Abstract
Gastric cancer is a rare condition affecting patients with familial adenomatous polyposis (FAP). The mainstay of treatment is total gastrectomy. Since duodenal cancer is the most common cause of death after total colectomy in FAP, endoscopic surveillance for duodenal cancer is mandatory. Here, we describe the use of an isoperistaltic jejunal loop interposition technique to reconstruct the digestive tract after total gastrectomy in 2 patients with FAP. There were no early or late complications. Both patients are still alive and in good clinical condition. They did not experience weight loss or symptoms of dumping syndrome. Duodenal endoscopic surveillance after this technique was easier than after the classical Roux-en-Y reconstruction. Hence, regular follow-up was possible for both patients.
Keywords: Surgery, Endoscopy, Pathology
INTRODUCTION
Familial adenomatous polyposis (FAP) is characterized by the development of numerous adenomatous polyps in the gastrointestinal (GI) tract, especially the colon. Extra-colonic polyps are usually benign, but an endoscopic surveillance is required for pathologic confirmation [1]. The duodenum is the second most common site of polyps in FAP [2,3,4,5,6,7,8]. Duodenal cancer is the most common cause of mortality in patients with FAP who have undergone prophylactic total colectomy [3,9,10]. Therefore, in FAP patients, endoscopic surveillance of the duodenum is necessary for the early detection of malignant duodenal polyps.
Gastric cancer is a rare condition affecting FAP patients [11]. The mainstay of treatment for gastric cancer is total gastrectomy. Approximately 70 digestive tract reconstruction techniques after total gastrectomy have been described in the literature [12]. The classical Roux-en-Y reconstruction is the most widely used procedure, although it makes the endoscopic visualization of the duodenum difficult. Therefore, there is a need for reconstruction techniques that facilitate endoscopic surveillance of the duodenum
Herein, we report 2 cases of gastric cancer patients with FAP and describe the use of the jejunal loop interposition technique for digestive tract reconstruction after total gastrectomy in these patients.
CASE REPORT
Here, we report the case of 2 women with FAP syndrome who were followed at our outpatient clinic for Hereditary GI tumors.
The first patient A was diagnosed with FAP at the age of 18 years (the 3081delT mutation was detected), and she underwent total colectomy in 1981. Regular upper-GI endoscopy showed gastric hyperplastic polyposis, and at the age of 42 years in 2005, more than one polyp with gastric carcinoma in situ were retrieved.
The second patient B was diagnosed with FAP at the age of 22 years (the dupl. es.4-5 mutation was detected), and she underwent a proctocolectomy in 1979. Regular upper-GI endoscopy showed autoimmune atrophic gastritis and hyperplastic polyposis, and at the age of 61 years in 2018, 5 polyps with gastric carcinoma in situ were retrieved.
Both patients underwent total gastrectomy.The preoperative computed tomography (CT) did not show extra-gastric localization of the tumor. Intra-operative procedure, postoperative recovery, and follow-up were the same for both patients. They were given oral bowel preparation with 2 L of polyethylene glycol solution and a free-residue diet the day before surgery. After a standard total gastrectomy with D2 lymphadenectomy, we chose the jejunal loop interposition technique as the reconstructive procedure to allow easy endoscopic surveillance of the duodenum during follow-up.
The technical procedure was as follows:
1. The jejunum was cut at 2 different points with a linear stapler, at 40 cm and 90 cm from the Treitz's ligament, to obtain a jejunal loop of 50 cm in length.
2. The 50 cm jejunal loop, with preserved vascularization, was then positioned in the sovra-mesocolic region, through a hole in the transverse mesocolon.
3. End-to-side anastomosis was created between the abdominal esophagus and the jejunal loop, with the use of a circular stapler; the service access on the jejunal loop was then closed with a double layer suture with absorbable (polydioxanone [PDS]) 3/0 interrupted stitches.
4. The jejunal loop was connected to the duodenum through an end-to-end anastomosis with 3/0 PDS interrupted stitches that created an isoperistaltic conduit between the esophagus and the duodenum.
5. Digestive continuity below Treitz's ligament was restored with an end-to-end jejuno-jejunal anastomosis with interrupted absorbable 3/0 stitches.
6. Two drains were placed: one behind the esophago-jejunal anastomosis and the second near the duodenal-jejunal anastomosis.
We usually followed the same postoperative management procedure as that for total gastrectomy with Roux-en-Y reconstruction, which was as follows:
• The 1st postoperative day: blood tests and endovenous fluid intake.
• The 5th postoperative day: first leak-test with oral administration of a vial of methylene blue was performed; if no methylene blue appeared from the drains, clear oral fluids were administered.
• The 7th postoperative day: second leak-test with oral administration of soluble contrast medium was performed along with chest and abdominal X-rays; if negative, patients received solid oral food.
• The 8th postoperative day: patients had an interview with the dietitian to learn how and what to eat after discharge.
• The 9th postoperative day: removal of drains and discharge.
The follow-up schedule was as follows:
• Clinical examination and blood tests (including hemoglobin, iron, and vitamin B12) 30 days after surgery and subsequently, every 6 months.
• Esophagogastroduodenoscopy every year.
• Chest and abdominal CT scan every 6 months for the first 3 years.
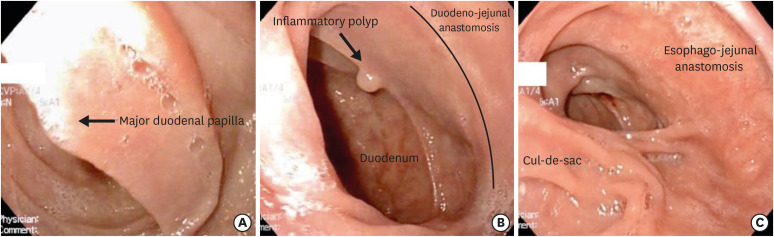
Operative time was 4 hours and 30 minutes and blood loss was less than 200 mL for both patients. We did not experience any intraoperative or postoperative complications. Leak-tests were negative in both patients (Fig. 1), and they received both fluids and a solid diet without difficulty. Pathological examination showed multiple carcinoma in situ without invasive carcinoma in both patients. Endoscopic follow-up was regular for both patients, and the duodenum was easily visualized through the interposed jejunal loop. Fig. 2 shows images from the gastroscopy of patient A, 1 year after the surgery: the duodenum, the interposed jejunal loop, the duodeno-jejunal anastomosis, and the esophago-jejunal anastomosis was clearly seen; an inflammatory polyp was retrieved at the level of the duodeno-jejunal anastomosis.
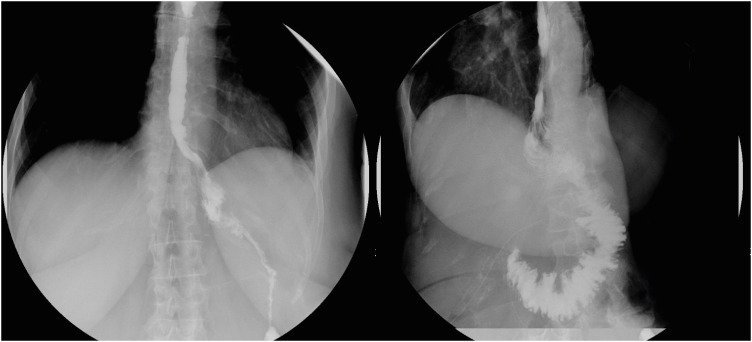
Fig. 1. X-ray imaging of the anastomosis of patient B (frontal and lateral view); the oral contrast medium passed regularly from the esophagus, through the jejunal loop (the esophago-jejunal anastomosis was visible in left side of the picture), and finally to the duodenum (the connective valves of the duodenum and the duodeno-jejunal anastomosis were visible on the right side of the figure). We observed no leakage of contrast medium which was a sign of tightness of both anastomosis.
Fig. 2. Endoscopic view of patient A, 1 year after surgery: (A) major duodenal papilla (B) duodeno-jejunal anastomosis (an inflammatory polyp was retrieved and resected near the anastomosis) (C) esophago-jejunal anastomosis (the cul-de-sac of jejunal loop in visible in the lower left).
Patient A and patient B were followed until December 2019 for a period of 14 years and 12 months, respectively. Both patients experienced a weight-loss of about 5 kg in the first month after surgery. Subsequently, the bodyweight was steady for both patients until the last follow-up (55 kg patient A and 64 kg patient B). Moreover, they did not experienced vomiting, symptoms of dumping syndrome, or anemia. Their hemoglobin level and levels of iron, folate, and vitamin B12 remained within normal limits during the follow-up period (13 g/dL for patient A and 12.3 g/dL for patient B at last follow-up).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all individual participants in the study.
DISCUSSION
Duodenal cancer is the most common cause of mortality in patients with FAP who have undergone prophylactic colectomy [3,9,10]. Therefore, the endoscopic surveillance of the duodenum is necessary for the early detection of duodenal adenomas and carcinomas. Presently, upper-GI endoscopy has been included in the surveillance of FAP patients starting at 25 years of age for staging duodenal polyposis, and the intervals are based on the Spigelman score which is determined by the number and grade of dysplasia of duodenal adenomas [13].
Gastric cancer is not a prominent extra-colonic manifestation of FAP in the Western world [11]. The types of gastric lesions associated with FAP include fundic gland polyps (FGPs), gastric foveolar-type gastric adenoma, gastric adenoma, and pyloric gland adenoma [14]. In addition, gastric cancer has been reported [15]. FGPs are present in more than 60% of FAP patients, although malignant progression is uncommon, and the lifetime risk of gastric cancer is reported to be in the range of 0.5%–1% [13].
Guidelines on gastric surveillance in FAP patients with diffuse FGPs have not yet been established. There is limited data in the literature that provides definitive conclusions, but previous reports suggest a suitable endoscopic surveillance procedure for long-term survival in patients with diffuse gastric FGPs in Western countries [13]. Upper-GI endoscopy for gastric polyps should be advised for individuals with FAP by the age of 25 years old or before colectomy, and endoscopy should be performed every 1–3 years [16]. Interestingly, a strong correlation between gastric adenomas and atrophic gastritis secondary to Helicobacter pylori infection was reported in Japanese FAP patients [17,18].
Out of 50–70 GI reconstruction procedures that have been described in the literature, the Roux-en-Y technique is the most commonly used. Several procedures have limitations in preserving the reservoir and digestive functions that leads to complications such as dietary restriction, early satiety, post-prandial fullness, vomiting, heartburn, and diarrhea [19,20,21].
Some studies suggested that a jejunal loop interposition could create a gastric reservoir that would allow food passage in the upper intestinal tract at a low speed and retain food storage volume, thus, improving the postoperative food capacity and nutritional status [22,23,24,25]. Jejunal loop interposition preserves the passage of food through the duodenum, and thus, stimulates the secretion of bile, pancreatic juice, and GI hormone production [26,27].
In this study, we describe the use of an isoperistaltic jejunal loop interposition technique to reconstruct the digestive tract after total gastrectomy in 2 patients with FAP. We chose this technique as it facilitates endoscopic surveillance of the duodenum after total gastrectomy. We experienced no early or late complications. The short and long-term outcomes were good. Both patients are still alive and in good clinical condition. Moreover, they did not experience symptoms of dumping syndrome or anemia. We observed minimal weight loss in the first few months after surgery, and subsequently, their bodyweight remained stable over time. Moreover, the duodenal endoscopic surveillance after the jejunal loop technique was easier than that after the classical Roux-en-Y reconstruction, and regular follow-up was possible in both patients.
A technical variant of the Roux-en-Y reconstruction that allows a simple endoscopic visualization of the duodenum is the double-tract reconstruction. By making an anastomosis between the duodenal stump and the jejunal limb, it is possible to visualize the duodenum without difficulty. Therefore, the follow-up of duodenal pathologies is possible. Otsuka et al. [28] described a series of 4 patients who underwent laparoscopic total gastrectomy with subsequent double-tract reconstruction and listed the benefits of this technique such as technical simplicity, preservation of food passage through the duodenum, lack of the duodenal stump which prevents risk of rupture, and possibility of simple endoscopic visualization of the duodenum. In our view, the jejunal loop interposition technique has the same advantages. Additionally, in our technique, food passes completely through the duodenum. However, in the double-tract reconstruction, food passes partially through the duodenum and partially through the jejunal limb, skipping the duodenum. We, therefore, believe that the jejunal loop technique leads to better nutritional results than the double-tract reconstruction. However, a randomized large-scale study would be necessary to verify this.
In conclusion, the jejunal loop interposition is a safe and effective reconstructive technique after total gastrectomy. It is associated with good short-term and long-term outcomes. Moreover, it allows easier endoscopic surveillance of the duodenum during follow-up in patients with FAP syndrome than the Roux-en-Y reconstruction. The greatest limitation of the study is the small number of patients; studies with a greater number, perhaps randomized, will lead to more comprehensive data.
Footnotes
- Conceptualization: U.E.D.L., Z.M.
- Data curation: Z.M., C.F.
- Formal analysis: Z.M., C.F.
- Investigation: Z.M., C.F.
- Writing - original draft: Z.M.
- Writing - review & editing: Z.M., U.E.D.L., C.F., P.S.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Srinivasa D, Wray CJ. Total gastrectomy with isoperistaltic jejunal interposition flap for symptomatic management of gastric polyposis from familial adenomatous polyposis. J Gastrointest Oncol. 2014;5:E18–E21. doi: 10.3978/j.issn.2078-6891.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1990;33:639–642. doi: 10.1007/BF02150736. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli F, Nardi F, Bechi P, Taddei G, Gozzo P, Romagnoli P. Extracolonic polyps in familial polyposis coli and Gardner's syndrome. Dis Colon Rectum. 1985;28:664–668. doi: 10.1007/BF02553447. [DOI] [PubMed] [Google Scholar]
- 5.Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306–314. doi: 10.1136/gut.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iida M, Yao T, Itoh H, Watanabe H, Matsui T, Iwashita A, et al. Natural history of duodenal lesions in Japanese patients with familial adenomatosis coli (Gardner's syndrome) Gastroenterology. 1989;96:1301–1306. doi: 10.1016/s0016-5085(89)80017-4. [DOI] [PubMed] [Google Scholar]
- 7.Domizio P, Talbot IC, Spigelman AD, Williams CB, Phillips RK. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–386. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–1062. doi: 10.1007/BF02047300. [DOI] [PubMed] [Google Scholar]
- 10.Bertario L, Presciuttini S, Sala P, Rossetti C, Pietroiusti M. Causes of death and postsurgical survival in familial adenomatous polyposis: results from the Italian Registry. Semin Surg Oncol. 1994;10:225–234. doi: 10.1002/ssu.2980100311. [DOI] [PubMed] [Google Scholar]
- 11.Walton SJ, Frayling IM, Clark SK, Latchford A. Gastric tumours in FAP. Fam Cancer. 2017;16:363–369. doi: 10.1007/s10689-017-9966-0. [DOI] [PubMed] [Google Scholar]
- 12.Fukuchi M, Mochiki E, Suzuki O, Ishiguro T, Sobajima J, Saito K, et al. Is gastric tube reconstruction the optimal surgical procedure for Siewert type II esophagogastric junction carcinoma? Anticancer Res. 2014;34:915–919. [PubMed] [Google Scholar]
- 13.Fornasarig M, Magris R, De Re V, Bidoli E, Canzonieri V, Maiero S, et al. Molecular and pathological features of gastric cancer in lynch syndrome and familial adenomatous polyposis. Int J Mol Sci. 2018;19:E1682. doi: 10.3390/ijms19061682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosens LA, Wood LD, Offerhaus GJ, Arnold CA, Lam-Himlin D, Giardiello FM, et al. Pathology and genetics of syndromic gastric polyps. Int J Surg Pathol. 2016;24:185–199. doi: 10.1177/1066896915620013. [DOI] [PubMed] [Google Scholar]
- 15.Mankaney G, Leone P, Cruise M, LaGuardia L, O'Malley M, Bhatt A, et al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer. 2017;16:371–376. doi: 10.1007/s10689-017-9971-3. [DOI] [PubMed] [Google Scholar]
- 16.Aihara H, Kumar N, Thompson CC. Diagnosis, surveillance, and treatment strategies for familial adenomatous polyposis: rationale and update. Eur J Gastroenterol Hepatol. 2014;26:255–262. doi: 10.1097/MEG.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Nonaka S, Nakajima T, Yachida T, Abe S, Sakamoto T, et al. Clinical outcomes of gastric polyps and neoplasms in patients with familial adenomatous polyposis. Endosc Int Open. 2017;5:E137–E145. doi: 10.1055/s-0042-119809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Iida M, Kobori Y, Mizuno M, Nakamura S, Hizawa K, et al. Serrated adenoma in familial adenomatous polyposis: relation to germline APC gene mutation. Gut. 2002;50:402–404. doi: 10.1136/gut.50.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs KH, Thiede A, Engemann R, Deltz E, Stremme O, Hamelmann H. Reconstruction of the food passage after total gastrectomy: randomized trial. World J Surg. 1995;19:698–705. doi: 10.1007/BF00295908. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz A, Büchler M, Usinger K, Rieger H, Glasbrenner B, Friess H, et al. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996;20:60–66. doi: 10.1007/s002689900011. [DOI] [PubMed] [Google Scholar]
- 21.Svedlund J, Sullivan M, Liedman B, Lundell L, Sjödin I. Quality of life after gastrectomy for gastric carcinoma: controlled study of reconstructive procedures. World J Surg. 1997;21:422–433. doi: 10.1007/pl00012265. [DOI] [PubMed] [Google Scholar]
- 22.Kono K, Iizuka H, Sekikawa T, Sugai H, Takahashi A, Fujii H, et al. Improved quality of life with jejunal pouch reconstruction after total gastrectomy. Am J Surg. 2003;185:150–154. doi: 10.1016/s0002-9610(02)01211-4. [DOI] [PubMed] [Google Scholar]
- 23.Lehnert T, Buhl K. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg. 2004;91:528–539. doi: 10.1002/bjs.4512. [DOI] [PubMed] [Google Scholar]
- 24.Liedman B, Bosaeus I, Hugosson I, Lundell L. Long-term beneficial effects of a gastric reservoir on weight control after total gastrectomy: a study of potential mechanisms. Br J Surg. 1998;85:542–547. doi: 10.1046/j.1365-2168.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 25.Svedlund J, Sullivan M, Liedman B, Lundell L. Long term consequences of gastrectomy for patient's quality of life: the impact of reconstructive techniques. Am J Gastroenterol. 1999;94:438–445. doi: 10.1111/j.1572-0241.1999.874_c.x. [DOI] [PubMed] [Google Scholar]
- 26.Ida S, Morita M, Hiyoshi Y, Ikeda K, Ando K, Kimura Y, et al. Surgical resection of hypopharynx and cervical esophageal cancer with a history of esophagectomy for thoracic esophageal cancer. Ann Surg Oncol. 2014;21:1175–1181. doi: 10.1245/s10434-013-3454-z. [DOI] [PubMed] [Google Scholar]
- 27.Kalmár K, Németh J, Kelemen D, Agoston E, Horváth OP. Postprandial gastrointestinal hormone production is different, depending on the type of reconstruction following total gastrectomy. Ann Surg. 2006;243:465–471. doi: 10.1097/01.sla.0000205740.12893.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otsuka R, Hayashi H, Hanari N, Gunji H, Hayano K, Kano M, et al. Laparoscopic double-tract reconstruction after total gastrectomy for postoperative duodenal surveillance: Case series. Ann Med Surg (Lond) 2017;21:105–108. doi: 10.1016/j.amsu.2017.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]