Abstract
Peritoneal metastasis (PM) frequently occurs in patients with gastric cancer (GC) and confers a dismal prognosis despite advances in systemic chemotherapy. While systemic chemotherapy has poor peritoneal penetration, intraperitoneal (IP) chemotherapy remains sequestered, resulting in high peritoneal drug concentrations with less systemic side-effects. The first application of IP treatment was hyperthermic intraperitoneal chemotherapy (HIPEC) with cytoreductive surgery (CRS) for gastric cancer peritoneal metastasis (GCPM); but was associated with an increased morbidity and mortality rate without significantly improving overall survival (OS). While CRS confers limited benefit, the potential role of prophylactic HIPEC and laparoscopic neoadjuvant HIPEC are currently being evaluated. Combination systemic and IP chemotherapy (SIPC) gained popularity in the 1990s, since it provided the benefits of IP treatment while reducing surgical morbidity, demonstrating promising early results in multiple Phase II trials. Unfortunately, these findings were not confirmed in the recent PHOENIX-GC randomized controlled trial; therefore, the appropriate treatment for GCPM remains controversial. Small observational studies from Japan and Singapore have reported successful downstaging of PM in GC patients receiving SIPC who subsequently underwent conversion gastrectomy with a median OS of 21.6–34.6 months. Recently, the most significant development in IP-directed therapy is pressurized IP aerosol chemotherapy (PIPAC). Given that aerosol chemotherapy achieves a wider distribution and deeper penetration, the outcomes of multiple ongoing trials assessing its efficacy are eagerly awaited. Indeed, IP-directed therapy has evolved rapidly in the last 3 decades, with an encouraging trend toward improved outcomes in GCPM, and may offer some hope for an otherwise fatal disease.
Keywords: Gastric cancer, Peritoneal metastasis, Intraperitoneal chemotherapy, HIPEC, PIPAC
INTRODUCTION
Peritoneal metastases (PMs) are detected in up to 30% of patients with advanced gastric cancer (GC) and are associated with a dismal prognosis [1,2]. From the time of diagnosis, patients with PM have a median overall survival (OS) of 3–6 months without any treatment and 6–12 months with systemic chemotherapy [3,4,5,6,7]. The limited effect of systemic chemotherapy was explained by early pharmacokinetic studies by demonstrating that the peritoneal permeability of chemotherapy agents was significantly reduced relative to plasma clearance, leading to low peritoneal concentration [8]. Conversely, the delivery of appropriate chemotherapy agents into the peritoneum may potentially be sequestered without crossing into the plasma, thereby increasing the efficacy of the treatment and possibly curing patients with gastric cancer peritoneal metastases (GCPMs) [8]. Indeed, multiple forms of intraperitoneal (IP) chemotherapy for GC have been developed and are currently in use. This review article aims to summarize the role of various forms of IP chemotherapy (IPC) that are pertinent to clinical practice today.
Historically, the first form of IPC involved a combination of hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) for GCPM and was first described by Koga et al. in the late 1980s; however, it was accompanied by morbidity and mortality rates of up to 55% and 10%, respectively, with marginal improvement in survival [9]. This led to the development of a system in which chemotherapy solution is introduced into the peritoneal cavity using an implantable port/catheter system in combination with systemic chemotherapy, which has been termed combination systemic and IP chemotherapy (SIPC) or bi-directional chemotherapy. While SIPC is associated with reduced morbidity and allows the biological response of GC to be assessed in vivo, there have been mixed results with regards to the efficacy of this approach. Further advances have led to a novel treatment modality, pressurized intraperitoneal aerosol chemotherapy (PIPAC), which has potentially improved peritoneal delivery and penetration and is currently the subject of intense research.
HIPEC
HIPEC for patients with GCPM
Based on the literature, the administration of hyperthermic chemotherapy achieves deeper penetration in the peritoneal cavity, enhances the cytotoxic effects of chemotherapy, and was most commonly employed in the setting of CRS [10]. The aim of this approach was to remove the primary tumor and all visible peritoneal disease, while the heated chemotherapy in HIPEC would treat residual microscopic disease. In a recent meta-analysis of 11 randomized controlled trials (RCTs) and 21 high quality comparative non-RCTs, Desiderio et al. [9] reported a significant, albeit modest, improvement in median OS with the addition of HIPEC to CRS for GC (HIPEC+CRS vs. CRS, median OS 11.1 vs. 7.1 months, P<0.001). Multiple individual studies have reported that patients with low volume PM and complete cytoreduction are most likely to benefit from HIPEC+CRS, advocating that careful patient selection is key to identifying appropriate patients for such radical treatment [11,12,13]. However, this was not consistently demonstrated in the meta-analysis by Desiderio et al. [9], which reported no difference in outcome with the addition of HIPEC to CRS in patients with a low peritoneal carcinomatosis index (PCI) score and a modest improvement in patients with a high PCI score. Conversely, the addition of HIPEC has been shown to increase the risk of morbidity (relative risk [RR], 2.15; 95% confidence interval [CI], 1.29–3.58; P<0.01), respiratory failure (RR, 3.67; 95% CI, 2.02–6.67; P<0.001), and renal dysfunction (RR, 4.46; 95% CI, 1.42–13.99; P=0.01), with large retrospective studies reporting a morbidity rate of 39%–43% and a mortality rate of 4.8%–5.1% [9,14,15]. While advocates of CRS+HIPEC suggest that such a radical approach represents the only treatment that can cure patients with GCPM, the rate of major morbidity (Clavien-Dindo grade IV or above) from CRS+HIPEC may be as high as 19% in tertiary referral centers and does not appear to dramatically increase OS [16]. In addition, sub-group analysis by Desiderio et al. [9] demonstrated a greater overall survival for CRS+HIPEC than standard gastrectomy, but not for systemic chemotherapy.
Laparoscopic HIPEC
An emerging application of HIPEC is the administration of neoadjuvant laparoscopic-HIPEC (NA-LHIPEC), with the aim of downstaging PM. Both Yonemura et al. [17] and Newhook et al. [18] have separately published their early experience with NA-LHIPEC since 2017 and have confirmed that it is an extremely safe procedure. In addition, between 15%–25% of patients who received NA-LHIPEC had a complete disappearance of PM after receiving treatment [17,18]. The increased benefit of laparoscopic HIPEC may be due to higher IP pressure and greater drug penetration induced by pneumoperitoneum when compared to conventional open HIPEC [19]. The median OS of patients who underwent NA-LHIPEC and NA-LHIPEC followed by CRS was 14.1 and 14.4, respectively [17]. To date, no study has compared NA-LHIPEC with other treatment modalities.
Prophylactic HIPEC for GC patients at risk for PM
Patients with GC >T2, diffuse histological subtype, and lymphovascular invasion are at increased risk of PM; however, the benefit of prophylactic HIPEC after radical gastrectomy in these patients remains controversial [20]. The meta-analysis by Desiderio et al. [9] examined the addition of HIPEC to patients with cT3–4 disease and no evidence of PM and demonstrated a decrease in overall disease recurrence (RR, 0.73; 95% CI, 0.59–0.89; P=0.002), 3-year (RR, 0.71; 95% CI, 0.53–0.96; P=0.03) and 5-year (RR, 0.82; 95% CI, 0.70–0.96; P=0.01); however, no significant difference was found in 1-year survival. The lack of survival benefit at 1-year may be due to the fact that the included studies did not routinely evaluate cytological assessment of GC cells and differing neoadjuvant and adjuvant chemotherapy regimens [9]. Also, the meta-analysis by Desiderio et al. [9] did not consider the extent of nodal disease, which is not addressed by IPC and may account for lack of significant difference in 1-year survival. Hence, patients with low nodal disease burden may benefit from HIPEC, while those with heavy nodal disease burden have a higher mortality risk and may not exhibit any improvement in OS from the addition of HIPEC [21]. However, the addition of HIPEC was associated with a higher risk of complications (RR, 2.17; 95% CI, 1.49–3.14; P<0.01) such as renal dysfunction (RR, 2.23; 95% CI, 1.21–4.11; P=0.01) [9].
These findings were corroborated by the meta-analysis performed by Sun et al. [22] based on 10 RCTs that evaluated the addition of HIPEC to surgery for T4A GC, with no gross evidence of PM, and reported a significant risk reduction in mortality (RR, 0. 73; 95% CI, 0.64–0.83; P<0.001) and peritoneal recurrence (RR, 0.45; 95% CI, 0.28–0.72; P=0.001), with follow-up ranging from 30–96 months. A similar improvement in OS was seen in subgroup analysis when mitomycin C (RR, 0.75; 95% CI, 0.65–0.86; P<0.001) and 5-fluorouracil (RR, 0. 69; 95% CI, 0.52–0.90; P<0.001) were used during HIPEC [22]. Sun et al. [22] also reported a significant risk reduction in development of PM (RR = 0.45, 95% CI 0.28–0.72; P=0.001), but no increased rate of adverse events, such as bone marrow suppression, anastomotic leak, bowel fistula formation, and liver dysfunction when compared to the study by Desiderio et al. [9]. These findings were again replicated in the systematic review by Feingold et al. [23] based on 17 studies evaluating either hyperthermic or normothermic IPC, demonstrating a significant overall reduction in 5-year mortality rates when mitomycin C (odds ratio [OR], 0.56; 95% CI, 0.41–0.77; P<0.005), but not cisplatin (OR, 0.79; 95% CI, 0.60–1.04; P=0.09), was used for intraoperative IPC. The risks and benefits of adding HIPEC to radical gastrectomy in high-risk patients with no PM remain controversial, with significant heterogeneity in patient characteristics in published literature. The ongoing GASTRICHIP trial (NCT01882933) is currently recruiting patients with T3/4, irrespective of nodal or peritoneal cytology status, and randomizing patients to undergo either gastrectomy alone or with HIPEC, which may shed some light on the role of pre-emptive HIPEC in high-risk patients [24].
CATHER-BASED IPC
Combination SIPC
Given the radical nature, high morbidity rate, and limited OS benefit following CRS+HIPEC, IPC delivered via an implantable peritoneal port system grew in popularity in the 1990s [25]. The implantation of a peritoneal port was much less invasive compared to CRS+HIPEC, and allowed for repeated IP administration of chemotherapy [25,26]. Over a period of 12.9 months, Emoto et al. [27] reported that minor complications, such as infections or port blockage, occurred in 20.6% of patients, but did not result in termination of chemotherapy nor affect survival. The early experience with IPC involved administering cisplatin and mitomycin C, based on the HIPEC experience, given their thermal stability and synergistic effects with heat [28]. Further pharmacokinetic studies demonstrated that the area under curve ratios of peritoneal to plasma concentration of both drugs were not high, suggesting that both drugs were absorbed in systemic circulation without reaching meaningful concentrations in the peritoneum [25]. This led to the use of taxanes such as paclitaxel (PTX) and docetaxel (DTX), hydrophobic and high-molecular-weight compounds that remain sequestered in the abdomen with minimal systemic absorption [25]. As the effect of IPC with taxane-based drugs remains localized to the peritoneum, they are generally combined with systemic chemotherapy and referred to as bi-directional chemotherapy.
The first favorable result from IP taxanes was obtained for ovarian cancer. A landmark study by Armstrong et al. [29] demonstrated that systemic and IP taxanes were significantly better than systemic chemotherapy alone for stage 3 ovarian cancer in terms of OS. For GC, a number of phase II trials, largely performed in Asian centers, have reported the efficacy of this strategy. Japanese centers administered systemic chemotherapy (S-1: combination tegafur/gimeracil/teracil with or without systemic PTX) with either IP PTX or DTX and reported a median OS of 16.2–24.6 months and 1-year OS of 69%–78% [25,30,31,32,33,34,35]. As S-1 is not widely available, a phase II study at our unit (based in the National University Health System, Singapore) was conducted to examine the efficacy of XELOX (combination of capecitabine and oxaliplatin) and IP PTX, with interim results showing a median OS of 18.8 months and 1-year survival of 72% [30,36]. Unfortunately, these encouraging results were not confirmed by the phase III RCT (PHOENIX-GC) comparing S-1/systemic PTX/IPC PTX vs. S-1/systemic cisplatin, which reported no statistical advantage of the IP PTX group (IP vs. non-IP group, median OS 17.7 vs. 15.2 months, P=0.080) [37]. However, in the sensitivity analysis adjusted for baseline ascites, the hazard ratio was 0.59 (95% CI, 0.39–0.87; P=0.008). The 3-year OS rate was 21.9% for the IP arm and 6% for the non-IP arm. The disappointing result of the median OS may be due to an overestimation of survival benefit of IPC based on the phase II data, which had not controlled for the extent of PM or the survival impact of conversion gastrectomy [38]. The interim results of an ongoing phase II trial (CY-PHOENIX) studying the effect of IP PTX+S-1/PTX for cytology-positive GC patients with no macroscopic PM reported 1-year OS of 84.2% and may address some of the shortcomings of the PHOENIX-GC study [25,39].
Conversion gastrectomy after combined SIPC may offer some hope to patients with GCPM and is considered in the following circumstances: 1) no extra-peritoneal disease; 2) demonstration of negative peritoneal cytology; and 3) confirmation of no PM on diagnostic laparoscopy. Ishigami et al. [40] reported successful downstaging of PM (disappearance of macroscopic disease and conversion to negative cytology) in 64/100 patients receiving combination SIPC who underwent conversion gastrectomy with a median OS of 34.6 months (95% CI, 26.8–39.4 months) and 1-year OS of 73.3%. In our study by Chan et al. [36], 25% of patients who received combination SIPC had successful downstaging and underwent conversion gastrectomy, with a median OS of 21.6 months (95% CI, 8.7–29.9 months) and 1-year survival of 100%. While a head-to-head comparison between neoadjuvant combination SIPC with successful conversion gastrectomy and CRS+HIPEC has not yet been performed, the anecdotal evidence suggests superior OS in the SIPC group, possibly due to the selection of patients with GCPM who present favorable biology and respond well to conversion gastrectomy. While there are fewer published reports on the outcomes of combined SIPC and conversion gastrectomy compared to CRS+HIPEC, the relatively low morbidity rate of 6% reported by Ishigami et al. [40] makes this approach one of the most exciting developments in the treatment of GCPM.
Early postoperative intraperitoneal chemotherapy (EPIC) as adjuvant therapy
The application of catheter-based chemotherapy was extended to an adjuvant setting, with reports of EPIC in the early 2000s. However, there is an extreme paucity of data reporting the benefit of EPIC for the management of GCPM. A Swedish phase II study examining neoadjuvant chemotherapy followed by CRS+HIPEC+EPIC for patients with macroscopic PM reported a median survival of 10.2 months (95% CI, 6.9–13.7 months) and a morbidity rate of 62.5%; therefore, this study demonstrated that EPIC had a limited role when gross PM was present, except if R0 resection could be achieved [41]. However, the role of EPIC as adjuvant therapy for patients with GC with no gross PM is far more ambiguous. In 2006, Cheong et al. [42] published the experience of 154 patients with stage IV GC undergoing either curative or palliative resections who received EPIC, reporting median OS of 11.4 months. While the initial result appeared dismal, subgroup analysis demonstrated that patients with no PM were more likely to have R0 resection, more cycles of EPIC, and increased median OS of 25.5 months compared to the R1/2 group (P<0.001) [42]. While this observation is tenuous, there was a suggestion that carefully selected patients with R0 resection may have an improved median OS with the addition of EPIC.
In the early- mid-2000s, Yu et al. [43,44] published a phase III RCT examining the benefit of EPIC for advanced GC without distant metastases. Patients enrolled in the trial had a wide range of pathological stages, with almost 30% of both arms presenting stage IV disease secondary to PM [43]. The 5-year OS of the entire cohort of patients was superior in the gastric resection+EPIC group (EPIC vs. surgery alone, 54 vs. 38%, P=0.0278) [43]. When stratified by stage, improved 5-year OS was also seen in patients with stage III GC (i.e., no PM) (EPIC vs. surgery alone, 57% and 23%, P=0.002) [43]. Further analysis demonstrated superior 10-year survival, particularly in stage III GC patients (stage IIIA: EPIC vs. surgery, 52.5% vs. 39.6%, stage IIIB: EPIC vs. surgery, 53.5% vs. 11.0%, P=0.003) [44]. However, the improvement in median OS proved costly, given the overall morbidity rate of 29% in the EPIC group, with a significantly increased risk of bleeding (EPIC vs. surgery alone, 10% vs. 1%, P=0.002) and intra-abdominal sepsis arising from abscess-formation or peritonitis (EPIC vs. surgery alone, 14% vs. 4%, P=0.008) [43].
While no RCT has examined the benefit of HIPEC relative to EPIC for patients with advanced GC without PM, Feingold et al. [23] reported that intraoperative IPC was associated with significant improvement in 5-year survival (OR, 0.54; 95% CI, 0.82–1.00; P=0.004) compared to EIPC. A phase II Japanese trial by Takahashi et al. [45] studied patients with advanced GC and positive cytology, or minimal peritoneal deposits, undergoing curative resection and randomized them to receive either systemic or IPC, demonstrating that IPC was not superior to systemic therapy. Hence, the role of EPIC as an alternative for adjuvant systemic chemotherapy in advanced GC without PM remains uncertain.
PIPAC
PIPAC is the latest development in IPC for PM, with Solass et al. [46] first reporting histological evidence of tumor regression in patients with PM of gastric, appendiceal, and ovarian origin in 2014. There are a number of procedural benefits associated with the use of PIPAC for IP-directed chemotherapy, namely: 1) PIPAC achieves a more uniform distribution and deeper peritoneal penetration in gaseous state when compared to liquid chemotherapy; 2) a minimally invasive procedure that is generally safe and well tolerated; and 3) allows for laparoscopic assessment of the extent of PM and obtaining of tissue/ascitic samples. Hence, the chemotherapy agent in PIPAC may reduce the need for systemic chemothrepy, decrease the systemic side-effects of these cytotoxic drugs and can be repeated multiple times in selected patients who respond well to the treatment [47,48].
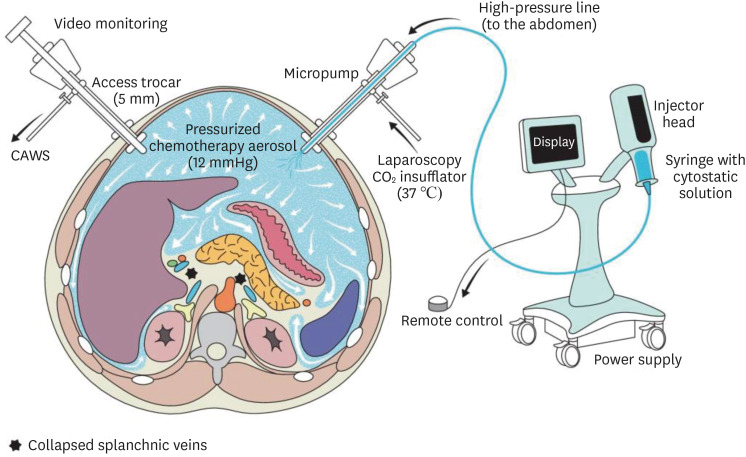
In current practice, most units use a 10–12 mm trocar for drug delivery and 5 mm optical trocar to administer PIPAC (Fig. 1). The procedure begins with creation of pneumoperitoneum with carbon dioxide at 12 mmHg, diagnostic laparoscopy and obtaining ascitic or tissue samples, and careful documentation of PCI score. Subsequently, chemotherapy drugs (either oxaliplatin alone or cisplatin and doxorubicin) are introduced into the peritoneal cavity as an aerosol with a high-pressure injector (maximum upstream pressure 290 PSI, flow rate 0.5–0.7 mL/s), with a proprietary disposable 9 mm microinjector (Capnopen®; Capnomed, Villingendorf, Germany). At this stage, capnoperitoneum is maintained at 12 mmHg with zero carbon dioxide flow for 30 minutes allowing nebulized chemotherapy agents to remain in the peritoneal cavity with no leakage. The remnant aerosol is then evacuated via a closed aerosol waste disposal system through 2 microparticle filters to protect medical and nursing staff from exposure to cytotoxic agents [48,49,50].
Fig. 1. Pressurized intraperitoneal aerosol chemotherapy (PIPAC). The abdominal cavity is accessed with 2 balloon trocars allowing hermetic seal. Liquid chemotherapy is dispersed as aerosol using a standard injector and a specific nebulizer (reproduced under the Creative Commons Attribution License, ©2017, Hübner et al. [49]).
CAWS = closed aerosol waste system.
While the ideal indications are difficult to define in the absence of large comparative studies, PIPAC may be considered in patients with PM who have failed standard chemotherapy and is administered alone or in combination with systemic chemotherapy [48]. Most centers recommend that no systemic therapy be administered 2 weeks before and 1 week after PIPAC, and there should be a 6-week interval before repeating PIPAC procedures in eligible patients [48]. Although generally safe, PIPAC is contraindicated in patients with a life-expectancy of <3 months, bowel obstruction, total dependence on parenteral nutrition, undergoing gastrointestinal resection in the same setting, prior anaphylaxis to chemotherapy agents, extra-peritoneal metastases, or poor performance status [48]. In the largest series to date, Alyami et al. [48] reviewed 1,810 PIPAC procedures in 838 patients and reported a mortality rate of 2.7% and adverse event rate (common terminology criteria for adverse events [CTCAE] >grade II) of 12%–15%, in which the most common included bowel obstruction (0%–5%), bleeding (0%–4%), and abdominal pain (0%–4%). Within this publication, the subgroup of patients with GCPM were reported to have primary, secondary non-access, and overall complication rates of 0%–11%, 8%–13% and 0%–11%, respectively [48]. Similarly, a systematic review by Grass et al. [51] reported ≥2 PIPAC procedures in 70%–71% of GC patients, with an overall complication rate of 12%. However, data on the efficacy of PIPAC for GCPM remains limited, with just 4 studies (2 retrospective studies and 2 phase II trials) comprising a total of 274 PIPAC procedures administered in 119 patients with GC, and with either PM or peritoneal recurrence, reporting median survival rates between 4.0–19.5 months [50,52,53,54,55]. In patients with GCPM undergoing PIPAC, major complication rates (CTCAE III–IV) and mortality rates ranged from 0%–29% and 0%–8.3%, respectively [50,52,53,54,55]. While these preliminary results are promising, the small sample size and considerable variation in inclusion criteria and chemotherapy regimens in these studies result in significant heterogeneity and make these early results difficult to generalize [56]. At the present moment, PIPAC should only be considered for GCPM in the setting of a clinical trial until its efficacy and associated morbidity is elucidated. There is currently no role for intraoperative PIPAC in patients with GC undergoing resection, with preliminary animal models demonstrating a high risk of anastomotic leak [57]. Multiple clinical trials carried out in Europe, Singapore, and the International PIPAC Registry are underway and may shed some light on the optimal role of PIPAC in GCPM [56]. We summarized the types of and indications for various IPC treatments for GC in Table 1.
Table 1. Types and indications of various modalities of IP chemotherapy for gastric cancer.
| Types of IP chemotherapy | Indication | Summary | |
|---|---|---|---|
| HIPEC | |||
| HIPEC+CRS | Curative treatment for patients with PM | Radical and invasive procedure with significant morbidity rate. May benefit patients who have limited PM (PCI ≤6) [9,14,15]. | |
| Laparoscopic HIPEC | Palliative treatment for patients with PM | Small number of studies report laparoscopic HIPEC to be relatively safe and having some efficacy in treating PM. Further studies are needed to verify the safety and efficacy of this modality [17,18,19]. | |
| Gastrectomy+HIPEC | Adjuvant treatment for patients at high risk of PM | Evidence suggests that the addition of HIPEC to gastrectomy reduces the risk of peritoneal recurrence, but has significant morbidity rate. Ongoing RCT (GASTRICHIP Trial, NCT01882933) aims to address these concerns [9,22,23,24]. | |
| Cather-based chemotherapy | |||
| SIPC | Palliative treatment for patients with PM | Safe procedure which allows for repeated dosing. A number of phase II studies have reported a median overall survival of 21–34 months following conversion gastrectomy in patients with complete resolution of PM. Unfortunately, these findings were not replicated in the only phase III trial to date comparing SIPC with systemic chemotherapy, possibly arising from issues with study methodology [36,37,38,40]. | |
| EPIC | Adjuvant treatment for patients at high risk of PM | Individual studies demonstrating improvement in overall survival, but with an increased risk of bleeding and intra-abdominal sepsis [41,42,43,44]. | |
| Emerging modalities | |||
| PIPAC | Palliative treatment for patients with PM | Safe procedure which allows for repeated dosing while preserving quality of life. Growing evidence suggests efficacy for PM, with multiple ongoing trials to verify these findings [52,53,54,55]. | |
IP = intraperitoneal; HIPEC = hyperthermic intraperitoneal chemotherapy; CRS = cytoreductive surgery; EPIC = early postoperative intraperitoneal chemotherapy; SIPC = systemic and intraperitoneal chemotherapy; PIPAC = pressurized intraperitoneal aerosol chemotherapy; PM = peritoneal metastasis; PCI = peritoneal carcinomatosis index.
IPC FOR RECURRENT PM AFTER PREVIOUS GASTRECTOMY
Currently all forms of treatment are considered palliative in patients with recurrent PM after previous gastrectomy, or with synchronous PM and extra-peritoneal metastases. The efficacy of IPC in improving OS, reducing symptomatic ascites, or malignant intestinal obstruction arising from PM has not yet been established. The potential benefit of IPC in these patients needs to be carefully weighed against the risk and morbidity in light of the poor prognosis and limited survival. With improvements in systemic chemotherapy regimens and the development of immunotherapy and targeted therapy, the median OS can be expected to improve in these patients. The interim results of an ongoing Singaporean phase II trial investigating the role of systemic XELOX and IP PTX examined a subgroup of 5 patients with peritoneal recurrence after gastrectomy and reported a median OS of 27.2 months and 1-year OS of 80% [36]. Larger studies are needed to verify these findings in the setting of an adequate clinical trial. As previously described, the application of PIPAC in these patients remains experimental, with a reported median OS of 4.0–19.5 months [50,51,52,53,54]. Further research is urgently required to identify patients who would benefit from these different modalities of IPC.
CONCLUSION
The role of IPC for patients with GCPM has evolved rapidly over the last 3 decades, with an encouraging trend toward improvement in OS and lower morbidity rates. While still considered experimental, peritoneal-directed treatment can offer some hope to patients with an otherwise fatal disease.
Footnotes
- Conceptualization: D.K.A.C, J.B.Y.S.
- Data curation: D.K.A.C, J.B.Y.S.
- Methodology: D.K.A.C, J.B.Y.S.
- Writing - original draft: D.K.A.C, J.B.Y.S.
- Writing - review & editing: D.K.A.C, J.B.Y.S.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier. Cancer Treat Res. 1996;82:53–63. doi: 10.1007/978-1-4613-1247-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Yonemura Y, Canbay E, Li Y, Coccolini F, Glehen O, Sugarbaker PH, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol. 2016;42:1123–1131. doi: 10.1016/j.ejso.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Goto M, et al. Randomized Phase III study of 5-fluorouracil continuous infusion vs. sequential methotrexate and 5-fluorouracil therapy in far advanced gastric cancer with peritoneal metastasis (JCOG0106) Jpn J Clin Oncol. 2013;43:972–980. doi: 10.1093/jjco/hyt114. [DOI] [PubMed] [Google Scholar]
- 6.Oh SY, Kwon HC, Lee S, Lee DM, Yoo HS, Kim SH, et al. A Phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37:930–935. doi: 10.1093/jjco/hym131. [DOI] [PubMed] [Google Scholar]
- 7.Yamao T, Shimada Y, Shirao K, Ohtsu A, Ikeda N, Hyodo I, et al. Phase II study of sequential methotrexate and 5-fluorouracil chemotherapy against peritoneally disseminated gastric cancer with malignant ascites: a report from the Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, JCOG 9603 Trial. Jpn J Clin Oncol. 2004;34:316–322. doi: 10.1093/jjco/hyh063. [DOI] [PubMed] [Google Scholar]
- 8.Dedrick RL, Myers CE, Bungay PM, DeVita VT., Jr Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 9.Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, et al. The 30-year experience-a meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1–14. doi: 10.1016/j.ejca.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27:365–374. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- 11.Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454–463. doi: 10.1016/j.gassur.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarema RR, Ohorchak MA, Zubarev GP, Mylyan YP, Oliynyk YY, Zubarev MG, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: results of a single-centre retrospective study. Int J Hyperthermia. 2014;30:159–165. doi: 10.3109/02656736.2014.893451. [DOI] [PubMed] [Google Scholar]
- 14.Yarema R, Mielko J, Fetsych T, Ohorchak M, Skorzewska M, Rawicz-Pruszyński K, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: a retrospective cooperative Central-Eastern European study. Cancer Med. 2019;8:2877–2885. doi: 10.1002/cam4.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine EA, Stewart JH, 4th, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635–644. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 17.Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol. 2017;24:478–485. doi: 10.1245/s10434-016-5487-6. [DOI] [PubMed] [Google Scholar]
- 18.Newhook TE, Agnes A, Blum M, Estrella JS, Das P, Ho L, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy is safe for patients with peritoneal metastases from gastric cancer and may lead to gastrectomy. Ann Surg Oncol. 2019;26:1394–1400. doi: 10.1245/s10434-018-07140-7. [DOI] [PubMed] [Google Scholar]
- 19.Thomas F, Ferron G, Gesson-Paute A, Hristova M, Lochon I, Chatelut E. Increased tissue diffusion of oxaliplatin during laparoscopically assisted versus open heated intraoperative intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:3623–3624. doi: 10.1245/s10434-008-0115-8. [DOI] [PubMed] [Google Scholar]
- 20.Pasqual EM, Bertozzi S, Londero AP, Brandolin D, Mariuzzi L, De Pellegrin A, et al. Microscopic peritoneal carcinomatosis in gastric cancer: prevalence, prognosis and predictive factors. Oncol Lett. 2018;15:710–716. doi: 10.3892/ol.2017.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeguchi M, Kondou A, Oka A, Tsujitani S, Maeta M, Kaibara N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg. 1995;161:581–586. [PubMed] [Google Scholar]
- 22.Sun J, Song Y, Wang Z, Gao P, Chen X, Xu Y, et al. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials. BMC Cancer. 2012;12:526. doi: 10.1186/1471-2407-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feingold PL, Kwong ML, Davis JL, Rudloff U. Adjuvant intraperitoneal chemotherapy for the treatment of gastric cancer at risk for peritoneal carcinomatosis: a systematic review. J Surg Oncol. 2017;115:192–201. doi: 10.1002/jso.24476. [DOI] [PubMed] [Google Scholar]
- 24.Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183. doi: 10.1186/1471-2407-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer. 2017;20:111–121. doi: 10.1007/s10120-016-0662-9. [DOI] [PubMed] [Google Scholar]
- 26.Kamei T, Kitayama J, Yamaguchi H, Soma D, Emoto S, Konno T, et al. Spatial distribution of intraperitoneally administrated paclitaxel nanoparticles solubilized with poly (2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) in peritoneal metastatic nodules. Cancer Sci. 2011;102:200–205. doi: 10.1111/j.1349-7006.2010.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emoto S, Ishigami H, Hidemura A, Yamaguchi H, Yamashita H, Kitayama J, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012;42:1013–1019. doi: 10.1093/jjco/hys129. [DOI] [PubMed] [Google Scholar]
- 28.Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7:29–44. doi: 10.3978/j.issn.2078-6891.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 30.Kono K, Yong WP, Okayama H, Shabbir A, Momma T, Ohki S, et al. Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: experience from Singapore and Japan. Gastric Cancer. 2017;20:122–127. doi: 10.1007/s10120-016-0660-y. [DOI] [PubMed] [Google Scholar]
- 31.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105:38–42. doi: 10.1002/jso.22057. [DOI] [PubMed] [Google Scholar]
- 33.Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32:4071–4075. [PubMed] [Google Scholar]
- 34.Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–3358. doi: 10.1002/cncr.28204. [DOI] [PubMed] [Google Scholar]
- 35.Fushida S, Kinoshita J, Kaji M, Hirono Y, Goda F, Yagi Y, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265–1272. doi: 10.1007/s00280-013-2122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan DY, Syn NL, Yap R, Phua JN, Soh TI, Chee CE, et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are we ready? J Gastrointest Surg. 2017;21:425–433. doi: 10.1007/s11605-016-3336-3. [DOI] [PubMed] [Google Scholar]
- 37.Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36:1922–1929. doi: 10.1200/JCO.2018.77.8613. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Xue K, Ying X, Ji J. PHOENIX-GC trial: underpowered for significant results? J Clin Oncol. 2019;37:167. doi: 10.1200/JCO.18.00364. [DOI] [PubMed] [Google Scholar]
- 39.Aizawa M, Ishigami H, Yabusaki H, Nashimoto A, Imamoto H, Imano M, et al. Phase II study of intraperitoneal paclitaxel plus S-1/paclitaxel for gastric cancer with positive peritoneal cytology: CY-PHOENIX trial. J Clin Oncol. 2017;35:96. [Google Scholar]
- 40.Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20:128–134. doi: 10.1007/s10120-016-0684-3. [DOI] [PubMed] [Google Scholar]
- 41.Hultman B, Lind P, Glimelius B, Sundbom M, Nygren P, Haglund U, et al. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2013;52:824–830. doi: 10.3109/0284186X.2012.702925. [DOI] [PubMed] [Google Scholar]
- 42.Cheong JH, Shen JY, Song CS, Hyung WJ, Shen JG, Choi SH, et al. Early postoperative intraperitoneal chemotherapy following cytoreductive surgery in patients with very advanced gastric cancer. Ann Surg Oncol. 2007;14:61–68. doi: 10.1245/s10434-006-9205-7. [DOI] [PubMed] [Google Scholar]
- 43.Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985–990. doi: 10.1007/s00268-001-0067-7. [DOI] [PubMed] [Google Scholar]
- 44.Yu W. A review of adjuvant therapy for resected primary gastric cancer with an update on Taegu's phase III trial with intraperitoneal chemotherapy. Eur J Surg Oncol. 2006;32:655–660. doi: 10.1016/j.ejso.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi N, Kanda M, Yoshikawa T, Takiguchi N, Fujitani K, Miyamoto K, et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: final results of the INPACT trial. Gastric Cancer. 2018;21:1014–1023. doi: 10.1007/s10120-018-0817-y. [DOI] [PubMed] [Google Scholar]
- 46.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–559. doi: 10.1245/s10434-013-3213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khosrawipour V, Khosrawipour T, Kern AJ, Osma A, Kabakci B, Diaz-Carballo D, et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol. 2016;142:2275–2280. doi: 10.1007/s00432-016-2234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–e377. doi: 10.1016/S1470-2045(19)30318-3. [DOI] [PubMed] [Google Scholar]
- 49.Hübner M, Teixeira Farinha H, Grass F, Wolfer A, Mathevet P, Hahnloser D, et al. Feasibility and safety of pressurized intraperitoneal aerosol chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract. 2017;2017:6852749. doi: 10.1155/2017/6852749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gockel I, Jansen-Winkeln B, Haase L, Rhode P, Mehdorn M, Niebisch S, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in gastric cancer patients with peritoneal metastasis (PM): results of a single-center experience and register study. J Gastric Cancer. 2018;18:379–391. doi: 10.5230/jgc.2018.18.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104:669–678. doi: 10.1002/bjs.10521. [DOI] [PubMed] [Google Scholar]
- 52.Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20:367–373. doi: 10.1007/s11605-015-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khomyakov V, Ryabov A, Ivanov A, Bolotina L, Utkina A, Volchenko N, et al. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2) Pleura Peritoneum. 2016;1:159–166. doi: 10.1515/pp-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alyami M, Bonnot PE, Villeneuve L, Bakrin N, Glehen O. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for nonresectable peritoneal carcinomatosis from gastric cancer. J Clin Oncol. 2018;36:149. [Google Scholar]
- 55.Struller F, Horvath P, Solass W, Weinreich FJ, Strumberg D, Kokkalis MK, et al. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol. 2019;11:1758835919846402. doi: 10.1177/1758835919846402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garg PK, Jara M, Alberto M, Rau B. The role of pressurized intraperitoneal aerosol chemotherapy in the management of gastric cancer: a systematic review. Pleura Peritoneum. 2019;4:20180127. doi: 10.1515/pp-2018-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tavernier C, Passot G, Vassal O, Allaouchiche B, Decullier E, Bakrin N, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) might increase the risk of anastomotic leakage compared to HIPEC: an experimental study. Surg Endosc. 2019 doi: 10.1007/s00464-019-07076-3. [DOI] [PubMed] [Google Scholar]