HCC cell proliferation, migration and invasion, respectively. (E and F) Cell proliferation assays. (G and H) Wound healing. (I and J) Invasion ability. (K and L) Colony formation (**p < 0.01). Original magnification: ×100 and ×200. Vector group transfected with empty vector; NC, infected with negative lentivirus; KD, infected with lenti-shRNA. GAPDH was used to normalize protein expression. Error bars represent the SD. *P < 0.05, **P < 0.01 and ***P < 0.001.
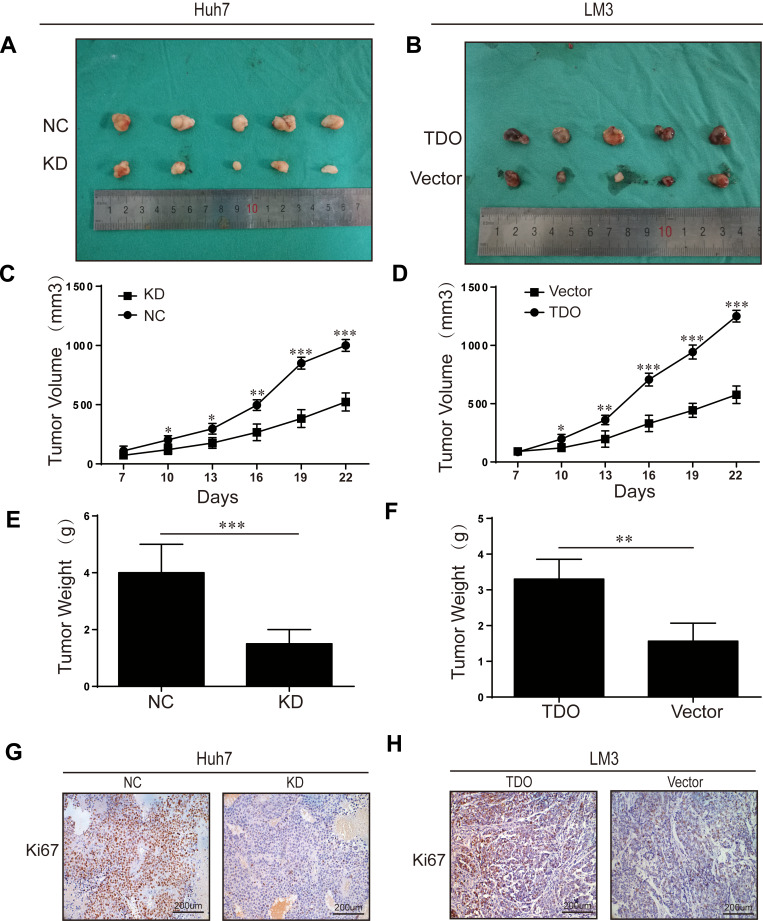
TDO Promotes Tumour Growth and Metastasis in Nude Mice
We performed a xenograft tumour growth assay to investigate the effect of TDO on tumorgenicity by measuring tumour weights and volumes in nude mice xenografted with control, TDO overexpression and knockdown cell lines. Tumour sizes were measured every 3 days from day 7 after implantation. Representative photographs of subcutaneous tumour xenografts were collected (Figure 5A and B), and growth measurements of tumour volume (Figure 5C and D) and weight (Figure 5E and F) showed that TDO-overexpressing cells generated larger tumours and grew faster than the control cells, while the TDO knockdown groups had opposite effects. IHC staining showed that TDO overexpression resulted in higher expression of the cell proliferation marker Ki-67 than that in the controls, while the knockdown group exhibited the opposite pattern, consistent with the results of the in vitro experiments (Figure 5G and H).
Figure 5.
Overexpression or knockdown of TDO inhibited or promoted tumour formation in nude mice. Representative photographs of subcutaneous tumour xenografts (A and B). Xenograft weight (C and D). Xenograft volume (E and F). Ki67 expression in subcutaneous xenograft tumors of Huh7 and LM3 cells was detected by IHC (G and H). Original magnification ×200. Error bars represent the SD. *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
HCC is characterized by a high incidence rate, a poor prognosis and a lack of effective targeted therapies due to its propensity for high recurrence and rapid metastasis. Although surgery, chemotherapy, intervention, etc. are used in treatment, the survival rate of HCC patients has not been improved. Therefore, it is extremely urgent to elucidate the molecular mechanisms underlying HCC development and progression and to discover novel molecular targets for early diagnosis and treatment. In this study, we documented that there is increased TDO expression in human HCC tissues and cells and that the increased TDO expression in HCC has a negative impact on survival. In addition, overexpression of TDO promotes HCC cell proliferation, colony formation, migration and invasion.
TDO was reported to be expressed in the liver but not in other tissues to regulate the Try level in the body.22,23 In our clinical study, we document that TDO expression is significantly higher in HCC tissues than in adjacent nonmalignant tissues (Figure 1) and in stage III–IV compared with stage I–II. TDO expression was related to the tumour size, stage and grade of HCC, indicating that TDO might act as a tumour promoter in the development and progression of HCC (Table 2). Kaplan–Meier analysis showed that HCC patients with high TDO expression had poorer OS and DFS than those with low TDO expression, suggesting that TDO may serve as a promising biomarker for prognosis (Figure 3). Consistently, publicly available data also revealed that high expression of TDO correlates with poor breast cancer clinical outcomes, such as poor overall survival and worse distant metastasis-free survival.24,25 TDO is highly expressed in other cancers, such as breast carcinoma, leiomyosarcoma, basal cell carcinoma, colorectal carcinoma, head and neck carcinoma, and lung carcinoma.12 Microarray analysis showed increased expression of TDO in basal cell carcinoma.26 TDO overexpression is associated with cancer stem cells and poor prognosis in oesophageal squamous cell carcinoma.27 TDO was significantly upregulated in metastases of leiomyosarcoma patients compared to primary tumours.28 In addition, high TDO was observed in triple-negative breast cancer (TNBC) compared with ER+ tumours and in stage IV compared with stage III tumours.24,29 In TNBC, TDO inhibits CD8 T-cell viability and then decreases anti-tumour CD8 T-cell activity, thereby leading to a poor prognosis.30 Considering that 75% of Try is metabolized in the liver by TDO, we propose that TDO may act as a tumour promoter and serve as an effective biomarker for diagnosis and prognosis and a promising target for therapy in HCC. In addition, 99mTc-N-pyridoxyl-5-methyl-tryptophan (PMT) scintigraphy, which is highly specific for the diagnosis of primary HCC, was performed for recurrent HCC following nonsurgical treatment, and D-tryptophan-6-luteinizing hormone-releasing hormone on tumoural growth and plasma sex steroid levels in cirrhotic patients with hepatocellular carcinoma, which might indicate that TDO is associated with HCC.31,32 However, the detailed biological functions and underlying molecular mechanisms of TDO warrant further study. Therefore, more endeavours and further studies are needed to reveal the functions and mechanisms of TDO in HCC.
Further, we demonstrated that TDO acts as an oncogene to promote HCC cell proliferation, colony formation, migration and invasion and HCC progression (Figure 3). First, we found that the protein and mRNA levels of TDO in HCC cells were higher than those in normal liver cells. Similarly, recent studies revealed that TDO was highly expressed in glioma cells and in TNBC cell lines (MDA-231, BT549 and SUM159).15,29 Second, we found that TDO overexpression promotes HCC cell proliferation, colony formation, migration and invasion in vitro. Inhibition of TDO expression decreased TNBC (BT549 and SUM159) cell growth, and knockdown of TDO2 also significantly increased apoptosis in TNBC (BT549 and MDA-MB-231) cells. Additionally, pharmacological inhibition and knockdown of TDO significantly reduced the migration and decreased the invasive capacity of MDA-MB-231 and BT549 cells.29 Supportively, the TDO protein level has been previously documented to be increased in glioma cells and correlates with the proliferation index. The knockdown of TDO in glioma cells restored allogeneic T cell proliferation and enhanced lysis of glioma cells.12 Finally, an in vivo xenograft tumour growth assay revealed that TDO overexpression promotes tumour growth (Figure 4).
These results obtained using animal tumour formation experiments are similar to those of following studies. After injection of MDA-MB-231 cells, triple negative breast cancer (TNBC) cells containing labelled luminescence, into nude mice, it was found that there were more luminescence markers in the TDO high expression group than the TDO low expression group. Three weeks later, lung tissues of nude mice were harvested, and it was found that the luminescence marker of the TDO high expression group was much more than that of the TDO inhibition group.29 Similarly, glioma cells with high TDO expression grew faster and had a higher proliferation index than TDO-deficient control tumours in vivo.12,33–35 Using the P815 mouse tumour model, Pilotte found that compared to control tumours, TDO-expressing tumours grew faster and more potently inhibited local T lymphocyte proliferation.12
In conclusion, our data show that overexpression of TDO promotes the proliferation and invasion of HCC cells in vitro and in vivo and indicate that TDO might serve as an effective biomarker for diagnosis and prognosis and a promising target for therapy in HCC.
Acknowledgments
This study was supported by funding from the National Natural Science Foundation of China (No.81670595) and the Scientific Research Planning Project of Science and Technology Commission of Shanghai (No.15411962700).
Disclosure
The authors declare no conflicts of interest.
Reference
- 1.Qin Y, Wang N, Zhang X, Han X, Zhai X, Lu Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab Brain Dis. 2018;33(6):1787–1800. doi: 10.1007/s11011-018-0290-7 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Banales JM, Inarrairaegui M, Arbelaiz A, et al. Serum metabolites as diagnostic biomarkers for cholangiocarcinoma, hepatocellular carcinoma, and primary sclerosing cholangitis. Hepatology. 2019;70(2):547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teufel M, Seidel H, Kochert K, et al. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology. 2019;156(6):1731–1741. doi: 10.1053/j.gastro.2019.01.261 [DOI] [PubMed] [Google Scholar]
- 6.Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem Rev. 1996;96(7):2841–2888. doi: 10.1021/cr9500500 [DOI] [PubMed] [Google Scholar]
- 7.Booth ES, Basran J, Lee M, Handa S, Raven EL. Substrate oxidation by indoleamine 2,3-Dioxygenase: evidence for a common reaction mechanism. J Biol Chem. 2015;290(52):30924–30930. doi: 10.1074/jbc.M115.695684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei Z, Mendonca R, Gazzard L, et al. Aminoisoxazoles as potent inhibitors of tryptophan 2,3-Dioxygenase 2 (TDO2). ACS Med Chem Lett. 2018;9(5):417–421. doi: 10.1021/acsmedchemlett.7b00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CP, Pan ZZ, Luo DY. TDO as a therapeutic target in brain diseases. Metab Brain Dis. 2016;31(4):737–747. doi: 10.1007/s11011-016-9824-z [DOI] [PubMed] [Google Scholar]
- 10.Schmidt SK, Muller A, Heseler K, et al. Antimicrobial and immunoregulatory properties of human tryptophan 2,3-dioxygenase. Eur J Immunol. 2009;39(10):2755–2764. doi: 10.1002/eji.200939535 [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann D, Dvorakova T, Stroobant V, Bouzin C. Tryptophan 2,3-dioxygenase expression identified in human hepatocellular carcinoma cells and in intratumoral pericytes of most cancers. Cancer Immunol Res. 2020;8(1):19–31. [DOI] [PubMed] [Google Scholar]
- 12.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(7):2497–2502. doi: 10.1073/pnas.1113873109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua S, Wang X, Chen F, Gou S. Novel conjugates with dual suppression of glutathione S-transferases and tryptophan-2,3-dioxygenase activities for improving hepatocellular carcinoma therapy. Bioorg Chem. 2019;92:103191. doi: 10.1016/j.bioorg.2019.103191 [DOI] [PubMed] [Google Scholar]
- 14.Chen DB, Zhao YJ, Wang XY, et al. Regulatory factor X5 promotes hepatocellular carcinoma progression by transactivating tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein theta and suppressing apoptosis. Chin Med J. 2019;132(13):1572–1581. doi: 10.1097/CM9.0000000000000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- 16.Guidelines on oncologic imaging. UICC Imaging committee. International union against cancer. Eur J Radiol. 1989;1(9 Suppl):1–28. [PubMed] [Google Scholar]
- 17.Sasaki M, Sato Y, Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70(3):423–434. doi: 10.1111/his.13084 [DOI] [PubMed] [Google Scholar]
- 18.Shi G-M, Xu Y, Fan J, et al. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J Cancer Res Clin Oncol. 2008;134(11):1155–1163. doi: 10.1007/s00432-008-0407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNairn AJ, Chuang C-H, Bloom JC, Wallace MD, Schimenti JC. Female-biased embryonic death from inflammation induced by genomic instability. Nature. 2019;567(7746):105–108. doi: 10.1038/s41586-019-0936-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin L, Feng T, Shih HP, et al. Colony-forming cells in the adult mouse pancreas are expandable in matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A. 2013;110(10):3907–3912. doi: 10.1073/pnas.1301889110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng J, Xiao J, Mi Y, et al. PCDHGA9 acts as a tumor suppressor to induce tumor cell apoptosis and autophagy and inhibit the EMT process in human gastric cancer. Cell Death Dis. 2018;9(2):27. doi: 10.1038/s41419-017-0189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng B, Wu D, Gu J, Ouyang S, Ding W, Liu Z-J. Structural and functional analyses of human tryptophan 2,3-dioxygenase. Proteins. 2014;82(11):3210–3216. doi: 10.1002/prot.24653 [DOI] [PubMed] [Google Scholar]
- 23.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x [DOI] [PubMed] [Google Scholar]
- 24.Novikov O, Wang Z, Stanford EA, et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER−/PR−/Her2−human breast cancer cells. Mol Pharmacol. 2016;90(5):674–688. doi: 10.1124/mol.116.105361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puccetti P, Fallarino F, Italiano A, et al. Accumulation of an endogenous tryptophan-derived metabolite in colorectal and breast cancers. PLoS One. 2015;10(4):e0122046. doi: 10.1371/journal.pone.0122046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tina E, Prosen S, Lennholm S, Gasparyan G, Lindberg M, Gothlin Eremo A. Expression profile of the amino acid transporters SLC7A5, SLC7A7, SLC7A8 and the enzyme TDO2 in basal cell carcinoma. Br J Dermatol. 2019;180(1):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham QT, Oue N, Sekino Y, et al. TDO2 overexpression is associated with cancer stem cells and poor prognosis in esophageal squamous cell carcinoma. Oncology. 2018;95(5):297–308. doi: 10.1159/000490725 [DOI] [PubMed] [Google Scholar]
- 28.Zhai L, Spranger S, Binder DC, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427–5433. doi: 10.1158/1078-0432.CCR-15-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Amato NC, Rogers TJ, Gordon MA, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75(21):4651–4664. doi: 10.1158/0008-5472.CAN-15-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greene LI, Bruno TC, Christenson JL, et al. A role for tryptophan-2,3-dioxygenase in CD8 T-cell suppression and evidence of tryptophan catabolism in breast cancer patient plasma. Mol Cancer Res. 2019;17(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto H, Matsuura M, Sakurai H, Nakajima N, Ito K. Radionuclear estimation with 99mTc-N-pyridoxyl-5-methyl-tryptophan (PMT) scintigraphy of recurrent hepatocellular carcinoma after non-surgical treatment. Nihon Igaku Hoshasen Gakkai Zasshi. 1997;57(12):805–811. [PubMed] [Google Scholar]
- 32.Guechot J, Peigney N, Ballet F, Vaubourdolle M, Giboudeau J, Poupon R. Effect of D-tryptophan-6-luteinizing hormone-releasing hormone on the tumoral growth and plasma sex steroid levels in cirrhotic patients with hepatocellular carcinoma. Hepatology. 1989;10(3):346–348. [DOI] [PubMed] [Google Scholar]
- 33.Wardhani LO, Matsushita M, Iwasaki T, et al. Expression of the IDO1/TDO2-AhR pathway in tumor cells or the tumor microenvironment is associated with Merkel cell polyomavirus status and prognosis in Merkel cell carcinoma. Hum Pathol. 2019;84:52–61. doi: 10.1016/j.humpath.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 34.Guastella AR, Michelhaugh SK, Klinger NV, et al. Investigation of the aryl hydrocarbon receptor and the intrinsic tumoral component of the kynurenine pathway of tryptophan metabolism in primary brain tumors. J Neuro Oncol. 2018;139(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ott M, Litzenburger UM, Rauschenbach KJ, et al. Suppression of TDO-mediated tryptophan catabolism in glioblastoma cells by a steroid-responsive FKBP52-dependent pathway. Glia. 2015;63(1):78–90. doi: 10.1002/glia.22734 [DOI] [PubMed] [Google Scholar]