Abstract
OBJECTIVE.
The purpose of this study was to compare in a multireader manner the diagnostic accuracies of 3-T multiparametric MRI interpretation and serial prostate-specific antigen (PSA) measurement in predicting the presence of residual clinically significant prostate cancer after focal laser ablation.
MATERIALS AND METHODS.
Eighteen men had undergone focal laser ablation for low- or intermediate-risk prostate cancer as part of two National Cancer Institute-funded phase 1 clinical trials. Multiparametric MRI was performed immediately after and 6 and 12 months after focal laser ablation. Serial PSA measurements after focal laser ablation were recorded, and MRI-ultrasound fusion biopsy was performed 6 and 12 months after ablation and served as the reference standard. Multiparametric MRI was performed at 3 T with pelvic phased-array coils. T2-weighted, DW, and dynamic contrast-enhanced MR images were retrospectively assessed by two blinded radiologists using a 3-point Likert scale (0–2). Interreader agreement was assessed with the Cohen kappa statistic. The diagnostic accuracies of multiparametric MRI and PSA measurement were compared.
RESULTS.
Residual clinically significant prostate cancer was identified in 11 of 18 (61%) men. Logistic regression analysis of serial PSA measurements yielded a correct classification rate of 61.1% (p > 0.05). Using a multiparametric MRI threshold score of 4 or greater, both radiologists made correct classifications for 16 of 18 men (89%) at 6 months and 15 of 17 men (88%) at 12 months. Interreader agreement was substantial to excellent for T2-weighted imaging, DWI, and dynamic contrast-enhanced MRI and improved uniformly from 6 to 12 months. Logistic regression analysis of the retrospectively reviewed multiparametric MR images yielded AUCs greater than 0.90 for each radiologist 6 and 12 months after focal laser ablation (p < 0.001).
CONCLUSION.
Multiparametric MRI 6 and 12 months after focal laser ablation significantly outperformed serial PSA measurements for predicting the presence of residual clinically significant prostate cancer.
Keywords: focal therapy, MRI, prostate
In selected men with localized prostate cancer (PCa), focal therapy with a variety of modalities is increasingly being investigated and used clinically. The primary objective of focal therapy is eradication of clinically significant PCa (csPCa) with preservation of genitourinary function. Despite being in widespread use for lung, liver, and renal cancers, the focal therapy paradigm has been controversial in PCa management because of concerns about multifocality and the utility of MRI in accurately identifying the index lesion and its margins [1, 2].
With improvements in MRI technology and interpretation, localization of the index lesion is possible in 80–90% of cases [3–5]. In addition to aiding in localization of the index lesion, the combination of 3-T MRI and targeted prostate biopsy can help effectively exclude csPCa in discrete regions [4], making focal therapy a potentially viable treatment strategy for selected men.
Focal therapy may be delivered by several different mechanisms, one of which is laser interstitial thermal therapy or focal laser ablation (FLA). FLA has been the subject of several small prospective trials [6–11], which have confirmed the safety of this technique, but long-term data on oncologic efficacy are lacking.
Unlike extirpative approaches in which biochemical surveillance is the standard means of posttherapy monitoring, posttreatment imaging surveillance with MRI will likely be critical for focal therapy, because varying volumes of and heterogeneity within residual prostate tissue after focal therapy may limit the diagnostic accuracy of serum prostate-specific antigen (PSA) measurement. The ability to use multiparametric MRI (mpMRI) to identify residual or recurrent PCa after focal therapy could help identify patients who need retreatment or more definitive therapy and would likely improve oncologic outcomes. To date there have been few published reports of studies evaluating the post-FLA appearance of the prostate gland on MR images [12–14]. None of the studies evaluated the diagnostic accuracy of MRI in this setting.
The purpose of this study was to compare the diagnostic accuracy of 3-T mpMRI and serial PSA measurements in predicting the presence of residual csPCa after FLA in a multireader manner with MRI-ultrasound fusion biopsy or whole-mount histopathologic analysis as the reference standard. A secondary aim was to characterize the temporal evolution of the ablation zone on dynamic contrast-enhanced (DCE) MR images.
Materials and Methods
Two phase 1 clinical trials evaluating FLA in men with intermediate-risk PCa have been completed at UCLA Medical Center. The first study (NCT02224911) [6] involved eight men who underwent FLA performed in the gantry of the MRI unit with MRI-compatible thermal probes. The second study (NCT02357121) [7] involved 10 men who underwent FLA in a clinic setting (out of bore) by means of MRI-ultrasound fusion and interstitial thermal probe monitoring. For both clinical trials, serum PSA measurements were obtained 1, 3, 6, and 12 months after treatment, and MRI was performed immediately after (within 30–60 minutes) and 6 and 12 months after ablation. At 6 and 12 months, all men in both trials underwent MRI-ultrasound fusion biopsy, which included sampling of the original tumor site, the ablation zone, the ablation zone margins, any new ROI (if present), and six template biopsy cores in the ipsilateral hemigland.
The current single-arm observational study was performed with institutional review board approval and was compliant with the 1996 U.S. HIPAA. For the purposes of this analysis, we retrospectively reassessed the diagnostic accuracy of 3-T mpMRI and PSA measurement for the prediction of residual or recurrent csPCa, which we defined as a Gleason score of 3 + 4 or greater in the ipsilateral hemigland based on the results of any post-FLA MRI-ultra-sound fusion biopsy. Among the three patients with a Gleason score of 3 + 3 before treatment, any residual PCa after FLA was deemed clinically significant. Four of 18 men (22%) went on to radical prostatectomy after FLA. Whole-mount histopathologic analysis supplanted MRI-ultrasound fusion biopsy as the reference standard for these four men.
Multiparametric MRI and Image Interpretation
All patients underwent 3-T mpMRI with an external phased-array coil on one of several MRI systems (Skyra, Prisma, Trio, and Verio, Siemens Healthcare). The mpMRI protocol included conventional multiplanar T2-weighted imaging, DWI, and DCE MRI, as has been described in detail previously [15].
Images were retrospectively reviewed at a workstation (DynaCAD 3.0, Invivo Philips Healthcare) by two fellowship-trained genitourinary radiologists (4 and 10 years of experience), each of whom had interpreted more than 1500 prostate mpMRI examinations. Both were blinded to histopathologic and PSA data at image review. T2-weighted and DW images and corresponding apparent diffusion coefficient maps and DCE MR images were scored on a 3-point Likert scale (0–2) as follows: 0, no suspicion of residual or recurrent tumor; 1, intermediate suspicion of residual or recurrent tumor; 2, high suspicion of residual or recurrent tumor. For each patient mpMRI examinations performed 6 and 12 months after ablation were scored with a composite score ranging from 0 to 6. The scoring system was based on previously published reports [16–19] of studies evaluating posttherapy mpMRI. These studies collectively identified early focal enhancement, diffusion restriction, and intermediate T2 signal intensity (higher than that of skeletal muscle) in the treatment zone as the findings raising greatest suspicion of residual or recurrent PCa. The scoring system is described in Table 1.
TABLE 1:
Multiparametric MRI Scoring System
| Score | |||
|---|---|---|---|
| Pulse Sequence | 0 | 1 | 2 |
| T2-weighted | Heterogeneous or homogeneous very low signal intensity | Uniform intermediate or low signal intensity; wedge-shaped or circumscribed | Uniform intermediate or low signal intensity; irregular or invasive margins |
| High-b-value DWI and ADC map | Low signal intensity | Intermediate signal intensity | High signal intensity |
| Dynamic contrast-enhanced | Nonenhancing or hypoenhancement | Isoenhancement or progressive enhancement | Early hyperenhancement with washout |
Note—Total composite score ranged from 0 to 6. For the purposes of this analysis, a threshold score of ≥ 4 was considered positive. ADC = apparent diffusion coefficient.
Three-dimensional delineation of the FLA zone of ablation, defined by the nonenhancing region on DCE images, was defined on DCE images by use of the workstation software. This step was performed at each of the three postablation time points by a third radiology fellow, also blinded to histopathologic and clinical data. The 3D ROI tool was used to manually contour the ablation zone in each sequential slice on which it was visualized to generate a 3D volume. DCE imaging was used to define the ablation zone, because FLA margins have been found to be most clearly delineated with this sequence [14].
Statistical Analysis
Descriptive statistics were generated. Logistic regression and ROC analysis were performed to obtain performance measures, such as total correct classification and AUC. Cohen kappa statistics were generated as measures of agreement. Simple linear regression was used to assess trend. IBM SPSS Statistics for Windows software (version 24.0, IBM) was used for database management and statistical analysis. Statistical significance was assessed at p ≤ 0.05.
Results
Patient Demographics
Among the 18 men (mean age, 63 years) treated in both clinical trials, the median pretreatment PSA level was 7.4 ng/mL. Fourteen patients had a pretreatment Gleason score of 3 + 4; three patients, 3 + 3; and one patient, 4 + 3. Residual csPCa was identified in 11 of 18 men (61%) (five [28%], Gleason 3 + 4; six [33%], ≥ 4 + 3). Among the seven men without residual csPCa after biopsy, four had no PCa detected at any post-FLA biopsy. The other three had residual small-volume Gleason 3 + 3 PCa in the hemigland ipsilateral to treatment (maximum cancer core lengths, 0.5, 1.0, and 2.0 mm). None of the three initially had Gleason 3 + 3 disease. Treated lesion locations and outcomes are shown in Table 2.
TABLE 2:
Treatment Outcome Based on Lesion Location
| Location of Treated Prostate Cancer | Residual Clinically Significant Prostate Cancera |
|---|---|
| Left anteriortransition zone base | Yes |
| Right posterior peripheral zone apex | Yes |
| Right anteriortransition zone base | No |
| Right posterior peripheral zone midgland | Yes |
| Right posterior peripheral zone apex | No |
| Left anterior peripheral zone apex | No |
| Left posterior peripheral zone midgland | Yes |
| Left anterior peripheral zone midgland | Yes |
| Right anteriortransition zone base | Yes |
| Right posterior peripheral zone midgland | Yes |
| Left anteriortransition zone base | Yes |
| Left posterior peripheral zone midgland | Yes |
| Right anteriortransition zone midgland | No |
| Left anteriortransition zone base | No |
| Left posterior transition zone base | Yes |
| Right posterior peripheral zone midgland | No |
| Left anteriortransition zone midgland | Yes |
| Left anterior peripheral zone apex | No |
Note—Lesion location defined according to Prostate Imaging Reporting and Data System version 2 sector map. For peripheral zone lesions in the apex and midgland, posteromedial and posterolateral locations are referred to as posterior.
Defined as Gleason score 3 + 4 or greater. Among the three patients with Gleason 3 + 3 at baseline, clinically significant prostate cancer was defined as any residual prostate cancer.
Serial Prostate-Specific Antigen Measurements
Among men with residual csPCa, the median change in PSA level over the 12 months (measurements at 1 month and 3, 6, and 12 months used to estimate change) after FLA was −0.81 ng/mL (interquartile range, 1.23 ng/mL), compared with −1.18 ng/mL (interquartile range, 1.79 ng/mL) in the group without residual csPCa. These values were not significantly different (p = 0.18). Descriptive PSA statistics for the entire study cohort are shown in Table 3.
TABLE 3:
Descriptive Prostate-Specific Antigen Statistics
| Prostate-Specific Antigen (ng/mL) | ||
|---|---|---|
| Time Frame | Mean | SD |
| Pretreatment | 8.2 | 4.5 |
| Posttreatment(mo) | ||
| 3 | 4.1 | 3.0 |
| 6 | 4.2 | 4.0 |
| 12 | 4.6 | 4.3 |
Note—n = 15 (three patients had at least one missing prostate-specific antigen data entry at one of the posttreatment time points).
Logistic regression analysis of the change in PSA level for predicting the presence of residual csPCa yielded a correct classification rate of 61.1%, which was not statistically significant (p = 0.19). Given lack of a statistically significant difference, no threshold PSA value to predict the presence of residual PCa was established.
Serial MRI Assessment
Using a threshold suspicion score of 4 or greater and the 6-month post-FLA MR images, both radiologists correctly classified 16 of 18 cases (89%) as to the presence of residual csPCa. The results based on the 12-month post-FLA MRI findings were the same as the 6-month results: both radiologists correctly classified 15 of 17 cases (88%). One patient did not undergo 12-month follow-up MRI, which accounts for the difference in denominators. Both radiologists incorrectly classified the same two cases at each time point: one false-positive and one false-negative. Figures 1 and 2 provide examples before and after focal laser ablation MRI and corresponding radiologist interpretations.
Fig. 1— 67-year-old man with prostate-specific antigen level of 8.4 ng/mL.
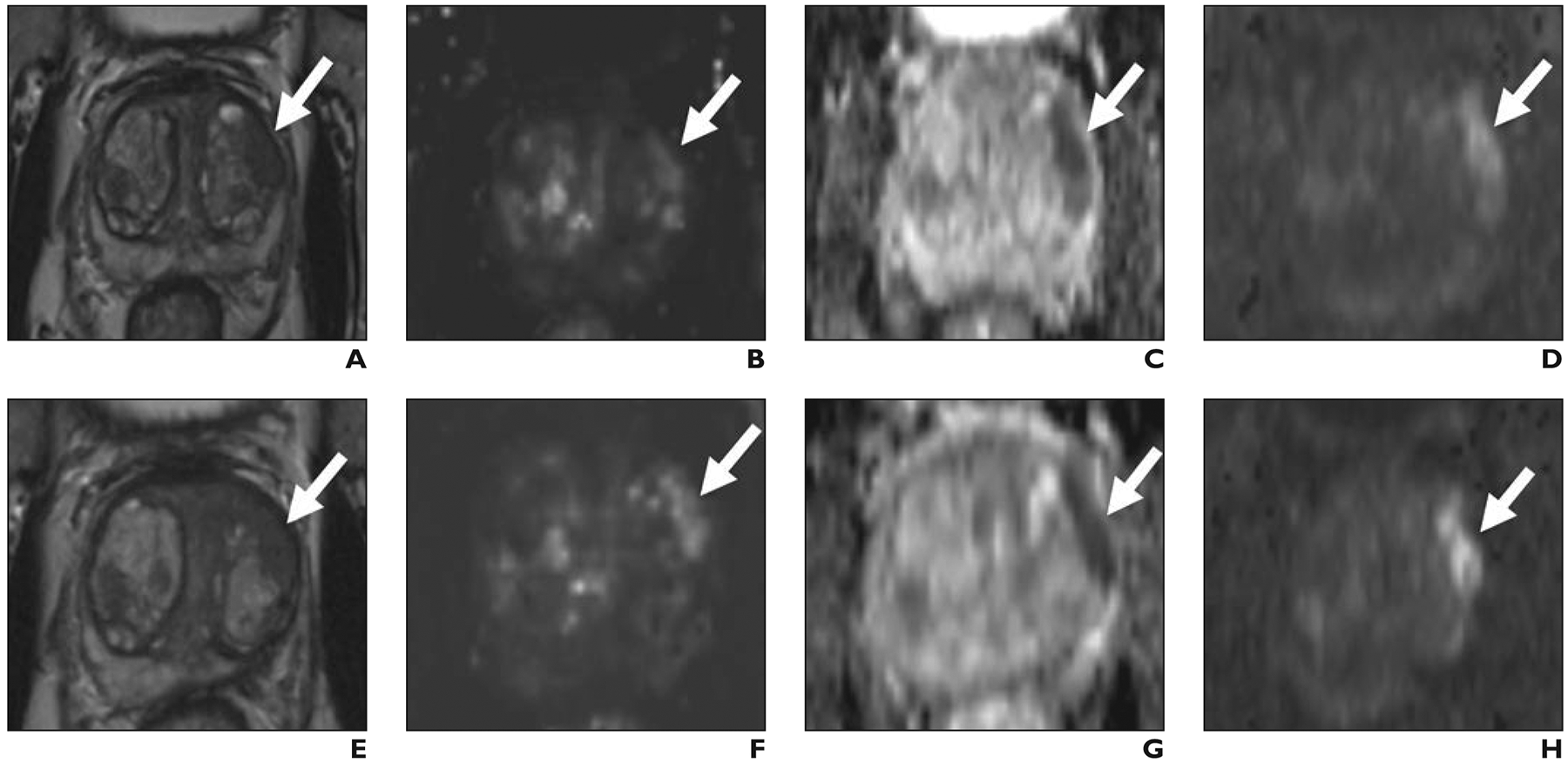
A–D, Before focal laser ablation, T2-weighted (A) and dynamic contrast-enhanced (B) images, apparent diffusion coefficient (ADC) map (C), and DW image (D) show Prostate Imaging Reporting and Data System version 2 5/5 lesion (arrow) in left anterior transition midgland corresponding to Gleason 3 + 4 prostate cancer at targeted biopsy.
E–H, Six months after focal laser ablation, T2-weighted (E) and dynamic contrast-enhanced (F) images, ADC map (G), and DW image (H) show persistent masslike low signal intensity, focal early enhancement, and markedly restricted diffusion (arrow). One radiologist scored T2-weighted, dynamic contrast-enhanced, and DW images 2, 2, 2; other radiologist scored them 1, 2, 2. Targeted biopsy 6 months after ablation revealed persistent Gleason 3 + 4 prostate cancer.
Fig. 2— 58-year-old man with prostate-specific antigen level of 11.7 ng/mL.
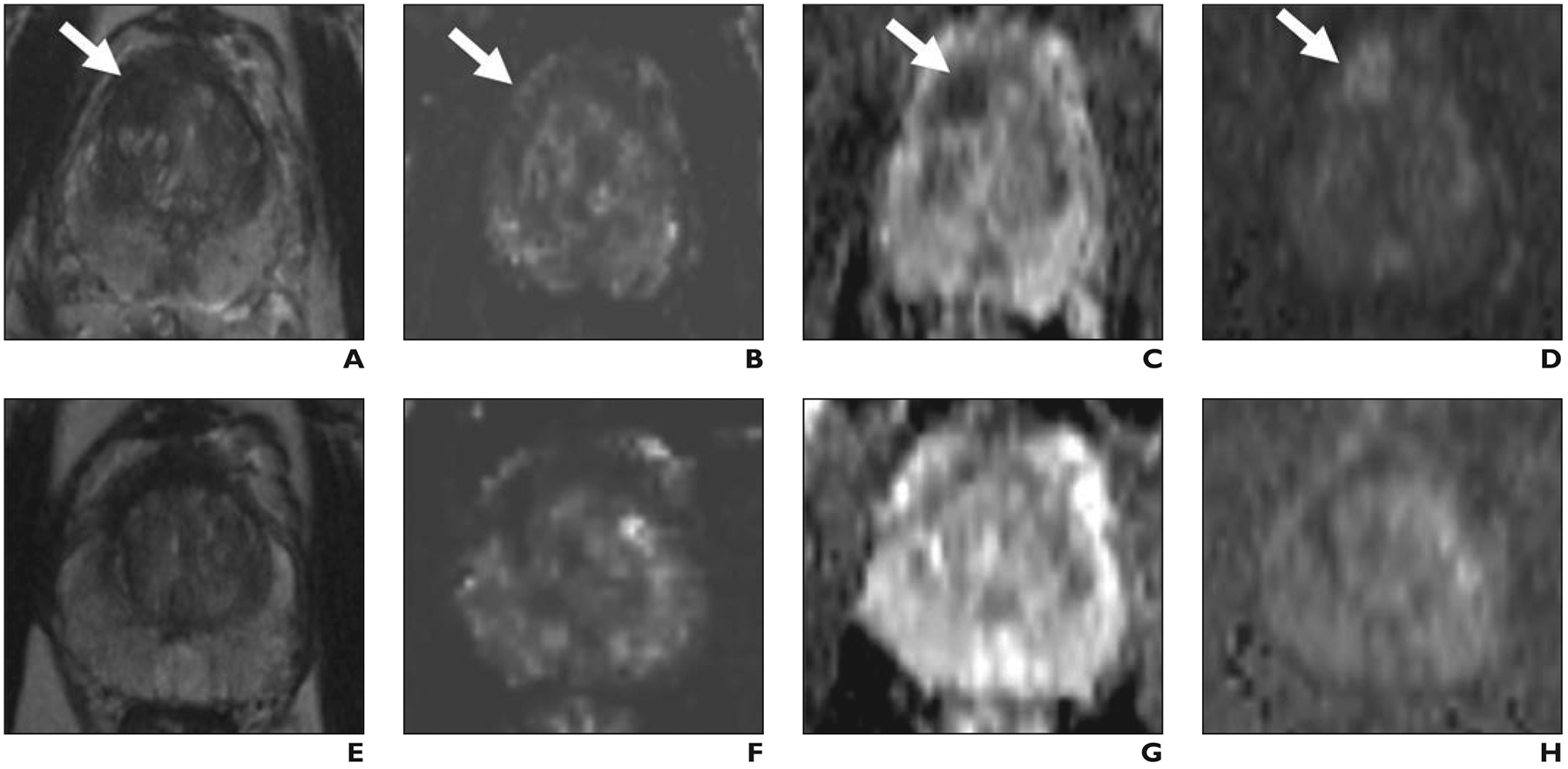
A–D, Before focal laser ablation, T2-weighted (A) and dynamic contrast-enhanced (B) images, apparent diffusion coefficient (ADC) map (C), and DW image (D) show Prostate Imaging Reporting and Data System version 2 5/5 lesion (arrow) in right anterior transition zone corresponding to Gleason 3 + 4 prostate cancer at MRI-targeted biopsy.
E–H, Twelve months after ablation, T2-weighted (E) and dynamic contrast-enhanced (F) images, ADC map (G), and DW image (H) show markedly low signal intensity, volume loss, and no early enhancement or diffusion restriction within treatment zone. Both radiologists scored T2-weighted, dynamic contrast-enhanced, and DW images 0 at 12 months. All postablation biopsy results were benign.
Logistic regression analysis was performed with MRI as the diagnostic test for predicting the presence of residual csPCa. For one reader the AUC at 6 months was 0.93 (95% CI, 0.79–1.0) and at 12 months was 0.96 (95% CI, 0.86–1.0). The corresponding AUCs for the other reader were 0.92 (95% CI, 0.78–1.0) and 0.97 (95% CI, 0.90–1.0) (p < 0.001 for both readers at each time point).
Interreader agreement was assessed with the Cohen kappa statistic. Each pulse sequence was evaluated separately. Agreement was substantial to excellent for T2-weighted imaging, DCE imaging, and DWI at both 6 and 12 months. It improved across all pulse sequences at 12 months and was the highest at both time points for DWI (Table 4).
TABLE 4:
Radiologist Interrater Agreement
| κ | |||
|---|---|---|---|
| Pulse Sequence | 6 mo | 12mo | P |
| T2-weighted | 0.68 | 0.77 | < 0.001 |
| DWI | 0.84 | 0.90 | < 0.001 |
| Dynamic contrast-enhanced | 0.76 | 0.81 | < 0.001 |
Note—κ = 0.2–0.4, fair to poor agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, substantial agreement; κ = 0.81–1.0, excellent agreement.
Volumetric Assessment of the MRI Focal Laser Ablation Zone
The ablation zone volume, as determined by the nonenhancing region on DCE images, decreased from immediately after to 6 and 12 months after treatment. Mean ablation zone volumes were significantly different at each time point, measuring 6.36 (SD, 3.16) cm3 immediately after treatment compared with 1.37 (SD, 1.48) cm3 at 6 months and 1.03 (SD, 1.26) cm3 at 12 months (p < 0.0001). The change in MRI ablation zone volume was described by means of linear regression (Fig. 3).
Fig. 3—
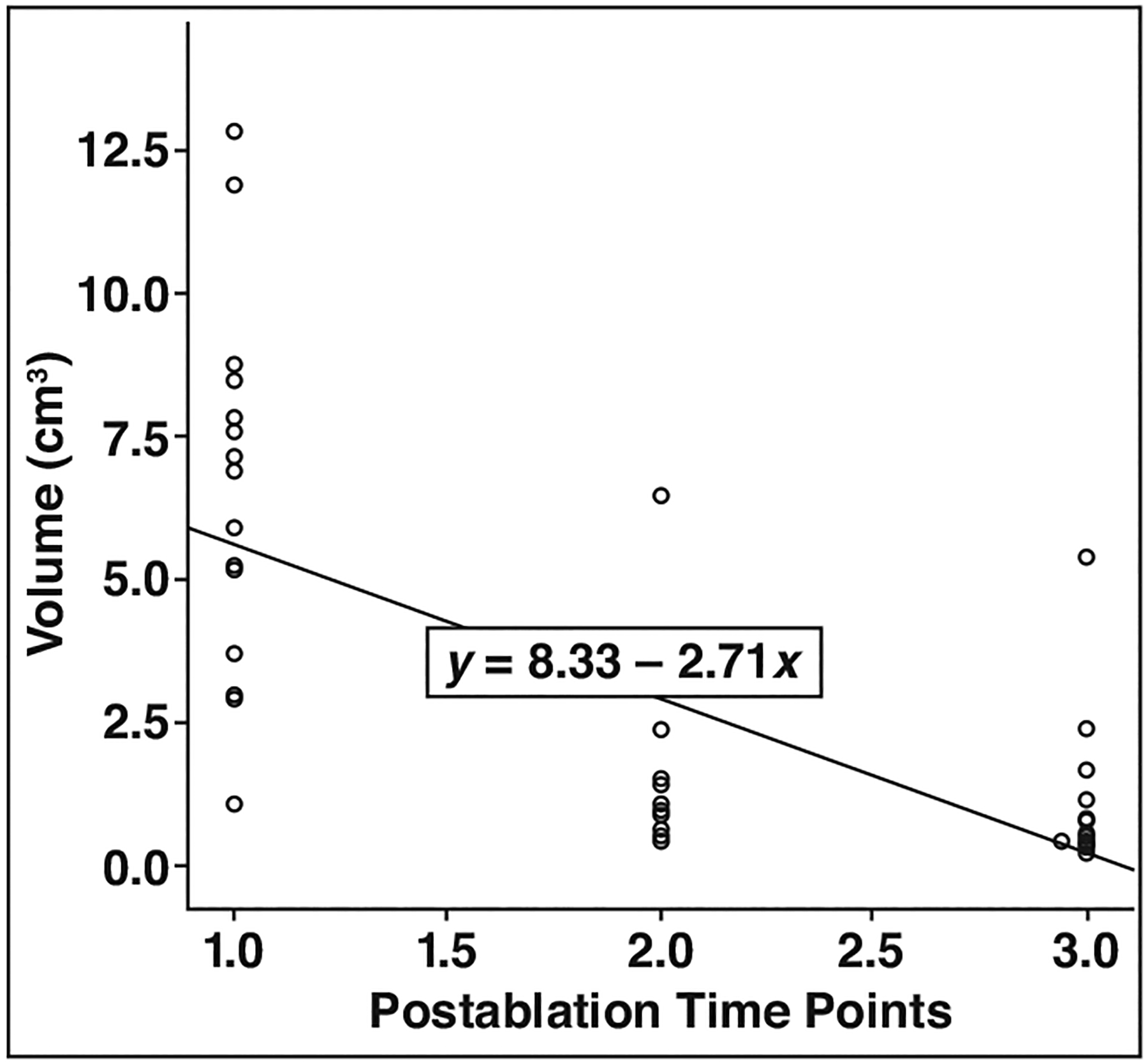
Linear regression plot shows volumetric change in focal laser ablation zone over time (1.0 = immediately, 2.0 = 6 mo, and 3.0 = 12 mo after ablation.
Discussion
In our study 3-T mpMRI findings interpreted by two experienced and blinded genitourinary radiologists significantly outperformed serial PSA measurements for predicting the presence of csPCa after FLA. This was true both 6 and 12 months after treatment and for two blinded radiologists, who interpreted the examinations separately. We observed that the combination of masslike intermediate to low T2 signal intensity, moderately or markedly restricted diffusion, and focal early enhancement was the group of imaging features raising the most suspicion of the presence of recurrent or residual csPCa. Using a novel scoring system composed of these elements, each radiologist achieved an AUC greater than 0.9 for predicting csPCa both 6 and 12 months after FLA. To our knowledge, this is the first study to assess the diagnostic performance of mpMRI in the post-FLA setting and the first to propose a scoring system and framework for such assessment, because the Prostate Imaging Reporting and Data System version 2 is not designed for use in the posttreatment setting.
In the largest post-FLA mpMRI study to date, Westin et al. [14] concluded that qualitative findings suspicious for recurrent cancer were apparent only at the 12-month follow-up mpMRI examination, which differs somewhat from our results. We found excellent diagnostic accuracy of mpMRI at both postablation time points. This may be because we performed mpMRI at 6 and 12 months, in accordance with expert consensus guidelines [20], whereas Westin et al. performed mpMRI at 3 and 12 months. Three months may not be enough time for suspicious findings to manifest themselves.
Our study differs from that by Westin et al. [14] in a few additional important ways. We conducted a blinded dual-reader review of the mpMRI examinations, whereas Westin et al. had one nonblinded radiologist perform image review. We performed more comprehensive post-FLA biopsies that included several targeted cores directed at the site of the original tumor and any additional MRI-suspicious region. Westin et al. used nontargeted prostate biopsy to detect recurrence. Finally, unlike Westin et al., we quantitatively assessed the change in ablation zone volume over time.
One potential reason that residual or recurrent PCa may not be apparent at earlier follow-up, such as at 3 months, is that during this time the immediate postablation changes are still evolving. This is borne out in our results of volumetric assessment of the ablation zone. We noted a statistically significant linear decrease in size of the ablation zone (nonenhancing region on DCE images) from immediately after the procedure through 6–12 months. The nonenhancing zone immediately after ablation was nearly five times the size of the nonenhancing zone on the image 6 months after FLA.
It is likely that as more time elapses after FLA, the postablation changes become less conspicuous and findings of recurrent PCa become more apparent. Our results indirectly support this hypothesis. The interreader agreement for T2-weighted MRI, DWI, and DCE MRI improved uniformly between 6 and 12 months after treatment, suggesting that the presence of recurrent or residual csPCa becomes clearer as more time passes after treatment.
Our study had several limitations, chief of which was a small sample size. This limitation was largely unavoidable, because FLA is still in its infancy. Second, the incidence of residual PCa was slightly higher in these two studies than in two subsequent FLA trials [8, 21]. The patients included in the current study were drawn from the first FLA prostate trial conducted with human subjects (n = 8) [6] and the first trial to use an office-based MRI-ultrasound fusion technique (n = 10) [7], so it is not surprising that the incidence of residual PCa was slightly higher than that reported in subsequent trials. Another limitation was that we performed a strictly qualitative assessment of the mpMRI examinations. We did not evaluate apparent diffusion coefficient or T2 texture features or perform a quantitative assessment of DCE pharmaco-kinetic parameters. These will be the subject of a future work. MRI-targeted biopsy was used as the pathologic reference standard after FLA rather than radical prostatectomy for most of the patients, so we could not directly correlate the nonenhancing zone on MR images with a pathologic ablation zone. Finally, we did not have longer-term clinical or imaging follow-up data beyond 12 months for most patients.
Conclusion
Dual-reader 3-T mpMRI interpretations performed by two independent blinded readers significantly outperformed serial PSA measurements for predicting the presence of residual or recurrent csPCa after FLA in this study of a novel mpMRI interpretation framework. Interreader agreement was substantial to excellent for T2-weighted, DW, and DCE images, and there was a significant linear decrease in the volume of nonenhancing tissue, as visualized on DCE images, from immediately after to 12 months after ablation. Treating physicians should therefore take care when making inferences about the total ablation zone volume at early post-treatment imaging. Nonetheless, our results suggest that mpMRI is well suited to playing a crucial role in the follow-up and monitoring of patients after FLA.
References
- 1.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 2002; 60:264–269 [DOI] [PubMed] [Google Scholar]
- 2.Giannarini G, Gandaglia G, Montorsi F, Briganti A. Will focal therapy remain only an attractive illusion for the primary treatment of prostate cancer? J Clin Oncol 2014; 32:1299–1301 [DOI] [PubMed] [Google Scholar]
- 3.Le JD, Tan N, Shkolyar E, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 2015; 67:569–576 [DOI] [PubMed] [Google Scholar]
- 4.Fütterer JJ, Briganti A, De Visschere P, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015; 68:1045–1053 [DOI] [PubMed] [Google Scholar]
- 5.Johnson DC, Raman SS, Mirak SA, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol 2019; 75:712–720 [DOI] [PubMed] [Google Scholar]
- 6.Natarajan S, Raman S, Priester AM, et al. Focal laser ablation of prostate cancer: phase I clinical trial. J Urol 2016; 196:68–75 [DOI] [PubMed] [Google Scholar]
- 7.Natarajan S, Jones TA, Priester AM, et al. Focal laser ablation of prostate cancer: feasibility of magnetic resonance imaging-ultrasound fusion for guidance. J Urol 2017; 198:839–847 [DOI] [PubMed] [Google Scholar]
- 8.Eggener SE, Yousuf A, Watson S, Wang S, Oto A. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol 2016; 196:1670–1675 [DOI] [PubMed] [Google Scholar]
- 9.Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol 2015; 68:924–926 [DOI] [PubMed] [Google Scholar]
- 10.Lindner U, Lawrentschuk N, Weersink RA, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol 2010; 57:1111–1114 [DOI] [PubMed] [Google Scholar]
- 11.Oto A, Sethi I, Karczmar G, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology 2013; 267:932–940 [DOI] [PubMed] [Google Scholar]
- 12.Bomers JG, Cornel EB, Fütterer JJ, et al. MRI-guided focal laser ablation for prostate cancer followed by radical prostatectomy: correlation of treatment effects with imaging. World J Urol 2017; 35:703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litjens GJ, Huisman HJ, Elliott RM, et al. Quantitative identification of magnetic resonance imaging features of prostate cancer response following laser ablation and radical prostatectomy. J Med Imaging (Bellingham) 2014; 1:035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westin C, Chatterjee A, Ku E, et al. MRI findings after MRI-guided focal laser ablation of prostate cancer. AJR 2018; 211:595–604 [DOI] [PubMed] [Google Scholar]
- 15.Tan N, Lin WC, Khoshnoodi P, et al. In-bore 3-T MR-guided transrectal targeted prostate biopsy: Prostate Imaging Reporting and Data System version 2–based diagnostic performance for detection of prostate cancer. Radiology 2017; 283:130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology 2004; 231:379–385 [DOI] [PubMed] [Google Scholar]
- 17.Kongnyuy M, Halpern DM, Liu CC, et al. 3-T multiparametric MRI characteristics of prostate cancer patients suspicious for biochemical recurrence after primary focal cryosurgery (hemiablation). Int Urol Nephrol 2017; 49:1947–1954 [DOI] [PubMed] [Google Scholar]
- 18.Kim CK, Park BK, Park W, Kim SS. Prostate MR imaging at 3T using a phased-arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdom Imaging 2010; 35:246–252 [DOI] [PubMed] [Google Scholar]
- 19.Dickinson L, Ahmed HU, Hindley RG, et al. Prostate-specific antigen vs. magnetic resonance imaging parameters for assessing oncological outcomes after high intensity-focused ultrasound focal therapy for localized prostate cancer. Urol Oncol 2017; 35:30.e9–30.e15 [DOI] [PubMed] [Google Scholar]
- 20.Muller BG, van den Bos W, Brausi M, et al. Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol 2015; 33:1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao B, Llukani E, Lepor H. Two-year outcomes following focal laser ablation of localized prostate cancer. Eur Urol Oncol 2018; 1:129–133 [DOI] [PubMed] [Google Scholar]


