Abstract
Background and Aims:
Limited and inconsistent data are available on the relation between egg consumption and risk of myocardial infarction (MI) and it is unclear if adiposity or type 2 diabetes modifies egg-MI relation. We tested the primary hypothesis that egg consumption is positively associated with incidence of MI among veterans. In secondary analyses, we examined potential effect modification of egg-MI relation by adiposity and type 2 diabetes.
Methods:
We analyzed data collected on 188,267 US veterans who were enrolled in the Million Veteran Program (MVP) from 2011 to 2018. Information on egg consumption was obtained via self-administered food frequency questionnaire and we used electronic health records to identify incident MI.
Results:
The mean age was 64.4 (SD=12.0) years and 9.9% of the population were female. We ascertained 10,260 new cases of non-fatal MI during an average follow up of 3.24 years (range: 0.002 to 7.49 y). Hazard ratio (95% CI) for non-fatal MI were 1.00 (ref), 0.93 (0.85–0.1.02), 0.96 (0.87–1.05), 0.98 (0.89–1.07), 1.08 (0.98–1.19), 1.11 (1.00–1.24), and 1.13 (1.00–1.28) for egg consumption of <1/month, 1–3/month, 1/week, 2–4/week, 5–6/week, 1/d, and 2+/d, respectively, controlling for age, sex, race, body mass index, smoking, exercise, alcohol intake, and overall dietary pattern (p non-linear trend 0.019). In secondary analyses, we observed similar results with a composite endpoint including fatal MI, coronary angioplasty and revascularization.
Conclusions:
Our data showed no association of infrequent consumption of eggs with non-fatal MI but a slightly elevated risk with intake of 1 or more eggs per day among US veterans.
Keywords: Diet, coronary artery disease, epidemiology, risk factors
Introduction
Coronary artery disease (CAD) is still associated with a high burden in the United States, despite steady decline in CAD death rate over the past decades.1 Previous studies have reported beneficial effects of healthy dietary patterns on the incidence of CAD,2–6 stroke4–6 and cardiovascular risk factors including diabetes,7–9 hypertension,10,11 and dyslipidemia.12 Consequently, adherence to healthy dietary patterns can complement pharmacological and other interventions that are currently being used to reduce financial and societal burden of CAD. While there is mounting evidence in support of protective effects of certain components of a healthy dietary pattern such as fatty fish, whole grains, and fruit and vegetables on CAD risk, data on the effects of egg consumption on CAD risk have been limited and inconsistent.13,14 Eggs are rich in protein, minerals, vitamins, and omega-3 fatty acids with known health benefits15 and are available worldwide at reasonable costs. Prior concerns about egg consumption and rate of cardiovascular disease have been partly derived from the cholesterol content of egg (about 200 mg of cholesterol in an average egg16) and choline content that can be metabolized to trimethylamine N-oxide (known to adversely affect cardiovascular risk17–19). However, short-term experimental studies, intervention with eggs did not raise LDL-cholesterol and in some studies,20 has been associated with increase in HDL-cholesterol.21,22 Furthermore, data have been inconsistent on the effects of egg consumption on glucose metabolism, incidence of type 2 diabetes, and other cardiovascular risk factors.23,24 While some studies reported no association of egg consumption with CAD, endothelial function, or subclinical disease, other investigators have documented a higher risk of CAD with frequent egg consumption.25–27 The heterogeneity across results could be partially due to inadequate adjustment for overall dietary patterns and/or limited statistical power to allow for subgroup analyses. In particular, adiposity is a risk factor for CAD and diabetes and it is possible that egg consumption may accelerate progression of atherosclerosis and exacerbate CAD event rate in diabetic or overweight/obese people. Hence, it is important to determine whether diabetes or body mass index modifies the relation of egg consumption with CAD risk. The Million Veteran Program by virtue of its large sample size and thousands of CAD events is suitable to address some of the gaps outlined above. Hence, we sought to test the hypothesis that egg consumption is associated with a higher risk of non-fatal myocardial infarction (MI) among US veterans after adjustment for major confounding factors including overall diet quality using Dietary Approach to Stop Hypertension (DASH) score. In a secondary analysis, we examined whether the relation of egg consumption with non-fatal MI risk is modified by body mass index and diabetes.
Method
Population:
Million Veteran Program (MVP) is an ongoing prospective cohort study designed to study genetic determinants of chronic diseases. All participants signed informed consent and the Veterans Affairs Central Institutional Review Board approved the study protocol. A detailed description of MVP design and methods has been published.28 As of January 2019, 702,740 veterans have been enrolled. Among enrollees 325,790 participated in the MVP Lifestyle Survey and provided data on egg consumption, and 250,950 of those with egg consumption data had complete demographic and clinical visit data. Current analyses were restricted to 188,267 out of 250,950 participants who were free of CAD at baseline.
Assessment of egg consumption and overall diet quality:
We used the Willett semi-quantitative food frequency questionnaire to collect baseline information on egg consumption and other dietary factors. Reproducibility and validity of the Willett food frequency questionnaire have been previously reported29,30. Study participants were asked the following question: “For each food listed, please mark the column indicating how often, on average, you have used the amount specified during the past year”. Possible answers were: “Never or less than once per month”; “1–3 per month”; “once (1) a week”; “2–4 per week”; “5–6 per week”; “once (1) a day”; “2–3 per day”; “4–5 per day”; and “6+ per day”. Food groups listed included eggs (one egg), dairy foods, fruit and vegetables, meats, sweets, baked goods, cereals, and beverages. We collapsed the last three categories of egg consumption due to limited number of events (22 MI events for 4–5 eggs per day and 31 MI events for 6+/d). We constructed a modified DASH score based on the following 7 components: fruits, vegetables, nuts and legumes, low-fat dairy products, wholegrain, sweetened beverages, and red and processed meats31. Due to a lack of nutrient database in MVP at the time of these analyses, we were unable to include estimate of sodium intake in the DASH score. We created quintiles for each of the seven components and assigned quintile ranking for foods that are encouraged in DASH (all but sweetened beverages and meats)31. For meats and sweetened beverages, where low intake is desired in DASH, we assigned a lowest value for quintile 5 and highest value for quintile 1 (example: a veteran in the first quintile of sweetened beverage was assigned a value of 5). Finally, we summed up the component scores to obtain an overall modified DASH score ranging from 7 to 35.32
Outcome assessment:
We defined non-fatal MI using ICD9 Codes 410–411, 413–414 and ICD10 codes I20 – I25 (excluding I25.2). In a secondary outcome, we also included coronary deaths (ICD10 I20-I25) and coronary angioplasty or revascularization (CPT Codes 33510–33536, 9292x, 9293x, 9294x, 92973, 92974, and 92975; ICD9 Procedure codes 36.x and 00.66). The validity of using ICD codes for the diagnosis of cardiovascular disease among veterans has been previously published33.
Important covariates:
We collected information on age, sex, race, education, body mass index, alcohol consumption, exercise, and smoking at baseline through self-reported survey. Comorbidities were derived through Veterans Health Administration (VHA) electronic medical records system, Corporate Data Warehouse (CDW), using ICD 9 and ICD10 codes.
Statistical analysis:
We calculated person-time of follow up from food frequency assessment until the first occurrence of MI event, death34, or last visit recorded in the CDW. We computed the incidence rate of MI within each category of egg consumption and used Cox proportional hazard models to estimate hazard ratios with 95% confidence interval of MI. Proportional hazard assumptions were tested by examining Schoenfeld residuals. We assessed confounding by building sequential models based on a priori knowledge of confounders of egg-MI relation. After adjustment for age in the initial model, the fully adjusted model controlled for age, sex, race, education, body mass index, smoking (never, former, current smokers), exercise, alcohol intake, and DASH score. In secondary analyses, we stratified by body mass index (<25 vs. 25+ kg/m2) and prevalence of diabetes (yes vs. no) because adiposity and diabetes are established CAD risk factors and their effects could be potentiated by egg consumption. We also examined the shape of the egg-MI relation by using restricted cubic splines with knots placed at 2, 3, 4 servings/week (using LGTPHCURV9 macro)35. Finally, we repeated main analyses using competing risk model to test the robustness of the findings. In sensitivity analysis, we excluded MI events that occurred within 1 year of follow up. All analyses were performed on SAS Enterprise Guide 7.1 and alpha level at 0.05.
Results
We analyzed data on 188,267 veterans of whom 90.1% were men and mean age was 64.4 (SD=12.0) years. Median frequency of egg consumption was 3 eggs per week (25th and 75th percentile: 1 and 3 eggs/week, respectively). Frequent consumption of egg was associated with male gender, higher body mass index, lower DASH score, former smoking, and a higher prevalence of diabetes and hypertension (Table 1). During a mean follow up of 3.24 years (range: 0.002 to 7.49 years), 10,260 new cases of non-fatal MI occurred. Crude incidence rates of non-fatal MI were 16.3, 15.5, 15.9, 16.7, 19.0, 20.5, and 19.1 cases per 1,000 person-years for egg consumption of <1/month, 1–3/month, 1/week, 2–4/week, 5–6/week, 1/day, and ≥2/day, respectively. Corresponding hazard ratios (95% CI) were 1.00 (ref), 0.93 (0.85–1.02), 0.96 (0.87–1.05), 0.98 (0.89–1.07), 1.08 (0.98–1.19), 1.11 (1.00–1.24), and 1.13 (1.00–1.28), respectively, controlling for age, sex, race, education, body mass index, exercise, smoking, alcohol intake, and DASH score, Table 2). Using restricted cubic spline, we found suggestive evidence of a threshold relation between egg consumption and non-fatal MI incidence (p non-linear 0.019, Fig 1); while egg consumption of up to 4 per week was not associated with CAD risk, intake above 4 per week was associated with a higher risk of CAD in a dose-response manner, Fig 1). In a sensitivity analysis using competing risk model, multivariable adjusted hazard ratios (95% CI) were 1.00 (ref), 0.93 (0.85–1.03), 0.96 (0.88–1.05), 0.98 (0.90–1.07), 1.08 (0.97–1.19), 1.11 (0.99, 1.23), and 1.13 (1.00–1.28) from the lowest to the highest category of egg consumption. We observed similar results when fatal and non-fatal MI and coronary angioplasty and revascularization were used as composite outcome (Table 2).
Table 1.
Baseline characteristics of 188,267 participants in the Million Veteran Program by frequency of egg intake
| Frequency of egg consumption | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | <1/Month (n=10,911) | 1–3/Month (n=30,909) | 1/Week (n=42,848) | 2–4/Week (n=64,510) | 5–6/Week (n=19,856) | 1/day (n=12,319) | 2+/day (n=6,914) | ||
| Age (y) | 63.5 (12) | 63.6 (11.7) | 64.4 (12.1) | 64.9 (11.9) | 64.6 (11.9) | 64.0 (12.4) | 63.6 (12.3) | ||
| Body mass index (kg/m2) | 28.1 (5.5) | 28.8 (5.3) | 28.8 (5.2) | 29.3 (5.5) | 29.7 (5.8) | 29.6 (5.9) | 30.3 (6.3) | ||
| Male (%) | 86.2 | 87.7 | 90.1 | 91.2 | 91.5 | 90.3 | 92.9 | ||
| White (%) | 78.9 | 80.5 | 84.3 | 82.0 | 80.7 | 78.4 | 80.9 | ||
| Black (%) | 13.0 | 11.9 | 8.7 | 10.2 | 10.7 | 12.1 | 9.7 | ||
| < High School (%) | 2.3 | 2.5 | 2.0 | 2.2 | 2.5 | 3.1 | 3.3 | ||
| ≥ High School (%) | 97.7 | 97.5 | 98.0 | 97.8 | 97.5 | 96.9 | 96.7 | ||
| DASH score* | 20.8 (5.5) | 20.2 (5.0) | 21.2 (4.9) | 21.7 (4.9) | 21.6 (4.9) | 22.0 (5.1) | 21.5 (5.1) | ||
| Current smokers (%) | 17.1 | 19.0 | 17.1 | 17.2 | 18.3 | 18.1 | 18.5 | ||
| Former smokers (%) | 48.5 | 49.4 | 51.9 | 53.0 | 53.4 | 53.3 | 53.4 | ||
| Current drinkers (%) | 45.2 | 53.4 | 58.9 | 59.3 | 57.5 | 53.0 | 53.8 | ||
| Diabetes (%) | 21.0 | 22.7 | 23.3 | 25.7 | 28.2 | 29.6 | 31.8 | ||
| Hypertension (%) | 57.4 | 59.3 | 59.1 | 62.0 | 63.4 | 63.5 | 64.8 | ||
| Exercise (%) | |||||||||
| <1 time/week | 43.0 | 44.3 | 39.5 | 39.5 | 41.6 | 40.5 | 43.0 | ||
| 1 time/week | 11.5 | 12.9 | 14.7 | 14.3 | 14.0 | 13.7 | 12.0 | ||
| 2–4 times/week | 26.7 | 28.5 | 31.3 | 32.6 | 30.2 | 28.8 | 27.6 | ||
| 5+ times/week | 18.7 | 14.4 | 14.5 | 13.7 | 14.2 | 17.1 | 17.4 | ||
| Total cholesterol (mmol/L) | 4.5 (1.0) | 4.6 (1.0) | 4.5 (1.0) | 4.5 (1.0) | 4.5 (1.0) | 4.5 (1.0) | 4.5 (1.0) | ||
| LDL-cholesterol (mmol/L) | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.9) | ||
| HDL-cholesterol (mmol/L) | 1.3 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.4) | ||
| Triglycerides (mmol/L) | 1.5 (1.0) | 1.6 (1.1) | 1.6 (1.1) | 1.6 (1.1) | 1.6 (1.2) | 1.6 (1.3) | 1.7 (1.2) | ||
DASH: dietary approach to stop hypertension
Table 2.
Hazard ratios (95% CI) for primary (non-fatal MI) and secondary (fatal/non-fatal MI, PCI & CABG) outcomes by frequency of egg consumption
| Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Frequency of egg intake | Cases/Total | Crude incidence (/1000PY) | Crude | Model 1* | Model 2Ɨ |
| Primary outcome (non-fatal myocardial infarction) | |||||
| <1/Month | 599/10,911 | 16.3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1–3/Month | 1,600/30,909 | 15.5 | 0.95 (0.87, 1.05) | 0.95 (0.87, 1.04) | 0.93 (0.85, 1.02) |
| 1/Week | 2,249/42,848 | 15.9 | 0.98 (0.90, 1.07) | 0.96 (0.87, 1.05) | 0.96 (0.87, 1.05) |
| 2–4/Week | 3,487/64,510 | 16.7 | 1.03 (0.94, 1.12) | 0.99 (0.90, 1.08) | 0.98 (0.89, 1.07) |
| 5–6/Week | 1,165/19,856 | 19.0 | 1.17 (1.06, 1.29) | 1.13 (1.02, 1.24) | 1.08 (0.98, 1.19) |
| 1/day | 731/12,319 | 20.5 | 1.18 (1.06, 1.31) | 1.16 (1.04, 1.29) | 1.11 (1.00, 1.24) |
| 2+/day | 429/6,914 | 19.1 | 1.26 (1.12, 1.43) | 1.24 (1.10, 1.41) | 1.13 (1.00, 1.28) |
| p for non-linear | 0.18 | 0.008 | 0.019 | ||
| Secondary outcome (fata/non-fatal myocardial infarction, CABG, and PCI) | |||||
| <1/Month | 633/10,911 | 17.2 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1–3/Month | 1,701/30,909 | 16.5 | 0.96 (0.87, 1.05) | 0.96 (0.87, 1.05) | 0.94 (0.85, 1.02) |
| 1/Week | 2,378/42,848 | 16.8 | 0.98 (0.90, 1.07) | 0.95 (0.87, 1.04) | 0.96 (0.88, 1.05) |
| 2–4/Week | 3,708/64,510 | 17.8 | 1.03 (0.95, 1.12) | 0.99 (0.91, 1.08) | 0.98 (0.90, 1.07) |
| 5–6/Week | 1,236/19,856 | 20.2 | 1.17 (1.07, 1.29) | 1.13 (1.02, 1.24) | 1.08 (0.98, 1.19) |
| 1/day | 780/12,319 | 22.1 | 1.19 (1.07, 1.32) | 1.17 (1.05, 1.30) | 1.12 (1.01, 1.24) |
| 2+/day | 461/6,914 | 20.4 | 1.28 (1.14, 1.45) | 1.26 (1.12, 1.42) | 1.15 (1.02, 1.30) |
| p for non-linear | 0.17 | 0.005 | 0.012 | ||
Model 1: Adjusted for age; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; MI: myocardial infarction
Model 2: Adjusted for age, sex, race, education, body mass index, exercise, smoking, alcohol intake, and DASH score
Figure 1.
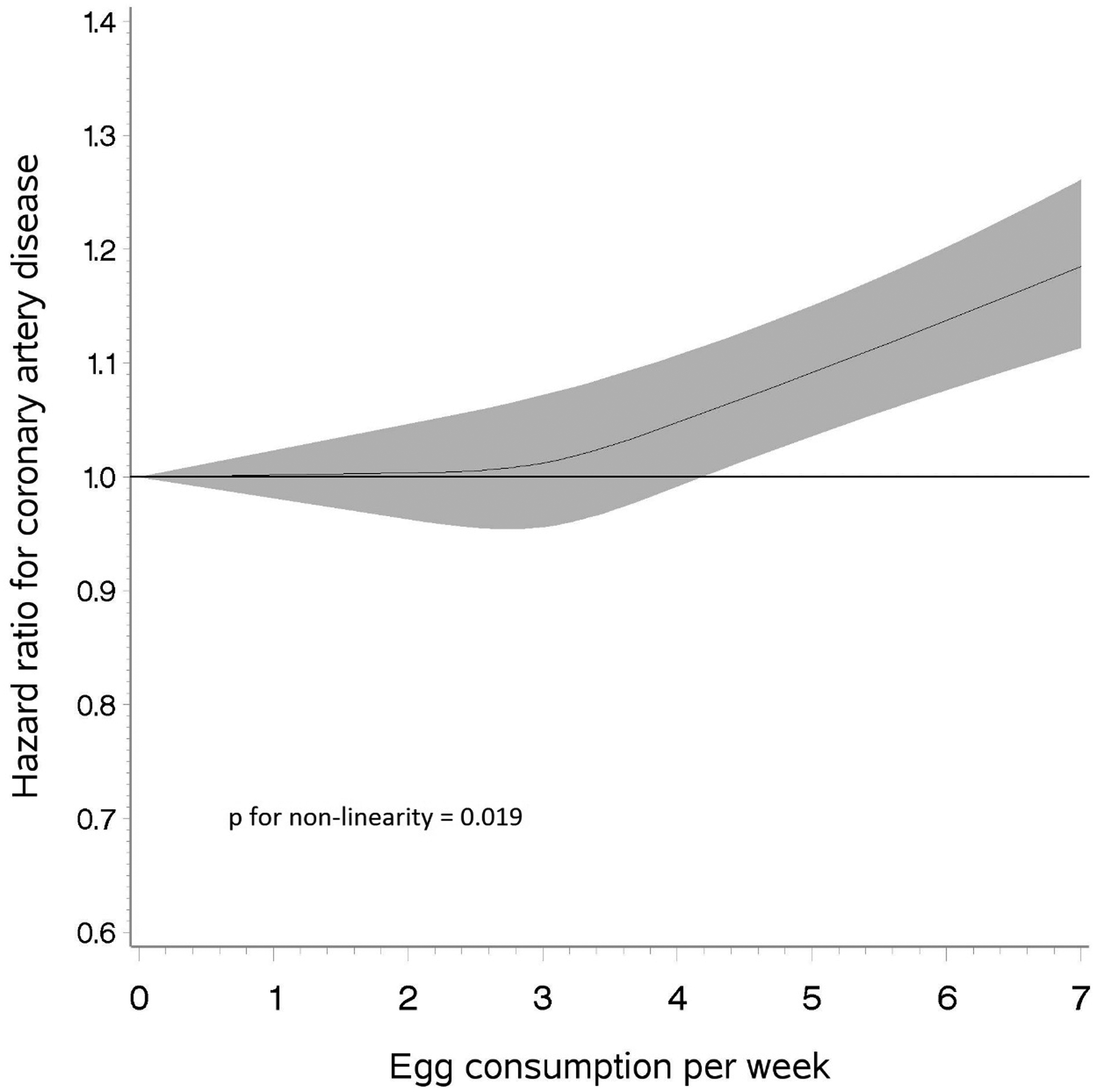
Restricted cubic spline depicting the relation of egg consumption with risk of non-fatal myocardial infarction among veterans
When stratified by body mass index and prevalent diabetes, there was no statistically significant interaction between egg consumption and body mass index (p interaction 0.53) or prevalent diabetes (p interaction 0.42) albeit slightly stronger results in overweight/obese and people with prevalent diabetes (Table 3). Additional adjustment for serum LDL-cholesterol and triglycerides yielded similar conclusion of non-linear relation of egg consumption with MI (p non-linear trend 0.017) and further adjustment for fried foods (p non-linear trend 0.016) or red meat (p non-linear trend 0.0058) did not alter the results. Lastly, to further assess residual confounding by dietary pattern, we repeated analyses among subjects with best dietary pattern (top 2 quintiles of DASH score) and those with poor dietary pattern (bottom 3 quintiles of DASH score) and found similar results in both groups (data not shown).
Table 3.
Hazard ratios (95% CI) for non-fatal MI by frequency of egg consumption stratified by body mass index and prevalent diabetes
| Frequency of egg intake | BMI <25 kg/m2 | BMI ≥25 kg/m2 | Diabetic | Non-Diabetic | |
|---|---|---|---|---|---|
| <1/Month | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| 1–3/Month | 0.85 (0.71, 1.03) | 0.95 (0.86, 1.06) | 0.94 (0.80, 1.10) | 0.92 (0.82, 1.03) | |
| 1/Week | 0.92 (0.77, 1.10) | 0.97 (0.88, 1.08) | 0.93 (0.79, 1.09) | 0.95 (0.85, 1.07) | |
| 2–4/Week | 0.88 (0.74, 1.05) | 1.00 (0.91, 1.11) | 0.95 (0.82, 1.11) | 0.96 (0.86, 1.07) | |
| 5–6/Week | 0.98 (0.79, 1.21) | 1.11 (0.99, 1.24) | 1.09 (0.93, 1.29) | 1.03 (0.91, 1.17) | |
| 1/day | 1.08 (0.87, 1.35) | 1.12 (0.99, 1.27) | 1.16 (0.97, 1.39) | 1.01 (0.89, 1.16) | |
| 2+/day | 1.05 (0.79, 1.40) | 1.16 (1.01–1.33) | 1.20 (0.98, 1.45) | 1.02 (0.86, 1.20) |
Adjusted for age, sex race, education, body mass index, exercise, smoking, alcohol intake, and dietary approach to stop hypertension score
BMI: body mass index; MI: myocardial infarction
Discussion
In this large prospective cohort of 188,267 US veterans, while egg consumption of up to 4 per week was not associated with the incidence of non-fatal MI, intake of 5 or more eggs per week was associated with about 10% elevated risk, especially among veterans with diabetes and those who were overweight or obese.
Our primary results are consistent with findings from a meta-analysis of 9 prospective studies which reported that for each additional increase in one egg per day, the multivariable adjusted relative risk was 0.99 (95% CI: 0.85 −1.15; P=0.88 for linear trend)14. In another meta-analysis of seven prospective studies, there was no association of egg consumption with incidence of CAD: pooled relative risk comparing high versus low egg consumption was 0.97 (95% CI: 0.88–1.07, p for heterogeneity = 0.67, I2 = 0.00) and no clear dose-response trend was apparent13. A prospective study of half a million Chinese reported a 12% lower risk of CAD (95% CI: 7% to 16%) comparing daily intake of 0.8 egg with none36 and that association was stronger in people with normal body mass index (p interaction BMI*egg = 0.05).36 Our findings were slightly stronger in subjects with body mass index ≥ 25 kg/m2 or prevalent diabetes and we observed no evidence for a statistically significant interaction between body mass index or diabetes and egg consumption on the risk of MI. Unfortunately, the China Kadoorie Biobank study36 did not data on egg intake of ≥1 eggs per day for comparison with our results.
In contrast, analyses of prospective data from 514 Western Australian Aborigines (256 women, 258 men) aged 15–88 years showed a high risk of fatal and non-fatal MI [HR: 2.59 (95% CI: 1.11– 6.04) when comparing >2 eggs/week with none in a model controlling for age, sex, total cholesterol, mean arterial pressure, and waist girth].37 A major limitation of this study is a lack of adjustment for overall dietary pattern as potential positive confounder. In fact, Burke et al.37 noted that egg consumption was associated with smoking, heavy drinking, adding salt to prepared foods, consumption of processed meats, and not trimming fat from meat; these factors could have led to an overestimation of the effect size associated with egg consumption and CAD. In a pooled analysis of six US prospective cohorts, consumption of each additional half egg was associated with an 8% higher risk of CAD (95% CI: 3% −14%) in a model adjusting for age, sex, race/ethnicity, and education; however, further control for dietary patterns attenuated the association [HR: 1.04 (95% CI: 0.99–1.10) in multivariable adjusted model plus alternate healthy eating index 2000; HR:1.05 (95% CI: 0.99–1.11) for multivariable model plus additional adjustment for dietary approach to stop hypertension; and HR: 1.05 (95% CI:0.99–1.10) for multivariable model with additional adjustment for alternate Mediterranean diet score.27 Taken together, these results underscore the importance of adequate adjustment for overall dietary patterns when evaluating the relation of eggs with MI and other outcomes as single foods are seldom consumed alone. Egg consumption appears to be clustered with intake of 1) other foods associated with a higher risk of MI (i.e., red meats, saturated fat, and salt intake38,39) and/or 2) behaviors known to adversely influence coronary disease risk including smoking.38–40 In our study, adjustment for red meat along with other food groups did not alter the conclusion. To further assess the possibility of residual confounding by unhealthy food items consumed with eggs, we repeated main analyses with subjects within the top 2 quintiles of DASH (most likely healthy eaters) versus bottom 3 quintiles and observed similar results in both groups.
Our secondary analyses showed a slightly elevated risk of non-fatal MI in people with diabetes or body mass index of 25+ kg/m2 consuming 5 or more eggs per week (despite no statistically significant interaction between diabetes/adiposity and egg intake). This is in contrast with findings from male physicians40 and from the NHANES41 which reported no association of egg consumption with incidence of MI and death from MI or stroke, respectively, among subjects with prevalent diabetes. In the PREDIMED study, egg consumption was not associated with cardiovascular disease among people with or without type 2 diabetes.42 In contrast, higher egg consumption was associated with a higher risk of MI in diabetic subjects in the Health Professionals Follow-up study [HR: 2.02 (95% CI: 1.05–3.87) comparing ≥ 1 egg/d with <1 egg/week] and in nurses with prevalent diabetes [HR:1.49 (95% CI: 0.88–2.52), p trend 0.008 comparing ≥1 egg/d vs. <1 egg/week).25 Additional studies are needed to further elucidate the role of frequent egg consumption on MI risk in people with diabetes or those who are overweight or obese.
Limitations of the current study include a single assessment of egg consumption which limits our ability to account for change in dietary habits over time as potential source of bias. Self-reported information on diet could have been inaccurate and biased the results towards no association if non-differential. Although most veterans are likely to receive their care at VA hospitals, we cannot exclude incomplete ascertainment of MI cases in current analyses. We did not have adequate number of fatal MI across categories of egg intake to examine the relation of egg intake with fatal MI separately. Lastly, we were not able to assess the influence of fried, boiled, or scrambled eggs on incidence of MI due to a lack of such information in MVP. Strengths of our study include a large sample size with adequate number of incident MI for sub-analyses; prospective design; adjustment for dietary patterns using DASH score; robustness of results in various sensitivity analyses; and the availability of data on several relevant confounders.
In conclusion, our data suggest no association of infrequent egg consumption with non-fatal MI but a slightly elevated risk of non-fatal MI with consumption of 5 or more eggs per week, especially among veterans with diabetes or overweight/obesity.
Acknowledgements
This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration. This publication does not represent the views of the Department of Veterans Affairs or the U.S. government. We are indebted to the MVP participants and staff. We thank Ms. Petra Schubert, MS, for her contribution in preparing data sets for current analyses.
Conflict of interest and sources of Funding: Dr. Djousse has received in the past an investigator-initiated grant from the American Egg Board. None of the co-authors has disclosures to declare.
This research was funded by the Department of Veterans Affairs Office of Research and Development, Million Veteran Program Grant # MVP001 and VA Merit Award I01 BX003340-01.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019:CIR0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation 2009;119:2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000;72:912–21. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XY, Shu L, Si CJ, et al. Dietary Patterns, Alcohol Consumption and Risk of Coronary Heart Disease in Adults: A Meta-Analysis. Nutrients 2015;7:6582–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 6.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition 2013;29:611–8. [DOI] [PubMed] [Google Scholar]
- 7.Schwingshackl L, Missbach B, Konig J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr 2014:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwingshackl L, Bogensberger B, Hoffmann G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J Acad Nutr Diet 2018;118:74–100 e11. [DOI] [PubMed] [Google Scholar]
- 9.Jannasch F, Kroger J, Schulze MB. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J Nutr 2017;147:1174–82. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Kim J, Kim J. Gender Differences in the Association between Dietary Pattern and the Incidence of Hypertension in Middle-Aged and Older Adults. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. NEnglJMed 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 13.Alexander DD, Miller PE, Vargas AJ, Weed DL, Cohen SS. Meta-analysis of Egg Consumption and Risk of Coronary Heart Disease and Stroke. J Am Coll Nutr 2016;35:704–16. [DOI] [PubMed] [Google Scholar]
- 14.Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda JM, Anton X, Redondo-Valbuena C, et al. Egg and egg-derived foods: effects on human health and use as functional foods. Nutrients 2015;7:706–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song WO, Kerver JM. Nutritional contribution of eggs to American diets. J Am CollNutr 2000;19:556S–62S. [DOI] [PubMed] [Google Scholar]
- 17.Qi J, You T, Li J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanitsoraphan C, Rattanawong P, Charoensri S, Senthong V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr Nutr Rep 2018;7:207–13. [DOI] [PubMed] [Google Scholar]
- 19.Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–56. [DOI] [PubMed] [Google Scholar]
- 20.Njike V, Faridi Z, Dutta S, Gonzalez-Simon AL, Katz DL. Daily egg consumption in hyperlipidemic adults--effects on endothelial function and cardiovascular risk. Nutr J 2010;9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013;62:400–10. [DOI] [PubMed] [Google Scholar]
- 22.Blesso CN, Andersen CJ, Barona J, Volk B, Volek JS, Fernandez ML. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. J Clin Lipidol 2013;7:463–71. [DOI] [PubMed] [Google Scholar]
- 23.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. AmJClinNutr 2013;98:146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: A meta-analysis. Atherosclerosis 2013;229:524–30. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Rimm EB, et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA 1999;281:1387–94. [DOI] [PubMed] [Google Scholar]
- 26.Katz DL, Evans MA, Nawaz H, et al. Egg consumption and endothelial function: a randomized controlled crossover trial. IntJ Cardiol 2005;99:65–70. [DOI] [PubMed] [Google Scholar]
- 27.Zhong VW, Van Horn L, Cornelis MC, et al. Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA 2019;321:1081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214–23. [DOI] [PubMed] [Google Scholar]
- 29.Stein AD, Shea S, Basch CE, Contento IR, Zybert P. Consistency of the Willett semiquantitative food frequency questionnaire and 24-hour dietary recalls in estimating nutrient intakes of preschool children. Am J Epidemiol 1992;135:667–77. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 31.Bertoia ML, Triche EW, Michaud DS, et al. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. AmJClinNutr 2014;99:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djousse L, Ho YL, Nguyen XT, et al. DASH Score and Subsequent Risk of Coronary Artery Disease: The Findings From Million Veteran Program. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf 2016;25:467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Center of Excellence for Suicide Prevention. Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository – National Death Index (NDI). http://vaww.virec.research.va.gov/Mortality/Overview.htm; Extract 2019 Mar 14.
- 35.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 36.Qin C, Lv J, Guo Y, et al. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke V, Zhao Y, Lee AH, et al. Health-related behaviours as predictors of mortality and morbidity in Australian Aborigines. PrevMed 2007;44:135–42. [DOI] [PubMed] [Google Scholar]
- 38.Djousse L, Gaziano JM, Buring JE, Lee IM. Egg Consumption and Risk of Type 2 Diabetes in Men and Women. Diabetes Care 2009;32:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djousse L, Kamineni A, Nelson TL, et al. Egg consumption and risk of type 2 diabetes in older adults. Am J Clin Nutr 2010;92:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djousse L, Gaziano JM. Egg consumption in relation to cardiovascular disease and mortality: The Physicians’ Health Study. Am J Clin Nutr 2008;87:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scrafford CG, Tran NL, Barraj LM, Mink PJ. Egg consumption and CHD and stroke mortality: a prospective study of US adults. Public Health Nutr 2011;14:261–70. [DOI] [PubMed] [Google Scholar]
- 42.Diez-Espino J, Basterra-Gortari FJ, Salas-Salvado J, et al. Egg consumption and cardiovascular disease according to diabetic status: The PREDIMED study. Clin Nutr 2017;36:1015–21. [DOI] [PubMed] [Google Scholar]


